Abstract
Background and Objectives:
Symptomatic uterine fibroids are a societal and healthcare burden with no clear consensus among medical professionals as to which procedural treatment is most appropriate for each symptomatic patient. Our purpose was to determine whether recommendations can be made regarding best practice based on review and analysis of the literature since 2006.
Database:
A systematic search of journal articles relevant to the treatment of symptomatic uterine fibroids was performed within PubMed, clinical society websites, and medical device manufacturers' websites. All clinical trials published in English, representing original research, and reporting clinical outcomes associated with interventions for the management of symptomatic uterine fibroids were considered. Each article was screened and selected based on study type, content, relevance, American College of Obstetricians and Gynecologists score, and internal/external validity. Outcomes of interest were patient baseline characteristics, fibroid characteristics, procedural details, complications, and long-term follow-up. Random-effects meta-analyses were used to test the quantitative data. Assessment of 143 full-length articles through January 2016 produced 45 articles for the quantitative analysis. The weighted combined results from hysterectomy trials were compared with those from uterine-preserving fibroid studies (myomectomy, uterine artery embolization, laparoscopic radiofrequency ablation, and magnetic resonance-guided focused ultrasound).
Conclusion:
We explored trends that might guide clinicians when counseling patients who need treatment of symptomatic fibroids. We found that fibroid therapy is trending toward uterine-conserving treatments and outcomes are comparable across those treatments. Since minimally invasive options are increasing, it is important for the clinician to provide the patient with evidence-based therapeutic strategies.
Keywords: Intervention, Leiomyoma, Symptomatic fibroid, Uterine
INTRODUCTION
Symptomatic uterine fibroids (leiomyomas or myomas) represent a significant societal and healthcare burden, and there is no clear consensus among medical professionals as to which treatment is appropriate for their symptomatic patients. These benign, solid myometrial tumors are the most common tumors found in women. They have an estimated cumulative incidence of up to 70% in white women and 80% in black women during the premenopausal years.1 Severe symptoms may develop in 15 to 30% of cases, and the extent of symptoms depends on fibroid location, number, and size. Submucosal and intramural fibroids typically manifest with abnormal uterine bleeding, whereas subserosal and pedunculated fibroids usually present with bulk-related symptoms of pelvic pain and bowel or bladder dysfunction. Symptomatic patients may miss work and, overall, have lower quality of life than asymptomatic patients.2 In addition, the presence of fibroids may lead to infertility and adverse pregnancy outcomes.3,4
Annual direct and indirect costs of symptomatic fibroids in the United States may exceed $34 billion.5 Of the more than 400,000 inpatient hysterectomies performed annually in the United States, the overwhelming indications are symptomatic leiomyomas.6 Although most women with symptomatic fibroids initially choose nonsurgical management, this approach fails in many, and patients may then attempt uterine-conserving therapy—myomectomy (abdominal, laparoscopic, hysteroscopic, and robot-assisted), uterine artery embolization (UAE), radiofrequency ablation (Lap-RFA), and magnetic resonance-guided focused ultrasound surgery (MRg-FUS)—or they may opt for hysterectomy.7
We performed a systematic review and meta-analysis of data from the last 10 years of clinical studies that described populations of premenopausal women seeking surgical management (both uterine-sparing and hysterectomy) of their symptomatic fibroids to determine if any recommendations can be made regarding best practice. Demographic and fibroid characteristics of patients who choose uterine-conserving therapy versus hysterectomy, as well as the perioperative and long-term clinical outcomes of the various surgical approaches in terms of safety and efficacy, are described and analyzed.
METHODS
Literature Search
MOOSE guidelines for meta-analysis and systematic review of observational studies76 were followed in describing the sources and in the study selection process, results, and discussion. A systematic electronic search of journal articles relevant to the treatment of symptomatic uterine fibroids was performed with the following MeSH key words (uterine fibroid, leiomyoma, symptomatic, interventions, English, humans, 2006–2016) within PubMed, clinical society websites, and medical device manufacturers' websites.8 We were guided by and established consensus regarding each article's relevance and comparability using standardized data collection forms (see the Appendix), and we scored the quality of publications in terms of the clarity of risk/benefit, methodological strength of supporting evidence, and clinical implications.9 Meta-analysis of data extracted from the publications from January 1, 2006 to January 31, 2016 was summarized in evidence tables (Supplemental Tables S1, S2, and S3)77 to address the following three research questions:
What are the general demographics of patients choosing to have a uterine-conserving therapy versus a hysterectomy in treating their symptomatic uterine fibroids?
What are the types of uterine fibroids treated with each of the uterine-conserving therapies versus a hysterectomy in the management of symptomatic uterine fibroids?
What are the short-term (≤90 days after the procedure) and long-term (>90 days after the procedure) clinical outcomes with a uterine-conserving approach versus a hysterectomy in the management of symptomatic uterine fibroids?
The literature search focused on the following procedures:
Hysterectomy: surgical removal of the uterus via vaginal or abdominal (open or laparoscopic) incision.
Myomectomy: uterine-sparing surgical removal of fibroids via abdominal, laparoscopic (including robot-assisted), and hysteroscopic approaches.
Uterine artery embolization (UAE): an interventional radiologist identifies the uterine vessels that supply the fibroids and occlude the vessels with trisacryl gelatin microspheres or polyvinyl alcohol particles.10,11 Usually, the approach is via the transcutaneous femoral artery. Fibroids undergo devascularization and ultimately involution.
Magnetic resonance-guided focused ultrasound (MRg-FUS): an interventional radiologist focuses magnetic resonance-guided high-frequency ultrasound energy to ablate fibroid tissue.12
Radiofrequency volumetric thermal ablation (Lap-RFA): laparoscopic ultrasound-guided treatment of fibroids. Thermal energy is delivered to fibroids, sparing normal tissue. Fibroids shrink and may be reabsorbed by the body over time.13
Classification of Complications
Peri- and postoperative complications that occurred during the first 90 days after fibroid treatment were reported. Major complications were defined as adverse events that carried moderate to significant clinical implications for the patient and included the following: bowel or bladder injury, pelvic abscess, wound infection, blood transfusion, pneumonia, prolonged hospital stay (>48 h after UAE, >72 h after a laparoscopic procedure, or >144 h after an open procedure), need for additional course of antibiotics, hematoma evacuation, pulmonary embolus, ileus, vaginal hemorrhage, sarcoma on final pathology, prolonged postoperative fever with need for antibiotics (>2 day), reoperation, sepsis, bowel obstruction, hernia at the incision site, conversion to laparotomy, emergency department evaluation, readmission, ICU admission, unanticipated medical therapy, amenorrhea, skin burn or ulcer, unstable angina, pyelonephritis, ischemic limb, and sciatic nerve palsy. Minor complications were atelectasis, urinary tract infection, headache after epidural placement, rash/urticaria/blister, postembolization syndrome, groin hematoma, abdominal wall bruising or hematoma that resolved spontaneously, urinary retention with or without short-term use of Foley catheter, vertigo, vaginal discharge, spontaneous fibroid expulsion, temporary amenorrhea, transient decrease in libido, and arterial spasms.
Study Selection
We selected studies based on objective criteria (Table A1), specified a priori to avoid bias in the analysis of clinical evidence regarding the effectiveness of uterine-preserving therapies in contrast to hysterectomies for the treatment of symptomatic uterine fibroids. The criteria were designed for selection of those trials most likely to have valid conclusions and be generalizable to routine use. Two gynecologic surgeons (YH and RS) first evaluated the study designs and reporting methods in each article without examining specific results. During the early stages of the review process, both surgeons reviewed the same 5 articles and completed the full-text data-extraction forms. After completing the form, the surgeons employed Delphi methods to review the completed forms together to establish consensus for the coding conventions and form completion. Because a high degree of agreement was found between the 2 reviewing surgeons during this early Delphi Method exercise, the 2 surgeons independently reviewed the remainder of the articles. Additional quality assurance of the review process and data extraction was achieved through a third independent data review. Our research also adopted the American Congress of Obstetrics and Gynecology (ACOG) procedures for grading the quality of the publications.9
Data Extraction
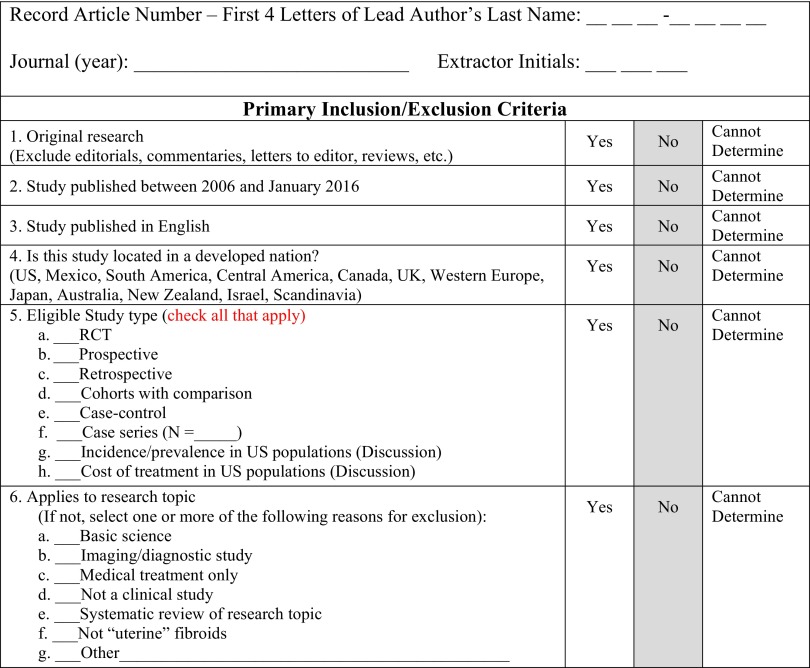
Data-extraction forms were used only for those articles that met all inclusion and exclusion criteria in Phase I of article abstract screening (Figure A1). After documentation of inclusion and exclusion criteria, we progressed to Phase II of full article screening (Figure A2). Data abstracted from each article were transferred directly into individual evidence tables. Supplemental Table S177 includes patient demographics such as average age, body mass index (BMI), race, parity, baseline Health-Related Quality of Life (HRQL) scores, baseline Symptom Severity Scores, and baseline EQ-5D scores stratified by intervention. Supplemental Table S277 consists of literature-reported fibroid and procedural information such as average operative time, estimated blood loss, complications, uterine volume, number of fibroids per patient, largest size fibroids, and type of fibroid stratified by intervention. Supplemental Table S377 comprises short and long-term outcomes such as average hospital stay, length of follow-up, HRQL scores, Symptom Severity Scores, EQ-5D scores, reintervention and hospital readmission rates stratified by intervention. Evidence table development and subsequent analyses were performed by an independent statistician.
Analytical Methods
Univariate random-effects meta-analysis methods were used to synthesize and test the quantitative data for single- and multi-arm trials. For continuous outcomes, a general formula weighted-average effect size (d+) determined the difference in mean scores. The Dwass, Steel, Critchlow-Fligner (DSCF) test, which is based on pairwise 2-sample Wilcoxon comparisons, was used for multiple comparison analysis.14–16 The DSCF analysis is appropriate when the number of interventions is greater than 2. Under the null hypothesis of no location differences among r samples, the distribution of the DSCF statistics can be approximated by the studentized range distribution for r independent standard normal variables. The P-value for a two-sample DSCF comparison is the percentile of the studentized range distribution that corresponds to the value of the DSCF statistic.17–19
For the analysis of 1-way ANOVA with multiple levels, the nonparametric alternative Friedman test was used to test the following hypothesis, where M is median of each group:
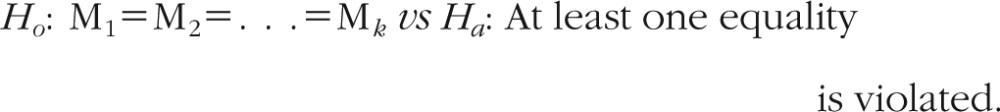 |
The test statistics under Ho follows a χ2 distribution with df = k – 1.
For binary outcomes, the DerSimonian and Laird method was used to combine 2 × 2 tables.20 Weighting for proportions reported in the studies was related to the inverse of the standard error and indirectly to the sample size. The studies with smaller standard errors and larger sample sizes were given more weight in the calculation of the pooled effect size. When zero counts occurred for study data, a continuity correction of 0.5 was added to every value for that study to test the difference in proportions. Statistical heterogeneity was assessed using Cochran's Q-Statistics.
The analyses were undertaken using MedCalc Statistical Software version 16.8.4 (MedCalc Software bvba, Ostend, Belgium) and JMP Statistical Software, Version 13.0 (SAS Institute, Cary, North Carolina, USA).
RESULTS
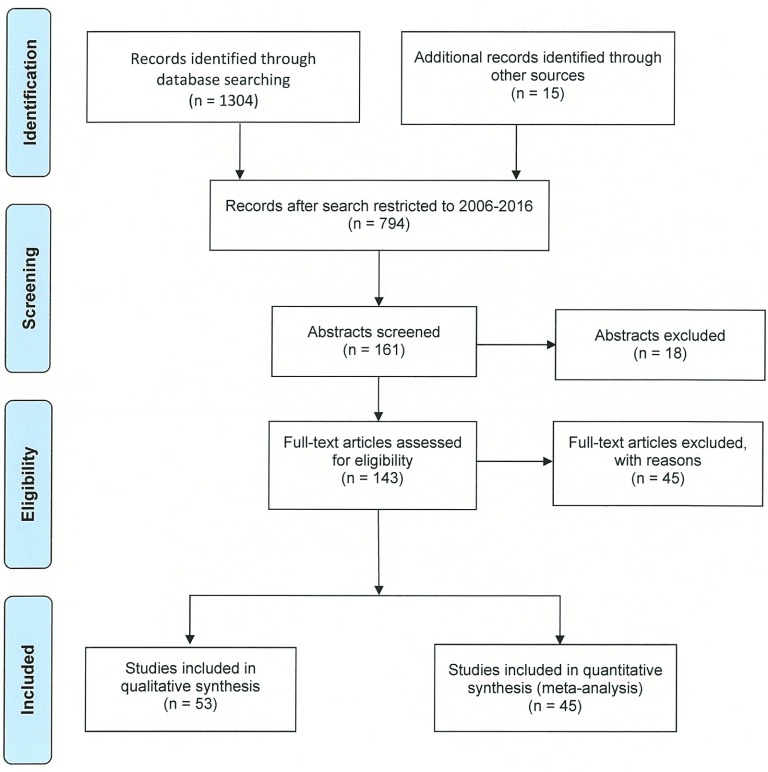
Article disposition through Phase I and Phase II of the screening process is presented in (Figure 1). The initial search resulted in 794 articles published between January 1, 2006 and January 31, 2016. Search of key words followed, and 161 abstracts were screened. From the 161 abstracts screened, 143 full-length articles were assessed for eligibility. Forty-five articles were excluded for several reasons, but mostly because there were no clinical data provided (27%), the article was a discussion paper on treatment options (24%), or was a theoretical position paper (13%). The full-length article screening resulted in 53 articles which provided qualitative information and 45 articles that met the inclusion criteria for the quantitative analysis as shown in the Evidence Tables (Supplemental Tables S1, S2, and S3)77 pertaining to the three Key Questions. Among those 45 articles used in the quantitative analysis, data were abstracted and analyzed from 26 myomectomy studies, 19 UAE studies, 13 Lap-RFA studies, 14 MRg-FUS studies, and 7 hysterectomy studies.
Figure 1.
Article disposition flow diagram.
Patient Characteristics
Baseline patient characteristics among different intervention groups (myomectomy, UAE, Lap-RFA, MRg-FUS and hysterectomy) are presented in Table 1. Age results were combined for as many as 3915 patients from 24 myomectomy studies and as few as 269 patients from six Lap-RFA publications. Women who had hysterectomy were significantly older (45.6 years of age) and patients who had myomectomy were on the average younger (37.4 years of age) than any of the other interventional groups. There were no significant differences in BMI except when contrasting the myomectomy group with the hysterectomy group where the difference in BMI was marginally significant (P = .083). The distribution of patients within each race category for each intervention group is uniform except for the MRg-FUS and Lap-RFA groups. Five of the eight MRg-FUS studies were conducted in an Asian country and 3 of the 6 studies in Lap-RFA group were conducted outside the United States. Reporting of patient parity was sparse, but it appears that patients who underwent hysterectomy had the highest parity (2.0) and those who underwent myomectomy had the lowest parity (0.6).
Table 1.
Baseline Demographics and Patient Characteristics
| Variable | Myomectomy | UAE | Lap-RFA | MRg-FUS | Hysterectomy |
|---|---|---|---|---|---|
| Mean age, y (range) | 37.4**** (32.0–43.2) | 41.7*** (32.4–47.1) | 41.5*** (39.2–43.6) | 43.3** (35.6–46.0) | 45.6 (44.2–49.3) |
| Cohorts, n | 24 | 16 | 6 | 10 | 10 |
| Patients, n | 3915 | 1248 | 269 | 801 | 519365 |
| References | 10, 23–36 | 10, 21, 22, 26, 30, 31, 37–43 | 36, 44–47 | 21, 22, 47–54 | 30, 31, 36, 37, 55, 56 |
| Mean BMI, kg/m2 (range) | 24.0* (20.4–27.2) | 26.6 (23.0–28.4) | 30.4 (29.5–30.9) | 25.2 (21.6–25.9) | 27.8 (24.8–30.5) |
| Cohorts, n | 18 | 4 | 2 | 5 | 6 |
| Patients, n | 1581 | 253 | 127 | 571 | 529 |
| References | 23, 24, 27, 28, 30, 32–36 | 30, 37, 43 | 44 | 30, 36, 37, 56 | 30, 36, 37, 56 |
| Race, % (95% CI) | |||||
| Caucasian | 39.5 (10.9, 72.9) | 58.3 (24.4, 88.2) | 26.3 (2.0, 64.6) | 20.0 (0.56, 56.7) | 70.4 (61.3, 78.7) |
| Black | 22.3 (5.7, 45.8) | 12.4 (1.1, 33.4) | 4.2 (0.9, 23.7) | 5.0 (0.46, 14.1) | 14.5 (8.1, 22.6) |
| Other | 29.3 (0.43, 77.7) | 22.5 (2.0, 55.8) | 63.6 (14.5, 98.7) | 72.2 (25.2, 99.6) | 14.3 (12.0, 16.8) |
| Cohorts, n | 9 | 8 | 6 | 8 | 5 |
| Patients, n | 858 | 589 | 269 | 715 | 519,023 |
| References | 23, 26, 27, 30, 35, 37 | 21, 26, 30, 37, 39, 42, 43 | 44–47, 57 | 21, 47–52, 54 | 30, 37, 55 |
| Mean parity (range) | 0.6 (0.07–1.1) | 0.8 | 1.1 (1.0–1.1) | 2.0 (1.9–2.2) | |
| Cohorts, n | 5 | 1 | 0 | 2 | 4 |
| Patients, n | 674 | 87 | 372 | 334 | |
| References | 23, 26, 32, 36 | 26 | 48, 51 | 36, 56 | |
| Mean baseline HRQL (range) | 42.2 (37.3–46.4) | 41.8 (40.2–42.9) | 40.9 (37.3–60.2) | 47.0 | 40.9 |
| Cohorts, n | 3 | 2 | 4 | 1 | 1 |
| Patients, n | 159 | 181 | 216 | 109 | 106 |
| References | 25, 30, 57 | 25, 30 | 44–46, 57 | 49 | 30 |
| Mean baseline SSS (range) | 61.2 (55.9–70.2) | 62.9 (59.8–65.1) | 60.8 (43.6–77.2) | 61.7 | 64.9 |
| Cohorts, n | 3 | 2 | 4 | 1 | 1 |
| Patients, n | 159 | 181 | 216 | 109 | 106 |
| References | 14, 52, 69 | 52, 69 | 14, 18, 27, 62 | 101 | 69 |
| Mean baseline EQ-5D | 72.3 | 70.0 | 76.4 | ||
| Cohorts, n | 1 | 1 | 2 | 0 | 0 |
| Patients, n | 25 | 106 | 151 | ||
| References | 57 | 38 | 44, 57 |
UAE = uterine artery embolization; Lap-RFA = laparoscopic radiofrequency ablation; MRg-FUS = magnetic resonance-guided focused ultrasound; BMI = body mass index; CI = confidence interval; HRQL = health-related quality of life; SSS = symptom severity score; EQ-5D = EuroQol-5D.
*P < .10,
**P < .05,
***P < .01,
****P < .001, hysterectomy results vs all other interventions.
The results from baseline HRQL assessments were reported sparingly. The largest cohort was four Lap-RFA studies with a combined total of 216 patients. Only 1 MRg-FUS study with 109 patients and 1 hysterectomy study with 106 patients reported administering baseline HRQL surveys. The differences in baseline HRQL scores between treatment groups were not statistically significant. The greatest difference in baseline HRQL scores was 6.1 points between the MRg-FUS group and the combined baseline HRQL scores for the 4 Lap-RFA studies. Only 1 hysterectomy study reported baseline HRQL scores. None of the other differences in baseline HRQL scores for the other interventions were statistically significant. Two papers reported median baseline HRQL scores and, thus, could not be incorporated into the meta-analysis.21,22 In addition, there was no significant difference between baseline symptom severity scores among the intervention groups. The largest difference of 4.2 points in the weighted average baseline symptom severity scores was between 1 hysterectomy study and four lap-RFA studies. No analysis was performed on baseline of EQ-5D since only 4 studies reported this parameter.
Fibroid Characteristics
Uterine and fibroid characteristics are presented in Table 2. The UAE treatment group reporting mean uterine volume was the largest cohort with 10 studies and 792 combined patients. The greatest difference in the weighted average baseline uterine volume was 341 cm3 between the MRg-FUS and Lap-RFA groups; the difference was not statically significant. The difference between the weighted average baseline uterine volume for the Lap-RFA group and any of the other treatment groups trended toward significance.
Table 2.
Fibroid Characteristics at Baseline
| Variable | Myomectomy | UAE | Lap-RFA | MRg-FUS | Hysterectomy |
|---|---|---|---|---|---|
| Mean uterine volume, cm3 (range) | 457.9 (321–707) | 540.5 (305–973) | 214.7 (194–232) | 555.8 (230–792) | 543.5 (484–594) |
| Cohorts, n | 4 | 10 | 2 | 3 | 2 |
| Patients, n | 258 | 792 | 67 | 210 | 194 |
| References | 23, 25, 30 | 21, 25, 30, 37, 38, 41, 43 | 45, 46 | 21, 22, 49 | 30, 37 |
| Mean fibroids/patient, n (range) | 4.5 (1.0–6.5) | 2.1 (2.1–2.2) | 3.9 (1.4–5.0) | 1.5 (1.1–3.9) | 4.0 (4.0–4.0) |
| Cohorts, n | 13 | 2 | 3 | 4 | 1 |
| Patients, n | 3451 | 77 | 212 | 154 | 8 |
| References | 23, 24, 29, 31, 33, 35, 57, 58 | 31, 42 | 44, 47, 57 | 47, 50–52 | 31 |
| Fibroid type, % (95% CI) | |||||
| Subserosal | 45.0 (24.4–63.3) | 16.7 (6.8–29.8) | 29.9 (18.1–43.2) | 22.2 (16.7–28.1) | 38.6 (26.5–51.4) |
| Intramural | 45.1 (35.5, 54.9) | 68.6 (57.1, 79.1) | 56.9 (47.3, 64.9) | 61.6 (50.2, 72.5) | 26.3 (0.8–84.5) |
| Submucosal | 16.1 (7.2–27.7) | 20.2 (14.0–27.3) | 10.3 (3.1–21.2) | 16.2 (4.7–32.8) | 24.1 (0.2–69.0) |
| Cohorts, n | 9 | 7 | 5 | 4 | 2 |
| Fibroids, n | 6020 | 316 | 1049 | 202 | 55 |
| References | 24, 29–32, 57, 58 | 21, 22, 30, 31, 42, 43 | 44–47, 57 | 21, 22, 47, 52 | 30, 31 |
| Largest mean fibroid diameter, cm (range) | 6.4 (4.7–9.2) | 7.0 (5.0–10.7) | 6.9 (4.7–10.0) | 6.7 (4.8–8.5) | 5.7 (5.4–5.9) |
| Cohorts, n | 14 | 10 | 4 | 2 | 2 |
| Patients, n | 1217 | 1003 | 142 | 101 | 194 |
| References | 10, 23, 25, 30, 32–34, 57, 59 | 10, 21, 25, 30, 37–39, 41, 42 | 45–47, 57 | 21, 47 | 30, 37 |
UAE = uterine artery embolization; Lap-RFA = laparoscopic radiofrequency ablation; MRg-FUS = magnetic resonance-guided focused ultrasound; CI = confidence interval.
*P < .10, **P < .05, ***P < .01, ****P < .001 when contrasting hysterectomy results with all other interventions.
The largest mean uterine volume of 973 cm3 was reported for patients in one UAE study25 whereas the largest Lap-RFA group uterine volume was 232.2 cm3.46 In terms of the number of uterine fibroids treated per patient, MRg-FUS group with 4 studies and 154 combined patients had the lowest average number of 1.5 and myomectomy group with 13 studies and 3451 combined patients had the most fibroids treated of 4.5 on the average, but the difference was not statistically significant.
Intramural fibroids were the most frequently treated fibroids in all intervention groups except hysterectomy. The largest diameter on the average ranged from a mean of 5.7 cm for the hysterectomy group (cohort of 2 studies and 194 combined patients) to a mean of 7.0 cm for the UAE group (cohort of 14 studies and 1217 combined patients). The largest fibroid diameter (10.7 cm) on average was from 1 UAE study41 and the smallest mean fibroid diameter reported from one myomectomy study24 and 1 Lap-RFA study47 was 4.7 cm. Differences in fibroid diameters between groups were not statistically significant.
Procedural Details
The greatest difference in weighted average operative time of 78.5 min was between MRg-FUS group (cohort of 3 studies and 98 combined patients) and UAE group (cohort of 5 studies and 697 combined patients) and trended toward statistical significance (P = .054; Table 3. The difference of 45.1 min between the hysterectomy group (cohort of 5 studies and 423 combined patients) and the UAE group was statistical significance (P = .042). The difference of 55.1 min between the myomectomy group (cohort of 16 studies and 3400 combined patients) and UAE group was also statistically significant (P = .017). The longest procedure time of 234 min was reported for one robotic myomectomy study.28 The shortest procedure time of 45 min was reported for one UAE study, which described treatment within uteri of <700 cm3.41
Table 3.
Procedural Details and Early Postoperative Follow-Up
| Variable | Myomectomy | UAE | Lap-RFA | MRg-FUS | Hysterectomy |
|---|---|---|---|---|---|
| Mean operative time, min (range) | 105.9 (68–234) | 50.8* (45–79) | 116.7 (66–126) | 129.3 (93–228) | 95.9 (80–133) |
| Cohorts, n | 16 | 5 | 2 | 3 | 5 |
| Patients, n | 3400 | 697 | 162 | 98 | 423 |
| References | 19, 24, 28, 29, 32–36, 57 | 10, 11, 37, 41 | 44, 57 | 51, 52, 54 | 36, 37, 56 |
| Mean EBL, mL (range) | 175.5 (16–459) | 35.4 (32.5–51.0) | 269.3 (181–474.8) | ||
| Cohorts, n | 15 | 0 | 2 | 0 | 4 |
| Patients, n | 1394 | 162 | 334 | ||
| References | 10, 23, 28, 32–36, 57 | 44, 57 | 36, 56 | ||
| Mean LOS, d (range) | 2.0 (0.5–6.0) | 2.4 (1.0–4.2) | 2.2 (1.8–4.0) | ||
| Cohorts, n | 14 | 8 | 0 | 0 | 5 |
| Patients, n | 3683 | 737 | 429 | ||
| References | 10, 23, 25, 28–30, 35, 36, 58 | 10, 22, 25, 30, 38, 39, 41 | 30, 36, 56 |
UAE = uterine artery embolization; Lap-RFA = laparoscopic radiofrequency ablation; MRg-FUS = magnetic resonance-guided focused ultrasound; EBL = estimated blood loss; LOS = hospital length of stay.
*P < .10, **P < .05, ***P < .01, ****P < .001, hysterectomy results vs all other interventions.
Complications
Complications rates, which ranged from 4.1% to 16.8% among groups, are reported in Table 4. Cohort sizes ranged from 16 studies and 3479 combined patients for the myomectomy group to 3 studies and 229 combined patients for the Lap-RFA treatment group. When contrasting the overall aggregated complication rates for the hysterectomy group (4.1%) with the UAE group (16.8%) and the myomectomy group (7.9%), statistical significance was found for both comparisons (P < .0001 and .004, respectively). The difference between the overall complication rate for the hysterectomy group and that found with the Lap-RFA group (6.3%) and the MRg-FUS group (6.0%) were not statistically significant. However, major complications were infrequent and ranged from 1.3% for MRg-FUS patients to 3.5% for myomectomy patients. Minor complications ranged from 1.6% for hysterectomy patients to 14% for UAE patients. Estimated blood loss (EBL) was greatest in the hysterectomy group at 269.3 mL and was the least at 35.4 mL in the Lap-RFA group. UAE and MRg-FUS studies, as expected, did not report EBL. Patients were hospitalized on average for 2.0–2.4 days after myomectomy, UAE, and hysterectomy. Zero patients who had Lap-RFA and MRg-FUS required hospitalization.
Table 4.
Complications
| Variable | Myomectomy | UAE | Lap-RFA | MRg-FUS | Hysterectomy |
|---|---|---|---|---|---|
| Complication rate, % (95% CI) | |||||
| Overall | 7.9*** (4.6–12.0) | 16.8**** (7.7–28.6) | 6.3 (2.7–11.2) | 6.0 (2.3–11.2) | 4.1 (0.9–9.3) |
| Major | 3.5 (1.8–5.9) | 2.7 (0.9–5.5) | 1.7 (0.4–3.8) | 1.3 (0.3–2.9) | 2.1 (1.0–3.7) |
| Minor | 3.7** (1.6–6.7) | 14.0**** (6.7–23.3) | 4.4** (1.1–9.7) | 5.1*** (1.9–9.7) | 1.6 (0.02–6.8) |
| Cohorts, n | 16 | 10 | 3 | 6 | 5 |
| Patients, n | 3479 | 1154 | 229 | 298 | 439 |
| References | 10, 24, 25, 28–30, 32, 33, 35, 36, 60 | 10, 11, 22, 25, 30, 38, 39, 41, 42 | 60, 61 | 22, 49–53 | 30, 36, 56 |
UAE = uterine artery embolization; Lap-RFA = laparoscopic radiofrequency ablation; MRg-FUS = magnetic resonance-guided focused ultrasound; CI = confidence interval.
*P < .10, **P < .05, ***P < .01, ****P < .001 hysterectomy results vs all other interventions.
Long-Term Follow-up
Follow-up among the different studies varied from 3 to 56 months (Table 5). The longest average follow-up was 34.7 months based on 9 myomectomy studies with 689 combined patients and the shortest average follow-up was 11.2 months for 5 hysterectomy studies with 334 combined patients. The weighted average reintervention rates during reported follow-up periods ranged from 4.2% for the myomectomy group to 30.5% for the MRg-FUS group (cohort of 4 studies and 145 combined patients); the difference of 26.3% was statistically significant (95% CI: 18.7%–34.6%; P < .0001). The difference of 25.3% (95% CI: 16.9%–33.9%; P < .0001) between MRg-FUS and Lap-RFA (cohort of 4 studies and 209 combined patients) was also statistically significant, as was the difference of 15.7% (95% CI: 8.0%–24.2%; P < .0001) between the MRg-FUS and UAE groups (cohort of 12 studies and 1021 combined patients).
Table 5.
Long-term Follow-Up
| Variable | Myomectomy | UAE | Lap-RFA | MRg-FUS | Hysterectomy |
|---|---|---|---|---|---|
| Mean follow-up, mo (range) | 34.7 (12–52) | 13.5 (3–56) | 27.0 (12–36) | 12.6 (6–24) | 11.2 (3–24) |
| Cohorts, n | 9 | 15 | 4 | 5 | 5 |
| Patients, n | 689 | 1423 | 209 | 253 | 334 |
| References | 10, 25, 30, 32, 33, 43, 62 | 10, 11, 21, 22, 25, 30, 37, 38, 40–42, 63 | 45, 46, 62, 64 | 21, 22, 49–51 | 30, 37, 56 |
| Reintervention rate, % (95% CI) | 4.2 (1.3, 8.5) | 14.8 (8.0, 23.1) | 5.2 (0.49, 14.5) | 30.5 (11.6, 53.7) | |
| Cohorts, n | 6 | 12 | 4 | 4 | 0 |
| Patients, n | 915 | 1021 | 209 | 145 | |
| References | 10, 25, 32, 43, 58, 62 | 10, 11, 21, 22, 25, 37, 38, 40, 63 | 45, 46, 62, 64 | 21, 22, 50, 51 | |
| Readmission rate ≤ 90 days of discharge, % (95% CI) | 2.7 (0.91, 5.4) | 3.4 (1.8, 5.6) | 0.74 (0.11, 4.1) | 7.4 | |
| Cohorts, n | 5 | 2 | 2 | 1 | 0 |
| Patients, n | 193 | 346 | 66 | 108 | |
| References | 10, 24, 28 | 10, 11 | 45, 46 | 49 | |
| Weighted mean HRQL (range) | 84.1 (81.1–86.3) | 78.9 (72.9–82.9) | 84.1 (77.8–97.8) | 67.9 | 92.3 |
| Cohorts, n | 3 | 2 | 4 | 1 | 1 |
| Patients, n | 139 | 157 | 193 | 108 | 95 |
| References | 25, 30, 62 | 25, 30 | 45, 46, 62, 64 | 49 | 30 |
| Weighted mean SSS (range) | 37.0 (22.3–55.9) | 38.0 (23.4–59.8) | 19.5 (5.5–27.6) | 37.7 | 7.6 |
| Cohorts, n | 3 | 2 | 4 | 1 | 1 |
| Patients, n | 139 | 157 | 193 | 108 | 95 |
| References | 25, 30, 62 | 25, 30 | 45, 46, 62, 64 | 49 | 30 |
| Weighted mean EQ-5D (range) | 79.3 | 82.0 | 86.2 (85.2–87.2) | ||
| Cohorts, n | 1 | 1 | 2 | 0 | 0 |
| Patients, n | 25 | 93 | 129 | ||
| References | 62 | 38 | 62, 64 |
UAE = uterine artery embolization; Lap-RFA = laparoscopic radiofrequency ablation; MRg-FUS = magnetic resonance-guided focused ultrasound; CI = confidence interval; HRQL = health-related quality of life; SSS = symptom severity score; EQ-5D = EuroQol-5D.
*P < .10, **P < .05, ***P < .01, ****P < .001, hysterectomy results vs all other interventions.
As with reinterventions, a hospital readmission for uterine fibroids among hysterectomy patients is not applicable. Only the difference of 2.7% (95% CI: –3.6%, 5.3%; P = .024) between the UAE group (cohort of 2 studies and 346 combined patients) and the Lap-RFA group (cohort of 2 studies and 66 combined patients) was statistically significant.
Percentage of patients reporting improvement in HRQL and symptom severity scores from baseline was significant between all intervention groups. Greatest improvement in HRQL and symptom severity scores was noted in the hysterectomy group at 92.3 and 7.6, respectively. HRQL scores were equal at 84.1 in the myomectomy and Lap-RFA groups and lowest in the MRg-FUS group at 67.9. Symptom severity scores secondary to hysterectomy were most improved in the Lap-RFA group to 19.5. The differences, nonetheless, in postoperative weighted-average HRQL scores and symptom severity scores between interventions were nonsignificant at the 0.05 significance level.
DISCUSSION
The purpose of this review was to offer practitioners, who provide care to women with fibroids, updated information on the different modalities that are available for interventional management of fibroids. We were looking for trends that might guide clinicians when counseling patients who needed treatment of their fibroids. We concluded that there was no definitive size, type or location of fibroids that favored one treatment approach over another. As the number of women with symptomatic fibroids, who seek minimally invasive options to address their symptoms with the least disruption to their routines increases, it is important to be current with available therapeutic strategies.
It was challenging to assess the outcomes of different available treatment options. From 2006 to 2016, selecting the optimal procedure for the patient was based on a multitude of factors ranging from size, number, and location of fibroids; symptoms; and fertility plans as well as cultural beliefs and perceptions. Hysterectomy is a curative procedure, and results of this analysis indicate that hysterectomy has overall low complication rates and the highest improvement in HRQL and symptom severity scores. However, there was a lack of long-term data on patients who underwent hysterectomy, as we identified only 1 study that assessed outcomes beyond the perioperative period. In the EMMY trial (Uterine Artery Embolization (UAE) Versus Hysterectomy for Uterine Fibroids) over the course of 5 years, 10.7% of patients who underwent hysterectomy needed reintervention because of development of adhesions, vesicovaginal fistula, or the need for reconstructive surgery.65
Increasingly, women are seeking alternatives to hysterectomy and desire uterine conservation even in peri- and postmenopausal age groups. Our review shows that there are several options available for them.
Myomectomy
Myomectomy is a uterine-conserving procedure traditionally provided for patients who desire future fertility. It was unclear whether small fibroids significantly impair fertility unless they were submucosal. Patients who had multiple fibroids or fibroids that were 5 cm and greater in size may be at higher risk for miscarriages, malpresentation of the fetus during pregnancy, cesarean delivery, and postpartum hemorrhage. As expected, women who underwent myomectomy were, on average, younger and had lower parity. Myomectomy was associated with some morbidity, as indicated by a 7.9% complication rate that was second highest after UAE, and moderate EBL (although EBL at myomectomy was less than that at hysterectomy). Traditionally, myomectomy has been by the open abdominal approach; however, in well-selected individuals, myomectomy can be performed laparoscopically, with or without robotic assistance, or using variations of techniques, such as minilaparotomy.33,58 It is a common perception that the abdominal approach for myomectomy provides the strongest repair of the uterine scar, because the defect is closed in multiple layers.23 Over the past decade, this belief has been challenged and debated. In 2004, the first published data came out on robotic-assisted laparoscopic myomectomy with the da Vinci robot (Intuitive Surgical, Sunnyvale, California, USA) and, since that time, an increasing number of providers are using this approach for myomectomy. The advantage of 360° movement of surgical instruments by the robot enables efficient multilayer closure of the uterine scar. Also, data from patients who had laparoscopic myomectomy did not show an increase in uterine scar dehiscence or rupture.58 Gynecologic surgeons must recognize that meticulous closure of hysterotomy incision is critical, especially in women who are considering future pregnancy. Reduced recovery times and reduced patient discomfort are obvious advantages of minimally invasive approaches for myomectomy.
The mean reintervention rate after myomectomy was 4.2% in the 6 studies that reported this event. Patients who had multiple myomas removed had a greater chance of fibroid recurrence, likely prompting further interventions, compared to patients who had fewer fibroids. In addition, practitioners need to counsel patients, who have had myomectomy, that they may need cesarean delivery in an event of pregnancy, if the muscle of the uterus was significantly disrupted and required extensive reconstruction. Vaginal delivery in those circumstances may not be an option because of increased risk of uterine rupture.29 Although cesarean delivery is a commonly performed procedure, it inherently carries additional surgical risks.
Uterine Artery Embolization
Uterine artery embolization became available for fibroid treatment in 1995.38 Since that time, it has become accepted as a minimally invasive, uterine-conserving approach. UAE had the highest reported complication rate of 16.8%. Most of the complications, however, were minor (14%), and only a few (2.7%) were considered to be major. Surprisingly, in the reviewed studies, the typical hospital stay after UAE was 2.4 days, although UAE had the shortest mean operating room time. The reintervention rate of 14.8% at the mean follow-up of 13.5 months was statistically and clinically significant. Patients reported, however, greater improvement of their fibroid symptoms as reflected by post-treatment high HRQL and EQ-5D scores and low symptom severity scores. It is noteworthy that patients who underwent UAE had the largest fibroid diameters compared to all other treatment groups and the largest proportion of intramural myomas; these factors may have contributed to the observed outcomes. In clinical practice, many women fear disruption of ovarian function and earlier onset of menopausal transition that may be associated with UAE; these fears may represent an inherent bias within this patient population. The chance of developing premature ovarian failure was very low in patients who were younger than 40 years of age; however, this risk increased in women older than 45 years as evident by the increase of gonadotropins to postmenopausal levels.66 Patients who desire to preserve their fertility should be carefully counseled as to the benefits and risks associated with UAE.
Laparoscopic Radiofrequency Ablation
In 2012, the U.S. Food and Drug Administration (FDA) approved Lap-RFA for the treatment of symptomatic fibroids. Well documented uses of radiofrequency ablation include treatment of cancers of the liver, kidney, prostate, breast, lung, and skin, as well as cardiac arrhythmias and neurologic and spinal conditions.67–75 Lap-RFA is the newest available minimally invasive, uterine-conserving technique for the treatment of fibroids. Laparoscopy is necessary to visualize fibroids and laparoscopic ultrasound guides correct placement of the ablation needle. However, there is no need for extensive dissection of the, which could lead to scar tissue formation, blood loss, and healing defects. At the time of Lap-RFA, gynecologic surgeons can potentially treat or diagnose other gynecologic conditions such as adnexal masses or endometriosis. Our analysis indicates that Lap-RFA is associated with low complication rates, minimal EBL, and low reintervention rates. In addition, patients reported major improvement in their HRQL and symptom severity scores compared to reports of more traditional interventions, such as hysterectomy, myomectomy, and UAE. Women who had Lap-RFA did not require hospitalization, similar to women who had MRg-FUS. Because of the precise placement of RF probe into a targeted myoma, which is confirmed by laparoscopic ultrasound before ablation, there is minimal disruption of normal myometrium and ovarian function.44 This is advantageous for patients who may desire future pregnancy. Pregnancy data are limited; however, normal full-term pregnancies resulting in vaginal deliveries have been reported after Lap-RFA.60 As of January 2017, a CPT-I code has been assigned. We are hopeful that this recognition of the Lap-RFA as an effective treatment of uterine fibroids will allow more patients to access this procedure.
Magnetic Resonance-Guided Focused Ultrasound
MRg-FUS is another noninvasive method for treatment of fibroids that was approved by the FDA in 2004. This method also carries low complication rates, no blood loss and moderate improvement in HRQL and symptom severity scores. The average reintervention rate, however, was the highest at 30.5%. During this procedure, focused ultrasound is applied through abdominal wall causing significant heat at the target area, therefore, there is significant concern for injury of organs that may be in the way of the focused ultrasound, such as bowel, bladder, and sacral nerves.49 This limits MRg-FUS use in patients whose fibroids may not be safely accessible and influences patient selection. On the other hand, MRg-FUS is FDA approved to treat patients who desire fertility, given that it is not associated with increased risks of spontaneous abortion and placental disorders. Data on successful term pregnancies after MRg-FUS are limited but appear comparable to surgical options.12 However, insurance coverage and reimbursement for MRg-FUS has been inconsistent. In many cases this procedure is approved on an individual basis, creating frustration for both patients and providers.
Limitations and Possible Bias
As this study was a meta-analysis, it was limited by the inherent heterogeneity among studies. We derived our conclusions from different study types with different designs and methodologies to provide a comprehensive review of the most current literature on uterine-sparing procedures. Although some of the included studies were randomized controlled trials, most were not and were assigned an ACOG quality score of B. The data extraction process was challenging for certain data categories, as there was a lack of uniformity in reporting conventions. For example, the same type of complication was considered “major” in one study but “minor” in another; consequently, we used our discretion to categorize complications uniformly to facilitate and validate the statistical analysis. In addition, surgeons may choose to treat larger size fibroids in procedures that have been studied extensively and have received clearance by the FDA, whereas fibroid sizes may be smaller in those premarket studies (because of protocol restrictions) as evident in many patients who underwent Lap-RFA and MRg-FUS.
We did not perform a comprehensive review of hysteroscopic myomectomy or endometrial ablation, as there was a paucity of related data during the study period. Abdominal, laparoscopic, and robotic approaches were included in the myomectomy category and may have influenced some of the measured outcomes. We also did not analyze fertility outcomes or adverse pregnancy outcomes, as these subjects were beyond the scope of this review.
Future Research
The currently available data regarding certain fibroid characteristics, such as size, location, or number are insufficient to assign specific cutoffs that favor one treatment modality over another. Further comprehensive prospective research, ideally in the form of well-powered randomized controlled trials, is needed to validate the specific treatment modality preferred for specific anatomical variances of fibroids.
Cost analysis of different technologies and procedures for fibroid treatment may add tremendous value to the way we view the different approaches. Procedures that have short or no hospital stay, low complication and reintervention rates, and high levels of patient satisfaction in controlling symptoms may become the first-line approaches for treating uterine fibroids.
Ultimately, our goal should be the development of an evidence-based algorithm or guideline, which would assist the clinician in recommending the optimum treatment for women with symptomatic fibroids who desire uterine preservation.
APPENDIX
Figure A1.
Data Extraction Form: Abstract Review Form.
Figure A2.
Data Extraction Form: Full Text Review Form.
Table A1.
Study Selection
| Selection Criteria |
| Study was reported in the English-language peer-reviewed literature, as a full article rather than an abstract. |
| Study reports treatment of symptomatic uterine fibroids information. |
| Comparative trials where interpretation of the study test was not blinded to the results of the reference standard were excluded. |
| Patients reported in one study are not reported in other included studies. |
| At least ten patients in the treatment group and the control group, if one used. |
| Studies were included irrespective of their prospective or retrospective design. |
| Studies were included irrespective of minor discrepancies in accounting for patients. |
| Studies were excluded if isolated case reports. |
| Studies were excluded if random experience reports lacking sufficient detail to permit scientific evaluation; unsubstantiated opinions. |
| Relevance of Data |
| Literature was selected which clinically, technically or biologically demonstrated relevance to the uterine preserving interventions as detailed below. |
| Assessment of Clinical Data |
| The identified literature was assessed for the following: |
| Relevance of author's background and expertise. |
| Whether the conclusion was substantiated by the available data. |
| Whether literature reflects the current medical practice. |
| Whether references were from recognized scientific publications. |
| Whether scientific principles in relation to study design, good clinical practices, and so forth, were followed. |
| American Congress of Obstetrics and Gynecology, ACOG, Publication Grading Criteria* |
| A. There is good evidence to support the recommendation. |
| B. There is fair evidence to support the recommendation. |
| C. There is insufficient evidence to support the recommendation; however, the recommendation may be made on other grounds. |
| D. There is fair evidence against the recommendation. |
| E. There is good evidence against the recommendation. |
*An ACOG grade of A or B was required for the publication to be included in the meta-analysis.
Contributor Information
Yelena Havryliuk, Department of Obstetrics and Gynecology, Weill Cornell Medical College, New York, New York, USA..
Robert Setton, Department of Obstetrics and Gynecology, Weill Cornell Medical College, New York, New York, USA..
John J. Carlow, Discovery Statistics, San Clemente, California, USA..
Barry D. Shaktman, Department of Obstetrics and Gynecology, Weill Cornell Medical College, New York, New York, USA..
References:
- 1. Baird DD, Dunson DB, Hill MV, Cousins D, Schechtman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. [DOI] [PubMed] [Google Scholar]
- 2. Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1–319.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pritts EA, Parker WH, Olive DL. Fibroids and Infertility: an updated systematic review of the literature. Fertil Steril. 2009;91:1215–1223. [DOI] [PubMed] [Google Scholar]
- 4. Lee HJ, Norwitz ER, Shaw J. Contemporary management of fibroids in pregnancy. Rev Obstet Gynecol. 2010;3:20–27. [PMC free article] [PubMed] [Google Scholar]
- 5. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211.e1–211.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacoby VL, Jacoby A, Learman LA, et al. Use of medical, surgical and complementary treatments among women with fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickerson, et al. , Systematic Reviews: Identifying relevant studies for systematic reviews. BMJ 1994;309:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Congress of Obstetricians and of Gynecologists. Reading the Medical Literature. Undated. Available at http://www.acog.org/Resources-And-Publications/Department-Publications/Reading-the-Medical-Literature Accessed November 15, 2015.
- 10. Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tropeano G, Amoroso S, Di Stasi C, et al. Incidence and predictive factors for complications after uterine leiomyoma embolization. Hum Reprod. 2014;29:1918–1924. [DOI] [PubMed] [Google Scholar]
- 12. Froeling V, Meckelburg K, Schreiter NF, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: long-term results. Eur J Radiol. 2013;82:2265–2269. [DOI] [PubMed] [Google Scholar]
- 13. Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768–773. [DOI] [PubMed] [Google Scholar]
- 14. Dwass M. Some k-sample rank-order tests. In: Olkin S., Ghurye G., Hoeffding W., Madow W. G., Mann H. B., eds Contributions to Probability and Statistics. Stanford, CA: Stanford University Press; 1960:198–202. [Google Scholar]
- 15. Steel RGD. A rank sum test for comparing all pairs of treatments. Technometrics. 1960;2:197–207. [Google Scholar]
- 16. Critchlow DE, Fligner MA. On distribution-free multiple comparisons in the one-way analysis of variance. Commun Stat Theory Method. 1991;20:127–139. [Google Scholar]
- 17. Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2nd Ed New York: John Wiley & Sons; 1999. [Google Scholar]
- 18. Juneau P. Simultaneous nonparametric inference in a one-way layout using the SAS System. In: Proceedings of PharmaSUG 2004 (Pharmaceutical Industry SAS User's Group). Cary, NC: SAS Institute Inc.; 2004;paper 4. [Google Scholar]
- 19. Juneau P. Nonparametric methods in pharmaceutical statistics. In: Dmitrienko A, Chuang-Stein C, D'Agostino R, eds. Pharmaceutical Statistics Using SAS: A Practical Guide. Cary, NC: SAS Institute Inc.; 2007:117–150. [Google Scholar]
- 20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trial. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 21. Ikink ME, Nijenhuis RJ, Verkooijen HM. Volumetric MR-guided high-intensity focused ultrasound versus uterine artery embolisation for treatment of symptomatic uterine fibroids: comparison of symptom improvement and reintervention rates. Eur Radiol. 2014;24:2649–2657. [DOI] [PubMed] [Google Scholar]
- 22. Froeling V, Meckelburg K, Scheurig-Muenkler C. Midterm results after uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for symptomatic uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:1508–1513. [DOI] [PubMed] [Google Scholar]
- 23. Ascher-Walsh CJ, Capes TL. Robot-assisted laparoscopic myomectomy is an improvement over laparotomy in women with a limited number of myomas. J Minim Invasive Gynecol. 2010;17:306–310. [DOI] [PubMed] [Google Scholar]
- 24. Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201:566.e1–566.e5. [DOI] [PubMed] [Google Scholar]
- 25. Manyonda IT, Bratby M, Horst JS. Uterine artery embolization versus myomectomy: impact on quality of life: results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) Trial. Cardiovasc Intervent Radiol. 2012;35:530–536. [DOI] [PubMed] [Google Scholar]
- 26. Narayan A, Lee AS, Kuo GP, Powe N, Kim HS. Uterine artery embolization versus abdominal myomectomy: a long-term clinical outcome comparison. J Vasc Interv Radiol. 2010;21:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435–440. [DOI] [PubMed] [Google Scholar]
- 28. Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy: a retrospective matched control study. Fertil Steril. 2009;91:556–559. [DOI] [PubMed] [Google Scholar]
- 29. Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14:453–462. [DOI] [PubMed] [Google Scholar]
- 30. Spies JB, Bradley LD, Guido R, Maxwell GL, Levine BA, Coyne K. Outcomes from leiomyoma therapies: comparison with normal controls. Obstet Gynecol. 2010;116:641–652. [DOI] [PubMed] [Google Scholar]
- 31. Tan N, Mcclure TD, Tarnay C, Johnson MT, Lu DS, Raman SS. Women seeking second opinion for symptomatic uterine leiomyoma: role of comprehensive fibroid center. J Ther Ultrasound. 2014;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tinelli A, Hurst BS, Hudelist G, et al. Laparoscopic myomectomy focusing on the myoma pseudocapsule: technical and outcome reports. Hum Reprod. 2012;27:427–435. [DOI] [PubMed] [Google Scholar]
- 33. Wen KC, Chen YJ, Sung PL, Wang PH. Comparing uterine fibroids treated by myomectomy through traditional laparotomy and 2 modified approaches: ultraminilaparotomy and laparoscopically assisted ultraminilaparotomy. Am J Obstet Gynecol. 2010;202:144.e1–144.e8. [DOI] [PubMed] [Google Scholar]
- 34. Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480–1484. [DOI] [PubMed] [Google Scholar]
- 35. Wang CJ, Lee JM, Yu HT, Huang CY, Chin HY, Wang SM. Comparison of morcellator and culdotomy for extraction of uterine fibroids laparoscopically. Eur J Obstet Gynecol Reprod Biol. 2014;183:183–187. [DOI] [PubMed] [Google Scholar]
- 36. Odejinmi F, Maclaran K, Agarwal N. Laparoscopic treatment of uterine fibroids: a comparison of peri-operative outcomes in laparoscopic hysterectomy and myomectomy. Arch Gynecol Obstet. 2015;291:579–584. [DOI] [PubMed] [Google Scholar]
- 37. Hehenkamp WJ, Volkers NA, Birnie E, et al. Pain and return to daily activities after uterine artery embolization and hysterectomy in the treatment of symptomatic uterine fibroids: results from the randomized EMMY trial. Cardiovasc Intervent Radiol. 2006;29:179–187. [DOI] [PubMed] [Google Scholar]
- 38. Edwards RD, Moss JG, Lumsden MA, et al. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360–370. [DOI] [PubMed] [Google Scholar]
- 39. Laios A, Baharuddin N, Iliou K, Gubara E, O'Sullivan G. Uterine artery embolization for treatment of symptomatic fibroids; a single institution experience. Hippokratia. 2014;18:258–261. [PMC free article] [PubMed] [Google Scholar]
- 40. Dueholm M, Langfeldt S, Mafi HM, Eriksen G, Marinovskij E. Re-intervention after uterine leiomyoma embolisation is related to incomplete infarction and presence of submucous leiomyomas. Eur J Obstet Gynecol Reprod Biol. 2014;178:100–106. [DOI] [PubMed] [Google Scholar]
- 41. Choi HJ, Jeon GS, Kim MD, Lee JT, Yoon JH. Is uterine artery embolization for patients with large myomas safe and effective? A retrospective comparative study in 323 patients. J Vasc Interv Radiol. 2013;24:772–778. [DOI] [PubMed] [Google Scholar]
- 42. Jun F, Yamin L, Xinli X, et al. Uterine artery embolization versus surgery for symptomatic uterine fibroids: a randomized controlled trial and a meta-analysis of the literature. Arch Gynecol Obstet. 2012;285:1407–1413. [DOI] [PubMed] [Google Scholar]
- 43. Hald K, Norent HJ, Istre O, et al. Uterine embolization versus laparoscopic occlusion of uterine arteries for leiomyomas: long-term results of a randomized comparative trial. Am J Obstet Gynecol. 2011;205:317.e1–317.e18. [DOI] [PubMed] [Google Scholar]
- 44. Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121:1075–1082. [DOI] [PubMed] [Google Scholar]
- 45. Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 Ablation System. J Minim Invasive Gynecol. 2011;18:364–371. [DOI] [PubMed] [Google Scholar]
- 46. Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric thermal ablation of uterine myomas with 12 months of follow-up. Int J Gynaecol Obstet. 2013;120:65–69. [DOI] [PubMed] [Google Scholar]
- 47. Meng X, He G, Zhang J, Han Z, Yu M, Zhang M, Tang Y, Fang L, Zhou X. A comparative study of fibroid ablation rates using radio frequency or high-intensity focused ultrasound. Cardiovasc Intervent Radiol. 2010;33:794–799. [DOI] [PubMed] [Google Scholar]
- 48. Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–287. [DOI] [PubMed] [Google Scholar]
- 49. Stewart EA, Rabinovici J, Tempany CMC, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–29. [DOI] [PubMed] [Google Scholar]
- 50. Morita Y, Ito N, Hikida H, et al. Noninvasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids: early experience. Eur J Obstet Gynecol Reprod Biol. 2008;139:199–203. [DOI] [PubMed] [Google Scholar]
- 51. Jacoby VL, Kohi MP, Poder L, et al. PROMISe trial: a pilot, randomized placebo-controlled trial of magnetic resonance guided focused ultrasound for uterine fibroids. Fertil Steril. 2016;105:773–780. [DOI] [PubMed] [Google Scholar]
- 52. Xu Y, Fu Z, Yang L, et al. Feasibility, safety, and efficacy of accurate uterine fibtroid ablation using magnetic resonance imaging-guided high-intensity focused ultrasound with shot sonication. J Ultrasound Med. 2015;34:2293–2303. [DOI] [PubMed] [Google Scholar]
- 53. Thiburce AC, Frulio N, Hocquelet A, et al. Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: Mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperthermia. 2015;31:764–770. [DOI] [PubMed] [Google Scholar]
- 54. Zhao WP, Han ZY, Zhang J, et al. A retrospective comparison of microwave ablation and high intensity focused ultrasound for treating symptomatic uterine fibroids. Eur J Radiol. 2015;84:413–417. [DOI] [PubMed] [Google Scholar]
- 55. Jacoby VL, Autry A, Jacobson G, Domush R, Nakagawa S, Jacoby A. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sesti F, Alonzo F, Ruggeri V, et al. A comparison of vaginal, laparoscopic-assisted vaginal, and minilaparotomy hysterectomies for enlarged myomatous uteri. Int J Gynaecol Obstet. 2008;103:227–231. [DOI] [PubMed] [Google Scholar]
- 57. Brucker SY, Hahn M, Kraemer D, Taran FA, Isaacson KB, Krämer B. Laparoscopic radiofrequency volumetric thermal ablation of fibroids versus laparoscopic myomectomy. Int J Gynaecol Obstet. 2014;125:261–265. [DOI] [PubMed] [Google Scholar]
- 58. Sinha R, Hegde A, Mahajan C, Dubey N, Sundaram M. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292–300. [DOI] [PubMed] [Google Scholar]
- 59. Chen L, Xiao X, Wang Q, Wu C, Zou M, Xiong Y. High-intensity focused ultrasound ablation for diffuse uterine leiomyomatosis: a case report. Ultrason Sonochem. 2015;27:717–721. [DOI] [PubMed] [Google Scholar]
- 60. Hahn M, Brucker S, Kraemer D, et al. Radiofrequency volumetric thermal ablation of fibroids and laparoscopic myomectomy: long-term follow-up from a randomized trial (abstract in English; article in German). Geburtshilfe Frauenheilkd. 2015;75:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Galen DI, Pemueller RR, Leal JG, Abbott KR, Falls JL, Macer J. Laparoscopic radiofrequency fibroid ablation: phase II and phase III results. JSLS. 2014;18:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Krämer B, Hahn M, Taran FA, Kraemer D, Isaacson KB, Brucker SY. Interim analysis of a randomized controlled trial comparing laparoscopic radiofrequency volumetric thermal ablation of uterine fibroids with laparoscopic myomectomy. Int J Gynaecol Obstet. 2015;75:442–449. [DOI] [PubMed] [Google Scholar]
- 63. Dueholm M, Langfeldt S, Mafi HM, Eriksen G, Marinovskij E. Re-intervention after uterine leiomyoma embolisation is related to incomplete infarction and presence of submucous leiomyomas. Eur J Obstet Gynecol Reprod Biol. 2014;178:100–106. [DOI] [PubMed] [Google Scholar]
- 64. Berman JM, Guido RS, Garza Leal JG, et al. Three-year outcome of the Halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. J Minim Invasive Gynecol. 2014;21:767–774. [DOI] [PubMed] [Google Scholar]
- 65. van der Kooij SM, Hehenkamp WJ, Volkers NA, et al. Uterine artery embolization versus hysterectomy in the treatment of symptomatic uterine fibroids: 5 years' outcome from the randomized EMMY trial. Am J Obstet Gynecol. 2010;203:105.e1–105.e.13. [DOI] [PubMed] [Google Scholar]
- 66. Moss JG, Cooper KG, Khaund A, et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011;118:936–944. [DOI] [PubMed] [Google Scholar]
- 67. Zhang CS, Zhang JL, Li XH, Li L, Li X, Zhou XY. Is radiofrequency ablation equal to surgical re-resection for recurrent hepatocellular carcinoma meeting the Milan criteria? A meta-analysis. J BUON. 2015;20:223–230. [PubMed] [Google Scholar]
- 68. Wagstaff P, Ingels A, Zondervan P, de la Rosette JJ, Laguna MP. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol. 2014;24:474–482. [DOI] [PubMed] [Google Scholar]
- 69. Marien A, Gill I, Ukimura O, Betrouni N, Villers A. Target ablation—image-guided therapy in prostate canser. Urol Oncol. 2014;32:912–23. [DOI] [PubMed] [Google Scholar]
- 70. Rubio IT, Landolfi S, Molla M, Cortes J, Xercavins J. Breast-conservative surgery followed by radiofrequency ablation of margins decreases the need for a second surgical procedure for close or positive margins. Clin Breast Cancer. 2014;14:346–351. [DOI] [PubMed] [Google Scholar]
- 71. Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Intervent Radiol. 2014;25:333–339. [DOI] [PubMed] [Google Scholar]
- 72. Almeida JP, Drezek RA, Foster AE. Controlling melanoma at local and systemic levels: is a combination of ablative therapy and immunotherapy the way forward? Immunotherapy. 2014;6:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary centre. Heart Rhythm. 2015;12:1756–1761. Accessed November 15, 2015. [DOI] [PubMed] [Google Scholar]
- 74. Strickland BA, Jimenez-Shahed J, Jankovic J, Viswanathan A. Radiofrequency lesioning through deep grain stimulation electrodes: a pilot study of lesion geometry and temperature characteristics. J Clin Neurosci. 2013;20:1709–1712. [DOI] [PubMed] [Google Scholar]
- 75. Eckmann MS, Martinez MA, Lindauer S, Khan A, Ramamurthy S. Radiofrequency ablation near the bone-muscle interface alters soft tissue lesion dimensions. Reg Anesth Pain Med. 2015;40:270–275. [DOI] [PubMed] [Google Scholar]
- 76. Stroup DF, Berlin JA, Morton SC. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 77. Carlow J. Supplemental data: Demographics of patients undergoing uterine conserving procedures vs hysterectomy. Available at: https://www.fibroiddata.com/ Accessed September 7, 2017.