Abstract
Rationale and Objective
Emphysema is characterized by airspace dilation, inflammation, and irregular deposition of elastin and collagen in the interstitium. Computed tomographic (CT) studies have reported that lung mass (LM) may be increased in smokers, a finding attributed to inflammatory and parenchymal remodeling processes observed on histopathology. We sought to examine the epidemiologic and clinical associations of LM in smokers.
Materials and Methods
Baseline epidemiologic, clinical, and CT data (n=8,156) from smokers enrolled into the COPDGene Study were analyzed. LM was calculated from the CT scan. Changes in lung function at five-year follow-up were available from 1,623 subjects. Regression analysis was performed to assess for associations of LM with forced expiratory volume in 1 second (FEV1) and FEV1 decline.
Results
Subjects with Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1 COPD had greater LM than either smokers with normal lung function or those with GOLD 2–4 COPD (P<0.001 for both comparisons). LM was predictive of rate of the decline in FEV1 (decline per 100 g, −4.7 ± 1.7 ml/yr, P=0.006).
Conclusion
Our cross sectional data suggest the presence of a biphasic radiologic remodeling process in smokers: the presence of such non-linearity must be accounted for in longitudinal CT studies. Baseline LM predicts the decline in lung function.
Keywords: lung mass, smoking, CT scan, COPD, emphysema
Introduction
Emphysema is defined as abnormal, permanent dilation of the distal airspaces.1 The development and progression of this pathologic process is associated with a decline in lung function and progressive clinical impairment.2 Spirometric measures of lung function have been the benchmark for monitoring progression of disease and response to therapeutic intervention but such investigations lack sensitivity and require large cohorts followed over relatively long periods of time.3 For these reasons, computed tomographic (CT) imaging of the chest is increasingly being leveraged as a source of intermediate study endpoints to objectively assess response to treatment.4–6
Densitometric measures of the lung parenchyma to detect and quantify emphysema have been utilized in cross-sectional investigations for almost 30 years7–9 including the percent low attenuation areas (%LAA – those regions of the lung less than a select attenuation value) and percentage of lung volume less than the 10th or 15th percentile.8,10 While each of these may have relative advantages when considering disease severity and progression11, they are all focused on the low attenuating regions of the lung histogram, the tail that may be most sensitive for the detection of airspace dilation. This may limit the ability of such metrics to fully assess the remodeling process characteristic of COPD.
Prior histological work by Vlahovic and colleagues demonstrated that airspace dilation in emphysema was also accompanied by inflammation and the deposition of excess elastin and collagen.12 The mean degree of airspace enlargement was directly related to interstitial thickness. Additional CT-based work suggests that macroscopic emphysema is not just an absence of tissue. In their series of 40 subjects, Guenard et al reported that 22 of the 24 patients with emphysema had normal or even increased lung mass (LM).13 These previous studies prompted us to more comprehensively explore the significance of lung mass in smokers where we hypothesized that such measures would be highly clinically relevant even after adjustment for CT-based estimates of emphysema. To do this we examined quantitative measures of emphysema and lung mass in CT scans obtained as part of the COPDGene Study.14
Methods
The COPDGene Study has been described in detail previously.14 Approximately 10,300 non-Hispanic White and African-American smokers aged 45–80 years were recruited for the purpose of identifying genetic and epidemiological predictors of the disease (thereafter referred to as the baseline cohort). At baseline, subjects underwent detailed characterization including volumetric inspiratory CT scans of the chest, questionnaires, and spirometric measures of lung function. Subjects with active lung diseases other than asthma, emphysema, or COPD were excluded. The COPDGene Study was approved by the Institutional Review Board (IRB) of each participating center, and all subjects provided written informed consent.
COPDGene subjects are returning for a 5 year interval visit to repeat the characterization performed at baseline. The first 2000 data set of smokers who returned to the second visit are the basis for the decline in lung function analysis using their clinical and CT data from their baseline visit.
Spirometric measurements and COPD definition
Spirometric measures of lung function including forced expiratory lung volume in a second (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio were performed using the Easy-One spirometer (ndd Medical Technologies Inc, Andover MA). Testing was performed before and after the administration of a short acting inhaled bronchodilator (albuterol) per American Thoracic Society recommendations and results were expressed as a percent of predicted values.15,16 Subjects were then classified into Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages of disease severity.17 Smokers with no evidence of spirometric obstruction (FEV1/FVC >0.7) were categorized as being “At risk” for the development of COPD while those with an FEV1/FVC <0.7 were categorized has having COPD. In our investigation, never-smoking subjects and smokers with a proportionally reduced FEV1 and FVC with preserved ratio were excluded from analysis.18
Clinical assessment
Demographics and clinical demographics data including smoking history and acute respiratory disease events were obtained with standardized questionnaires, which are available at www.COPDGene.org. Acute respiratory diseases episodes were defined as an increase of respiratory symptoms including cough, sputum production, and dyspnea in smokers with and without COPD. The episodes were counted if the subject had an episode lasting 48 hours or more and it was associated with antibiotic or corticosteroid use.19
CT assessment
Volumetric CT scans of the chest were performed at both maximal inflation and relaxed exhalation.14 Baseline inspiratory CT scans were used in this analysis. Images were acquired with the following CT protocol: for General Electric (GE) LightSpeed-16, GE VCT-64, Siemens Sensation-16 and -64, and Philips 40- and 60-slice scanners with 120kVp, 200mAs, and 0.5s rotation time. Images were reconstructed using a standard algorithm at 0.625mm slice thickness and 0.625mm intervals for GE scanners; using a B31f algorithm at 0.625 (Sensation-16) or 0.75mm slice thickness and 0.5mm intervals for Siemens scanners; and using a B algorithm at 0.9mm slice thickness and 0.45mm intervals for Philips scanners.20 Densitometric assessments of the lung parenchyma were performed on the inspiratory scans using in-house software. Attenuation areas thought to reflect emphysematous destruction of the lung parenchyma were defined as the percent of lung attenuation areas less than -950 Hounsfield Unit (HU) (%LAA-950). LM was calculated on a voxel by voxel basis as described and validated previously.21,22 Briefly, we used the following equation to calculate LM:
Statistical Analysis
Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). Data are presented as means ± standard deviation (SD) or median (interquartile range) for continuous variables according to their distribution type and as frequency (%) for categorical variables. All references to LM in this manuscript pertain to measures obtained from the baseline CT scans. Comparisons of LM and %LAA-950 across GOLD groups were performed using analysis of the variance (GLM procedure of SAS). Between-group comparisons were carried out using appropriate contrast statements as well as an interaction term between GOLD stage and current smoking status. This latter was done to test differences in LM by smoking status across disease stages. In subjects with COPD, multivariable linear regression models were used to assess the relationship between LM and both baseline FEV1 as well as the annual change in FEV1 over the 5 year interval between baseline and second visits. This change in FEV1 was calculated as the difference between baseline visit and second visit measures divided by follow-up time. A negative value for ml/year is to be interpreted as the ml/year decrease in FEV1 from baseline with a larger number signifying a more rapid decline in lung function. In the cross-sectional analysis of outcome FEV1, covariate selection for model construction was done based on prior work by Vestbo and colleagues.23 Models included age, sex, height, weight, current smoking status, %LAA-950, and exacerbation in the year prior to enrollment. In preliminary analysis, height and weight were the body size measures most strongly related to a subject’s lung mass. In addition to those covariates, baseline FEV1 was included in models for decline in FEV1. For both outcomes there was an additional adjustment for scanner brand/make as differences in section thickness and reconstruction kernel influence CT densitometric measures of lung parenchyma.24. P values <0.05 were considered statistically significant.
Results
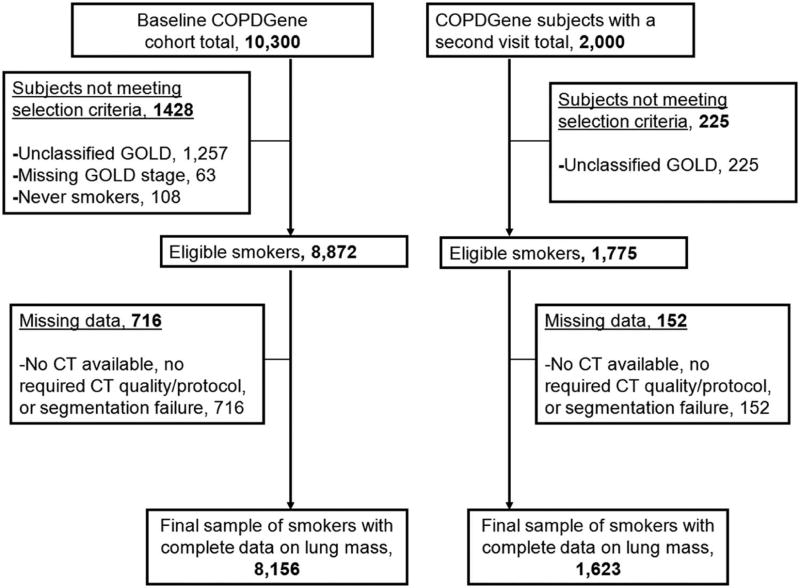
There were 10,300 subjects enrolled in COPDGene at baseline with 8,872 of these subjects eligible for the primary analyses of LM. LM data was available on 8,156 (92%) of this cohort (Figure 1). Characteristics of the subjects in the baseline and second visit are presented in Table 1. At the baseline visit 51% of this cohort was current smokers and 50% had COPD. Participants’ characteristics in the baseline by GOLD stage are shown in Table 2.
Figure 1.
Flowchart showing subject selection and final samples.
Table 1.
Characteristics of the subjects in the baseline and of those with a second visit.
| Characteristic | Baseline (N=8,156) |
Subjects with a second visit (N=1,623) |
|---|---|---|
| Age, y | 60 ± 9 | 61± 9 |
| Male gender, % | 55 | 52 |
| African-American race, % | 31 | 27 |
| Height, cm | 170 ± 9 | 170 ± 9 |
| Weight, kg | 82 ± 19 | 83 ± 18 |
| Pack-years of smoking | 44 ± 25 | 44 ± 24 |
| Current smoking status, % | 51 | 43 |
| FEV1, L | 2.3 ± 1.0 | 2.3 ± 0.9 |
| FEV1 change, ml/yr | - | −41 ± 52 |
| FEV1, % predicted | 78 ± 27 | 80 ± 25 |
| FVC, L | 3.4 ± 1.0 | 3.4 ± 1.0 |
| FVC, % predicted | 89 ± 18 | 91 ± 17 |
| FEV1/FVC ratio | 0.65 ± 0.17 | 0.66 ± 0.16 |
| %LAA-950, % | 6.8 ± 10.0 | 7.1 ± 9.3 |
| Lung mass, g | 902 ± 183 | 884 ± 174 |
| Subjects with COPD, % | 50 | 50 |
| One or more acute respiratory disease episode in the prior yr. to enrollment, % | 21 | 18 |
Data are presented as mean ± SD or proportion (%)
Table 2.
Characteristics of the subjects in the baseline by GOLD stage
| GOLD STAGE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 0 (N= 4,047) | 1 (N=747) | 2 (N=1,786) | 3 (N=1,042) | 4 (N=534) | |||||
| Age, y | 57 | ± 8 | 62 | ± 9 | 63 | ± 9 | 64 | ± 8 | 64 | ± 8 |
| Male gender, % | 53 | 58 | 54 | 58 | 59 | |||||
| African-American race, % | 41 | 22 | 24 | 20 | 18 | |||||
| Height, cm | 170 | ± 9 | 170 | ± 10 | 170 | ± 9 | 170 | ± 9 | 170 | ± 9 |
| Weight, kg | 84 | ± 18 | 78 | ± 16 | 83 | ± 20 | 81 | ± 20 | 73 | ± 18 |
| Pack-years of smoking | 37 | ± 20 | 45 | ± 24 | 51 | ± 27 | 55 | ± 27 | 57 | ± 29 |
| Current smoking status, % | 59 | 55 | 49 | 36 | 23 | |||||
| FEV1, L | 2.9 | ± 0.7 | 2.7 | ± 0.7 | 1.9 | ± 0.5 | 1.2 | ± 0.3 | 0.7 | ± 0.2 |
| FEV1, % predicted | 98 | ± 12 | 91 | ± 9 | 65 | ± 8 | 40 | ± 6 | 23 | ± 5 |
| FVC, L | 3.7 | ± 0.9 | 4.1 | ± 1.0 | 3.2 | ± 0.9 | 2.7 | ± 0.8 | 2.1 | ± 0.7 |
| FVC, % predicted | 97 | ± 12 | 108 | ± 12 | 86 | ± 13 | 71 | ± 13 | 56 | ± 14 |
| FEV1/FVC ratio | 0.79 | ± 0.05 | 0.65 | ± 0.04 | 0.58 | ± 0.08 | 0.44 | ± 0.09 | 0.32 | ± 0.07 |
| One or more acute respiratory disease episode in the prior yr to enrollment, % | 9 | 12 | 30 | 43 | 57 | |||||
Data are presented as mean ± SD or proportion (%)
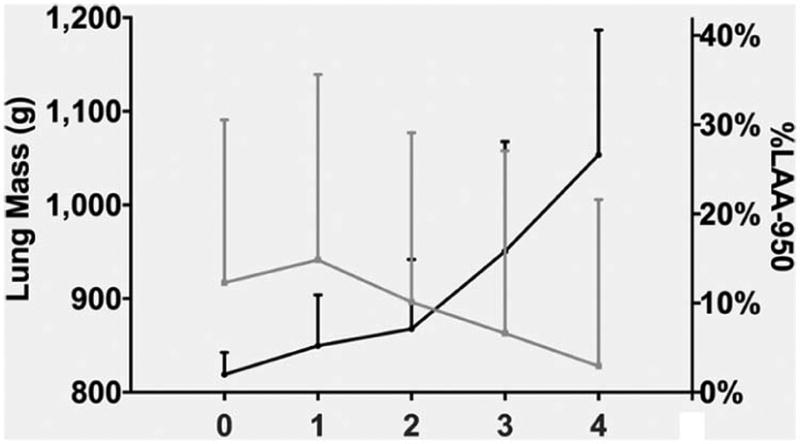
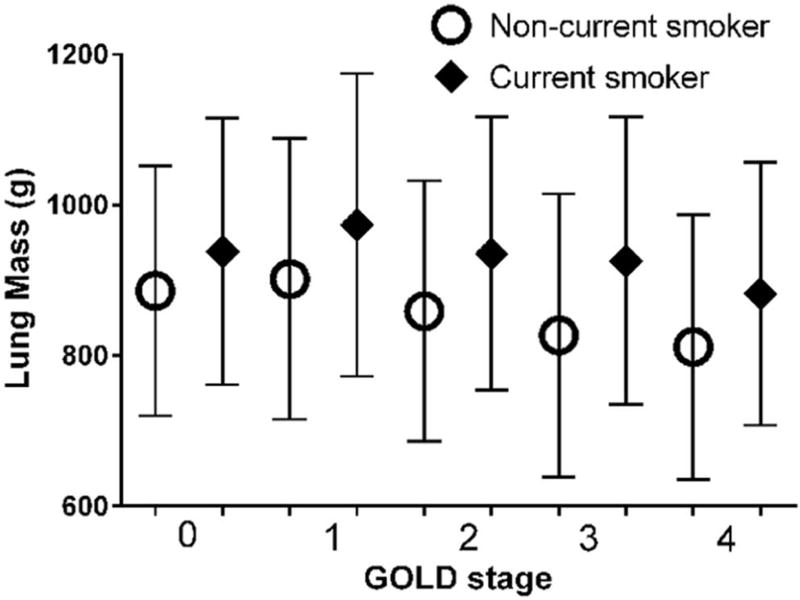
The difference in LM between smokers with and without COPD was 30 g (887 ± 190 g vs. 917 ± 165 g P<0.0001). LM was the greatest in subjects with GOLD Stage 1 COPD (941 ± 198) and they had greater LM than either smokers without COPD (P=0.0007) or GOLD 2–4 COPD subjects (P<0.0001). LM tended to decline with disease severity (P for trend <0.0001) (Figure 2). In contrast, %LAA-950 slightly increased from GOLD 0 (2.0 ± 2.5%) to GOLD 1 (5.2 ± 5.7%) and to GOLD 2 (7.1 ± 7.8%) and then it showed a larger increase from GOLD 2 to GOLD 3 (15.8 ± 12.3%) and GOLD 4 (26.9 ± 14%) (Figure 2). LM was greater in current than former smokers (P<0.0001 for both GOLD and smoking status effects; P=0.003 for interaction GOLD×Smoking status) (Figure 3).
Figure 2.
Lung mass and %LAA-950 as a function of COPD GOLD stages. The differences in lung mass (mean ± SD) between GOLD 0 (or smokers at risk) and GOLD 1 and between GOLD 1 and GOLD 2–4 were significant (P=0.0007 and P<0.0001, respectively). The difference in %LAA-950 (mean ± SD) between GOLD 0 and GOLD 1–4 was significant (P<0.0001).
Figure 3.
Lung mass as a function of COPD GOLD stages and current smoking status. The difference in lung mass (mean ± SD) between current (black diamond) and non-current smokers was significant (interaction term between GOLD stage and smoking status, P=0.003).
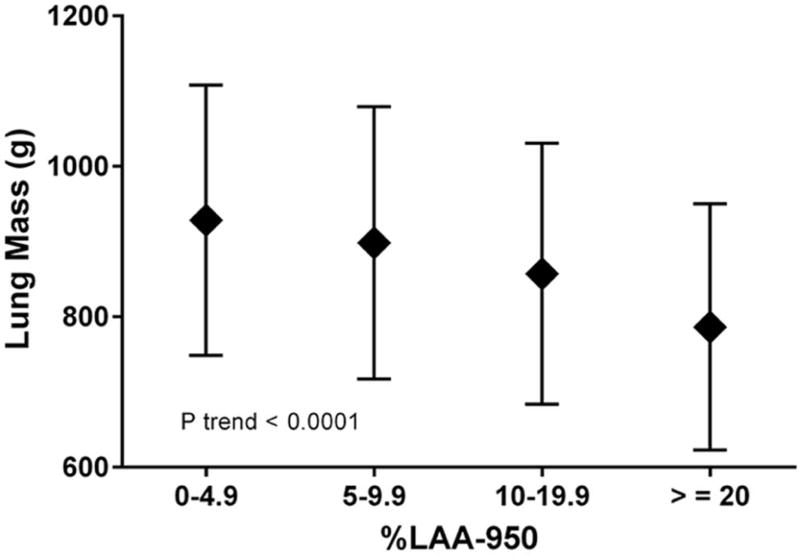
As expected from the observed trend of LM by GOLD stage among COPD subjects, LM was directly associated with FEV1 in adjusted models as shown in Table 3. When the analysis was restricted to smokers at risk, LM remained significantly associated with FEV1 (Beta = 144; P <0.0001). LM was inversely related to emphysema when %LAA-950 was considered as a continuous covariate (P<0.0001) in the adjusted model (Table 3) or a categorical variable (P<0.0001 for trend) (Figure 4).
Table 3.
Effect of COPD Subjects’ Characteristics on FEV1 at Baseline (ml) and on the Rate of Change in FEV1 (ml/yr)*
| Characteristic | Baseline FEV1 (N=4,109) (ml) |
Rate of change in FEV1 (N=811) (ml/yr) |
||||
|---|---|---|---|---|---|---|
| Estimate | SE | P value | Estimate** | SE | P value | |
| Lung mass (per 100 g) | 73.4 | 7.6 | <0.0001 | −4.7 | 1.7 | 0.006 |
| Age (per 10 yr) | −142.3 | 11.9 | <0.0001 | 2.7 | 2.6 | 0.30 |
| Male sex | 156.0 | 27.0 | <0.0001 | 7.6 | 5.9 | 0.20 |
| Height (per 5 cm) | 115.2 | 7.6 | <0.0001 | 3.2 | 1.6 | 0.051 |
| Weight (per 5 kg) | −22.0 | 2.9 | <0.0001 | −0.5 | 0.7 | 0.48 |
| Current Smoker | −51.2 | 22.5 | 0.02 | −12.5 | 4.8 | 0.009 |
| Log %LAA-950 | −272.1 | 10.7 | <0.0001 | −18.8 | 2.5 | <0.0001 |
| One or more acute respiratory disease episode in the prior yr to enrollment | −310.5 | 19.5 | <0.0001 | −4.3 | 4.4 | 0.33 |
| Baseline FEV1 (ml) | - | - | - | −3.0 | 0.3 | <0.0001 |
Both multivariate models were additionally adjusted for scanner brand/make.
Negative estimates represent decline in FEV1
Figure 4.
Lung mass as a function of emphysema groups on CT scans in smokers. The decline in lung mass (mean ± SD) as %LAA-950 emphysema increases was significant (P trend <0.0001).
Relationship between lung mass and change in FEV1 in COPD subjects
Two thousand subjects had completed their second visit of which 1,775 were eligible for this analysis (Figure 1) and 1623 (91%) had available data with a mean baseline LM of 884 ± 174 g. Approximately 43% of these subjects were current smokers and 50% had COPD (Table 1). Greater LM and higher percentage of emphysema at baseline were associated with a more rapid decline in FEV1 in the multivariable model even after adjusting for baseline demographics, FEV1 level, current smoking status, acute respiratory disease episodes in the prior year to enrollment, and CT scanner brand/make (Table 3).
Discussion
This investigation consisted of a cross-sectional examination of CT based estimates of LM of smokers enrolled in COPDGene as well as a secondary analysis of the relationship of baseline LM and subsequent rate of decline in lung function. Our analyses revealed that GOLD 1 subjects had the greatest mean LM, greater than either the smokers with normal lung function or the smokers with GOLD 2–4 COPD. The GOLD 4 subjects had the lowest mean lung mass. These trends were observed in both pooled analyses and analyses stratified by current smoking status. LM was also related to rate of decline in FEV1.
Smoking status is a known confounder of the densitometric assessment of the lung parenchyma.25 Prior investigation has demonstrated that smokers with GOLD 1 to 2 COPD had greater lung density than never smokers, current smokers (vs. former) have increased lung density,26 and that smoking cessation, presumably through resolution of parenchymal inflammation, may in fact lead to a paradoxical increase in the amount of low attenuation areas evident on CT scan.27 For this reason we stratified our initial analysis of LM by smoking status. Visual inspection of data in Figure 3 and subsequent statistical analyses corroborates this supposition and current smokers tended to have greater LM than former smokers at all GOLD stages. Even after such stratification however, GOLD 1 former smokers still had the greatest LM suggesting that our findings cannot be attributed to current smoking status alone.
The histopathologic evolution of emphysema is not a monotonic loss of tissue but rather a biphasic trajectory. There is an increase in regional lung tissue in early disease (inflammation and obstruction of the terminal and respiratory bronchioles) followed by dilation of the adjacent alveoli and destruction of the acinus.28,29 Our cross-sectional CT analysis also suggests the presence of a biphasic nature to parenchymal remodeling in smokers. In the earliest stages (GOLD 0 to GOLD 1), chronic tobacco smoke exposure, in susceptible smokers, results in an increase in overall lung mass, possibly secondary to inflammation and subsequent remodeling. In those smokers with advanced disease (GOLD 2–4), the loss of tissue may have outpaced inflammation and attempts at repair, with a resultant net decrease in lung mass.
CT scanning is looked to as a tool that may provide quantitative COPD biomarkers to serve as intermediate study endpoints in clinical investigation. The most common current application of this tool is in the objective assessment of emphysema progression. To date, studies of such have demonstrated mixed results. While Stockley et al reported that CT may be useful for monitoring the therapeutic benefit of anti-protease augmentation in patients with alpha one antitrypsin deficiency,30 recent data from the ECLIPSE Study suggested that the heterogeneity of longitudinal CT data precludes its utilization in small clinical cohorts.31 While these discrepant observations may be due to the enrollment criteria, number of participating research centers, standardization of the CT data, or biologic basis for the development of COPD, a biphasic change in lung mass, if present, would also confound longitudinal densitometry in a manner dependent upon the sampling interval. Subjects with earlier stages of COPD may manifest a gain of tissue on serial imaging, which will obscure low attenuation areas and result in the appearance of less emphysema. This may in part explain why a small but significant portion of ELCIPSE subjects “lost” emphysema from their baseline to year 3 CT scan.32 In contrast, subjects with latter stages of COPD may behave in a more expected fashion by losing lung tissue and gaining low attenuation areas on CT scan. The implications for such would be significant when utilizing CT as an intermediate endpoint for a pharmaceutical agent that may slow the progression of emphysema. An efficacious compound may appropriately reduce the parenchymal inflammation promoting the progression of emphysema but on longitudinal CT analysis this reduction could even result in a paradoxical increase in low attenuation areas. Such a phenomenon would not obviate the use of CT to evaluate disease progression but may further add to the intricacy of its interpretation.
Our study is focused on the complexity of CT based assessments of parenchymal remodeling in smokers. Since we did not have histopathologic validation of our findings we sought to substantiate the relevance of CT LM by exploring its clinical implications. In cross-sectional analyses LM was directly related to the absolute measures of FEV1 and FVC (r=0.62, P=<0.0001), likely reflecting the association between lung mass and lung size. Subjects with larger lungs (and therefore greater LM) have greater spirometric measures of lung function.
LM was also predictive of the rate of decline in lung function. The reasons for this are not clear but persisted after multivariable adjustment including anthropomorphics and the %LAA-950. While highly speculative, these findings may suggest that greater LM reflects a heightened inflammatory state of the lung with excessive remodeling and subsequent worsening of lung function. Further comprehensive exploration of this observation of the decline in lung function is warranted but is beyond the scope of our current study.
A great limitation to the analyses presented herein is the lack of longitudinal CT data on LM and the tenuous assertion that cross-sectional data (trend of lung mass by GOLD Stage) reflects disease progression. While this assertion may indeed be true, potential verification or repudiation of our interpretation of the CT data awaits the ongoing collection and objective analyses of the follow up COPDGene CT scans. Even a second CT scan will not, however, be enough to validate our hypothesis since data from 2 time points cannot be readily used to define a non-linear trajectory. Our study also lacks an in vivo assessment of pulmonary parenchymal inflammation or histological validation by explanted tissue. Finally, our large sample size allowed us to detect statistically significant small differences in lung mass across GOLD stages as well as according to smoking and COPD statuses. Whether or not the observed differences have clinical relevance requires further investigation.
Computed tomographic assessments of the lung parenchymal in smokers are increasingly being examined as tools that may serve as intermediate study endpoints for clinical investigation. While current smoking status has been rather convincingly shown to confound such efforts,25 our data suggests that there is a biphasic remodeling process found in the parenchyma of former smokers. Further, this biphasic process may hinder our ability to assess emphysema progression in early stage disease. The extrapolation of longitudinal trajectories from cross sectional data is subject to clear limitations, but our findings may in part explain previously published reports on the progression/regression of emphysema. Validation of our results would suggest that CT scanning can provide new insight into parenchymal remodeling in smokers and possibly even the initiation of emphysema. It would also suggest that densitometric assessments of the change in emphysema are confounded by this complex process and longitudinal studies must account for this when quantifying disease progression.
Acknowledgments
Dr. Diaz has received speaker fees from Novartis Inc. unrelated to this work.
Funding
This work was supported by NIH Grants: COPDGene, R01HL089897 (Dr. Crapo), R01HL089856 (Dr. Silverman); Dr. San José Estépar, 1K25HL104085 and R01 HL116473; Dr Washko, R01 HL116473 and R01 HL107246; Dr. Diaz, K01HL118714-01 and the Brigham and Women’s Hospital Minority Faculty Career Development Award. The sponsors had no role on any aspects of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Guarantors:
Drs. Washko and Diaz take responsibility for the content of this manuscript including the data and analysis.
Authors’ Contributions:
Conception and design of this study and creation, revision, and final approval of this manuscript: GW, GK, JR, RSJ, MH, MD, VK, HH, CC, RB, ES, JC, DL, JH, AD.
Analysis and interpretation: GW, AD.
Data acquisition: GW, AD, JR, RSJ, ES, JC, DL.
Drafting the manuscript for important intellectual content: GW, GK, MD, VK, HH, CC, RB, ES, AD.
Conflict of Interest
Drs Washko, Kinney, Ross, San Jose Estepar, Han, Dransfield, Kim, Hatabu, Kim, Come, Bowler, Silverman, Crapo, Lynch, Hokanson and Diaz have no conflict of interest to disclose related to this manuscript.
References
- 1.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M, Makita H, Nagai K, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 3.Croxton TL, Weinmann GG, Senior RM, Wise RA, Crapo JD, Buist AS. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med. 2003;167(8):1142–1149. doi: 10.1164/rccm.200207-756WS. [DOI] [PubMed] [Google Scholar]
- 4.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coxson HO, Leipsic J, Parraga G, Sin DD. Using pulmonary imaging to move chronic obstructive pulmonary disease beyond FEV1. Am J Respir Crit Care Med. 2014;190(2):135–144. doi: 10.1164/rccm.201402-0256PP. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkrantz AB, Mendiratta-Lala M, Bartholmai BJ, et al. Clinical utility of quantitative imaging. Acad Radiol. 2015;22(1):33–49. doi: 10.1016/j.acra.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayhurst MD, MacNee W, Flenley DC, et al. Diagnosis of pulmonary emphysema by computerised tomography. Lancet. 1984;2(8398):320–322. doi: 10.1016/s0140-6736(84)92689-8. [DOI] [PubMed] [Google Scholar]
- 8.Muller NL, Staples CA, Miller RR, Abboud RT. "Density mask". An objective method to quantitate emphysema using computed tomography. Chest. 1988;94(4):782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 9.Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 10.Dirksen A, Dijkman JH, Madsen F, et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1468–1472. doi: 10.1164/ajrccm.160.5.9901055. [DOI] [PubMed] [Google Scholar]
- 11.Parr DG, Sevenoaks M, Deng C, Stoel BC, Stockley RA. Detection of emphysema progression in alpha 1-antitrypsin deficiency using CT densitometry; methodological advances. Respir Res. 2008;9:21. doi: 10.1186/1465-9921-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlahovic G, Russell ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal walls in emphysema. Am J Respir Crit Care Med. 1999;160(6):2086–2092. doi: 10.1164/ajrccm.160.6.9706031. [DOI] [PubMed] [Google Scholar]
- 13.Guenard H, Diallo MH, Laurent F, Vergeret J. Lung density and lung mass in emphysema. Chest. 1992;102(1):198–203. doi: 10.1378/chest.102.1.198. [DOI] [PubMed] [Google Scholar]
- 14.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang EY, Miller RR, Muller NL. Bronchiectasis: comparison of preoperative thin-section CT and pathologic findings in resected specimens. Radiology. 1995;195(3):649–654. doi: 10.1148/radiology.195.3.7753989. [DOI] [PubMed] [Google Scholar]
- 16.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 17.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 18.Wan ES, Hokanson JE, Murphy JR, et al. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184(1):57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowler RP, Kim V, Regan E, et al. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146(4):941–950. doi: 10.1378/chest.13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz AA, Han MK, Come CE, et al. Effect of emphysema on CT scan measures of airway dimensions in smokers. Chest. 2013;143(3):687–693. doi: 10.1378/chest.12-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coxson HO, Rogers RM, Whittall KP, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med. 1999;159(3):851–856. doi: 10.1164/ajrccm.159.3.9805067. [DOI] [PubMed] [Google Scholar]
- 22.Henne E, Anderson JC, Lowe N, Kesten S. Comparison of human lung tissue mass measurements from ex vivo lungs and high resolution CT software analysis. BMC pulmonary medicine. 2012;12:18. doi: 10.1186/1471-2466-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 24.Bartel ST, Bierhals AJ, Pilgram TK, et al. Equating quantitative emphysema measurements on different CT image reconstructions. Medical physics. 2011;38(8):4894–4902. doi: 10.1118/1.3615624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaker SB, Dirksen A, Lo P, Skovgaard LT, de Bruijne M, Pedersen JH. Factors influencing the decline in lung density in a Danish lung cancer screening cohort. Eur Respir J. 2012;40(5):1142–1148. doi: 10.1183/09031936.00207911. [DOI] [PubMed] [Google Scholar]
- 26.Karimi R, Tornling G, Forsslund H, et al. Lung density on high resolution computer tomography (HRCT) reflects degree of inflammation in smokers. Respir Res. 2014;15:23. doi: 10.1186/1465-9921-15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD. 2011;8(1):2–7. doi: 10.3109/15412555.2010.541306. [DOI] [PubMed] [Google Scholar]
- 28.Leopold JG, Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957;12(3):219–235. doi: 10.1136/thx.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 30.Stockley RA, Parr DG, Piitulainen E, Stolk J, Stoel BC, Dirksen A. Therapeutic efficacy of alpha-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;11:136. doi: 10.1186/1465-9921-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vestbo J, Agusti A, Wouters EF, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189(9):1022–1030. doi: 10.1164/rccm.201311-2006PP. [DOI] [PubMed] [Google Scholar]
- 32.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]