Summary
Variation in aerobic capacity has far reaching consequences for the physiology, ecology, and evolution of vertebrates. Whether at rest or active, animals are constrained to operate within the energetic bounds determined by their minimum (minMR) and sustained or maximum metabolic rates (upperMR). MinMR and upperMR can differ considerably among individuals and species but are often presumed to be mechanistically linked to one another. Specifically, minMR is thought to reflect the idling cost of the machinery needed to support upperMR. However, previous analyses based on limited datasets have come to conflicting conclusions regarding the generality and strength of their association.
Here we conduct the first comprehensive assessment of their relationship, based on a large number of published estimates of both the intra‐specific (n = 176) and inter‐specific (n = 41) phenotypic correlations between minMR and upperMR, estimated as either exercise‐induced maximum metabolic rate (VO 2max), cold‐induced summit metabolic rate (Msum), or daily energy expenditure (DEE).
Our meta‐analysis shows that there is a general positive association between minMR and upperMR that is shared among vertebrate taxonomic classes. However, there was stronger evidence for intra‐specific correlations between minMR and Msum and between minMR and DEE than there was for a correlation between minMR and VO 2max across different taxa. As expected, inter‐specific correlation estimates were consistently higher than intra‐specific estimates across all traits and vertebrate classes.
An interesting exception to this general trend was observed in mammals, which contrast with birds and exhibit no correlation between minMR and Msum. We speculate that this is due to the evolution and recruitment of brown fat as a thermogenic tissue, which illustrates how some species and lineages might circumvent this seemingly general association.
We conclude that, in spite of some variability across taxa and traits, the contention that minMR and upperMR are positively correlated generally holds true both within and across vertebrate species. Ecological and comparative studies should therefore take into consideration the possibility that variation in any one of these traits might partly reflect correlated responses to selection on other metabolic parameters.
A lay summary is available for this article.
Keywords: aerobic capacity, daily energy expenditure, locomotion, maximum thermogenesis, resting metabolic rate, standard metabolic rate
Introduction
Metabolism is the ‘fire of life’ that fuels processes at all levels of biological organization (Kleiber 1961) and has far reaching consequences for the physiology, behaviour, ecology, and evolution of organisms (Chown & Gaston 1999; Brown et al. 2004; Anderson & Jetz 2005; Buckley, Rodda & Jetz 2008). Metabolic rates vary widely across individuals, populations, and species (Burton et al. 2011; White & Kearney 2013; Killen et al. 2016). They are heritable to a certain extent (Nespolo et al. 2005; Nilsson, Åkesson & Nilsson 2009; Wone et al. 2009) and can evolve in both laboratory (Książek, Konarzewski & Łapo 2004; Wone et al. 2015) and wild populations (Boratyński & Koteja 2010). Metabolic rates are often under selection (Hayes & O'Connor 1999; Bochdansky et al. 2005) and their variation among individuals has been linked to components of fitness such as growth (Steyermark 2002; Auer et al. 2015b), reproduction (Blackmer et al. 2005; Boratyński & Koteja 2010) and survival (Artacho & Nespolo 2009; Larivee et al. 2010). Indeed, inter‐specific variation in metabolic rates has been attributed to a wide range of extrinsic factors such as climate, lifestyle, habitat productivity, and diet (Mueller & Diamond 2001; Rezende et al. 2004; Anderson & Jetz 2005; Bozinovic et al. 2009; White & Kearney 2013; Killen et al. 2016).
The links between the lower and upper limits to energy expenditure (minMR and upperMR hereafter) have garnered significant interest over the last half century. The baseline energetic costs of living are set by minMR (Hulbert & Else 2004), which have been quantified as standard metabolic rate (SMR) in ectotherms, basal metabolic rate (BMR) in endotherms, or simply resting metabolic rate (RMR) as a proxy for the previous estimates under less restrictive conditions (e.g., allowing for low levels of spontaneous activity and some digestion; Jobling 1994). In contrast, upperMR sets the limit for the energy available to finance locomotion, digestion, growth, reproduction, and thermoregulation. UpperMR has been quantified acutely as maximum metabolism during strenuous exercise (VO2max) or cold‐exposure for endothermic organisms (summit metabolism or Msum) and, over longer time spans, as sustained metabolic rates and daily energy expenditure (DEE). Early observations that minMR appears to be a relatively constant proportion of both sustained and maximum metabolic rates (Bennett & Ruben 1979; Drent & Daan 1980; Hammond & Diamond 1997) led to the hypothesis that they are mechanistically linked (Packard 1968; Bennett & Ruben 1979; Drent & Daan 1980; Taigen 1983; Hayes & Garland 1995) and may evolve together in a correlated fashion (Hayes 2010; Nespolo et al. 2017). This premise underlies various models such as the ‘aerobic capacity model for the evolution of endothermy’ (Bennett & Ruben 1979), the ‘assimilation capacity model for the evolution of endothermy’ (Koteja 2000), and the ‘sustained maximal limit model’ (Drent & Daan 1980; Speakman, Król & Johnson 2004), which posit that minMR reflects the idling cost of maintaining the machinery required to support total energy expenditure.
An association between minMR and upperMR across vertebrate lineages has important implications for their ecological and evolutionary physiology. Not only does it open up the question of which cellular or tissue‐level mechanisms determine or limit different aspects of aerobic performance (Chappell et al. 2007; Norin & Malte 2012), but also how organisms might respond to different and often antagonistic selective pressures (e.g. Rezende et al. 2004; Killen et al. 2016). Not surprisingly, many studies have estimated the correlation between these traits at both the intra‐ and inter‐specific level, but with mixed results. For instance, studies have reported positive and nonsignificant phenotypic correlations at the inter‐specific level (e.g. Ricklefs, Konarzewski & Daan 1996; Rezende et al. 2004) and positive, negative, and nonsignificant correlations at the intra‐specific level (e.g. Gomes et al. 2004; Rezende et al. 2005; Dlugosz et al. 2012). Genetic correlations between minMR and upperMR (Dohm, Hayes & Garland 2001; Sadowska et al. 2005; Wone et al. 2009) and correlated responses to selection (Książek, Konarzewski & Łapo 2004; Sadowska et al. 2015) also provide equivocal results. No consensus seems to emerge because studies focus on different taxonomic groups (from fish to birds and mammals), metabolic traits (VO2max, Msum, and DEE) and levels of organization (intra‐ vs. inter‐specific). Hence, it is currently unclear whether there are systematic factors that explain variation in the degree to which minMR and upperMR are linked.
Whether the association between minMR and upperMR is driven by physiological constraint or statistical artefact is also not well understood. Selection for high aerobic performance is thought to result in increased metabolic expenditure at rest, which inherently assumes that a positive correlation emerges from a physiological constraint (i.e., upperMR drives the correlation; Bennett & Ruben 1979; Drent & Daan 1980; Taigen 1983; Hayes & Garland 1995). However, part‐whole correlation between minMR and upperMR may also give rise to a positive correlation (for a simple algebraic formulation, see Chayes 1971); if energy requirements for different processes such as locomotion are additive to minMR (i.e. minMR is not suppressed during periods of high energy expenditure), then upperMR could simply reflect minMR rather than aerobic performance that is independent of minMR (Ricklefs, Konarzewski & Daan 1996; Speakman 2000; Pontzer, Brown & Raichlen 2016). These alternatives can be disentangled by assessing whether correlations exhibit a positive vs. negative association with FAS (Fig. 1a). If species with a high FAS are closer to a physiological limit in which upperMR may not increase any further without also increasing minMR, as proposed by the aerobic capacity model (Bennett & Ruben 1979), then correlations are expected to be positively associated with FAS (stretched coil spring in Fig. 1a). Alternatively, if the relationship between minMR and upperMR is due to part‐whole correlation, then correlations are expected to be negatively associated with FAS because minMR becomes increasingly correlated with itself as FAS decreases (compressed coil spring in Fig. 1a).
Figure 1.
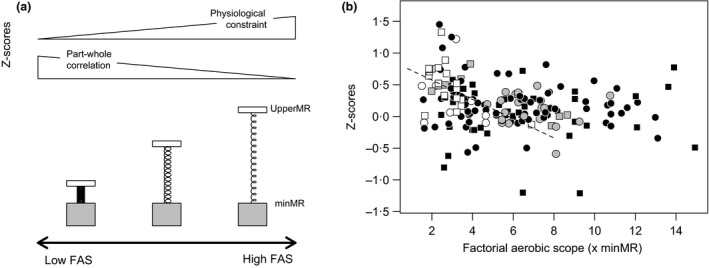
(a) Conceptual model: The strength of association between minMR and upperMR should vary predictably with factorial aerobic scope (FAS = upperMR/minMR), as shown with the coil spring model, either because species with high FAS are near a physiological limit (stretched spring) or due to part‐whole correlation because minMR encompasses an increasingly large fraction of upperMR in species with lower FAS (compressed spring). (b) Empirical evaluation: Z‐scores of the correlation between minMR and each of exercise‐induced maximum metabolic rate (black), cold‐induced summit metabolic rate (grey) and daily energy expenditure (white). Data are from this study. Correlations were assessed using different measures of minMR: standard and basal metabolic rate (circles) or resting metabolic rate (squares). Our analyses support the later alternative for correlations involving DEE, as shown by the dotted line.
Here we address these issues by using a meta‐analytical approach to examine the large number of published estimates of both the intra‐specific and inter‐specific phenotypic correlations between minMR and upperMR. Our specific objectives were to determine: (i) the magnitude and direction of intra‐ and inter‐specific correlations and whether they vary across traits since VO2max, Msum, and DEE represent very different measures of metabolism that are not necessarily correlated across individuals (Peterson, Walton & Bennett 1998; Chappell et al. 2004, 2007; Swanson et al. 2012) or species (Wiersma, Chappell & Williams 2007); (ii) whether correlations vary predictably among vertebrate taxonomic classes; (iii) the level of agreement between correlations reported at intra‐ and inter‐specific levels; and (iv) whether the strength of the association between minMR and upperMR is driven by physiological constraint or statistical artefact, by looking for directional trends between the magnitude of the correlation and the factorial difference between minMR and upperMR.
Materials and methods
Literature review and selection criteria
We searched for published estimates of intra‐specific and inter‐specific phenotypic correlations between the different types of minMR and upperMR using both Google Scholar and Web of Science. We used the following search terms: intra‐specific, inter‐specific, correlation, metabolic rate, metabolism, energy expenditure, standard metabolism, standard metabolic rate, basal metabolism, basal metabolic rate, resting metabolism, resting metabolic rate, daily energy expenditure, field metabolism, field metabolic rate, maximum metabolism, maximum metabolic rate, summit metabolism, summit metabolic rate, peak metabolism, and the related acronyms RMR, SMR, BMR, DEE, FMR, MMR, VO2max and Msum. We also searched the reference list of each paper to identify additional studies missed in our initial search. Finally, several unpublished estimates of intra‐specific correlations were obtained from colleagues at the University of Glasgow. Correlations among measures of metabolic rate may be positive simply because they are all highly dependent on body mass. Thus, only phenotypic correlations that accounted for variation in body mass were considered.
For each study, we recorded the species name (for intra‐specific correlations) and taxonomic class (fish, amphibian, reptile, bird or mammal). We also recorded the sample size of the study, the type of minMR (RMR vs. SMR or BMR) and upperMR (VO2max, Msum and DEE) measured, the estimate of their correlation (Pearson's r) and the factorial aerobic scope associated with each correlation as an estimate of the factorial difference between minMR and upperMR (FAS = upperMR/minMR). The respirometry methods for many studies were not detailed enough for us to assess whether RMR vs. SMR or BMR was being measured, so labels provided in the original studies were used. DEE was typically measured using the doubly labelled water technique, but several studies measured the average daily oxygen consumption of animals living in the laboratory (e.g. Chappell et al. 2004). For studies that did not provide the correlation estimate, we obtained it from reported P‐values and t or F statistics, by contacting the authors directly, or by using data grabbing software (Graphclick; http://www.arizona-software.ch/graphclick/). For inter‐specific studies, we recorded whether analyses accounted for phylogenetic history.
Statistical analyses
Meta‐analyses of intra‐specific and inter‐specific correlations were performed with the statistical package metafor for r (Viechtbauer 2010), employing Fisher's r‐to‐z transformation to obtain unbiased estimates of effect sizes and sampling variances (Hedges & Olkin 1985). We used Akaike's information criterion (AIC) to compare models with different fixed effects, and a multi‐step approach employing ML for model selection and REML for the estimation of variance components of the best candidate models (see Ngo & Brand 1997 and references therein). We used the AICc corrected for small sample sizes for model selection, and quantified the relative support of each model with Akaike's weights (w i). The best models were those whose Akaike weights were within 10% of the highest value in each set (see below), a minimum cut‐off point comparable to that suggested by Royall (1997). Given their different underlying physiology and ecological significance (McKechnie & Swanson 2010), VO2max, Msum, and DEE were analyzed separately and the 95% confidence intervals for intra‐specific and inter‐specific effect sizes were estimated from these models for each taxonomic class.
For analyses of intra‐specific correlations, we assembled a dated phylogeny based on different sources in the literature (Fig. 2, see Appendix S1, Supporting Information). The impact of phylogeny was determined by estimating the λ (Pagel 1999) that best fit the residual variation of models including species as a random factor (since multiple studies reported correlations for the same species). For each of VO2max, Msum, and DEE, a standard model with the type of minMR measured (SMR or BMR vs. RMR) included as a categorical factor was compared against more complex models including taxonomic class and FAS. Because minMR type (SMR or BMR vs. RMR) had a negligible impact, the mean effect of this factor was employed to calculate adjusted effect sizes and their 95% confidence intervals. Some studies provided multiple estimates, so study was initially included as a random effect in all analyses. However, it was subsequently removed since in all cases it did not improve the model fit.
Figure 2.
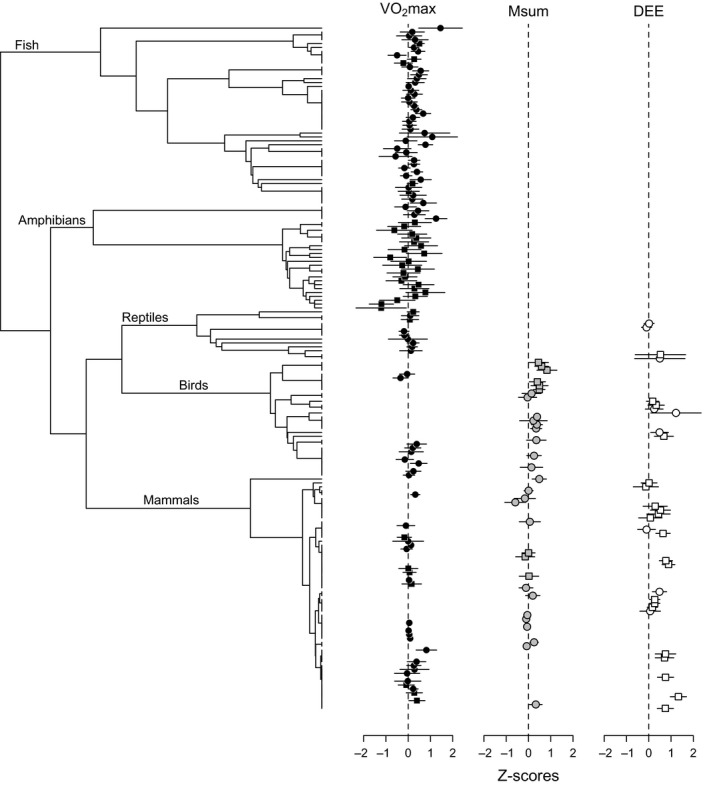
Phylogeny and distribution of effect sizes for the intra‐specific correlation between minMR and exercise‐induced maximum metabolic rate (VO 2max), cold‐induced summit metabolic rate (Msum), and daily energy expenditure (DEE). Correlations were assessed using different measures of minMR: standard and basal metabolic rate (circles) or resting metabolic rate (squares). See Appendix S1 for details on species and their phylogenetic relationships and Appendix S2 for species’ correlations and references.
For inter‐specific analyses, we examined the effects of taxonomic class and a categorical variable coding phylogenetic vs. non‐phylogenetic analyses. In this case, we did not use a dummy variable coding for RMR vs. BMR or SMR since some studies included both estimates and controlled for them statistically in their analyses (e.g. Rezende et al. 2004). Importantly, some studies may have included the same species data in their analyses (e.g., the data for passerines in Dutenhoffer & Swanson 1996 constitutes a subset of the dataset compiled by Rezende et al. 2002 for birds), and therefore some degree of pseudo‐replication is expected between results. Because the degree of overlap between datasets varies from study to study and may not be readily removed without the raw data, we opted to include all analyses compiled for completeness. Nonetheless, the adjusted estimates and confidence intervals reported here must be interpreted with caution in light of this limitation.
Results
We obtained a total of 176 estimates of intra‐specific phenotypic correlations and 41 estimates of inter‐specific correlations between minMR and either VO2max, Msum, or DEE (Table 1, Appendices S2 and S3). For the intra‐specific dataset, we obtained estimates for 73 different species from a total of 75 studies, which included 115 correlations with VO2max across all taxa, 31 correlations with Msum across birds and mammals, and 30 correlations with DEE across reptiles, birds, and mammals (Fig. 2, Appendix S2). In contrast, the inter‐specific dataset contained a total of 15 published papers that included 14 correlation estimates for VO2max, 12 for Msum, and 15 for DEE (Fig. 3, Appendix S3). Funnel plots of effect size as a function of log10‐transformed sample size were symmetrical for both intra‐specific and inter‐specific correlations estimates (Appendix S4), suggesting a lack of publication bias.
Table 1.
Summary for each taxonomic class of studies examining the intra‐specific and inter‐specific correlations between minimum metabolism and exercise‐induced maximum metabolic rate (VO2max), cold‐induced summit metabolic rate (Msum), and daily energy expenditure (DEE)
| Studies | Species | N | VO2max | Msum | DEE | |
|---|---|---|---|---|---|---|
| Intra‐specific | ||||||
| Fish | 22 | 19 | 6–452 | 46 | 0 | 0 |
| Amphibians | 3 | 20 | 6–19 | 27 | 0 | 0 |
| Reptiles | 8 | 8 | 6–250 | 9 | 0 | 4 |
| Birds | 15 | 12 | 6–200 | 9 | 15 | 6 |
| Mammals | 27 | 14 | 11–1334 | 24 | 16 | 20 |
| Inter‐specific | ||||||
| Fish | 1 | 131 | – | 1 | 0 | 0 |
| Amphibians | 5 | 8–17 | – | 5 | 0 | 0 |
| Reptiles | 1 | 9 | – | 1 | 0 | 0 |
| Birds | 9 | 8–45 | – | 1 | 3 | 5 |
| Mammalsa | 25 | 4–60 | – | 6 | 9 | 10 |
See Appendices S2 and S3 for details of species and references. Listed are the numbers of studies, species, individuals per correlation (N), and correlations based on each of VO2max, Msum and DEE.
One correlation for DEE and one for VO2max were not included in inter‐specific analyses because they were calculated employing four species, hence z‐scores and variance estimates could not be calculated (see Table 3).
Figure 3.
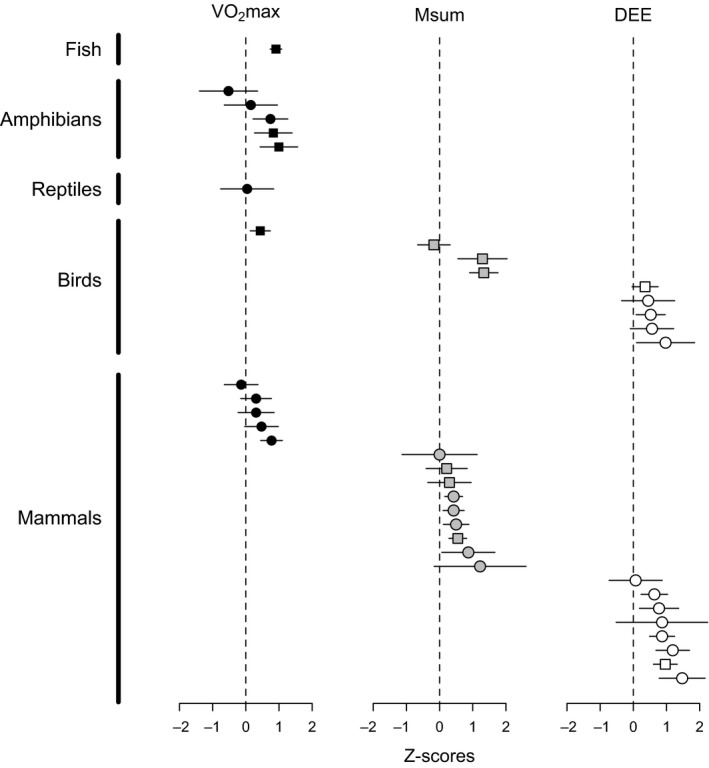
Effect sizes for the inter‐specific correlation between minimum metabolic rate and exercise‐induced maximum metabolic rate (VO 2max), cold‐induced summit metabolic rate (Msum) or daily energy expenditure (DEE). Correlations in original studies were assessed with phylogenetic (squares) and non‐phylogenetic analyses (circles). See Appendix S3 for details of taxonomic classes and references.
Intra‐specific correlations
Pearson's correlation coefficients for the intra‐specific dataset ranged from −0·837 to 0·896 for VO2max, from −0·530 to 0·680 for Msum, and from −0·129 to 0·869 for DEE (Figs 2 and 4). For VO2max, comparison between models suggests that there is a small amount of phylogenetic signal in effect sizes (λ = 0·1) that seems to partly reflect differences among taxonomic classes, since λ = 0·0 in models including class as a predictor (Table 2). Based on AICc estimates, neither the inclusion of class nor FAS improved overall fit, and the distribution of effect sizes suggests the correlation between minMR and VO2max is very close to zero in all taxonomic groups (Fig. 5). In contrast, effect sizes with Msum exhibited strong phylogenetic signal (λ = 0·5 in the standard model) and were consistently positive for birds but not mammals (Fig. 5). Accordingly, based on AICc estimates, the two models with the best fit include taxonomic class as a predictor (Table 2). For DEE, effect sizes did not exhibit phylogenetic signal (λ = 0·0) and were generally positive (Fig. 5). Even though no differences were evident between taxonomic classes, comparisons between models suggest that effect sizes involving DEE may be influenced by FAS (Table 2). Accordingly, there was a significant negative relationship between effect sizes and FAS (Fig. 1b; z = −2·82, P = 0·005) in the model including this predictor. Taken together, our analyses suggest that intra‐specific correlations are generally positive for Msum and DEE but not VO2max (Fig. 5). In addition, the magnitude of correlations between minMR and DEE decreases with FAS whereas no association is evident for VO2max or Msum (Fig. 1b).
Figure 4.
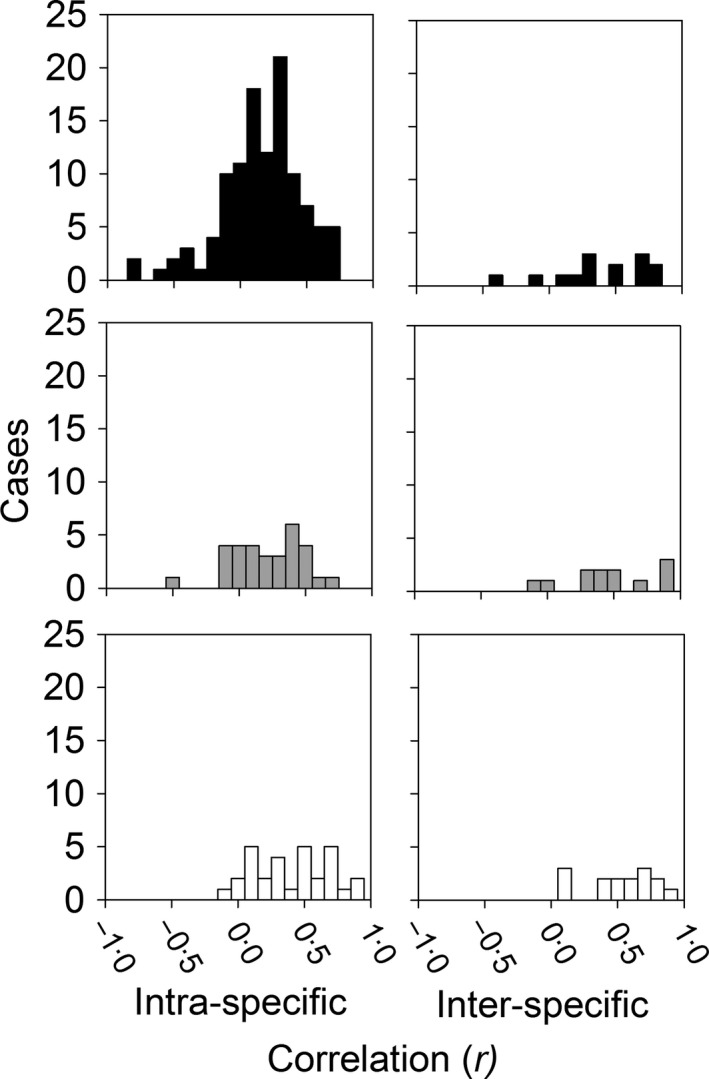
Frequency distributions of the intra‐ and inter‐specific correlations between minimum metabolic rate (standard, basal, and resting) and each of exercise‐induced maximum metabolic rate (VO 2max = black), cold‐induced maximum metabolic rate (Msum = grey), and daily energy expenditure (DEE = white) in vertebrates.
Table 2.
AICc rankings and weights of models describing the effects of taxonomic class (fish, amphibian, reptile, bird, mammal) and factorial aerobic scope (FAS) on the intra‐specific correlation between minimum metabolism and exercise‐induced maximum metabolic rate (VO2max), cold‐induced summit metabolic rate (Msum), and daily energy expenditure (DEE)
| Modela | k | λ | LogLik | AICc | ΔAICc | w i | |
|---|---|---|---|---|---|---|---|
| VO2max (n = 115) | Std | 3 | 0·1 | −35·81 | 77·85 | 0·00 | 0·70 |
| Std + Class | 7 | 0·0 | −34·01 | 83·07 | 5·22 | 0·05 | |
| Std + FAS | 4 | 0·1 | −35·86 | 80·08 | 2·23 | 0·23 | |
| Std + Class + FAS | 8 | 0·0 | −34·22 | 85·80 | 7·95 | 0·01 | |
| Msum (n = 31) | Std | 3 | 0·5 | 3·70 | −0·51 | 5·44 | 0·05 |
| Std + Class | 4 | 0·6 | 7·75 | −5·95 | 0·00 | 0·70 | |
| Std + FAS | 4 | 0·5 | 5·54 | −1·54 | 4·41 | 0·08 | |
| Std + Class + FAS | 5 | 0·0 | 7·82 | −3·24 | 2·71 | 0·18 | |
| DEE (n = 30) | Std | 3 | 0·0 | −6·69 | 20·30 | 0·00 | 0·50 |
| Std + Class | 5 | 0·0 | −6·16 | 24·83 | 4·53 | 0·05 | |
| Std + FAS | 4 | 0·0 | −5·49 | 20·59 | 0·29 | 0·43 | |
| Std + Class + FAS | 6 | 0·0 | −5·65 | 26·96 | 6·66 | 0·02 |
Shown are the number of correlations in the analysis (n), the amount of phylogenetic signal (λ), the number of parameters (k), the log likelihood (LogLik) of the model, the difference in Akaike's information criterion (ΔAICc) between each model and the top‐ranked model (in bold), and the Akaike weights (w i) of each model.
Standard model = Intercept + minMR, where minMR is categorical standard metabolic rate (SMR) or basal metabolic rate (BMR) vs. resting metabolic rate (RMR).
Figure 5.
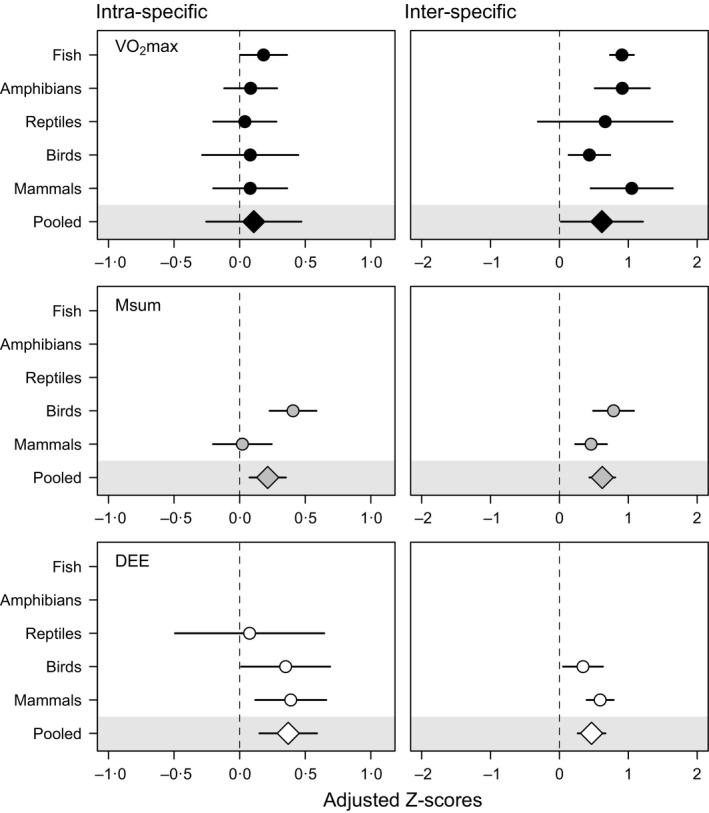
Means and 95% confidence intervals for effect sizes of the intra‐ and inter‐specific correlation between minimum metabolic rate and exercise‐induced maximum metabolic rate (VO 2max), cold‐induced summit metabolic rate (Msum), and daily energy expenditure (DEE). Because inter‐specific estimates often differed between phylogenetic vs. non‐phylogenetic analyses (see Results), adjusted effect sizes were calculated for phylogenetic analyses.
Inter‐specific correlations
Pearson's correlation coefficients for the inter‐specific correlation between minMR and upperMR ranged from −0·48 to 0·76 for VO2max, from −0·17 to 0·87 for Msum, and from 0·07 to 0·92 for DEE (Figs 3 and 4). The meta‐analyses performed separately for these variables suggest that effects sizes, and therefore the inter‐specific correlations, are consistently positive (Fig. 5). In fact, 95% confidence intervals were greater than zero for all estimates and taxonomic classes, with the notable exception of VO2max in reptiles for which a single estimate was available (n = 9 species, Pearson's r = 0·04; Appendix S2). Comparison between models for VO2max suggests that differences between effects sizes can be partly attributed to the statistical analyses employed to estimate inter‐specific correlations (Table 3), with analyses that corrected for phylogeny providing higher estimates than those that did not (z = 3·78, P = 0·0002). In contrast, the model with the best fit for Msum provides some support for differences between birds and mammals (z = −1·89, P = 0·059), whereas comparisons between models for DEE give similar weights for different models and therefore had very little discriminatory power (Table 3).
Table 3.
AICc rankings and weights of models describing the effects of analysis (phylogenetic vs. non‐phylogenetic) and taxonomic class (fish, amphibian, reptile, bird, mammal) on the inter‐specific correlation between minimum and exercise‐induced maximum metabolic rate (VO2max), cold‐induced summit metabolic rate (Msum), and daily energy expenditure (DEE)
| Model | k | LogLik | AICc | ΔAICc | w i | |
|---|---|---|---|---|---|---|
| VO2max (n = 13) | Int | 1 | −12·61 | 27·60 | 9·89 | 0·01 |
| Int + Analysis | 2 | −6·25 | 17·71 | 0·00 | 0·98 | |
| Int + Class | 5 | −4·04 | 26·66 | 8·95 | 0·01 | |
| Int + Analysis + Class | 6 | −2·08 | 30·16 | 12·45 | 0·00 | |
| Msum (n = 12) | Int | 1 | −10·95 | 24·29 | 0·63 | 0·34 |
| Int + Analysis | 2 | −10·46 | 26·27 | 2·61 | 0·13 | |
| Int + Class | 2 | −9·16 | 23·66 | 0·00 | 0·46 | |
| Int + Analysis + Class | 3 | −9·18 | 27·35 | 3·69 | 0·07 | |
| DEE (n = 14) | Int | 1 | −7·03 | 16·39 | 0·62 | 0·23 |
| Int + Analysis | 2 | −5·34 | 15·77 | 0·00 | 0·31 | |
| Int + Class | 2 | −5·79 | 16·67 | 0·90 | 0·20 | |
| Int + Analysis + Class | 3 | −3·81 | 16·03 | 0·26 | 0·27 |
Shown are the number of correlations in the analysis (n), the number of parameters (k), the log likelihood (LogLik) of the model, the difference in Akaike's information criterion (ΔAICc) between each model and the top‐ranked model (in bold), and the Akaike weights (w i) of each model.
Int, intercept; Analysis, dummy variable comparing phylogenetically vs. non‐phylogenetically corrected analyses.
Intra‐specific vs. inter‐specific correlations
The magnitude of the association between minMR and upperMR was significantly higher at the inter‐specific compared to intra‐specific level when adjusted effect sizes for the different taxonomic classes were compared (Fig. 5; paired t‐test, t 8 = 4·81, P = 0·001), with grand mean effect sizes and 95% confidence intervals back‐transformed into Pearson's r corresponding to 0·180 (intervals between 0·025 and 0·383) and 0·594 (0·346–0·771) for intra‐specific and inter‐specific analyses, respectively. Nonetheless, intra‐specific and inter‐specific effect sizes were not correlated (Pearson's r 7 = −0·159, P = 0·683).
Discussion
Our meta‐analysis reveals some important generalities. First, the results support a general association between minMR and upperMR that is evident in most cases. Mean adjusted effect sizes were positive for all traits across all taxonomic classes, and 95% confidence intervals for pooled estimates were consistently higher than zero in all analyses, with the notable exception of intra‐specific correlations involving VO2max. Second, our analyses demonstrate that the magnitude of the association between minMR and upperMR is also generally consistent across taxa. And third, the association between minMR and upperMR at the inter‐specific level is consistently higher and appears to be unrelated to estimates at the intra‐specific level, suggesting that correlations at these two levels are affected by different factors and convey different types of information regarding the overall relationship between minMR and upperMR.
The positive correlation between minMR and upperMR is in line with physiological models that posit a mechanistic link between these traits (Packard 1968; Bennett & Ruben 1979; Drent & Daan 1980; Taigen 1983; Hayes & Garland 1995; Koteja 2000). However, part‐whole correlation may also give rise to this pattern (Chayes 1971). Here, the intra‐specific correlation between minMR and DEE decreases with increasing FAS. Hence, we find partial support for the association between minMR and upperMR being driven by part‐whole correlation but no tangible evidence for a physiological limit. This outcome may partly explain why intra‐specific effect sizes are significantly higher for DEE than for VO2max (see Results), since VO2max is typically much higher than either Msum (Chappell et al. 2004; McKechnie & Swanson 2010; Swanson et al. 2012) or DEE (Song & Wang 2002; Chappell et al. 2007) and therefore a greater multiple of minMR.
Other factors may also account for the overall low intra‐specific correlations observed for VO2max. First, motivation can be an issue in measurements of exercise‐induced maximal performance (Losos, Creer & Schulte 2002) and could have a major impact on estimates of VO2max, but not Msum or DEE. Second, studies of VO2max often included a small number of individuals (Fig. 2, Appendix S4). For instance, 39% of the correlations compiled for VO2max were obtained with N < 20, compared to 6% for Msum and 23% for DEE; when these estimates are removed, the mean pooled effect size increases (and the ±95% confidence intervals drop) from 0·108 ± 0·363 shown in Fig. 4 to 0·152 ± 0·194. Consequently, minMR and VO2max might exhibit a positive intra‐specific correlation more often than reported, simply because it may be more difficult to establish such an association for this particular trait. Third, maximum metabolic rates are typically measured during or after intense exercise in postprandial individuals, but there is evidence that digestion can increase oxygen consumption rates during exhaustive exercise in some species (Bennett & Hicks 2001; Fu et al. 2009) but not others (Alsop & Wood 1997; Fu et al. 2009; Jackson et al. 2015). Thus, measures at peak exercise alone may underestimate the total maximum aerobic capacity of some species and therefore explain why, relative to DEE, there was weaker evidence for a positive correlation between minMR and VO2max. Finally, there is some evidence that the direction and magnitude of the correlation between minMR and upperMR can change due to individual variation in plasticity in response to environmental factors such as temperature, hypoxia, and salinity (Careau, Gifford & Biro 2014; Norin, Malte & Clark 2016). However, the majority of studies thus far test for a correlation between minMR and VO2max in only a single environment, so at present we are unable to tease apart the relative effects of these extrinsic factors on observed intra‐specific correlations.
An alternative, but not mutually exclusive, explanation is that the strength of this association is malleable and evolves along the phylogeny, as recently described by Nespolo et al. (2017). Hence, the negligible or even negative correlation reported in some studies may be an accurate representation for those species and taxonomic groups. The notion that the association between minMR and upperMR evolves is supported by several lines of evidence. First, a clear phylogenetic signal was detected in our analyses of correlations between minMR and each of VO2max and Msum (Table 2). Second, the comparison between the intra‐specific effect sizes of Msum for birds vs. mammals provides a very compelling example of how physiological differences between lineages may emerge. Whereas small mammals employ brown adipose tissue and non‐shivering thermogenesis to thermoregulate in the cold (Nespolo et al. 2001), birds lack this specialized tissue and rely more heavily on shivering to produce heat (Swanson 2010). A higher positive correlation between minMR and Msum in birds could therefore reflect larger maintenance costs of muscles, since the contribution of brown fat to BMR is negligible (Cannon & Nedergaard 2011), and/or tighter directional selection on reduced body mass due to flight restrictions. Consequently, the evolution of brown fat in mammals, with its inherently low maintenance costs and no mechanical power output, may underlie the disruption of an otherwise general constraint imposed by the association between minMR and upperMR. Finally, there is some evidence that the direction and magnitude of the correlation between minMR and VO2max can differ among species according to their respective life styles and thermal ecology (Gomes et al. 2004). However, given that minMR and VO2max are plastic traits and their intra‐specific association can change as a function of the environment (see above), further study is needed to measure and compare the metabolic rates of different species acclimated to common garden conditions to better elucidate the degree to which these metabolic traits are coupled across different environments.
In the light of these results, the higher effect sizes observed in inter‐specific studies is not entirely surprising. The range of variation in minimum, sustained, and maximum metabolic rates is larger across species, and mass‐corrected metabolic rates can vary up to an order of magnitude among species (Weibel et al. 2004; Hillman, Hancock & Hedrick 2013; Killen et al. 2016) compared to the three to fourfold variation typically observed among individuals within a species (Kvist & Lindström 2001; Labocha et al. 2004; Steyermark et al. 2005; Careau, Gifford & Biro 2014). Whereas it is generally unclear to what extent genetic variation underlies observed phenotypic trends in intra‐specific analyses, inter‐specific comparisons involve by definition a higher degree of genetic differentiation that should partly account for the increased variation in metabolic rates observed across species. Consequently, plastic responses to environmental factors such as food and temperature (McKechnie, Chetty & Lovegrove 2007; Auer et al. 2015a), which will effectively add noise to metabolic estimates, are expected to have a higher impact on the phenotypic variation across individuals than across species. Indeed, there is evidence at the intra‐specific level that environmental and genetic effects can cancel one another out, leading to no phenotypic correlation despite a strong positive genetic one between minMR and VO2max (Sadowska et al. 2005). As such, the statistical power to detect a phenotypic correlation between these variables is expected to be higher in inter‐specific relative to intra‐specific analyses (Konarzewski, Książek & Łapo 2005). Our results confirm this prediction for all estimates across all taxonomic classes (this is apparent in Fig. 5 after noting that the x‐axis range differs between intra‐ and inter‐specific analyses).
In conclusion, our meta‐analyses suggest that a positive association between minMR and upperMR – estimated as VO2max, Msum, or DEE – is pervasive across vertebrate lineages. This is in line with the observation that despite enormous variation in metabolic rates, FAS in vertebrates generally falls within a very narrow range (Bennett & Ruben 1979; Hinds et al. 1993; Killen et al. 2016). Our results, in combination with the relatively low variation in FAS, provide compelling evidence that minMR and upperMR often evolve in tandem. However, more studies are needed to assess the genetic basis of their association since phenotypic correlations do not always mirror genetic ones (Dohm, Hayes & Garland 2001; Sadowska et al. 2005). In addition, the mechanistic causes underlying this observation remain a matter of debate, partly because some taxonomic groups remain very poorly studied (e.g., sustained metabolism and DEE in ectotherms have received little attention in the literature; see Table 1). Whereas research at subordinate levels may reveal the physiological basis of such an association (Hulbert & Else 1999, 2000), more studies of organismal aerobic performance may shed light on the evolutionary causes and ecological consequences of this general constraint.
Authors’ contributions
S.K.A. conceived of the study with input on its design from S.S.K. and E.L.R.; S.K.A. and S.S.K. surveyed the literature and collected the data; S.K.A. and E.L.R. analysed the data; S.K.A. drafted the manuscript. All authors contributed to manuscript revisions and gave final approval for publication.
Data accessibility
Data and associated references are provided in Appendices S2 and S3.
Supporting information
Lay Summary
Appendix S1. Vertebrate phylogeny.
Appendix S2. Intra‐specific studies.
Appendix S3. Inter‐specific studies.
Appendix S4. Funnel plots of effect sizes.
Acknowledgements
The authors would like to thank N.B. Metcalfe for his helpful comments on an initial draft of the manuscript. The manuscript was also improved by the comments of three anonymous referees. S.K.A. was supported by a European Research Council Advanced Grant (number 322784). S.S.K. was supported by a NERC Advanced Fellowship (NE/J019100/1) and European Research Council Starting grant (640004).
References
- Alsop, D. & Wood, C. (1997) The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). Journal of Experimental Biology, 200, 2337–2346. [DOI] [PubMed] [Google Scholar]
- Anderson, K.J. & Jetz, W. (2005) The broad‐scale ecology of energy expenditure of endotherms. Ecology Letters, 8, 310–318. [Google Scholar]
- Artacho, P. & Nespolo, R.F. (2009) Natural selection reduces energy metabolism in the garden snail, Helix aspersa (Cornu aspersum). Evolution, 63, 1044–1050. [DOI] [PubMed] [Google Scholar]
- Auer, S.K. , Salin, K. , Rudolf, A.M. , Anderson, G.J. & Metcalfe, N.B. (2015a) Flexibility in metabolic rate confers a growth advantage under changing food availability. Journal of Animal Ecology, 84, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer, S.K. , Salin, K. , Rudolf, A.M. , Anderson, G.J. & Metcalfe, N.B. (2015b) The optimal combination of standard metabolic rate and aerobic scope for somatic growth depends on food availability. Functional Ecology, 29, 479–486. [Google Scholar]
- Bennett, A.F. & Hicks, J.W. (2001) Postprandial exercise: prioritization or additivity of the metabolic responses? Journal of Experimental Biology, 204, 2127–2132. [DOI] [PubMed] [Google Scholar]
- Bennett, A.F. & Ruben, J.A. (1979) Endothermy and activity in vertebrates. Science, 206, 649–654. [DOI] [PubMed] [Google Scholar]
- Blackmer, A.L. , Mauck, R.A. , Ackerman, J.T. , Huntington, C.E. , Nevitt, G.A. & Williams, J.B. (2005) Exploring individual quality: basal metabolic rate and reproductive performance in storm‐petrels. Behavioral Ecology, 16, 906–913. [Google Scholar]
- Bochdansky, A. , Grønkjær, P. , Herra, T. & Leggett, W. (2005) Experimental evidence for selection against fish larvae with high metabolic rates in a food limited environment. Marine Biology, 147, 1413–1417. [Google Scholar]
- Boratyński, Z. & Koteja, P. (2010) Sexual and natural selection on body mass and metabolic rates in free‐living bank voles. Functional Ecology, 24, 1252–1261. [Google Scholar]
- Bozinovic, F. , Rojas, J.M. , Broitman, B.R. & Vásquez, R.A. (2009) Basal metabolism is correlated with habitat productivity among populations of degus (Octodon degus). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 152, 560–564. [DOI] [PubMed] [Google Scholar]
- Brown, J.H. , Gillooly, J.F. , Allen, A.P. , Savage, V.M. & West, G.B. (2004) Toward a metabolic theory of ecology. Ecology, 85, 1771–1789. [Google Scholar]
- Buckley, L.B. , Rodda, G.H. & Jetz, W. (2008) Thermal and energetic constraints on ectotherm abundance: a global test using lizards. Ecology, 89, 48–55. [DOI] [PubMed] [Google Scholar]
- Burton, T. , Killen, S. , Armstrong, J. & Metcalfe, N. (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proceedings of the Royal Society B: Biological Sciences, 278, 3465–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, B. & Nedergaard, J. (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology, 214, 242–253. [DOI] [PubMed] [Google Scholar]
- Careau, V. , Gifford, M.E. & Biro, P.A. (2014) Individual (co) variation in thermal reaction norms of standard and maximal metabolic rates in wild‐caught slimy salamanders. Functional Ecology, 28, 1175–1186. [Google Scholar]
- Chappell, M.A. , Garland, T. , Rezende, E.L. & Gomes, F.R. (2004) Voluntary running in deer mice: speed, distance, energy costs and temperature effects. Journal of Experimental Biology, 207, 3839–3854. [DOI] [PubMed] [Google Scholar]
- Chappell, M.A. , Garland, T. , Robertson, G.F. & Saltzman, W. (2007) Relationships among running performance, aerobic physiology and organ mass in male Mongolian gerbils. Journal of Experimental Biology, 210, 4179–4197. [DOI] [PubMed] [Google Scholar]
- Chayes, F. (1971) Ratio Correlation: A Manual for Students of Petrology and Geochemistry. University of Chicago Press, Chicago, IL, USA. [Google Scholar]
- Chown, S.L. & Gaston, K.J. (1999) Exploring links between physiology and ecology at macro‐scales: the role of respiratory metabolism in insects. Biological Reviews of the Cambridge Philosophical Society, 74, 87–120. [Google Scholar]
- Dlugosz, E.M. , Harris, B.N. , Saltzman, W. & Chappell, M.A. (2012) Glucocorticoids, aerobic physiology, and locomotor behavior in California mice. Physiological and Biochemical Zoology, 85, 671–683. [DOI] [PubMed] [Google Scholar]
- Dohm, M.R. , Hayes, J.P. & Garland, T. (2001) The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics, 159, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drent, R. & Daan, S. (1980) The prudent parent: energetic adjustments in avian breeding. Ardea, 68, 225–252. [Google Scholar]
- Dutenhoffer, M.S. & Swanson, D.L. (1996) Relationship of basal to summit metabolic rate in passerine birds and the aerobic capacity model for the evolution of endothermy. Physiological Zoology, 65, 1232–1254. [Google Scholar]
- Fu, S.‐J. , Zeng, L.‐Q. , Li, X.‐M. , Pang, X. , Cao, Z.‐D. , Peng, J.‐L. & Wang, Y.‐X. (2009) Effect of meal size on excess post‐exercise oxygen consumption in fishes with different locomotive and digestive performance. Journal of Comparative Physiology B, 179, 509–517. [DOI] [PubMed] [Google Scholar]
- Gomes, F.R. , Chauí‐Berlinck, J.G. , Bicudo, J.E.P. & Navas, C.A. (2004) Intraspecific relationships between resting and activity metabolism in anuran amphibians: influence of ecology and behavior. Physiological and Biochemical Zoology, 77, 197–208. [DOI] [PubMed] [Google Scholar]
- Hammond, K.A. & Diamond, J. (1997) Maximal sustained energy budgets in humans and animals. Nature, 386, 457. [DOI] [PubMed] [Google Scholar]
- Hayes, J. (2010) Metabolic rates, genetic constraints, and the evolution of endothermy. Journal of Evolutionary Biology, 23, 1868–1877. [DOI] [PubMed] [Google Scholar]
- Hayes, J.P. & Garland, T. Jr (1995) The evolution of endothermy: testing the aerobic capacity model. Evolution, 49, 836–847. [DOI] [PubMed] [Google Scholar]
- Hayes, J.P. & O'Connor, C.S. (1999) Natural selection on thermogenic capacity of high‐altitude deer mice. Evolution, 53, 1280–1287. [DOI] [PubMed] [Google Scholar]
- Hedges, L.V. & Olkin, I. (1985) Statistical Methods for Meta‐Analysis. Academic Press Inc, San Diego, CA, USA. [Google Scholar]
- Hillman, S.S. , Hancock, T.V. & Hedrick, M.S. (2013) A comparative meta‐analysis of maximal aerobic metabolism of vertebrates: implications for respiratory and cardiovascular limits to gas exchange. Journal of Comparative Physiology B, 183, 167–179. [DOI] [PubMed] [Google Scholar]
- Hinds, D.S. , Baudinette, R. , Macmillen, R.E. & Halpern, E.A. (1993) Maximum metabolism and the aerobic factorial scope of endotherms. Journal of Experimental Biology, 182, 41–56. [DOI] [PubMed] [Google Scholar]
- Hulbert, A. & Else, P.L. (1999) Membranes as possible pacemakers of metabolism. Journal of Theoretical Biology, 199, 257–274. [DOI] [PubMed] [Google Scholar]
- Hulbert, A. & Else, P.L. (2000) Mechanisms underlying the cost of living in animals. Annual Review of Physiology, 62, 207–235. [DOI] [PubMed] [Google Scholar]
- Hulbert, A.J. & Else, P.L. (2004) Basal metabolic rate: history, composition, regulation, and usefulness. Physiological and Biochemical Zoology, 77, 869–876. [DOI] [PubMed] [Google Scholar]
- Jackson, A.G. , Leu, S.‐Y. , Ford, N.B. & Hicks, J.W. (2015) Patterns of oxygen consumption during simultaneously occurring elevated metabolic states in the viviparous snake Thamnophis marcianus . Journal of Experimental Biology, 218, 3570–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling, M. (1994) Respiration and metabolism Fish Bioenergetics (ed. Jobling M.), pp. 121–145. Chapman & Hall, London, UK. [Google Scholar]
- Killen, S.S. , Glazier, D.S. , Rezende, E.L. , Clark, T.D. , Atkinson, D. , Willener, A.S.T. & Halsey, L.G. (2016) Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. American Naturalist, 187, 592–606. [DOI] [PubMed] [Google Scholar]
- Kleiber, M. (1961) The Fire of Life. An Introduction to Animal Energetics. John Wiley & Sons Inc, New York, NY, USA. [Google Scholar]
- Konarzewski, M. , Książek, A. & Łapo, I.B. (2005) Artificial selection on metabolic rates and related traits in rodents. Integrative and Comparative Biology, 45, 416–425. [DOI] [PubMed] [Google Scholar]
- Koteja, P. (2000) Energy assimilation, parental care and the evolution of endothermy. Proceedings of the Royal Society of London B: Biological Sciences, 267, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Książek, A. , Konarzewski, M. & Łapo, I.B. (2004) Anatomic and energetic correlates of divergent selection for basal metabolic rate in laboratory mice. Physiological and Biochemical Zoology, 77, 890–899. [DOI] [PubMed] [Google Scholar]
- Kvist, A. & Lindström, Å. (2001) Basal metabolic rate in migratory waders: intra‐individual, intraspecific, interspecific and seasonal variation. Functional Ecology, 15, 465–473. [Google Scholar]
- Labocha, M.K. , Sadowska, E.T. , Baliga, K. , Semer, A.K. & Koteja, P. (2004) Individual variation and repeatability of basal metabolism in the bank vole, Clethrionomys glareolus. Proceedings of the Royal Society of London B: Biological Sciences, 271, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larivee, M.L. , Boutin, S. , Speakman, J.R. , McAdam, A.G. & Humphries, M.M. (2010) Associations between over‐winter survival and resting metabolic rate in juvenile North American red squirrels. Functional Ecology, 24, 597–607. [Google Scholar]
- Losos, J.B. , Creer, D.A. & Schulte, J.A. II (2002) Cautionary comments on the measurement of maximum locomotor capabilities. Journal of Zoology, 258, 57–61. [Google Scholar]
- McKechnie, A.E. , Chetty, K. & Lovegrove, B.G. (2007) Phenotypic flexibility in the basal metabolic rate of laughing doves: responses to short‐term thermal acclimation. Journal of Experimental Biology, 210, 97–106. [DOI] [PubMed] [Google Scholar]
- McKechnie, A.E. & Swanson, D.L. (2010) Sources and significance of variation in basal, summit and maximal metabolic rates in birds. Current Zoology, 56, 741–758. [Google Scholar]
- Mueller, P. & Diamond, J. (2001) Metabolic rate and environmental productivity: well‐provisioned animals evolved to run and idle fast. Proceedings of the National Academy of Sciences, 98, 12550–12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespolo, R.F. , Bacigalupe, L.D. , Rezende, E.L. & Bozinovic, F. (2001) When nonshivering thermogenesis equals maximum metabolic rate: thermal acclimation and phenotypic plasticity of fossorial Spalacopus cyanus (Rodentia). Physiological and Biochemical Zoology, 74, 325–332. [DOI] [PubMed] [Google Scholar]
- Nespolo, R.F. , Bustamante, D.M. , Bacigalupe, L.D. & Bozinovic, F. (2005) Quantitative genetics of bioenergetics and growth‐related traits in the wild mammal, Phyllotis darwini . Evolution, 59, 1829–1837. [PubMed] [Google Scholar]
- Nespolo, R.F. , Solano‐Iguaran, J.J. , Bozinovic, F. , Williams, T.D. & Bronstein, J.L. (2017) Phylogenetic analysis supports the aerobic‐capacity model for the evolution of endothermy. The American Naturalist, 189, 13–27. [DOI] [PubMed] [Google Scholar]
- Ngo, L. & Brand, R. (1997) Model Selection in linear mixed effects models using SAS. Proc Mixed SAS Users Group International. San Diego, CA, March 16–19.
- Nilsson, J.Å. , Åkesson, M. & Nilsson, J. (2009) Heritability of resting metabolic rate in a wild population of blue tits. Journal of Evolutionary Biology, 22, 1867–1874. [DOI] [PubMed] [Google Scholar]
- Norin, T. & Malte, H. (2012) Intraspecific variation in aerobic metabolic rate of fish: relations with organ size and enzyme activity in Brown Trout. Physiological and Biochemical Zoology, 85, 645–656. [DOI] [PubMed] [Google Scholar]
- Norin, T. , Malte, H. & Clark, T.D. (2016) Differential plasticity of metabolic rate phenotypes in a tropical fish facing environmental change. Functional Ecology, 30, 369–378. [Google Scholar]
- Packard, G.C. (1968) Oxygen consumption of Microtus montanus in relation to ambient temperature. Journal of Mammalogy, 49, 215–220. [PubMed] [Google Scholar]
- Pagel, M. (1999) Inferring the historical patterns of biological evolution. Nature, 401, 877–884. [DOI] [PubMed] [Google Scholar]
- Peterson, C.C. , Walton, B.M. & Bennett, A.F. (1998) Intrapopulation variation in ecological energetics of the garter snake Thamnophis sirtalis, with analysis of the precision of doubly labeled water measurements. Physiological and Biochemical Zoology, 71, 333–349. [DOI] [PubMed] [Google Scholar]
- Pontzer, H. , Brown, M.H. , Raichlen, D.A. et al (2016) Metabolic acceleration and the evolution of human brain size and life history. Nature, 533, 390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende, E.L. , Bozinovic, F. , Garland, T. Jr & Merilä, J. (2004) Climatic adaptation and the evolution of basal and maximum rates of metabolism in rodents. Evolution, 58, 1361–1374. [DOI] [PubMed] [Google Scholar]
- Rezende, E.L. , Chappell, M.A. , Gomes, F.R. , Malisch, J.L. & Garland, T. (2005) Maximal metabolic rates during voluntary exercise, forced exercise, and cold exposure in house mice selectively bred for high wheel‐running. Journal of Experimental Biology, 208, 2447–2458. [DOI] [PubMed] [Google Scholar]
- Rezende, E.L. , Swanson, D.L. , Novoa, F.F. & Bozinovic, F. (2002) Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. Journal of Experimental Biology, 205, 101–107. [DOI] [PubMed] [Google Scholar]
- Ricklefs, R.E. , Konarzewski, M. & Daan, S. (1996) The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. American Naturalist, 147, 1047–1071. [Google Scholar]
- Royall, R.M. (1997) Statistical Evidence: A Likelihood Paradigm. Chapman & Hall, London, UK. [Google Scholar]
- Sadowska, E.T. , Labocha, M.K. , Baliga, K. , Stanisz, A. , Wróblewska, A.K. , Jagusiak, W. & Koteja, P. (2005) Genetic correlations between basal and maximum metabolic rates in a wild rodent: consequences for evolution of endothermy. Evolution, 59, 672–681. [PubMed] [Google Scholar]
- Sadowska, E.T. , Stawski, C. , Rudolf, A. , Dheyongera, G. , Chrząścik, K.M. , Baliga‐Klimczyk, K. & Koteja, P. (2015) Evolution of basal metabolic rate in bank voles from a multidirectional selection experiment. Proceedings of the Royal Society B, 282, 20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Z. & Wang, D. (2002) Relationship between metabolic rate and organ size in Brandt's voles (Microtus brandti). Acta Theriologica Sinica, 23, 230–234. [Google Scholar]
- Speakman, J.R. (2000) The cost of living: field metabolic rates of small mammals. Advances in Ecological Research, 30, 177–297. [Google Scholar]
- Speakman, J.R. , Król, E. & Johnson, M.S. (2004) The functional significance of individual variation in basal metabolic rate. Physiological and Biochemical Zoology, 77, 900–915. [DOI] [PubMed] [Google Scholar]
- Steyermark, A.C. (2002) A high standard metabolic rate constrains juvenile growth. Zoology, 105, 147–151. [DOI] [PubMed] [Google Scholar]
- Steyermark, A.C. , Miamen, A.G. , Feghahati, H.S. & Lewno, A.W. (2005) Physiological and morphological correlates of among‐individual variation in standard metabolic rate in the leopard frog Rana pipiens . Journal of Experimental Biology, 208, 1201–1208. [DOI] [PubMed] [Google Scholar]
- Swanson, D.L. (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. Current Ornithology, 17, 75–129. [Google Scholar]
- Swanson, D.L. , Thomas, N.E. , Liknes, E.T. & Cooper, S.J. (2012) Intraspecific correlations of basal and maximal metabolic rates in birds and the aerobic capacity model for the evolution of endothermy. PLoS ONE, 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taigen, T.L. (1983) Activity metabolism of anuran amphibians: implications for the origin of endothermy. American Naturalist, 121, 94–109. [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010) Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- Weibel, E.R. , Bacigalupe, L.D. , Schmitt, B. & Hoppeler, H. (2004) Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respiratory Physiology & Neurobiology, 140, 115–132. [DOI] [PubMed] [Google Scholar]
- White, C.R. & Kearney, M.R. (2013) Determinants of inter‐specific variation in basal metabolic rate. Journal of Comparative Physiology B, 183, 1–26. [DOI] [PubMed] [Google Scholar]
- Wiersma, P. , Chappell, M.A. & Williams, J.B. (2007) Cold‐and exercise‐induced peak metabolic rates in tropical birds. Proceedings of the National Academy of Sciences, 104, 20866–20871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wone, B. , Madsen, P. , Donovan, E. , Labocha, M. , Sears, M. , Downs, C. , Sorensen, D. & Hayes, J. (2015) A strong response to selection on mass‐independent maximal metabolic rate without a correlated response in basal metabolic rate. Heredity, 114, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wone, B. , Sears, M.W. , Labocha, M.K. , Donovan, E.R. & Hayes, J.P. (2009) Genetic variances and covariances of aerobic metabolic rates in laboratory mice. Proceedings of the Royal Society B: Biological Sciences, 276, 3695–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lay Summary
Appendix S1. Vertebrate phylogeny.
Appendix S2. Intra‐specific studies.
Appendix S3. Inter‐specific studies.
Appendix S4. Funnel plots of effect sizes.
Data Availability Statement
Data and associated references are provided in Appendices S2 and S3.


