Summary
Romiplostim can improve platelet counts in about 50% of patients with low‐ or intermediate 1‐risk (lower risk) myelodysplastic syndromes (MDS) and thrombocytopenia, but its long‐term toxicity and efficacy are not known. This open‐label extension study evaluated the long‐term safety and efficacy of romiplostim in 60 patients with lower risk MDS and platelet counts ≤50 × 109/l. The primary endpoint was adverse event (AE) incidence. Secondary endpoints were efficacy parameters, including bleeding events and platelet response. Median (range) treatment time in the extension study and the median observation times thereafter were 25 (2–181) and 57 (11–209) weeks, respectively. Treatment‐related AEs and serious AEs were reported in 14/60 (23%) and 4/60 (7%) patients, respectively. Progression to acute myeloid leukaemia (AML) occurred in two patients after 44 and 46 weeks. Patients (n = 34, 57%) with a platelet response were further evaluated for length of response. Median (range) response duration was 33 (7–174) weeks; 28/34 (82%) patients had a continuous response. Five of 34 patients (15%) had grade ≥3 bleeding events; three when the platelet count was >50 × 109/l. There were no new safety concerns and the rate of progression to AML was low; response to romiplostim was maintained for most patients.
Keywords: romiplostim, myelodysplastic syndromes, platelets, thrombocytopenia, thrombopoietin receptor agonist
Myelodysplastic syndromes (MDS) are haematological malignancies characterised by ineffective clonal haematopoiesis, cytopenia and, for some patients, progression to acute myeloid leukaemia (AML) (Vardiman et al, 2009; Neukirchen et al, 2011). Thrombocytopenia is found in 40–65% of MDS patients and is associated with reduced survival and increased bleeding risk (Kantarjian et al, 2007, 2008). The incidence of thrombocytopenia in patients with MDS increases with increasing International Prognostic Scoring System (IPSS) risk group (Kantarjian et al, 2007). Patients with MDS who have thrombocytopenia are at risk for a range of bleeding events, from minor bleeding that does not affect survival but does impact quality of life (i.e., petechiae, gingival bleeding and retinal haemorrhages), to more serious and potentially life‐threatening bleeding, such as intracranial or pulmonary haemorrhage (Hofmann & Koeffler, 2005).
Therapeutic options to treat thrombocytopenia in MDS patients with lower risk of death or progression to AML are limited (National Comprehensive Cancer Network, 2015). Most patients do not have serious enough disease to justify allogeneic haematopoietic cell transplantation (National Comprehensive Cancer Network, 2015). Other treatments for thrombocytopenia in MDS include anabolic steroids, immunosuppressive therapy and hypomethylating agents (National Comprehensive Cancer Network, 2015; Gangat et al, 2016), although these have limited efficacy. Platelet transfusions therefore remain the standard approach to thrombocytopenia associated with MDS (National Comprehensive Cancer Network, 2015), and 6–33% of patients with MDS are platelet transfusion‐dependent (Sekeres et al, 2008).
Romiplostim (Nplate®; Amgen Inc., Thousand Oaks, CA) is a recombinant peptibody that targets the thrombopoietin (TPO) receptor to increase platelet production and is approved for use in adults with chronic immune thrombocytopenia (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000942/WC500039537.pdf; https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125268s155s156lbl.pdf). Treatment with romiplostim improves platelet counts in 40–50% of lower‐risk MDS patients, decreases bleeding, and reduces the need for platelet transfusions (Kantarjian et al, 2010; Giagounidis et al, 2014), but its long‐term safety (in terms of increased risk of progression to AML, bone marrow fibrosis, and thromboembolic events) and long‐term efficacy are not known.
In this open‐label extension study, we examined the long‐term safety and efficacy of romiplostim for the treatment of thrombocytopenia in patients with MDS who completed parent studies in which they received monotherapy with romiplostim or placebo. This trial was prematurely discontinued because of a suspected safety concern for AML progression, and because transient increases in blast cell counts could lead to mistaken diagnosis and treatment for AML; therefore, the rates of progression to AML and increases in blast cells were of particular interest.
Methods
Patients
Patients included in the present analysis had completed one of two parent trials that evaluated romiplostim monotherapy in a romiplostim versus placebo (Giagounidis et al, 2014) or open‐label romiplostim study (Kantarjian et al, 2010; Sekeres et al, 2011) for thrombocytopenia in adults with IPSS low‐ or intermediate 1‐risk MDS at diagnosis (Fig 1; methods and outcomes of these trials have been published (Giagounidis et al, 2014; Kantarjian et al, 2010; Sekeres et al, 2011). Patients were eligible to enrol in the extension trial provided they met the eligibility criteria, which included a platelet count ≤50 × 109/l for at least 4 weeks since the last dose of romiplostim in the parent study and Eastern Cooperative Oncology Group performance status of 0–2. Key exclusion criteria were marrow blast count ≥10%, history of progression to AML, haematopoietic stem cell transplantation or thrombosis. The study was approved by the independent ethics committee or institutional review board for each site; all patients provided written informed consent.
Figure 1.
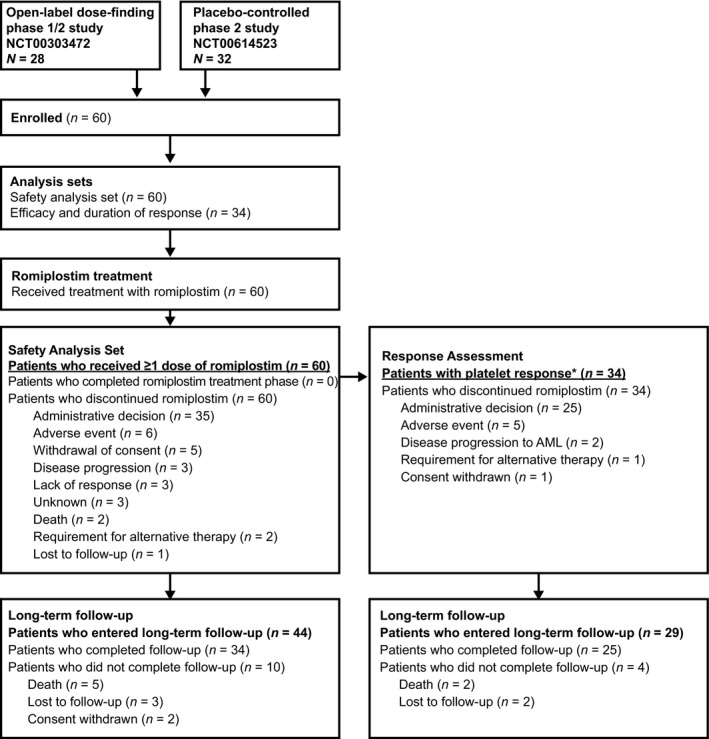
Study design and treatment schema. Patients were enrolled from one of two parent studies: (i) a 52‐week, phase 1/2 study of once‐weekly romiplostim 300–1500 μg (Kantarjian et al, 2010; Sekeres et al, 2011); (ii) a 58‐week, randomised, phase 2, placebo‐controlled study of once‐weekly romiplostim 750 μg.(Giagounidis et al, 2014) *Response per IWG 2006 criteria (Cheson et al, 2006) (Table 2). AML, acute myeloid leukaemia.
Study design and treatment
This open‐label extension study was conducted at 14 centres in the United States, Europe, Canada and Australia (first patient enrolled on July 17, 2007). Eligible patients received subcutaneous romiplostim at the same dose as in their parent study or were transitioned to the romiplostim regimen closest to the parent study: i.e., weekly or biweekly 250, 500, 750, 1000 or 1500 μg). Dosing was adjusted if a platelet response was not achieved or if platelet counts were >450 × 109/l (Table SI) (Cheson et al, 2006). Treatment continued until either transformation to AML, toxicity, withdrawal of consent, administrative decision or no platelet response after 4 weeks at the maximum romiplostim dose of 1000 μg (patients with a previous dose of 1500 μg in their parent study were permitted to continue to receive this dose amount); no additional MDS therapy was permitted. Concomitant medications or treatments necessary to provide adequate supportive care, including platelet transfusions, were permitted. Romiplostim administration in the extension study was ended prematurely on 25 May 2011, because of suspected concerns from the Data Monitoring Committee (DMC) about the potential risk of disease progression to AML, and that transient increases in blast cell counts might put patients at risk for the mistaken diagnosis of and treatment for AML. Patients remained in the study for long‐term follow‐up observation until 31 December 2011.
Assessments
The primary endpoint was the incidence of adverse events (AEs) http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf; Cheson et al, 2006; Vardiman et al, 2002). Key secondary endpoints included incidence of bleeding events, platelet transfusions and duration of platelet response, based on International Working Group (IWG) 2006 criteria (Cheson et al, 2006) (Table SII). Response duration was independently evaluated by two clinical haematology experts. Responses were considered sustained if platelet counts remained ≥50 × 109/l (IWG criteria are established for patients with platelets <50 × 109/l) or if platelet counts were only transiently decreased by >50% or to <50 × 109/l because of missed doses (i.e., not considered relapses per IWG 2006 criteria). Response duration was examined until patients discontinued romiplostim for any reason.
In this extension study, adverse events, bleeding events, platelet counts and concomitant medications were assessed at screening, weekly throughout romiplostim treatment, and at the end‐of‐study visit (all patients completed an end‐of‐study visit 4 weeks after their last dose of romiplostim). Antibodies to TPO and romiplostim were assessed at 12‐week intervals. Bone marrow biopsies and aspirates were analysed by a central laboratory at screening and at end of study. Following the end‐of‐study visit, patients were monitored by telephone in long‐term follow‐up every 24 weeks until 31 December 2011 to assess disease status and survival.
Statistical analysis
Patients receiving ≥1 dose of romiplostim in the extension study were included in the safety analysis set. For the analysis of the response duration, we included only the 34 patients who achieved a response per IWG 2006 criteria (Cheson et al, 2006). Safety data, demographics and baseline characteristics were summarised using descriptive statistics for all patients (means, medians, and interquartile ranges).
Results
Patients
In total, 60 patients from the two parent monotherapy trials entered the open‐label extension study (Fig 1). This population included 50 patients who previously received romiplostim for a median (range) duration of 52 (7–74) weeks and 10 patients who previously received placebo. The median (range) study duration for the 60 patients in their parent studies was 56 (8–75) weeks. Because of varying times of enrolment in the extension study and the administrative decision on 25 May 2011 to end the treatment, time spent in the treatment phase of the open‐label extension study varied widely among patients, from 2 to 181 weeks.
Baseline characteristics of patients at the time of entering the parent studies did not differ between those patients who did and those who did not enter the extension study (data not shown). Demographics and disease characteristics at baseline of the extension study are listed in Table 1. The median (range) age was 71 (32–84) years. The mean (standard deviation, SD) time from MDS diagnosis to entering the open‐label extension study was 172 (192) weeks (median 104 weeks). Median interquartile range (Q1, Q3) baseline platelet count was 22 (14, 35) × 109/l. The median (range) total observation time in the open‐label study treatment phase and long‐term survival follow‐up phase was 57 (11–209) weeks. The treatment phase (romiplostim exposure) in this extension study lasted a median (range) of 25 (2–181) weeks, number of doses was 22 (2–178), and the average weekly dose was 760 (144–1488) μg. The combined median romiplostim treatment duration was approximately 1·5 years and for up to 5 years.
Table 1.
Baseline demographics and disease characteristicsa
| Romiplostim (N = 60) | |
|---|---|
| Male, n (%) | 31 (51·7) |
| Age, years, median (range) | 71 (32–84) |
| Age ≥65 years, n (%) | 46 (76·7) |
| Race, n (%) | |
| White | 52 (86·7) |
| Black | 3 (5·0) |
| Other | 5 (8·3) |
| IPSS status,b n (%) | |
| Low | 19 (31·7) |
| Intermediate‐1 | 39 (65·0) |
| Intermediate‐2 | 0 |
| Unknown | 2 (3·3) |
| WHO classification at screening, n (%) | |
| RCMD | 20 (33·3) |
| RA | 17 (28·3) |
| RAEB‐1 | 4 (6·7) |
| RAEB‐2 | 1 (1·7) |
| RARS | 1 (1·7) |
| RCMD‐RS | 3 (5·0) |
| MDS‐U | 14 (23·3) |
| Baseline platelet count, n (%) | |
| <20 × 109/l | 26 (43·3) |
| ≥20 × 109/l | 34 (56·7) |
| ECOG performance status, n (%) | |
| 0 | 38 (63·3) |
| 1 | 17 (28·3) |
| 2 | 5 (8·3) |
ECOG, Eastern Cooperative Oncology Group; IPSS, International Prognostic Scoring System; MDS‐U, myelodysplastic syndrome unclassified; RA, refractory anaemia; RAEB, refractory anaemia with excessive blasts; RARS, refractory anaemia with ringed sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD‐RS, refractory cytopenia with multilineage dysplasia with ringed sideroblasts; WHO, World Health Organisation.
Baseline at screening for the extension study.
At parent study baseline.
All 60 patients discontinued romiplostim in the extension study. The majority (n = 35, 58%) of patients discontinued romiplostim treatment because of the administrative decision of the DMC due to the suspected safety concern of progression to AML; other reasons for discontinuation are listed in Fig 1. After the end‐of‐study visit, 44 patients entered long‐term follow‐up, of whom 34 (77%) were followed until the final study date (31 December 2011). Reasons for discontinuing the long‐term follow‐up phase before this date were death (n = 5), loss to follow‐up (n = 3) and withdrawal of consent (n = 2).
Adverse events and serious adverse events
The most commonly reported AEs (those occurring after initiation of romiplostim in the extension but not necessarily related to treatment) were epistaxis, cough, arthralgia, haematoma and contusion (Table 2).(http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf) Fourteen patients (23%) experienced AEs considered treatment‐related by the investigator. AEs that led to study withdrawal occurred in six patients (10%) and included B‐cell lymphoma and pancytopenia (not considered related to investigational product), and chronic myeloid leukaemia (BCR‐ABL1 confirmed by fluorescence in situ hybridisation), pulmonary fibrosis, splenomegaly and transient increase of blast cells in the bone marrow (considered by investigators to be related to investigational product). Serious AEs, regardless of their relationship to treatment, occurred in 22 patients (37%), the most common of which were pneumonia (reported in three patients), followed by arthralgia and urinary tract infection (reported in two patients each). Treatment‐related serious AEs, reported in four patients (7%), were chronic myeloid leukaemia (leading to study withdrawal), cerebral ischaemia/intracranial haemorrhage (leading to withdrawal of romiplostim), pulmonary fibrosis (leading to study withdrawal) and speech disorder (leading to change in romiplostim dose) (Table 2). Three patients (5%) died (muscular dystrophy, pulmonary fibrosis and congestive cardiac failure) during the study period; pulmonary fibrosis was considered by investigators to be related to investigational product. Eight patients (13%) had grade ≥3 bleeding events.
Table 2.
Overall summary of treatment‐emergent AEsa occurring during the study period
| AEs, n (%) | Romiplostim (N = 60) |
|---|---|
| Total AEs | 59 (98·3) |
| Grade 3 | 31 (52·5) |
| Grade 4 | 5 (8·3) |
| Grade 5 | 3 (5·0) |
| AEs reported in ≥10% of patients | |
| Epistaxis | 20 (33·3) |
| Cough | 13 (21·7) |
| Arthralgia | 12 (20·0) |
| Haematoma | 12 (20·0) |
| Contusion | 11 (18·3) |
| Anaemia | 9 (15·0) |
| Abdominal pain | 9 (15·0) |
| Pyrexia | 8 (13·3) |
| Fatigue | 7 (11·7) |
| Headache | 7 (11·7) |
| Back pain | 7 (11·7) |
| Constipation | 6 (10·0) |
| Eye haemorrhage | 6 (10·0) |
| Bronchitis | 6 (10·0) |
| Nasopharyngitis | 6 (10·0) |
| Gingival bleeding | 6 (10·0) |
| Treatment‐related AEs | 14 (23·3) |
| Grade 3 | 2 (3·3) |
| Grade 4 | 1 (1·7) |
| Grade 5 | 1 (1·7) |
| Serious AEs | 22 (36·7) |
| Treatment‐related serious AEsb | 4 (6·7) |
| Fatal AEsc | 3 (5·0) |
| AEs leading to study withdrawal | 6 (10·0) |
AE, adverse event.
AEs were classified according to the Common Terminology Criteria for Adverse Events version 3.0 severity grading scale (http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf).
Treatment‐related serious AEs were cerebral ischaemia (grade 4)/intracranial haemorrhage (grade 2), chronic myeloid leukaemia (grade 3), pulmonary fibrosis (grade 5) and speech disorder (grade 3).
Fatal events were congestive heart failure, muscular dystrophy and pulmonary fibrosis.
Progression of myelodysplastic syndrome to acute myeloid leukaemia
Patients who had MDS progression, transient increases in peripheral blasts, or developed AML are listed in Table SIII. Two of 60 patients (3%) had progressed to AML during the treatment phase of the extension; respective romiplostim exposure was 4 doses in the parent study and 19 doses in the extension (every other week dosing) in one patient, and 49 doses in the parent study and 47 doses in the extension (weekly dosing) in the other. Two other patients (3%) had transient increases in peripheral blast cell counts from 0% at baseline to <10% at weeks 11 and 25 without disease progression to AML, respectively, with 13 and 38 weeks of romiplostim exposure, and 14 and 41 weeks of observation time. One patient had MDS progression on week 174 after 172 weeks of romiplostim treatment, without progression to AML through 195 weeks of observation. The annualised rate of progression to AML in the 60 patients was 2% (95% confidence interval [CI]; 0·2%, 7%).
Thromboembolic events and increased bone marrow reticulin and collagen
Four patients (7%) had thromboembolic events (one instance each of cerebral ischaemia, myocardial infarction, thrombophlebitis and thrombosis). Of the 6 patients (10%) available for assessment, none developed bone marrow reticulin. Similarly, of the 17 patients (28%) available for assessment, no patients developed collagen fibrosis.
Binding and neutralising antibodies
No neutralising antibodies to romiplostim or TPO were detected (data not shown).
Response duration per international working group 2006 criteria (haematological improvement in platelets)
Fifty patients (83%) had a platelet response, meeting the criteria for either sustained or transient response (Table SII) during romiplostim treatment. To examine the durability of responses to romiplostim, we focused our analysis on the 34 of 60 patients (57%) who achieved a platelet response (defined as a response ≥8 weeks per IWG 2006 criteria (Cheson et al, 2006)) during this open‐label extension study (Fig 1). Of the 34 patients with a response, three had received placebo and the remaining 31 had received romiplostim for a median (range) duration of 52 (7–74) weeks in the parent study. All 34 patients discontinued the treatment phase in the open‐label extension, 25 (74%) owing to administrative decision, five (15%) owing to AEs, two (6%) to disease progression to AML, one (3%) for requirement for alternative therapy, and one (3%) because of consent withdrawal. The median weekly dose was 750 μg, and median (Q1, Q3) number of doses was 43 (21, 125). The median (Q1, Q3) time to first week satisfying response criteria was 2 (1, 3) weeks, and the median (Q1, Q3) longest response observed was 28 (14, 56) weeks (Table 3).(Cheson et al, 2006) The median (Q1, Q3) duration of romiplostim treatment was 44 (23, 160) weeks. Median (Q1, Q3) percentage of observation time with a response was 86% (77%, 93%), and the median (Q1, Q3) dose at first response was 750 (750, 750) μg. Median platelet counts for patients with a response (Fig 2A) were consistent over time. At the time of the last dose of romiplostim, 32 of the 34 patients were still meeting response criteria; neither of the two patients who were not meeting haematological improvement with platelet response (HI‐P) criteria at the last platelet count had developed AML or had progression of their MDS during their long‐term follow‐up (68 and 89 weeks of follow‐up).
Table 3.
Response duration in the open‐label extension study per IWG 2006 criteriaa
| Romiplostim n = 34 | |
|---|---|
| Time to first week satisfying criteria,b week | 2·1 (1·1, 3·0) |
| Longest continuous response,c week | 28 (14, 56) |
| Periods ≥8 consecutive weeks satisfying response criteria, n | 1 (1, 3) |
| Total number of weeks satisfying response criteria, n | 37 (17, 126) |
| Percentage of study time meeting response criteria | 86 (77, 93) |
| Dose at first response, μg | 750 (750, 750) |
| Duration of romiplostim treatment, weeks | 44 (23, 160) |
| Not meeting criteria at last platelet count, n (%) | 2 (6·3) |
All data are median (interquartile range [Q1, Q3]) unless otherwise noted.
Platelet response was based on International Working Group (IWG) 2006 criteria and was defined as (in the absence of platelet transfusion) an absolute increase of ≥30 × 109/l for patients with an initial platelet count of >20 × 109/l but <100 × 109/l or an increase to >20 × 109/l and by at least 100% for patients with an initial platelet count <20 × 109/l for ≥8 weeks.(Cheson et al, 2006).
Time to the first of 8 weeks to establish response.
Defined as continuous response per IWG 2006 criteria.
Figure 2.
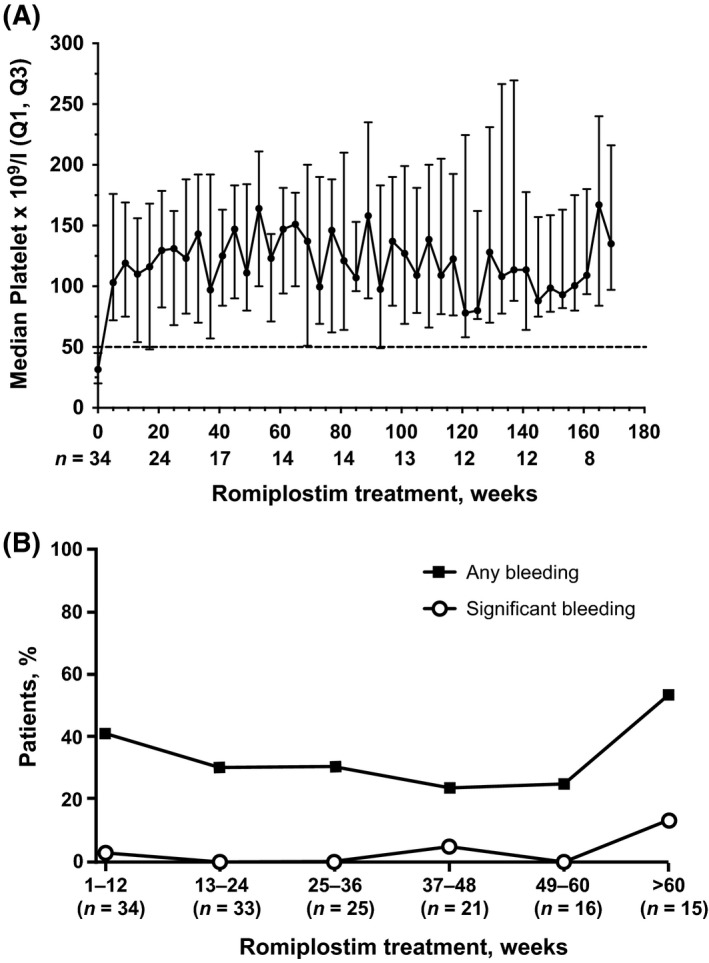
(A) Median platelet counts over time and (B) incidence of bleeding events among patients with platelet response in the open‐label extension exposed to romiplostim during the time interval. Q1, Q3 = interquartile range.
Response duration per modified criteria
Among the responders, some fluctuations in platelet counts were observed. Transiently decreased platelet counts that occurred after a single missed dose, as independently assessed by two investigators were not considered clinically meaningful. Overall, 28 of 34 patients (82%) with platelet response per IWG 2006 criteria had a sustained response based on our modified criteria during romiplostim treatment. Their median (range) response duration was 33 (7–174) weeks. Nine patients received romiplostim for <6 months (range, 11–22 weeks); all had a continuous response during treatment. Ten patients received romiplostim for 6–12 months (range, 27–48 weeks), with seven of 10 patients (70%) having a continuous response during treatment. Finally, 15 patients received romiplostim for >12 months (range, 63–181 weeks), with 12 of 15 (80%) having a continuous response during treatment (range, 61–174 weeks). The other three patients with >12 months of treatment had intermittent responses with cumulative response lengths/total treatment times of 15/181, 35/149, and 52/177 weeks. Of the 15 patients who received romiplostim for more than 12 months, 10 received romiplostim for >156 weeks (3 years); eight of these patients (53%) responded continuously. By the end of the treatment period, two patients had lost their response: one after 11 out of 48 total weeks of treatment and the other after 12 out of 38 total weeks of treatment. Overall, these data indicate that long‐term platelet responses per modified IWG 2006 criteria with romiplostim treatment are possible in patients with MDS (28 of 34 patients responded as long as they received treatment), although our analysis was limited by the premature discontinuation of romiplostim treatment.
Incidence of bleeding events and platelet transfusions
In the patients with responses, the incidence of any bleeding event during the treatment phase of the extension was approximately 30% (Fig 2B). Five patients (15%) experienced grade ≥3 bleeding events, and in three of the five patients, the bleeding events were associated with platelet counts >50 × 109/l; platelet function and coagulation status/function were not available in these instances. Twenty of the 34 responding patients (59%) had a mean (SD) of 16 (18) platelet transfusions during the treatment phase; 14 (41%) had no transfusions, seven (21%) had one to four transfusions, and 13 (38%) had more than five transfusions.
Discussion
This open‐label extension study examined the safety and efficacy of long‐term romiplostim monotherapy in patients with low‐ to intermediate 1‐risk MDS. Of the 60 patients included in the safety analysis set, 50 had previously received romiplostim for a median (range) duration of 52 (7–74) weeks during their parent studies and then received romiplostim for a median (range) of 25 (2–181) weeks in this extension study (combined median romiplostim treatment duration of approximately 1·5 years and for up to 5 years). The safety profile observed in this study is consistent with previous studies. As in previous trials, few patients discontinued because of AEs, and no patients developed neutralising antibodies to romiplostim or TPO (Kantarjian et al, 2010; Giagounidis et al, 2014). Three patients died during the study period; one was considered as possibly related to treatment. Patients achieved durable responses with only two relapses at the last available platelet count. Most patients (82%) with responses per IWG 2006 criteria experienced continuous response with romiplostim treatment as judged by the clinical experts. Although the IWG 2006 criteria provided an objective measurement of platelet response, these results highlight the utility of looking at platelet counts over time for establishing clinical benefit. Twelve patients with romiplostim treatment for >1 year in the extension study had continuous responses ranging from 61 to 174 weeks. Thus, platelet responses appear to be maintained with romiplostim in the vast majority of patients who respond according to the IWG 2006 criteria. Grade ≥3 bleeding events occurred in 16% of all 60 patients in this analysis and in 15% of the 34 patients with a response, with their incidence decreasing over the course of the study.
The primary reason for drug discontinuation was the DMC's safety concern for the potential risk for disease progression to AML, and that transient increases in blast cells might put patients at risk of diagnosis of and treatment for AML. In this study, the rate of progression to AML was low. During the extension study phase, only two patients had transient appearance of peripheral blasts, which disappeared after discontinuation of treatment. One patient had progression of MDS during the treatment phase of this study without further progression to AML, and two patients progressed to AML, for an annualised event rate of progression to AML of 2% (95% CI; 0·2%, 7%).
Information on patients from the parent studies who progressed to AML (3 patients in the open‐label dose‐finding study [N = 72] (Kantarjian et al, 2010; Sekeres et al, 2011) and 12 patients in the double‐blind study [N = 250] (Giagounidis et al, 2014) is summarised in Table SIV. The annualised rate of progression to AML in the double‐blind parent study (N = 250) was 8·0% (95% CI; 3·9%, 14·8%) for patients receiving romiplostim (n = 10) and 3·2% (95% CI; 0·4%, 11·6%) for those receiving placebo (n = 2) (Giagounidis et al, 2014). In the parent studies, 17 patients (10·1%) receiving romiplostim and 7 patients (8·5%) receiving placebo met the criteria for worsening MDS (Giagounidis et al, 2014). Among the 25 patients with maximum peripheral blast counts >10%, 12 patients had decreases to <10% without treatment, two had decreases with treatment, two had sustained increases and 9 patients did not have end‐of‐study peripheral blood blast assessments, so their status is not known (Giagounidis et al, 2014). Among three placebo recipients with elevated peripheral blast counts, two had deceases without treatment and one did not have an end‐of‐study peripheral blood blast assessment (Giagounidis et al, 2014). Four patients in the open‐label dose‐finding study had transient increases in blast cells ≥20% that subsequently fell to below 20% (Sekeres et al, 2011).
There are a number of study limitations to consider. Importantly, 25 of the 34 patients with a platelet response discontinued treatment because of regulatory agency and/or DMC recommendation, thereby limiting the assessment of more prolonged romiplostim treatment. Because of differing times of enrolment, approximately one‐third of patients with a response received romiplostim for <6 months; however, 15 of 34 patients with a response received romiplostim for >12 months, including 10 patients with treatment >156 weeks (3 years), helping to inform safety and efficacy over multiple years of treatment. In addition, this extension trial enrolled only patients who had not progressed to AML and most had received romiplostim in the parent study, which introduces potential selection bias. Patients with IPSS low‐ to intermediate 1‐risk MDS have a lower risk of AML progression than patients with intermediate‐2 or high‐risk MDS; the incidence of progression to AML (median follow‐up, 22 months) has been reported to be 13%, 22%, 46%, and 65% in patients with low‐risk, intermediate 1‐risk, intermediate 2‐risk and high‐risk MDS, respectively (Shukron et al, 2012). The median romiplostim treatment exposure in this study, combining parent and extension studies, was approximately 1·5 years (up to 5 years). Therefore, the recruitment of patients at low risk for AML progression, combined with a relatively short duration of follow‐up, may have underestimated the risk of progression to secondary AML compared with a more general MDS population.
In conclusion, platelet counts increased and bleeding events decreased in patients with low‐ or intermediate 1‐risk MDS and thrombocytopenia treated long term with romiplostim monotherapy. Most patients with HI‐P maintained platelet responses while receiving romiplostim. The overall safety and efficacy profiles were similar to those seen in other romiplostim studies; no new safety signals were identified and the rate of progression to AML was low.
Authorship contributions
All authors meet the criteria for authorship, and have reviewed and approved the submitted version. Patient data collection/Data acquisition: PF, PM, HK, RML, RAL, MAS, PSB, AO, JF. Analysis and interpretation of data: PF, PM, HK, RML, RAL, MAS, PSB, AO, JF. Writing and/or critical revision of the manuscript: PF, PM, HK, RML, RAL, MAS, PSB, AO, JF. Post hoc HI‐P analyses: PF, PM.
Disclosure of conflict of interest
PF has received honoraria from Amgen Inc; PM served on advisory boards for Alexion and Ra Pharma; HK has received research grants from Amgen Inc.; RML is a consultant for Amgen Inc. and has received research support from Amgen Inc.; RAL is a consultant for Amgen, Inc. and has received research support from Amgen, Inc.; PSB has received research support from Amgen Inc.; MAS has served on advisory boards for Celgene and Amgen Inc.; AO was a consultant with Amgen Inc.; JF is an employee and shareholder with Amgen Inc.
Supporting information
Table SI. Dose adjustments during the treatment phase.
Table SII. Assessments.
Table SIII. Romiplostim exposure and times to event for patients with acute myeloid leukaemia, transient peripheral blast increases, or progression of myelodysplastic syndrome.
Table SIV. Romiplostim exposure and times to event for patients with AML in the parent studies.
Acknowledgements
The study was sponsored by Amgen Inc. Rick Davis and Miranda Tradewell (Complete Healthcare Communications, LLC) and Susanna Mac (Amgen Inc.) provided medical writing support. Helen Wei (Amgen Inc.) provided assistance with statistical analyses.
ClinicalTrials.gov identifier: NCT00472290
References
- Cheson, B.D. , Greenberg, P.L. , Bennett, J.M. , Lowenberg, B. , Wijermans, P.W. , Nimer, S.D. , Pinto, A. , Beran, M. , de Witte, T.M. , Stone, R.M. , Mittelman, M. , Sanz, G.F. , Gore, S.D. , Schiffer, C.A. & Kantarjian, H. (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood, 108, 419–425. [DOI] [PubMed] [Google Scholar]
- Gangat, N. , Patnaik, M.M. & Tefferi, A. (2016) Myelodysplastic syndromes: contemporary review and how we treat. American Journal of Hematology, 91, 76–89. [DOI] [PubMed] [Google Scholar]
- Giagounidis, A. , Mufti, G.J. , Fenaux, P. , Sekeres, M.A. , Szer, J. , Platzbecker, U. , Kuendgen, A. , Gaidano, G. , Wiktor‐Jedrzejczak, W. , Hu, K. , Woodard, P. , Yang, A.S. & Kantarjian, H.M. (2014) Results of a randomized, double‐blind study of romiplostim versus placebo in patients with low/intermediate‐1‐risk myelodysplastic syndrome and thrombocytopenia. Cancer, 120, 1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, W.K. & Koeffler, H.P. (2005) Myelodysplastic syndrome. Annual Review of Medicine, 56, 1–16. [DOI] [PubMed] [Google Scholar]
- Kantarjian, H. , Giles, F. , List, A. , Lyons, R. , Sekeres, M.A. , Pierce, S. , Deuson, R. & Leveque, J. (2007) The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer, 109, 1705–1714. [DOI] [PubMed] [Google Scholar]
- Kantarjian, H. , O'Brien, S. , Ravandi, F. , Cortes, J. , Shan, J. , Bennett, J.M. , List, A. , Fenaux, P. , Sanz, G. , Issa, J.P. , Freireich, E.J. & Garcia‐Manero, G. (2008) Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer, 113, 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian, H. , Fenaux, P. , Sekeres, M.A. , Becker, P.S. , Boruchov, A. , Bowen, D. , Hellstrom‐Lindberg, E. , Larson, R.A. , Lyons, R.M. , Muus, P. , Shammo, J. , Siegel, R. , Hu, K. , Franklin, J. & Berger, D.P. (2010) Safety and efficacy of romiplostim in patients with lower‐risk myelodysplastic syndrome and thrombocytopenia. Journal of Clinical Oncology, 28, 437–444. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network . (2015) NCCN Clinical Practice Guidelines in Oncology: Myelodysplastic Syndromes v2.2015. National Comprehensive Cancer Network. Available at: https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf Accessed July 29, 2016. [DOI] [PMC free article] [PubMed]
- Neukirchen, J. , Schoonen, W.M. , Strupp, C. , Gattermann, N. , Aul, C. , Haas, R. & Germing, U. (2011) Incidence and prevalence of myelodysplastic syndromes: data from the Dusseldorf MDS‐registry. Leukemia Research, 35, 1591–1596. [DOI] [PubMed] [Google Scholar]
- Sekeres, M.A. , Schoonen, W.M. , Kantarjian, H. , List, A. , Fryzek, J. , Paquette, R. & Maciejewski, J.P. (2008) Characteristics of US patients with myelodysplastic syndromes: results of six cross‐sectional physician surveys. Journal of the National Cancer Institute, 100, 1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres, M.A. , Kantarjian, H. , Fenaux, P. , Becker, P. , Boruchov, A. , Guerci‐Bresler, A. , Hu, K. , Franklin, J. , Wang, Y.M. & Berger, D. (2011) Subcutaneous or intravenous administration of romiplostim in thrombocytopenic patients with lower risk myelodysplastic syndromes. Cancer, 117, 992–1000. [DOI] [PubMed] [Google Scholar]
- Shukron, O. , Vainstein, V. , Kundgen, A. , Germing, U. & Agur, Z. (2012) Analyzing transformation of myelodysplastic syndrome to secondary acute myeloid leukemia using a large patient database. American Journal of Hematology, 87, 853–860. [DOI] [PubMed] [Google Scholar]
- Vardiman, J.W. , Harris, N.L. & Brunning, R.D. (2002) The World Health Organization (WHO) classification of the myeloid neoplasms. Blood, 100, 2292–2302. [DOI] [PubMed] [Google Scholar]
- Vardiman, J.W. , Thiele, J. , Arber, D.A. , Brunning, R.D. , Borowitz, M.J. , Porwit, A. , Harris, N.L. , Le Beau, M.M. , Hellstrom‐Lindberg, E. , Tefferi, A. & Bloomfield, C.D. (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood, 114, 937–951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Dose adjustments during the treatment phase.
Table SII. Assessments.
Table SIII. Romiplostim exposure and times to event for patients with acute myeloid leukaemia, transient peripheral blast increases, or progression of myelodysplastic syndrome.
Table SIV. Romiplostim exposure and times to event for patients with AML in the parent studies.


