Abstract
Porphyromonas gingivalis is a Gram‐negative black pigmenting anaerobe that is unable to synthesize heme [Fe(II)‐protoporphyrin IX] or hemin [Fe(III)‐protoporphyrin IX‐Cl], which are important growth/virulence factors, and must therefore derive them from the host. Porphyromonas gingivalis expresses several proteinaceous hemin‐binding sites, which are important in the binding/transport of heme/hemin from the host. It also synthesizes several virulence factors, namely cysteine‐proteases Arg‐ and Lys‐gingipains and two lipopolysaccharides (LPS), O‐LPS and A‐LPS. The gingipains are required for the production of the black pigment, μ‐oxo‐bisheme {[Fe(III)PPIX]2 O}, which is derived from hemoglobin and deposited on the bacterial cell‐surface leading to the characteristic black colonies when grown on blood agar. In this study we investigated the role of LPS in the deposition of μ‐oxo‐bisheme on the cell‐surface. A P. gingivalis mutant defective in the biosynthesis of Arg‐gingipains, namely rgpA/rgpB, produces brown colonies on blood agar and mutants defective in Lys‐gingipain (kgp) and LPS biosynthesis namely porR, waaL, wzy, and pg0129 (α‐1, 3‐mannosyltransferase) produce non‐pigmented colonies. However, only those mutants lacking A‐LPS showed reduced hemin‐binding when cells in suspension were incubated with hemin. Using native, de‐O‐phosphorylated and de‐lipidated LPS from P. gingivalis W50 and porR strains, we demonstrated that hemin‐binding to O‐polysaccharide (PS) and to the lipid A moiety of LPS was reduced compared with hemin‐binding to A‐PS. We conclude that A‐LPS in the outer‐membrane of P. gingivalis serves as a scaffold/anchor for the retention of μ‐oxo‐bisheme on the cell surface and pigmentation is dependent on the presence of A‐LPS.
Keywords: A‐LPS, hemin binding, lipopolysaccharides, pigmentation, Porpyhromonas gingivalis
1. INTRODUCTION
The black pigmenting anaerobe Porphyromonas gingivalis is a major pathogen in chronic adult periodontal disease1 and has recently been described as a “keystone pathogen” with wide‐ranging effects critical for the development of dysbiosis and disease progression.2 The main habitat of this organism is the human gingival crevice where nutrients are gained from gingival crevicular fluid ‐ a plasma exudate. Hemin [Fe(III)‐protoporphyrin IX‐Cl] is an important requirement for growth of P. gingivalis 3, 4 and as the organism is not able to synthesize protoporphyrin IX ring5, 6 and does not contain any siderophores,7, 8 the major source of heme [Fe(II)‐protoporphyrin IX] is therefore the host.
Black‐pigmenting Bacteroides species have been shown to degrade plasma proteins involved in the transport and conservation of body iron, namely albumin, hemopexin, haptoglobin, and transferrin, to varying degrees, with Bacteroides gingivalis (P. gingivalis) being the most effective.9 The cysteine protease Lys‐gingipain (Kgp) of P. gingivalis can cleave free hemoglobin,10 haptoglobin, hemopexin, and transferrin in human serum but was not able to degrade hemoglobin, or the β‐chain of haptoglobin when these were present in a haptoglobin‐hemoglobin complex in serum.11
Porphyromonas gingivalis possesses additional outer membrane proteins that are important in the binding and transport of heme and which form part of the hmu hemin‐uptake locus,12 namely HmuY,13 and HBP35 protein has been described as an important hemin‐binding protein.14 Several putative TonB‐dependent outer‐membrane receptors have been described including Tlr,15 IhtA (iron heme transport),16 HmuR (hemin utilization receptor),6, 17, 18 and HemR (hemin‐regulated receptor).19 HmuR exhibited amino acid sequence homology to TonB‐dependent receptors involved in heme, vitamin B12 or iron‐siderophore transport in other bacteria.17 A P. gingivalis hmuR isogenic mutant strain was shown to have impaired growth on hemin and hemoglobin as sole source of iron and showed decreased ability to bind hemin and hemoglobin. Escherichia coli cells overexpressing P. gingivalis HmuR as well as purified recombinant HmuR were able to bind hemin, hemoglobin and serum albumin‐hemin complex.17
Porphyromonas gingivalis W50 produces several virulence factors including gingipain proteases and two lipopolysaccharides (LPSs), namely O‐LPS20 and A‐LPS.21
In this study, we addressed the question whether the high abundance low‐affinity hemin‐binding site described by Tompkins et al.22 may be one of the LPS of P. gingivalis. To test this hypothesis, we examined a variety of isogenic mutant strains of P. gingivalis lacking Arg‐gingipains, Lys‐gingipain and defective in the biosynthesis of O‐LPS and A‐LPS for their ability to pigment and to bind hemin not only to whole cells but also to LPS, de‐phosphorylated LPS and de‐lipidated LPS. Mutant strains of P. gingivalis: porR (PG1138), which is defective in A‐LPS synthesis, and galE (PG0347), which synthesizes a truncated O‐PS repeating unit of O‐LPS, are described in greater detail here in this manuscript. Shoji et al.23 described the porR mutant strain in P. gingivalis ATCC33277 isolated by transposon and targeted mutagenesis and Gallagher et al.24 have referred to a porR mutant strain isolated by inactivation of PG1138 in P. gingivalis W50.
Porphyromonas gingivalis galE (PG0347) shares homology with galE of E. coli, which encodes UDP‐galactose‐4‐epimerase, responsible for the conversion of UDP‐Glc to UDP‐Gal. Galactose is a component of the repeating unit of O‐PS20 and in galE (ΔPG0347), O‐LPS is still synthesized, but its repeating unit is shortened by one residue, namely Gal (Unpublished data, N. Paramonov, J. Aduse‐Opoku, M. Rangarajan and M.A. Curtis).
The results of hemin‐binding to the mutant strains of P. gingivalis exhibit a consistent pattern, which suggests that the deposition of μ‐oxo‐bisheme on the cell surface of the P. gingivalis strains appears to be related to the synthesis/presence of A‐LPS in the outer leaflet of the outer membrane. We propose that the presence of A‐LPS serves as a matrix for the deposition of μ‐oxo‐bisheme on the P. gingivalis cell surface.
2. MATERIALS AND METHODS
2.1. Materials
A solution containing 30% acrylamide‐N,N‐methylenebisacrylamide (BIS; 37.5:1) was obtained from Bio‐Rad Laboratories (Hercules, CA, USA). Horseradish peroxide‐labeled mouse immunoglobulin was purchased from Dako A/S (High Wycombe, UK). All other chemicals were from VWR (Lutterworth, UK) or from Sigma‐Aldrich Co. Ltd (Poole, UK) and were the purest grades available. Nα‐acetyl‐Lys‐p‐nitroanilide was obtained from Bachem Feinchemikalein AG (Bubendorf, Switzerland). Hemin was obtained from Roche (Burgess Hill, UK). Restriction and modification enzymes were purchased from New England BioLabs (Ipswich, MA, USA), and DNA purification reagents were obtained from Qiagen (Hilden, Germany).
2.2. Bacterial strains and growth conditions
Porphyromonas gingivalis W50 and mutant strains were grown either on blood agar plates containing 5% defibrinated horse blood or in brain‐heart infusion broth (Oxoid, Basingstoke, UK) supplemented with hemin (5 μg/mL) in an anaerobic atmosphere consisting of 80% N2, 10% H2 and 10% CO2.25 Clindamycin HCl and tetracycline HCl were added to 5 μg/mL and 1 μg/mL respectively, for selection of ermF and tet Q in P. gingivalis. Ampicillin (Na+ salt; 100 μg/mL) or erythromycin (300 μg/mL) was added to the growth medium to select for pUC‐derived or ermAM‐containing plasmids respectively, in Escherichia coli.
2.3. Generation of P. gingivalis mutants
Purification and general manipulation of DNA, restriction mapping of plasmids and transformation of E. coli were as described previously.25, 26
A list of P. gingivalis strains used in this study is shown in the Table S1.
For the generation of P. gingivalis mutant strains porR and galE, chromosomal DNA from P. gingivalis W50 was used as the template for amplification/cloning purposes. The nomenclature originally used by TIGR is used throughout the manuscript. The genes encoding UDP‐glucose‐4‐epimerase galE and porR 23, 24, 27, 28 in P. gingivalis W50 were insertionally inactivated with ermF‐ermAM by allelic exchange following electro‐transformation and are described in detail in the Figure S1. The primers used in this study are listed in detail in Supplemental Methods.
2.4. Measurement of enzyme activity
Arg‐gingipain and Lys‐gingipain activities in whole cultures and culture supernatants of P. gingivalis and isogenic mutant strains were measured using N‐benzoyl‐dl‐arginine‐p‐nitroanilide (dl‐BRpNA) and N‐α‐acetyl‐l‐lysine‐p‐nitroanilide (l‐AcKpNA) respectively as substrates, in spectrophotometric assays, as previously described.29 Units of enzyme activity are expressed as change in absorbance at 405 nm/min per optical density at 600 nm (OD600) at 30°C. Enzyme activities were usually measured in triplicate using batches of bacterial cultures grown on different days. Student's t test for paired samples was used and the data were considered to be significant at a P value <.05.
2.5. SDS‐PAGE and SDS‐Urea‐PAGE
Sodium dodecyl sulfate (SDS)‐urea‐polyacrylamide gel electrophoresis (PAGE) of LPS was performed according to Inzana and Apicella.30 Samples were transferred onto nitrocellulose membranes and probed with MAb1B5, which recognizes the epitope Manα1‐2‐Manα1‐phosphate fragment in A‐PS of A‐LPS, as described previously.27 Silver staining of gels was performed using the Silver staining kit (Sigma‐Aldrich Co. Ltd.) according to the manufacturer's instructions.
2.6. Isolation of LPS and Lipid A
Lipopolysaccharide from P. gingivalis W50 and mutant strains for use in SDS‐urea‐PAGE experiments was prepared using an LPS extraction kit from Intron Biotechnology (South Korea).
Lipopolysaccharides used in hemin‐binding studies was prepared as described previously.31 De‐O‐phosphorylated LPS samples used in hemin‐binding experiments were prepared by dissolving LPS (10‐15 mg) in 0.5 mL of 48% aqueous hydrofluoric acid at 4°C and incubating at 4°C for 16 hours. Excess hydrofluoric acid was removed by dialysis against distilled water (6000‐8000 MWCO tubing) at 4°C followed by freeze‐drying.
De‐lipidation of LPS samples was carried out by treatment with 1.5% aqueous acetic acid at 100°C for 2‐4 hours in a heating block. Insoluble lipid A and traces of undegraded LPS were removed by ultracentrifugation at 30,000 g for 30 minutes at 10°C. The water‐soluble supernatant was lyophilized twice to remove all traces of acetic acid.
2.7. Hemin binding to whole cells of P. gingivalis
Porphyromonas gingivalis W50 and mutant strains were grown for 48 hours and cells were harvested by centrifugation (13 300 g) for 20 minutes at 4°C in Eppendorf tubes. The cells were washed with ice‐cold sterile PBS (3×1 mL) and stored at −70°C until required. Frozen cells were thawed and washed twice with 1 mL of sterile PBS. The cells were resuspended in PBS to give an OD600 of 1.25. Cell suspensions (0.8 mL) in triplicate were mixed with 0.2 mL of hemin solution containing 5 μg or 10 μg hemin and incubated at 37°C for 1 hour. Control samples contained 0.8 mL of PBS mixed with 0.2 mL of hemin solution containing 5 μg or 10 μg hemin (as above) for each set of experiments. The reaction mixture was centrifuged at 13,300 g for 20 minutes at 4°C, the supernatant was transferred to 1‐mL plastic disposable cuvettes and the OD400 was measured. Concentration of hemin in the supernatant was calculated from standard curves for hemin. The hemin bound (μg/OD600 of cells) was equal to the difference between the values for the control samples (hemin solution with no added cells, zero binding) and the supernatant from the experimental samples (bound hemin). The standard deviation was calculated.
For statistical analysis, a Student's t test for paired values was used, and data were considered to be significant at a P value <.05.
2.8. Binding of hemin to LPS
Freeze‐dried native LPS, de‐O‐phosphorylated LPS and de‐lipidated LPS samples from P. gingivalis W50 and porR were dissolved/resuspended in 0.05 m Tris‐HCl, pH 7.2 at a concentration of 1 mg/mL. Aliquots (50 μL) of LPS containing 50 μg was added to PBS (0.95 mL) containing 20 μg or 30 μg of hemin, in duplicate, in an Eppendorf tube and incubated at 37°C with shaking. LPS‐, de‐O‐phosphorylated LPS‐ and de‐lipidated‐LPS‐hemin complexes were pelletted by high speed centrifugation (30,000 g) for 60 minutes at 14°C.32 The amount of unbound hemin in the supernatant was determined by measuring the OD400 and the concentration was determined using a standard curve for hemin. The amount of hemin bound was calculated as the difference between the total hemin added to the reaction mixture and the amount present in the supernatant. The mean of two separate determinations±standard error of the mean was calculated ( http://www.upscale.utoronto.ca/PVB/Harrison/ErrorAnalysis/ ).
3. RESULTS
The P. gingivalis mutant strains used in this study have been described elsewhere (see Table S1). These include strains in which the genes encoding the proteases Rgps (rgpA/rgpB) and Kgp (kgp) have been inactivated, leading to loss of Arg‐gingipains and Lys‐gingipain, respectively,33 both of which have been strongly implicated in hemin acquisition by P. gingivalis.34
Inactivation of PG1051(waaL,O‐antigen ligase),21, 31 PG1142 (wzy, O‐antigen polymerase),31 and PG0129 (α‐1,3‐mannosyl transferase)35 lead to defects in LPS synthesis and have been described in detail elsewhere. In this manuscript, we have also studied porR where there is no A‐LPS synthesis and galE, which synthesizes O‐LPS, where the O‐PS repeating unit is shortened by a residue of Gal, in greater detail.
PorR is a putative transaminase and is homologous to the RfbE orthologue of P. gingivalis and belongs to the DegT Clusters of Orthologous Groups (COGs), the prototype of which is DegT of Geobacillus (Bacillus) stearothermophilus,36 which is involved in a range of biochemical functions including glycan synthesis, regulation of extracellular enzymes, altered control of sporulation, abnormal cell division, and loss of flagella.36 Proteins homologous to PorR have been found in several microorganisms involved in the biosynthesis of sugars present in capsular polysaccharide and aminoglycosides. In P. gingivalis, the inactivation of porR leads to pleiotropic effects involving loss of pigmentation, lack of synthesis of A‐LPS,27 processing of other proteins including fimbriae, and major alteration to the surface of the cell without perceptible effect on O‐LPS.23, 24, 27, 28 In addition, the Rgp isoforms, namely HRgpA and RgpB, which do not acquire the MAb1B5‐reactive glycan are present in the porR mutant strain whereas the isoforms that usually contain the MAb1B5 cross‐reactive epitope, namely RgpAcat and mt‐Rgps27 are not synthesized. However, the synthesis of O‐LPS is not affected in the porR mutant strain and [1H]NMR spectroscopy of the O‐PS isolated from O‐LPS of this strain showed an identical [1H]NMR spectrum to that of O‐PS from the P. gingivalis W50 parent strain.27 Biologically, these effects translate to cell fragility, loss of recognition by antibodies of the periodontal patients’ sera, and an enhanced complement‐mediated killing as a result of the inability to synthesize A‐LPS.23, 24, 27, 28
3.1. Pigmentation and hemolysis of P. gingivalis strains
Porphyromonas gingivalis W50, rgpA/rgpB and galE form brown‐ or black‐pigmented colonies on blood agar plates (Figure 1), whereas colonies of kgp, porR, waaL, wzy, and pg0129 are non‐pigmented. Also shown is the P. gingivalis mutant strain wbpB, which has been described in detail elsewhere28, 37 and gives non‐pigmented colonies on blood agar plates.
Figure 1.
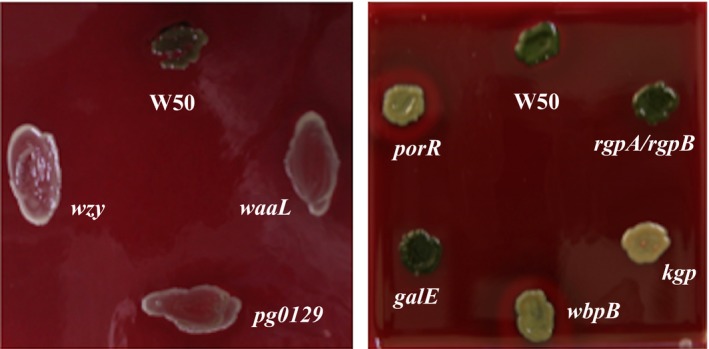
Pigmentation of Porphyromonas gingivalis strains on blood agar plates. P. gingivalis W50 and mutant strains were grown on blood agar plates for 7 d
3.2. Arg‐ and Lys‐gingipain activities in P. gingivalis strains
Arg‐gingipain and Lys‐gingipain activities in whole cultures and culture supernatants of P. gingivalis W50 and isogenic mutant strains were measured after either 24 or 48 hours and the results are shown in Figure 2A. The Arg‐gingipain (Rgp) activities present in the P. gingivalis strains vary widely. Total Rgp activity (100%) and activities present in cell‐associated and secreted forms in P. gingivalis W50 and kgp mutant strain are similar (~80% and ~20%, respectively) after 24 hours of growth, as expected. Porphyromonas gingivalis rgpA/rgpB mutant strain contains no Rgp activity, as expected. However, mutant strains galE, porR, waaL, wzy, and pg0129 in which LPS synthesis is affected, contain lower levels of Rgp activity compared with the parent W50 strain (P values <.0003). In addition, in mutant strains porR, waaL, wzy, and pg0129, almost all the enzyme activity (~90%‐100%) is secreted into the supernatant after 24 hours of growth compared with the parent W50 strain, in which only ~20% of Rgp activity is shed into the supernatant. Although galE contains ~50% of total Rgp activity compared with the W50 parent strain after 48 hours of growth, the distribution of enzyme activity between cell‐associated and secreted forms was similar to that of the parent W50 strain: ~30% and ~20% of Rgp activity in cell‐associated and supernatant forms in galE compared with ~60% and ~40% of Rgp activity in cell‐associated and supernatant forms in P. gingivalis W50.
Figure 2.
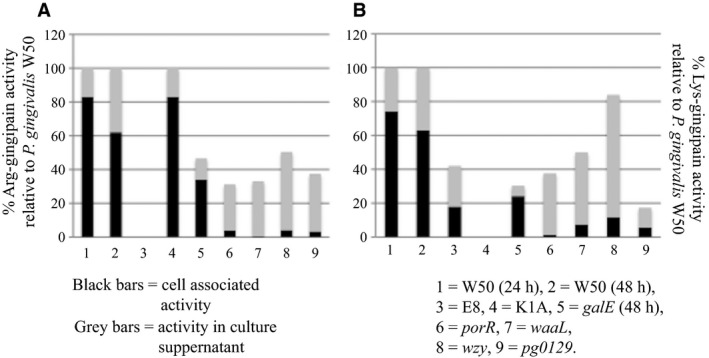
Arg‐gingipain and Lys‐gingipain activities in Porphyromonas gingivalis strains. Porphyromonas gingivalis W50 and isogenic mutant strains were grown in brain‐heart infusion broth for 24 or 48 h. Arg‐X (A) and Lys‐X (B) activities were measured using substrates dl‐BR p NA and L‐AcKp NA respectively as described in Methods. Enzyme activities are expressed as % activity relative to that of the parent P. gingivalis W50 strain (Absorbance405 nm units/min/OD 600). Black bars represent cell‐associated activities and grey bars represent enzyme activities in the culture supernatants
Similarly, the Lys‐gingipain (Kgp) activities (Figure 2B) in whole cultures of the P. gingivalis mutant strains also show wide variation. P. gingivalis W50 contains the highest amount of Kgp activity. As expected, kgp shows no detectable Kgp activity. The amounts of Kgp activity in cell‐associated and culture supernatants also show wide variation (Figure 2B). However, in P. gingivalis mutant strains, namely porR, waaL, wzy, and pg0129, almost all the Kgp activity is present in the culture supernatant after 24 hours of growth, which is very similar to that observed for Rgp activity. Although the Rgp and Kgp activities of the P. gingivalis mutant strains show great variation, these results highlight the properties of the isogenic mutant strains porR, waaL, wzy, and pg0129, where almost all the Rgp and Kgp activities are released into the culture supernatant after 24‐48 hours of growth indicating the lack of tethering/anchoring molecules on the cell surface of these strains, which would otherwise enable these enzymes from being shed into the culture medium. As the mutant strains porR, waaL, wzy, and pg0129 are defective in LPS biosynthesis, the inability to retain the gingipains on the cell surface could be a direct result of this deficiency.
3.3. Cross‐streaking experiments
Porphyromonas gingivalis W50 was initially streaked on a blood agar plate and following the formation of a zone of hemolysis (3 days), the cells were removed with a swab containing clindamycin to suppress regrowth of the wild‐type strain and the plates were cross‐streaked with P. gingivalis mutant strains (Figure 3). Although rgpA/rgpB and kgp give brown and non‐pigmenting colonies when grown on blood agar plates because of the lack of Rgps and Kgp, respectively, they do pigment when cross‐streaked on plates on which P. gingivalis W50 has been previously grown and caused hemolysis (Figure 3). This suggests that rgpA/rgpB and kgp have the ability to pigment if supplied with externally added hemin. However, cross‐streaking of P. gingivalis porR, waaL, wzy, and pg0129 strains on blood agar plates as above did not cause the deposition of hemin/black pigment on the surfaces of these cells (Figure 3). This indicates that the mutant strains are unable to harness any available hemin in the environment and retain it on their cell surface.
Figure 3.
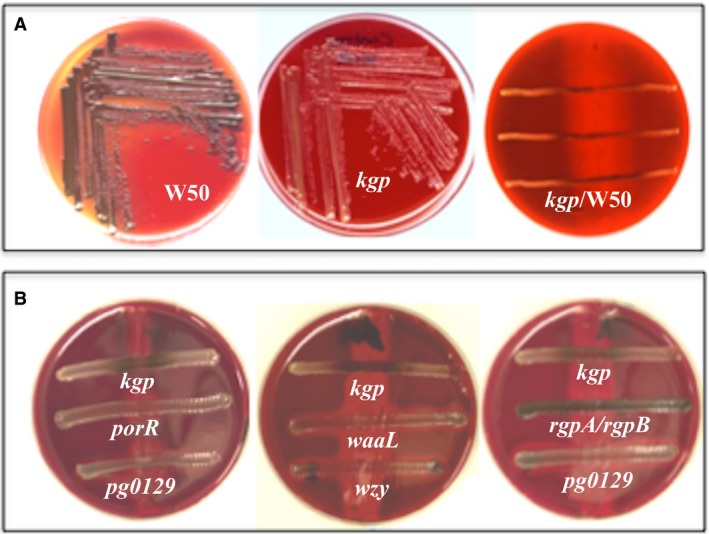
Cross‐streaking of Porphyromonas gingivalis kgp mutant strain on blood agar. (A) P. gingivalis W50 on blood agar, kgp on blood agar, P. gingivalis W50 was initially streaked on a blood agar plate and following the formation of a zone of hemolysis (3 d), the cells were removed with a swab containing clindamycin to suppress regrowth of the wild‐type strain and the plates were cross‐streaked with kgp. Note pigmentation of the kgp mutant cells takes place only on the zone of hemolysis produced by the parent strain. (B) Blood agar plates were initially streaked with W50 as in (A). Plates were cross‐streaked with rgpA/rgpB, kgp, porR, pg0129, waaL, and wzy. Pigmentation of kgp takes place on the zone of hemolysis whereas the other strains do not pigment even after 6 d of growth
3.4. Analysis of LPS
The SDS‐urea‐PAGE followed by silver staining of LPS purified from P. gingivalis W50 and mutant strains rgpA/rgpB, kgp, porR, and galE show the characteristic laddering pattern (Figure 4A). However, in porR and galE, the O‐LPS shows a higher intensity of bands in the core‐, core‐plus one repeating unit and core‐plus two repeating units (Figure 4A). In the P. gingivalis galE mutant strain, the O‐PS repeating unit, [→3)‐α‐D‐Galp‐(1→6)‐α‐D‐Glcp‐(1→4)‐α‐L‐Rhap‐(1→3)‐β‐D‐GalNAcp‐(1→] is shortened by one Gal residue (unpublished data). The SDS‐urea‐PAGE of LPS from P. gingivalis waaL, wzy, and pg0129 mutant strains have been described elsewhere21, 31, 35 and are not shown here.
Figure 4.
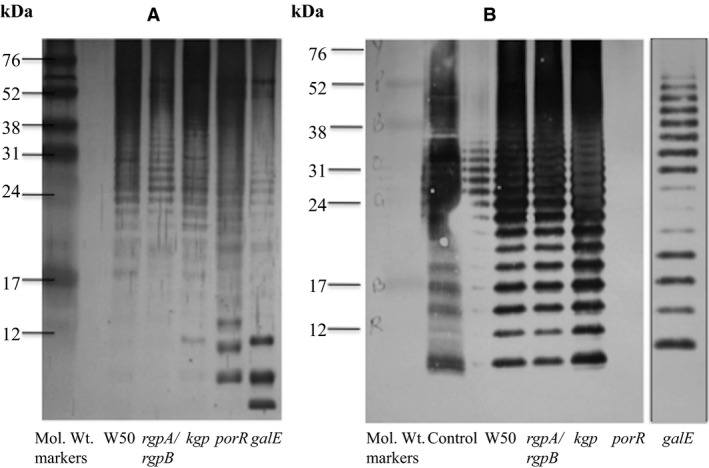
Sodium dodecyl sulfate‐urea‐PAGE and silver staining of lipopolysaccharide (LPS) from Porphyromonas gingivalis W50 and mutant strains (A). Western blotting vs MAb 1B5 of LPS from P. gingivalis W50 and mutant strains (B). LPS purified as described in Methods were subjected to SDS‐urea‐PAGE. The P. gingivalis strains used in the isolation of LPS are indicated below the lanes. The control sample in (B) is the phenol extract of P. gingivalis W50 cells containing predominantly LPS
Sodium dodecyl sulfate‐urea‐PAGE of LPS from P. gingivalis W50 and mutant strains followed by silver staining indicate that all these strains synthesize O‐LPS. SDS‐urea‐PAGE of LPS followed by Western blotting vs MAb 1B5, which recognizes the epitope Manα1‐2‐Manα1‐phosphate fragment in A‐PS of A‐LPS27 show that W50, rgpA/rgpB, kgp, and galE also synthesize A‐LPS (Figure 4B) as indicated by the laddering pattern and immunoreactivity with MAb 1B5. However, porR synthesizes only O‐LPS and A‐LPS is absent as shown by the lack of cross‐reactivity with MAb 1B5 (Figure 4B).
3.5. Hemin binding
3.5.1. Hemin binding by whole cells
Hemin binding by whole cells of P. gingivalis W50, rgpA/rgpB, kgp, galE, porR, waaL, wzy, and pg0129 was measured as described in the Methods section and the results obtained are shown in Figure 5. Porphyromonas gingivalis W50 and mutant strains rgpA/rgpB and galE, which pigmented brown and black on blood agar plates, respectively (Figure 1) showed hemin binding values (5.6 μg/OD600, 6.8 μg/OD600 and 5.9 μg/OD600, respectively), at the highest concentration (10 μg/mL) of hemin used in the binding experiments. Although kgp was non‐pigmenting on blood agar plates due to the absence of Kgp, it shows hemin binding (6.1 μg/OD600) when supplied with hemin (also observed when kgp is cross‐streaked on blood agar plates on which P. gingivalis W50 was previously grown (Figure 3). The P. gingivalis mutant strains porR, waaL, wzy, and pg0129, which were non‐pigmenting on blood agar plates, were able to bind between ~2.5 and 3.7 μg of hemin/OD600 of cells, which is ~45%‐65% of hemin bound by the parent W50 strain. Hence, P. gingivalis mutant strains that do not synthesize A‐LPS show reduced hemin binding.
Figure 5.
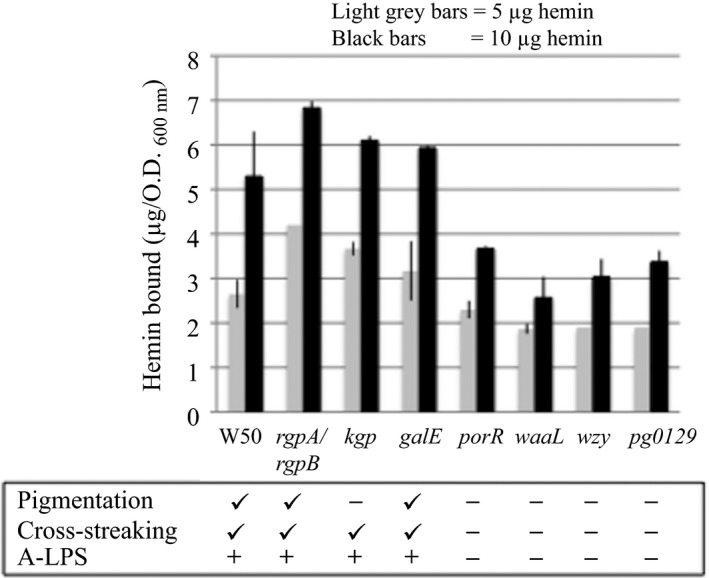
Hemin binding by whole cells of Porphyromonas gingivalis W50 and isogenic mutant strains. The P. gingivalis W50 and isogenic mutant strains were grown in brain‐heart infusion broth for 48 h. Details of hemin binding are as described in Methods. The amount of hemin bound by the cells (μg hemin bound/OD 600 cells) at the added concentrations of hemin of 5 μg and 10 μg are shown. The characteristics of the P. gingivalis strains are indicated below the figure. Statistical analyses (P values by Student's t test) of the amount of hemin bound by W50 and P. gingivalis mutant strains are also indicated. P‐values for hemin‐binding by strains rgpA/rgpB, kgp and galE compared with W50 were >.05 whereas P‐values for hemin‐binding by strains porR, waaL, wzy, and pg0129 compared with W50 were <.0003
3.5.2. Hemin binding by LPS
Hemin binding to LPS isolated from P. gingivalis W50 and mutant strain porR grown in brain‐heart infusion were measured at two different concentrations of hemin, namely 20 μg/mL and 30 μg/mL and are shown in Figure 6. At 30 μg/mL of added hemin, there is a slightly higher amount of hemin bound by all the LPS (Figure 6) compared with the amounts bound at 20 μg/mL of hemin. Henceforth, all the values for hemin binding to LPS will only refer to those obtained at the higher concentration of hemin used in the experiment, namely 30 μg/mL. LPS from P. gingivalis W50 is able to bind hemin at ~10.3 μg/50 μg of LPS. However, LPS from porR, which is devoid of A‐LPS27 was able to bind 3.6 μg hemin/50 μg of LPS, which is considerably lower than that of LPS from the parent W50 strain.
Figure 6.
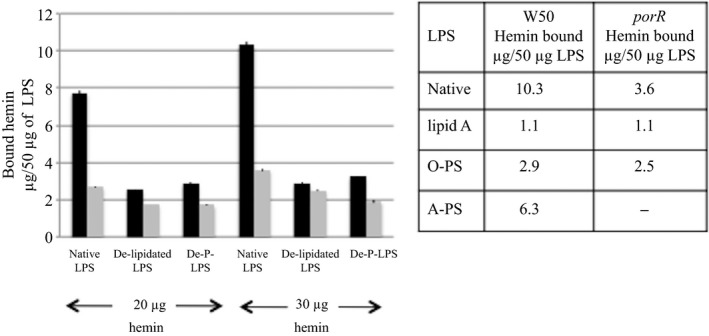
Hemin binding to lipopolysaccharide (LPS), de‐lipidated LPS and de‐O‐phosphorylated LPS derived from Porphyromonas gingivalis strains. The hemin‐binding studies were performed as described in the Methods. Black bars=LPS and derivatives from P. gingivalis W50 and grey bars=LPS and derivatives from porR mutant strain. Inset: hemin binding to native LPS, lipid A, O‐PS and A‐PS derived from P. gingivalis and porR mutant strains
To determine the extent of hemin binding to the lipid A portion of the LPS molecule, the LPS was delipidated and the binding of hemin to the resulting polysaccharide (PS) was measured. In addition to removal of lipid A, delipidation of LPS causes the destruction of A‐PS27 whereas O‐PS is largely unaffected.20 The binding of hemin by de‐lipidated LPS (Figure 6) from P. gingivalis W50 is reduced to ~2.9 μg/50 μg of LPS and hemin binding by de‐lipidated porR LPS is reduced to 2.5 μg hemin/50 μg of LPS. These results enable us to apportion the extent of hemin binding by A‐LPS, O‐LPS and lipid A. As porR LPS contains only O‐LPS, the hemin bound (~3.6 μg/50 μg of LPS) must be due to O‐LPS. De‐lipidation of porR LPS reduces the hemin bound to ~2.5 μg/50 μg of LPS suggesting that ~1.1 μg of hemin bound to de‐lipidated LPS must be due to binding to lipid A and the remaining ~2.5 μg of hemin bound to 50 μg of LPS must be due to binding to the O‐PS component of O‐LPS. The hemin binding to de‐lipidated LPS from P. gingivalis W50 and porR strains is remarkably similar (2.9 μg vs 2.5 μg hemin bound to 50 μg of de‐lipidated LPS, respectively) whereas hemin bound to native LPS differs greatly, 10.3 μg vs 3.6 μg hemin bound to W50 and porR LPS respectively. Hence, it can be deduced that ~6.3 μg of hemin bound/50 μg of native LPS in P. gingivalis W50 must be due to binding to A‐LPS.
We also investigated the binding of hemin to de‐O‐phosphorylated LPS derived from the parent W50 and porR mutant strain. De‐O‐phosphorylation of O‐LPS and A‐LPS does not cause de‐polymerization of the PS chains, but results in loss of phosphoethanolamine in O‐PS20 and in the loss of the cross‐reacting epitope Manα1‐2Manα1‐phosphate in A‐LPS.21, 27 The results (Figure 6) show that the binding of hemin is reduced in de‐O‐phosphorylated LPS from the parent and porR mutant strain to ~3.3 μg and 1.9 μg, respectively. Hence, the reduction in binding of hemin caused by de‐O‐phosphorylation of LPS from P. gingivalis W50 compared with that for LPS from porR mutant strain suggests that negatively‐charged A‐LPS may have an important role to play in the binding/deposition of hemin on the cell surface of P. gingivalis.
4. DISCUSSION
The hemin binding properties of P. gingivalis have been a major area of study and interest for several years. Iron utilization systems in P. gingivalis are quite complex and several proteins have been implicated in hemin release from the host, to its transport and deposition on the bacterial cell surface. Virulence of P. gingivalis is closely associated with the ability of the organism to pigment, namely to the deposition of the μ‐oxo‐bisheme on the cell surface. The requirement for the dimeric Arg‐gingipain (Rgp), HRgpA, and Lys‐gingipain (Kgp) in the release of heme groups from hemoglobin and the formation of the μ‐oxo‐bisheme complex is very well characterized.38 However, the cell‐surface molecules required for retention of μ‐oxo‐bisheme and pigmentation have not been fully elucidated.
Haem‐starved P. gingivalis ATCC33277 and WT40 expressed two binding sites for hemin, a low‐abundance high‐affinity site (1000‐1500 sites/cell) of K d between 3.6×10−11 and 9.6×10−11 m and a high‐abundance low‐affinity site (1.9×105 to 6.3×105 sites/cell) where the estimated K d ranged between 2.6×10−7 and 6.5×10−8 m.22 Treatment with N‐bromosuccinimide inactivated hemin binding by both sites in P. gingivalis, whereas pronase treatment caused only a limited reduction in hemin binding probably because only one of the two sites was sensitive to pronase. Tompkins et al.22 concluded that the higher‐affinity site was probably exposed on the surface of P. gingivalis and sensitive to pronase whereas the lower‐affinity site may be sequestered within the outer membrane, especially if it functioned to store hemin.
The black heme‐pigment deposited on the cell surface of P. gingivalis and which serves as an iron source for this organism, is composed of μ‐oxo‐bisheme [Fe(III)PPIX]2O, and the multidomain cysteine proteases Arg‐gingipains and Lys‐gingipain acting in concert have been shown to be important in the production of μ‐oxo‐bisheme from oxyhemoglobin.34 HRgpA, the dimeric isoform of RgpA, promotes the formation of methemoglobin from oxyhemoglobin, which is degraded by Kgp to form the black pigment μ‐oxo‐bisheme.10 Hence, both Arg and Lys gingipains are required for the production of the black pigment in P. gingivalis.
In the absence of Rgps, no μ‐oxo‐bisheme is produced, although the double knockout P. gingivalis rgpA/rgpB strain, which lacks Rgps, gave brown‐colored colonies even after prolonged incubation on blood agar plates. The brown pigment contained an Fe(III) hemoglobin‐hemichrome complex as the major heme‐containing species.38 The heme from the complex was transferred to albumin after prolonged incubation of cells with oxyhemoglobin in the presence of albumin and this was tightly bound to the cell surface in the P. gingivalis (rgpA/rgpB) strain.
Porphyromonas gingivalis W50 does not pigment when grown in liquid broth cultures with added hemin (5 mg/L), but gives black‐pigmented colonies when grown on blood agar plates due to the deposition of μ‐oxo‐bisheme, derived from hemoglobin, on the cell surface. This difference may suggest that the source of hemin is critical for the pigmentation process. Here, we have shown that P. gingivalis mutants rgpA/rgpB and kgp do not normally pigment, but produce black‐pigmented colonies when cross‐streaked on plates on which P. gingivalis W50 was previously grown and caused hemolysis. These observations show that the ability to retain the pigment on the P. gingivalis cell surface can be uncoupled from the ability to release heme from hemoglobin (with the concomitant formation of μ‐oxo‐bisheme) by the combined action of Rgps and Kgp. This behavior mirrors the ability of the cells of rgpA/rgpB and kgp mutant strains to bind externally added hemin to the same extent as the parent W50 strain when hemin‐binding was measured in liquid suspensions (Figure 5). Therefore, we propose that the ability of the bacterial cells to bind hemin may parallel the retention of μ‐oxo‐bisheme on the cell surface when the strains are grown on blood agar plates. The inability of P. gingivalis mutant strains porR, waaL, wzy, and pg0129 to produce black‐pigmented colonies in cross‐streaking experiments is supported by the reduced binding of hemin to cells of these strains. The major difference between the P. gingivalis mutant strains, which have the ability to acquire μ‐oxo‐bisheme (rgpA/rgpB and kgp) on cross‐streaking and those mutant strains that lack this property (porR, waaL, wzy, and pg0129) is the production of A‐LPS by pigmenting strains.
This suggests that the P. gingivalis cell surface must contain a molecule that provides a scaffold/matrix for the deposition and retention of any hemin or pigment that is produced/acquired by the organism. Figure 7 shows a simplified diagram of the pigmentation characteristics and the types of LPS synthesized by the P. gingivalis strains used in this study.
Figure 7.
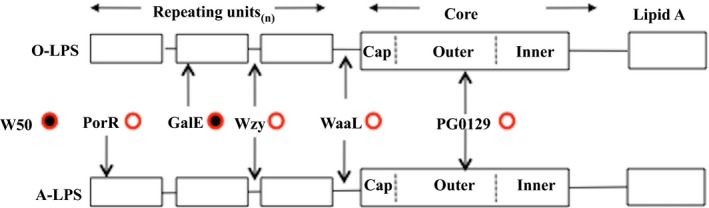
Structure of Porphyromonas gingivalis lipopolysaccharide (LPS) and role of A‐LPS in pigmentation. The P. gingivalis W50 parent strain synthesizes two LPS: O‐LPS and A‐LPS and is black‐pigmented on blood agar (filled circle). Inactivation of Wzy (O‐antigen polymerase) leads to a core‐plus‐one repeating unit structure for both LPS. Inactivation of either WaaL (O‐antigen ligase) or PG0129 (alpha‐1,3‐mannosyl‐transferase) leads to the absence of A‐PS and O‐PS. In all three cases, the mutants lose the ability to pigment (open circles). Inactivation of GalE affects the synthesis of the O‐PS but not A‐PS and pigmentation is unaffected. Inactivation of PorR abolishes the synthesis of A‐LPS but not O‐LPS and this mutant fails to pigment
Transmission electron microscopy of P. gingivalis porR 27, 28 and waaL mutant strains (which lack A‐LPS)21 show that their extracellular surface layers are of reduced thickness compared with the W50 parent and rgpA/rgpB mutant strains (which do synthesize A‐LPS) and the cells appear more fragile based on the rate of decrease of the culture OD in the stationary phase.21, 23, 27 Shoji et al.23 suggested that strains that were unable to synthesize A‐LPS probably lack a tethering/anchoring molecule(s) on their cell surface, which retain gingipains and this could explain the release of Arg‐ and Lys‐gingipains into the culture supernatants in P. gingivalis porR, waaL, wzy, and pg0129 mutant strains, which are defective in the LPS‐biosynthetic pathway.
Grenier reported that the lipid A component of LPS mediated the binding of uncomplexed hemin by P. gingivalis.39 As hemin is a lipophilic molecule, it would be expected to bind to Lipid A/LPS. Escherichia coli, which does not require exogenous heme when grown in iron‐replete conditions, was shown to bind as much uncomplexed hemin as Prevotella intermedia. This effect was inhibited by albumin, which indicated that when heme is provided in the free form, most of it binds to the bacterium with an affinity lower than that for albumin. However, Tompkins et al.22 concluded that most gram‐negative bacteria would exhibit similar non‐specific hemin binding and that the LPS‐mediated hemin binding is probably not biologically relevant because of the low affinity of the interaction and the presence of large amounts of host plasma proteins, which function to counter the lipophilic disposition of hemin. They showed that treatment of P. gingivalis cells with pronase caused a slight reduction in, but did not eliminate, hemin‐binding and the authors suggested that this was probably due to the pronase‐sensitive hemin‐binding sites not being exposed on the surface of the cell and therefore not digested by pronase treatment.22 However, it seems more plausible that it is the presence of A‐LPS (which is not sensitive to pronase treatment) on the surface of P. gingivalis that acts as a site for the deposition/binding of hemin.
Studies on hemin binding to whole cells of P. gingivalis W50 and mutant strains and hemin binding to native LPS and de‐lipidated LPS from P. gingivalis W50 and porR strains show that absence of A‐LPS causes a reduction in hemin binding. Hence, absence of A‐LPS in the extracellular surface of P. gingivalis eliminates or reduces a scaffold/anchoring mechanism not only for retention of Arg‐ and Lys‐gingipains but also for the deposition of μ‐oxo‐bisheme pigment or hemin derived from the environment and highlights the importance of A‐LPS in the virulence of this organism.
Supporting information
ACKNOWLEDGEMENTS
We thank Alexandra Gallagher for technical assistance. This investigation was supported by the Medical Research Council (U.K) Grant G0501478; Research Advisory Board of the Barts and The London Charity Grant RAB06/PJ/14.
Rangarajan M, Aduse‐Opoku J, Paramonov NA, Hashim A, Curtis MA. Hemin binding by Porphyromonas gingivalis strains is dependent on the presence of A‐LPS. Mol Oral Microbiol. 2017;32:365–374. https://doi.org/10.1111/omi.12178
REFERENCES
- 1. Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. [DOI] [PubMed] [Google Scholar]
- 2. Hajishengalis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibbons RJ, Macdonald JB. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus . J Bacteriol. 1960;80:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah HN, Bonnett R, Matten B, Williams RA. The porphyrin pigmentation of subspecies of Bacteroides melaninogenicus . Biochem J. 1979;180:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schifferle RE, Shostad SA, Bayers‐Thering MT, Dyer DW, Neiders ME. Effect of protoporphyrin IX limitation on Porphyromonas gingivalis . J Endod. 1996;22:352–355. [DOI] [PubMed] [Google Scholar]
- 6. Olczak T, Simpson W, Liu X, Genco CA. Iron and heme utilization in Porphyromonas gingivalis . FEMS Microbiol Rev. 2005;29:119–144. [DOI] [PubMed] [Google Scholar]
- 7. Bramanti TE, Holt SC. Roles of porphyrins and host iron transport proteins in regulation of growth of Porphyromonas gingivalis W50. J Bacteriol. 1991;173:7330–7339.1657888 [Google Scholar]
- 8. Genco CA. Regulation of hemin and iron transport in Porphyromonas gingivalis . Adv Dent Res. 1995;9:41–47. [DOI] [PubMed] [Google Scholar]
- 9. Carlsson J, Höfling JF. Sundqvist GK Degradation of albumin, haemopexin, haptoglobulin and transferrin, by black pigmented Bacteroides species. J Med Microbiol. 1984;18:39–46. [DOI] [PubMed] [Google Scholar]
- 10. Lewis JP, Dawson JA, Hannis JC, Muddiman D, Macrina FL. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J Bacteriol. 1999;181:4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sroka A, Sztukowska M, Potempa J, Travis J, Genco CA. Degradation of host heme proteins by lysine‐ and arginine‐specific cysteine proteinases (gingipains) of Porphyromonas gingivalis . J Bacteriol. 2001;183:5609–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis JP, Plata K, Yu F, Rosato A, Anaya C. Transcriptional organization, regulation and role of the Porphyromonas gingivalis W83 hmu haemin‐uptake locus. Microbiology. 2006;152:3367–3382. [DOI] [PubMed] [Google Scholar]
- 13. Wojtowicz H, Guevara T, Tallant C, et al. Unique structure and stability of HmuY, a novel heme‐binding protein of Porphyromonas gingivalis . PLoS Pathog. 2009;5:e1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shoji M, Shibata Y, Shiroza T, et al. Characterization of hemin‐binding protein 35 (HBP 35) in Porphyromonas gingivalis: its cellular distribution, thioredoxin activity and role in heme utilization. BMC Microbiol. 2010;10:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slakeski N, Dashper SG, Cook P, Poon C, Moore C, Reynolds EC. A Porphyromonas gingivalis genetic locus encoding a heme transport system. Oral Microbiol Immunol. 2000;15:388–392. [DOI] [PubMed] [Google Scholar]
- 16. Dashper SG, Hendtlass A, Slakeski N, et al. Characterization of a novel outer membrane hemin‐binding protein of Porphyromonas gingivalis . J Bacteriol. 2000;182:6456–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simpson W, Olczak T, Genco CA. Characterization and expression of HmuR, a TonB‐dependent hemoglobin receptor of Porphyromonas gingivalis . J Bacteriol. 2000;182:5737–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olczak T, Sroka A, Potempa J, Olczak M. Porphyromonas gingivalis HmuY and HmuR: further characterization of a novel mechanism of heme utilization. Arch Microbiol. 2008;189:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karunakaran T, Madden T, Kuramitsu H. Isolation and characterization of a hemin‐regulated gene, hemR, from Porphyromonas gingivalis . J Bacteriol. 1997;179:1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paramonov N, Bailey D, Rangarajan M, et al. Structural analysis of the polysaccharide from the lipopolysaccharide of Porphyromonas gingivalis strain W50. Eur J Biochem (FEBS J). 2001;268:4698–4707. [DOI] [PubMed] [Google Scholar]
- 21. Rangarajan M, Aduse‐Opoku J, Paramonov N, et al. Identification of a Second Lipopolysaccharide in Porphyromonas gingivalis W50. J Bacteriol. 2008;190:2920–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tompkins GR, Wood DP, Birchmeier KR. Detection and comparison of specific hemin binding by Porphyromonas gingivalis and Prevotella intermedia . J Bacteriol. 1997;179:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shoji M, Ratnayake DB, Shi Y, et al. Construction and characterization of a non‐pigmented mutant of Porphyromoinas gingivalis: cell surface polysaccharide as an anchorage for gingipains. Microbiology. 2002;148:1183–1191. [DOI] [PubMed] [Google Scholar]
- 24. Gallagher A, Aduse‐Opoku J, Rangarajan M, Slaney JM, Curtis MA. Glycosylation of the Arg‐gingipains of Porphyromonas gingivalis and comparison with glycoconjugate structure and synthesis in other bacteria. Curr Protein Pept Sci. 2003;4:427–441. [DOI] [PubMed] [Google Scholar]
- 25. Aduse‐Opoku J, Muir J, Slaney JM, Rangarajan M, Curtis MA. Characterization, genetic analysis, and expression of a protease antigen (PrpRI) of Porphyromonas gingivalis W50. Infect Immun. 1995;63:4744–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27. Paramonov N, Rangarajan M, Hashim A, et al. Structural analysis of a novel anionic polysaccharide from Porphyromonas gingivalis strain W50 related to Arg‐gingipain glycans. Mol Microbiol. 2005;58:847–863. [DOI] [PubMed] [Google Scholar]
- 28. Slaney JM, Gallagher A, Aduse‐Opoku J, Pell K, Curtis MA. Mechanisms of resistance of Porphyromonas gingivalis to killing by serum complement. Infect Immun. 2006;74:5352–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rangarajan M, Smith SJ, U S, Curtis MA. Biochemical characterization of the arginine‐specific proteases of Porphyromonas gingivalis W50 suggests a common precursor. Biochem J. 1997;323:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inzana TJ, Apicella MA. Use of a bilayer stacking gel to improve resolution of lipopolysaccharides and lipooligosaccharides in polyacrylamide gels. Electrophoresis. 1999;20:462–465. [DOI] [PubMed] [Google Scholar]
- 31. Paramonov NA, Aduse‐Opoku J, Hashim A, Rangarajan M, Curtis MA. Structural analysis of the core region of O‐lipopolysaccharide of Porphyromonas gingivalis from mutants defective in O‐antigen ligase and O‐antigen polymerase. J Bacteriol. 2009;191:5272–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cutler CW, Eke PI, Genco CA, Van Dyke TE, Arnold RA. Hemin‐induced modifications of the antigenicity and hemin‐binding capacity of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 1996;64:2282–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aduse‐Opoku J, Davies NN, Gallagher A, et al. Generation of Lys‐gingipain protease activity in Porphyromonas gingivalis W50 is independent of Arg‐gingipain protease activities. Microbiology. 2000;146:1933–1940. [DOI] [PubMed] [Google Scholar]
- 34. Smalley JW, Birss AJ, Szmigielsji B, Potempa J. Sequential action of R‐ and K‐specific gingipains of Porphyromonas gingivalis in the generation of the haem‐containing pigment from oxyhaemoglobin. Arch Biochem Biophys. 2007;465:44–49. [DOI] [PubMed] [Google Scholar]
- 35. Paramonov N, Aduse‐Opoku J, Hashim A, Rangarajan M, Curtis MA. Identification of the linkage between A‐polysaccharide and the core in the A‐lipopolysaccharide of Porphyromonas gingivalis W50. J Bacteriol. 2015;197:1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takagi M, Takada H, Imanaka T. Nucleotide sequence and cloning in Bacillus subtilis of the Bacillus stearothermophilus pleiotropic regulatory gene degT . J Bacteriol. 1990;172:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shoji M, Sato K, Yukitake H, Naito M, Nakayama K. Involvement of the Wbp pathway in the biosynthesis of Porphyromonas gingivalis lipopolysaccharide with anionic polysaccharide. Sci Rep. 2014;4:5056–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smalley JW, Thomas MF, Birss AJ, Withnall R, Silver J. A combination of both arginine‐ and lysine‐specific gingipain activity of Porphyromonas gingivalis is necessary for the generation of μ‐oxo bishaem‐containing pigment from haemoglobin. Biochem J. 2004;379:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grenier D. Hemin‐binding property of Porphyromonas gingivalis outer membranes. FEMS Microbiol Lett. 1991;77:45–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


