Abstract
Background
Chlamydia trachomatis infection is highly prevalent among young women in the United States. Prevention of long-term sequelae of infection, including tubal factor infertility, is a primary goal of chlamydia screening and treatment activities. However, the population attributable fraction of tubal factor infertility associated with chlamydia is unclear, and optimal measures for assessing tubal factor infertility and prior chlamydia in epidemiologic studies have not been established. Black women have increased rates of chlamydia and tubal factor infertility compared to white women, but have been underrepresented in prior studies of the association of chlamydia and tubal factor infertility.
Objectives
To estimate the population attributable fraction of tubal factor infertility associated with Chlamydia trachomatis infection by race (black, non-black), and assess how different definitions of C. trachomatis seropositivity and tubal factor infertility affect population attributable fraction estimates.
Study Design
We conducted a case-control study, enrolling infertile women attending infertility practices in Birmingham, AL and Pittsburgh, PA during October 2012–June 2015. Tubal factor infertility case status was primarily defined by unilateral or bilateral fallopian tube occlusion (cases) or bilateral fallopian tube patency (controls) on hysterosalpingogram. Alternate tubal factor infertility definitions incorporated history suggestive of tubal damage or were based on laparoscopic evidence of tubal damage. We aimed to enroll all eligible women, with an expected ratio of one and three controls per case for black and non-black women, respectively. We assessed C. trachomatis seropositivity with a commercial assay and a more sensitive research assay; our primary measure of seropositivity was defined as positivity on either assay. We estimated C. trachomatis seropositivity and calculated C. trachomatis-TFI odds ratios and population attributable fraction, stratified by race.
Results
We enrolled 107 black women (47 cases, 60 controls) and 620 non-black women (140 cases, 480 controls). C. trachomatis seropositivity by either assay was 81% (95% confidence interval 73%, 89%) among black and 31% (95% confidence interval 28%, 35%) among non-black participants (P<0.001). Using the primary C. trachomatis seropositivity and tubal factor infertility definitions, no significant association was detected between chlamydia and tubal factor infertility among blacks (odds ratio 1.22, 95% confidence interval 0.45, 3.28) or non-blacks (odds ratio 1.41, 95% confidence interval 0.95, 2.09), and the estimated population attributable fraction was 15% (95% confidence interval −97%, 68%) among blacks and 11% (95% confidence interval −3%, 23%) among non-blacks. Use of alternate serologic measures and tubal factor infertility definitions impacted the magnitude of the chlamydia-tubal factor infertility association, and resulted in a significant association among non-blacks.
Conclusions
Low population attributable fraction estimates suggest factors in addition to chlamydia contribute to tubal factor infertility in the study population. However, high background C. trachomatis seropositivity among controls, most striking among black participants, could have obscured an association with tubal factor infertility and resulted in a population attributable fraction that underestimates the true etiologic role of chlamydia. Choice of chlamydia and tubal factor infertility definitions also impacts odds ratio and population attributable fraction estimates.
Keywords: Chlamydia trachomatis, population attributable fraction, serology, tubal factor infertility
INTRODUCTION
Infertility affects millions of people in the United States,1 with tubal factor infertility (TFI) contributing to a substantial proportion of these cases.2 Sexually transmitted infections such as Chlamydia trachomatis infection (“chlamydia”), which is highly prevalent among young women in the United States,3 are a primary risk factor for TFI. While frequently asymptomatic, chlamydia can ascend to the upper genital tract, causing acute or subclinical pelvic inflammatory disease (PID), fallopian tube damage, and increased risk for ectopic pregnancy and TFI.4,5 Prevention of these sequelae is a primary goal of chlamydia screening and treatment activities. However, the population attributable fraction (PAF)6 of TFI associated with chlamydia, which provides a measure of TFI burden that might be prevented by eliminating chlamydial infection, is unclear. Furthermore, optimal measures for assessing TFI and prior chlamydia in epidemiologic studies have not been established. Black women have increased rates of chlamydia3,7 and TFI8,9 compared to white women, but have been underrepresented in prior studies of the association of chlamydia and TFI. An estimate of the PAF of TFI associated with chlamydia would inform evaluation of chlamydia prevention efforts.
The primary objective of this case-control study was to estimate the PAF of TFI associated with chlamydia by race among infertile women assessed for fallopian tube patency. Secondary objectives were to: 1) determine C. trachomatis seropositivity rates in infertile women using a commercial assay and a newer, more sensitive research assay, 2) calculate the odds of C. trachomatis seropositivity in infertile women with obstructed versus patent fallopian tubes (TFI versus non-TFI, respectively), and 3) assess how estimates of odds ratio (OR) and PAF are affected by different definitions of C. trachomatis seropositivity and TFI.
MATERIALS AND METHODS
Between October, 2012 and June, 2015, women were recruited from a private, community-based infertility practice in Birmingham, AL and a university-affiliated infertility practice in Pittsburgh, PA. Eligibility criteria included: age 19–42 years, infertility (inability to achieve an intrauterine pregnancy after ≥12 months of regular sexual intercourse without contraception), having a hysterosalpingogram (HSG) within 12 months of enrollment, ability to provide informed consent, and a current U.S. mailing address. Eligible women were referred to the study by clinical staff in Pittsburgh, and identified directly by clinician investigators in Birmingham. We aimed to enroll all eligible women, with an expected ratio of one control per case for black participants and three controls per case for non-black participants, based on historical prevalence of TFI diagnosis among women evaluated for infertility at the enrollment sites. Information on demographic and clinical risk factors was collected by participant interview and infertility clinic record review. Serum specimens were collected for C. trachomatis antibody testing.
Written informed consent was obtained from all participants. Study approval was obtained from the Institutional Review Boards (IRBs) of the University of Alabama at Birmingham and the University of Pittsburgh. CDC Human Subjects review determined that CDC investigators were not engaged in Human Subjects research for this study and CDC IRB approval was not required.
Sera were evaluated at CDC for IgG antibody to the C. trachomatis major outer membrane protein (OmpA) peptide using the ccany, catalogue #497-PLUS) according to the manufacturer’s instructions (“Medac IgG MOMP”). In addition, sera were evaluated for IgG1 and IgG3 antibodies to C. trachomatis elementary bodies (EBs) at UAB according to a previously published protocol (“EB ELISA”).10,11
For our a priori selected primary definition of TFI (“HSG Case Definition”), participants were categorized as TFI case-patients if their enrollment HSG showed unilateral or bilateral fallopian tube blockage (as defined by lack of free spill of dye into the pelvic cavity), or as non-TFI infertile controls if their enrollment HSG showed bilateral patent fallopian tubes with no other tubal abnormalities and they had no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes. As an exploratory analysis, two other TFI definitions were used. Firstly, participants who could not be categorized as case-patients according to the primary case definition, but who had HSG or historical evidence suggestive of tubal damage (tubal abnormalities other than obstruction on HSG, bilateral patent tubes on HSG with prior history of tubal ectopic pregnancy or surgery to repair blocked tubes, or presence of a single patent tube on HSG with history of contralateral tube removal due to tubal ectopic pregnancy or hydrosalpinx) were categorized as TFI case-patients using an “Expanded Case Definition”. Secondly, a “Laparoscopy Case Definition” was used to categorize participants, independently of HSG results, as TFI case-patients if they had a laparoscopy with evidence of tubal damage (tubal occlusion or fibrosis, fragmented fimbriae, hydrosalpinx, peritubal adhesions) or as non-TFI infertile controls if they had a laparoscopy within one year of study enrollment with no evidence of tubal damage.
Our a priori selected primary measure of C. trachomatis seropositivity was defined as seropositivity by either Medac IgG MOMP or EB ELISA (IgG1 or IgG3). To explore the impact of substituting different C. trachomatis seropositivity definitions on study findings, C. trachomatis seropositivity was alternatively defined in five additional ways: a positive result by the Medac IgG MOMP assay (regardless of EB ELISA results), a positive result by EB ELISA (IgG1 or IgG3, regardless of Medac IgG MOMP results), a positive result by both Medac IgG MOMP and EB ELISA (IgG1 or IgG3), a positive IgG1 response by EB ELISA (regardless of other results), and a positive IgG3 response by EB ELISA (regardless of other results).
Analyses were performed stratified by race (black, non-black). The target sample size of 784 (118 black and 666 non-black) was determined based on range of values for C. trachomatis seroprevalence among infertile women with and without TFI reported in the literature,12 along with anticipated number of eligible participants identified during the enrollment period. Based on C. trachomatis seroprevalence measured among the first 300 enrolled participants (70% of black controls and 12% of non-black controls were seropositive by EB ELISA), we anticipated achieving 80% power to detect a significant (two-sided alpha=0.05) association between C. trachomatis seropositivity and TFI, assuming an odds ratio of at least 3 among blacks and at least 2 among non-blacks.
Chi-square, Fisher’s exact, Cochran-Mantel-Haenszel, and Wilcoxon Mann Whitney tests were used to compare characteristics of cases and controls. C. trachomatis seropositivity with 95% confidence intervals (CIs) was calculated under the binomial distribution. We used logistic regression to assess relationships between exposure variables and TFI. Age, age at first vaginal sex, and lifetime number of male sex partners were categorized as less than or equal to versus greater than the median value, and household income was categorized using pre-defined categories included on the questionnaire. Multivariable analyses were performed using the primary TFI case definition and C. trachomatis seropositivity definition, and adjusted for study site, participant age, household income, and other variables associated with TFI at P<0.1 in bivariate analysis. Associations with P<0.05 were considered statistically significant. Crude and adjusted PAFs and 95% CIs (stratified by race) were calculated based on methods described by Bruzzi et al13 and Efron and Tibshirani.14 We examined the impact of substituting different C. trachomatis seropositivity and TFI case definitions on estimated crude odds ratios (ORs) and PAFs. Statistical analyses were performed using SAS (version 9.3, SAS Institute, Cary, NC).
RESULTS
Figure 1 illustrates enrollment and inclusion in analyses. All eligible women approached were enrolled in the study. Of 784 patients enrolled, 13 were excluded because they did not meet inclusion criteria (n=6) or could not be categorized by any of the case definitions (n=7). Of the remaining 771, 44 had history or HSG findings suggestive of TFI, but could not be categorized using the HSG case definition; these participants were included as cases in analyses using the expanded case definition. Laparoscopy was performed within the designated timeframe among 169 participants.
Figure 1.
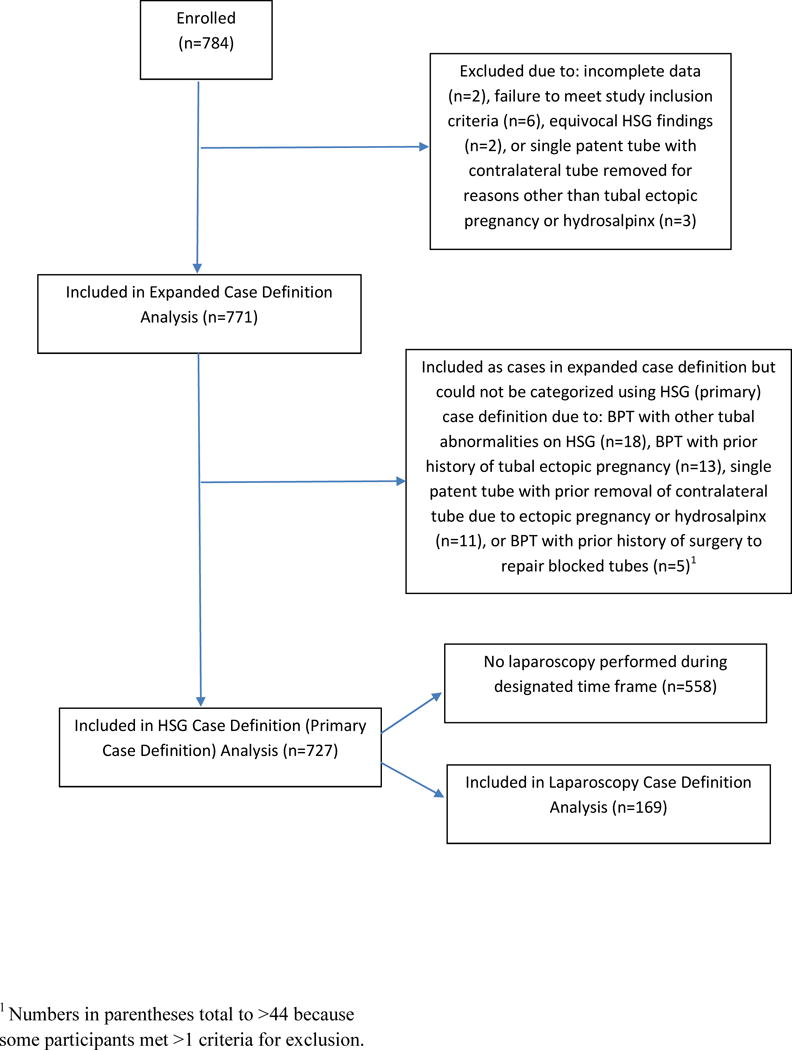
Flow Chart of Study Enrollment and Inclusion in Analyses Using Three TFI Case Definitions.
TFI, tubal factor infertility; HSG, hysterosalpingogram; BPT, bilateral patent tubes.
Characteristics of the 727 study participants (107 black and 620 non-black) categorized according to the HSG case definition are presented in Table 1. Participants ranged in age from 19 through 42 years. Participants of non-black race were predominantly (96%) white. Case-patients were significantly more likely than controls to report household income <$50,000, and history of chlamydia, trichomoniasis, PID, surgically-confirmed endometriosis, and prior abdominal or pelvic surgery, but significantly less likely to report history of combined hormonal contraceptive use. Case-patients had a significantly longer median duration of infertility than controls (28 versus 23 months).
Table 1.
Description of Study Participants by Race and Case Status, HSG Case Definitiona
| All Participants | Black Race | Non-Black Raceb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Cases (n=187) n (%) |
Controls (n=540) n (%) |
Pc | Cases (n=47) n (%) |
Controls (n=60) n (%) |
Pc | Cases (n=140) n (%) |
Controls (n=480) n (%) |
Pc |
| Study site | 0.654 | 0.707 | 0.671 | ||||||
| Birmingham | 72 (38.5) | 198 (36.7) | 26 (55.3) | 31 (51.7) | 46 (32.9) | 167 (34.8) | |||
| Pittsburgh | 115 (61.5) | 342 (63.3) | 21 (44.7) | 29 (48.3) | 94 (67.1) | 313 (65.2) | |||
| Age (median, IQR) | 32 (29–36) | 31 (29–35) | 0.246 | 33 (29–38) | 32 (28–37) | 0.441 | 32 (29–36) | 31 (29–35) | 0.520 |
| Household incomed | 0.001 | 0.031 | 0.134 | ||||||
| <$50,000 | 49 (29.7) | 66 (13.2) | 22 (56.4) | 15 (30.6) | 27 (21.4) | 51 (11.3) | |||
| $50,000–<$75,000 | 27 (16.4) | 116 (23.1) | 5 (12.8) | 11 (22.4) | 22 (17.5) | 105 (23.2) | |||
| ≥$75,000 | 89 (53.9) | 319 (63.7) | 12 (30.8) | 23 (46.9) | 77 (61.1) | 296 (65.5) | |||
| Education | 0.135 | 0.527 | 0.551 | ||||||
| <Bachelor’s degree | 78 (41.7) | 177 (32.8) | 27 (57.4) | 31 (51.7) | 51 (36.4) | 146 (30.4) | |||
| Bachelor’s/4-yr degree | 55 (29.4) | 202 (37.4) | 11 (23.4) | 15 (25.0) | 44 (31.4) | 187 (39.0) | |||
| Master’s degree or higher | 54 (28.9) | 161 (29.8) | 9 (19.2) | 14 (23.3) | 45 (32.1) | 147 (30.6) | |||
| Duration of infertility, months (median, IQR) | 28 (17–61) | 23 (15–38) | <0.001 | 44 (24–72) | 25 (17–54) | 0.034 | 25 (16–57) | 22 (15–37) | 0.026 |
| History of intrauterine pregnancy without ART | 74 (39.6) | 188 (34.8) | 0.244 | 25 (53.2) | 31 (51.7) | 0.875 | 49 (35.0) | 157 (32.7) | 0.612 |
| History of live birth without ART | 37 (19.8) | 101 (18.7) | 0.745 | 14 (29.8) | 15 (25.0) | 0.580 | 23 (16.4) | 86 (17.9) | 0.684 |
| History of smoking | 67 (35.8) | 169 (31.3) | 0.255 | 15 (31.9) | 14 (23.3) | 0.322 | 52 (37.1) | 155 (32.3) | 0.285 |
| History of marijuana use | 93 (49.7) | 250 (46.3) | 0.417 | 28 (59.6) | 29 (48.3) | 0.247 | 65 (46.4) | 221 (46.0) | 0.936 |
| History of other illicit drug use | 26 (13.9) | 64 (11.8) | 0.463 | 9 (19.1) | 3 (5.0) | 0.021 | 17 (12.1) | 61 (12.7) | 0.859 |
| History of combined hormonal contraception | 154 (83.7) | 486 (90.5) | 0.012 | 33 (71.7) | 54 (90.0) | 0.015 | 121 (87.7) | 432 (90.6) | 0.321 |
| History of progestin-only contraception | 34 (18.6) | 80 (15.1) | 0.263 | 14 (30.4) | 18 (30.0) | 0.961 | 20 (14.6) | 62 (13.2) | 0.665 |
| Age at first vaginal sex (median, IQR) | 17 (16–20) | 18 (16–20) | 0.122 | 16 (15–18) | 16 (15–18) | 0.596 | 18 (16–20) | 18 (16–20) | 0.626 |
| Lifetime number of male sex partners (median, IQR) | 5 (3–9) | 5 (2–8) | 0.124 | 6 (4–8) | 7 (4–10) | 0.652 | 4 (2–9) | 4 (2–7) | 0.291 |
| History of chlamydia | 29 (15.5) | 44 (8.1) | 0.004 | 17 (36.2) | 17 (28.3) | 0.387 | 12 (8.6) | 27 (5.6) | 0.206 |
| History of gonorrhea | 6 (3.2) | 12 (2.2) | 0.424 | 5 (10.6) | 8 (13.3) | 0.672 | 1 (0.7) | 4 (0.8) | 1.000 |
| History of bacterial vaginosis | 15 (8.0) | 27 (5.0) | 0.127 | 9 (19.1) | 14 (23.3) | 0.601 | 6 (4.3) | 13 (2.7) | 0.401 |
| History of trichomoniasis | 13 (6.9) | 9 (1.7) | <0.001 | 8 (17.0) | 7 (11.7) | 0.428 | 5 (3.6) | 2 (0.4) | 0.008 |
| History of PID | 14 (7.5) | 14 (2.6) | 0.003 | 9 (19.1) | 5 (8.3) | 0.100 | 5 (3.6) | 9 (1.9) | 0.327 |
| History of ectopic pregnancy | 20 (10.7) | N/A | N/A | 11 (23.4) | N/A | N/A | 9 (6.4) | N/A | N/A |
| Surgically-confirmed endometriosis | 37 (19.8) | 63 (11.7) | 0.005 | 7 (14.9) | 6 (10.0) | 0.442 | 30 (21.4) | 57 (11.9) | 0.004 |
| History of abdominal/pelvic surgery | 53 (28.3) | 100 (18.5) | 0.004 | 17 (36.2) | 14 (23.3) | 0.146 | 36 (25.7) | 86 (17.9) | 0.041 |
| History of abdominal/pelvic inflammation | 16 (8.6) | 37 (6.8) | 0.440 | 4 (8.5) | 0 (0.0) | 0.035 | 12 (8.6) | 37 (7.7) | 0.739 |
IQR, interquartile range; ART, assisted reproductive technology; PID, pelvic inflammatory disease; HSG, hysterosalpingogram
TFI case=unilateral or bilateral fallopian tube blockage on HSG; Control=bilateral patent tubes and no other tubal abnormalities on HSG, no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes
Non-black race included: 595 white, 19 Asian, 6 other race
Chi-square, Fisher’s Exact, or Cochran-Mantel-Haenszel test for categorical variables and Wilcoxon Mann Whitney test for continuous variables. Statistically significant (P<0.05) results are indicated by bold print.
Income data unavailable for 18% of black participants (17% of cases, 18% of controls) and 7% of non-black participants (10% of cases, 6% of controls)
Characteristics of the 771 participants included in the expanded case definition analysis were similar to those included in the HSG case definition analysis (Table A.1). Characteristics of the 169 participants categorized according to the laparoscopy case definition are presented in Table A.2. Distribution of cases and controls contributed by the two sites differed for the laparoscopy case definition, with the Birmingham site contributing 56% of cases and 77% of controls.
C. trachomatis seropositivity by race, case status, and serologic measure used is presented in Table 2 for the 727 participants categorized according to the HSG case definition. Using the primary definition of C. trachomatis seropositivity, 81% (95% CI 73%, 89%) of black and 31% (95% CI 28%, 35%) of non-black participants were seropositive (P<0.001). Using different definitions of C. trachomatis seropositivity, point estimates of seropositivity were consistently higher among case-patients versus controls for non-black participants, although 95% CIs often overlapped. In contrast, among black participants, point estimates of C. trachomatis seropositivity were not consistently higher or lower in case-patients versus controls. A larger proportion of participants were seropositive by EB ELISA than by Medac IgG MOMP, particularly among blacks.
Table 2.
Chlamydia trachomatis Seropositivity by Race, Serologic Measure, and Tubal Factor Infertility (TFI) Case Status based on HSG Case Definitiona
| Serologic Measure used to Determine Chlamydia trachomatis Seropositivity | |||||||
|---|---|---|---|---|---|---|---|
| Study Group | N | Positive by Medac IgG MOMP n (%, 95% CI) |
Positive by EB-ELISA (IgG1 or IgG3) n (%, 95% CI) |
Positive by EB-ELISA IgG1 n (%, 95% CI) |
Positive by EB ELISA IgG3 n (%, 95% CI) |
Positive by Medac IgG MOMP or EB ELISAb n (%, 95% CI) |
Positive by Medac IgG MOMP and EB ELISA n (%, 95% CI) |
| Black Race | |||||||
| Overall | 107c | 51 48% (38%–58%) |
85 79% (71%–88%) |
83 78% (69%–86%) |
67 63% (53%–72%) |
87 81% (73%–89%) |
49 46% (36%–56%) |
| Cases | 47c | 22 48% (32%–63%) |
38 81% (69%–93%) |
36 77% (63%–90%) |
27 57% (42%–73%) |
39 83% (71%–95%) |
21 46% (30%–61%) |
| Controls | 60 | 29 48% (35%–62%) |
47 78% (67%–90%) |
47 78% (67%–90%) |
40 67% (54%–79%) |
48 80% (69%–91%) |
28 47% (33%–60%) |
| Non-Black Race | |||||||
| Overall | 620 | 106 17% (14%–20%) |
146 24% (20%–27%) |
97 16% (13%–19%) |
95 15% (12%–18%) |
194 31% (28%–35%) |
58 9% (7%–12%) |
| Cases | 140 | 31 22% (15%–29%) |
43 31% (23%–39%) |
29 21% (14%–28%) |
35 25% (17%–33%) |
52 37% (29%–46%) |
22 16% (9%–22%) |
| Controls | 480 | 75 16% (12%–19%) |
103 21% (18%–25%) |
68 14% (11%–17%) |
60 13% (9%–16%) |
142 30% (25%–34%) |
36 8% (5%–10%) |
HSG, hysterosalpingogram; MOMP, major outer membrane protein; CI, confidence interval; EB, elementary body; ELISA, enzyme-linked immunosorbent assay
TFI case=unilateral or bilateral fallopian tube blockage on HSG; Control=bilateral patent tubes and no other tubal abnormalities on HSG, no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes
Primary definition of C. trachomatis seropositivity
Medac IgG MOMP result was not available for one participant.
Crude ORs and 95% CIs for associations between C. trachomatis seropositivity and TFI by race, TFI case definition, and chlamydia serologic measure are presented in Table 3. Using the primary definitions for TFI and seropositivity, ORs were less than 1.5, and no significant association was detected. Among blacks, no significant association was detected regardless of case definition used; however, the magnitude of association was greatest using the laparoscopy case definition. Among non-blacks, a significant association was consistently detected using the expanded case definition, and the magnitude of association was increased using the laparoscopy case definition. Definition of C. trachomatis seropositivity also impacted the findings, with a significant association detected consistently when defining seropositivity based on EB ELISA (IgG1 or IgG3 response), EB ELISA IgG3, or a positive response on both EB ELISA and Medac IgG MOMP.
Table 3.
Crude Odds Ratios for Association between Chlamydia trachomatis Seropositivity and Tubal Factor Infertility (TFI), by Race, Serologic Measure, and TFI Case Definition
| Serologic Measure used to Determine Chlamydia trachomatis Seropositivity | |||||||
|---|---|---|---|---|---|---|---|
| Case Definition (by Race) | N | Positive by Medac IgG MOMP OR (95% CI)a |
Positive by EB-ELISA (IgG1 or IgG3) OR (95% CI)a |
Positive by EB-ELISA IgG1 OR (95% CI)a |
Positive by EB ELISA IgG3 OR (95% CI)a |
Positive by Medac IgG MOMP or EB ELISA OR (95% CI)a |
Positive by Medac IgG MOMP and EB ELISA OR (95% CI)a |
| Black Race | |||||||
| HSG case definitionb | 107c | 0.98 (0.45–2.11) |
1.17 (0.45–3.02) |
0.91 (0.36–2.26) |
0.68 (0.31–1.49) |
1.22 (0.45–3.28) |
0.96 (0.44–2.07) |
| Expanded case definitiond | 117c | 0.93 (0.45–1.92) |
1.04 (0.43–2.51) |
0.85 (0.36–2.01) |
0.74 (0.35–1.57) |
1.18 (0.46–2.98) |
0.86 (0.41–1.78) |
| Laparoscopy case definitione | 31 | 1.13 | 2.95 (0.48–18.3) |
2.95 (0.48–18.3) |
1.50 (0.36–6.23) |
1.50 (0.21–10.5) |
1.93 (0.44–8.33) |
| Non-Black Race | |||||||
| HSG case definitionb | 620 | 1.54 (0.96–2.45) |
1.62 (1.07–2.47) |
1.58 (0.98–2.56) |
2.33 (1.46–3.73) |
1.41 (0.95–2.09) |
2.30 (1.30–4.06) |
| Expanded case definitiond | 654 |
1.61 (1.05–2.48) |
1.78 (1.21–2.62) |
1.81 (1.17–2.80) |
2.37 (1.53–3.66) |
1.56 (1.09–2.25) |
2.37 (1.39–4.01) |
| Laparoscopy case definitione | 138 |
3.04 (1.27–7.29) |
2.13 (1.01–4.50) |
2.24 (0.91–5.49) |
3.74 (1.58–8.87) |
1.90 (0.94–3.85) |
5.44 (1.71–17.3) |
OR, odds ratio; HSG, hysterosalpingogram; MOMP, major outer membrane protein; CI, confidence interval; EB, elementary body; ELISA, enzyme-linked immunosorbent assay
Statistically significant results (P<0.05) are indicated by bold print.
TFI case=unilateral or bilateral fallopian tube blockage on HSG; Control=bilateral patent tubes and no other tubal abnormalities on HSG and no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes
Medac IgG MOMP result was not available for one participant
TFI case=unilateral or bilateral fallopian tube blockage or other tubal abnormalities on HSG, or prior history of tubal ectopic pregnancy, hydrosalpinx, or surgery to repair blocked tubes; Control=bilateral patent tubes and no other tubal abnormalities on HSG and no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes
TFI case=laparoscopy showing evidence of tubal damage; Control=laparoscopy within one year of study showing no evidence of tubal damage
Table 4 presents ORs and 95% CIs for bivariate associations between measured covariates and TFI and multivariable associations between C. trachomatis seropositivity and TFI adjusting for these covariates. Among black participants, history of illicit drug use, income, and history of combined hormonal contraception were independently associated with TFI, with the latter two being protective associations. Among non-blacks, history of trichomoniasis, surgically-confirmed endometriosis, and income were independently associated with TFI, with income being a protective association. No significant association between C. trachomatis seropositivity and TFI was detected in the multivariable models for either blacks or non-blacks, although the association approached statistical significance in non-blacks.
Table 4.
Bivariate and Multivariable Associations with Tubal Factor Infertility (TFI)a by Race
| Black Race | Non-Black Race | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Bivariate (n=107) | Multivariableb (n=87) | Bivariate (n=620) | Multivariableb (n=578) | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| C. trachomatis seropositivity | 1.22 (0.45,3.28) | 0.695 | 1.00 (0.31,3.24) | 0.998 | 1.41 (0.95,2.09) | 0.090 | 1.42 (0.93,2.17) | 0.109 |
| Study site | ||||||||
| Birmingham | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Pittsburgh | 0.86 (0.40–1.86) | 0.707 | 0.48 (0.15–1.52) | 0.212 | 1.09 (0.73–1.63) | 0.671 | 1.45 (0.90–2.35) | 0.126 |
| Agec | 1.28 (0.59–2.74) | 0.533 | 0.94 (0.33–2.65) | 0.900 | 1.16 (0.79–1.68) | 0.454 | 1.12 (0.74–1.69) | 0.606 |
| Household income | ||||||||
| <$50,000 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| $50,000–<$75,000 | 0.31 (0.09–1.08) | 0.065 | 0.14 (0.03–0.67) | 0.015 | 0.40 (0.21–0.76) | 0.005 | 0.38 (0.20–0.75) | 0.005 |
| ≥$75,000 | 0.36 (0.14–0.93) | 0.034 | 0.41 (0.13–1.31) | 0.133 | 0.49 (0.29–0.83) | 0.008 | 0.49 (0.28–0.86) | 0.013 |
| Education | ||||||||
| <Bachelor’s degree | Reference | Reference | Reference | Reference | ||||
| Bachelor’s/4-yr degree | 0.84 (0.33–2.14) | 0.718 | 0.67 (0.43–1.06) | 0.091 | ||||
| Master’s degree or higher | 0.74 (0.28–1.97 | 0.545 | 0.88 (0.55–1.39) | 0.575 | ||||
| Smoking | 1.54 (0.65–3.63) | 0.322 | 1.24 (0.84–1.83) | 0.284 | ||||
| Illicit drug use (other than marijuana) | 4.50 (1.14–17.7) | 0.031 | 7.73 (1.31–45.6) | 0.024 | 0.95 (0.53–1.69) | 0.859 | ||
| Combined hormonal contraception | 0.28 (0.10–0.81) | 0.013 | 0.13 (0.03–0.65) | 0.013 | 0.74 (0.41–1.34) | 0.323 | ||
| Progestin-only contraception | 1.02 (0.44–2.36) | 0.961 | 1.13 (0.65–1.94) | 0.665 | ||||
| Age at first vaginal sexc | 0.79 (0.36–1.71) | 0.552 | 0.83 (0.56–1.23) | 0.350 | ||||
| Lifetime # of male sex partnersc | 0.73 (0.34–1.57) | 0.414 | 1.00 (0.68–1.45) | 0.985 | ||||
| History of gonorrhea | 0.77 (0.24–2.54) | 0.672 | 0.86 (0.09–7.72) | 0.890 | ||||
| History of bacterial vaginosis | 0.78 (0.30–1.99) | 0.601 | 1.61 (0.60–4.31) | 0.344 | ||||
| History of trichomoniasis | 1.55 (0.52–4.64) | 0.431 | 8.85 (1.70–46.1) | 0.010 | 6.96 (1.30–37.2) | 0.023 | ||
| Surgically-confirmed endometriosis | 1.57 (0.49–5.05) | 0.445 | 2.02 (1.24–3.30) | 0.005 | 2.30 (1.38–3.85) | 0.002 | ||
| Abdominal/pelvic surgery | 1.86 (0.80–4.33) | 0.149 | 1.59 (1.02–2.48) | 0.042d | ||||
| Abdominal/pelvic inflammation | >9999.99 | 0.971 | 1.12 (0.57–2.22) | 0.739 | ||||
OR, odds ratio; CI, confidence interval, C. trachomatis, Chlamydia trachomatis; #, number
Based on hysterosalpingogram (HSG) case definition (primary case definition): TFI case=unilateral or bilateral fallopian tube blockage on HSG; Control=bilateral patent tubes and no other tubal abnormalities on HSG and no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes
Multivariable model included only those variables with ORs displayed in table
Less than or equal to versus greater than the median value
History of abdominal or pelvic surgery was strongly correlated with history of surgically-confirmed endometriosis and was not included in the multivariable model.
Crude fractions of TFI attributable to chlamydia and associated 95% CIs are presented in Table 5. Among blacks, PAF point estimates varied widely, and results were not statistically significant regardless of the serologic measure and case definition used. Among non-blacks, results were statistically significant using the expanded case definition or C. trachomatis seropositivity based on EB ELISA (IgG1 or IgG3 response), EB ELISA IgG3, or a positive response on both EB ELISA and Medac IgG MOMP, but PAF point estimates were consistently largest (and generally statistically significant) when based on the laparoscopy case definition. Adjusted PAF estimates using the HSG case definition yielded attenuated results compared to the corresponding unadjusted estimates (Table A.3).
Table 5.
Crude Fraction of Tubal Factor Infertility (TFI) Attributable to Chlamydia, by Race, Serologic Measure, and TFI Case Definition
| Serologic Measure used to Determine Chlamydia trachomatis Seropositivity | |||||||
|---|---|---|---|---|---|---|---|
| Case Definition (by Race) | N | Positive by Medac IgG MOMP PAF (95% CI) |
Positive by EB-ELISA (IgG1 or IgG3) PAF (95% CI) |
Positive by EB-ELISA IgG1 PAF (95% CI) |
Positive by EB ELISA IgG3 PAF (95% CI) |
Positive by Medac IgG MOMP or EB ELISA PAF (95% CI) |
Positive by Medac IgG MOMP and EB ELISA PAF (95% CI) |
| Black Race | |||||||
| HSG case definitiona | 107b | −1% (−48%, 31%) |
12% (−97%, 63%) |
−8% (−100%, 51%) |
−28% (−100%, 24%) |
15% (−97%, 68%) |
−2% (−47%, 30%) |
| Expanded case definitionc | 117b | −4% (−49%, 28%) |
3% (−100%, 55%) |
−13% (−100%, 44%) |
−21% (−100%, 26%) |
12% (−99%, 63%) |
−7% (−52%, 23%) |
| Laparoscopy case definitiond | 31 | 5% (−91%, 55%) |
57% (−82%, 87%) |
57% (−82%, 87%) |
20% (−88%, 70%) |
29% (−100%, 80%) |
22% (−39%, 61%) |
| Non-Black Race | |||||||
| HSG case definitiona | 620 | 8% (−1%, 17%) |
12% (1%, 22%) |
8% (−1%, 16%) |
14% (6%, 23%) |
11% (−3%, 23%) |
9% (2%, 16%) |
| Expanded case definitionc | 654 | 9% (0%, 17%) |
14% (5%, 24%) |
10% (2%, 18%) |
15% (7%, 23%) |
14% (3%, 26%) |
9% (3%, 16%) |
| Laparoscopy case definitiond | 138 | 20% (5%, 35%) |
20% (0%, 37%) |
13% (−1%, 27%) |
26% (10%, 40%) |
21% (−2%, 40%) |
20% (8%, 31%) |
PAF, population attributable fraction; CI, confidence interval; MOMP, major outer membrane protein; EB, elementary body; ELISA, enzyme-linked immunosorbent assay; HSG, hysterosalpingogram
TFI case=unilateral or bilateral fallopian tube blockage on HSG; Control=bilateral patent tubes and no other tubal abnormalities on HSG and no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes
Medac IgG MOMP result was not available for one subject.
TFI case=unilateral or bilateral fallopian tube blockage or other tubal abnormalities on HSG, or prior history of tubal ectopic pregnancy, hydrosalpinx, or surgery to repair blocked tubes; Control=bilateral patent tubes and no other tubal abnormalities on HSG and no prior history of tubal ectopic pregnancy or surgery to repair blocked tubes
TFI case=laparoscopy showing evidence of tubal damage; Control=laparoscopy within one year of study showing no evidence of tubal damage
COMMENT
In this study, designed to estimate the PAF of TFI associated with chlamydia, we found that race, C. trachomatis serologic measure, and TFI case definition substantially impacted our findings. Due to a limited number of black participants, our PAF estimates for blacks were imprecise, with an estimate of 15% (95% CI-97%, 68%) based on primary measures of seropositivity and TFI. Among non-blacks, estimated PAF was 11% (95% CI−3%, 23%) using the primary seropositivity measure and case definition; however, substitution of alternative measures resulted in PAF estimates as high as 26% (95% CI 10%, 40%). High C. trachomatis seroprevalence among infertile women with patent tubes, which was most striking (80%) among black participants, might have obscured an association between chlamydia and TFI and resulted in a PAF that underestimates the true etiologic role of chlamydia in TFI.
High C. trachomatis seropositivity in infertile black women, regardless of tubal patency, likely reflects high infection rates among black women in the communities served by our study sites. This is consistent with U.S. national data on distribution of chlamydia by race.3,7 High seropositivity in black controls might represent a high background prevalence of uncomplicated lower genital tract chlamydia, unrelated to infertility, or could indicate the presence of chlamydia-associated non-occlusive (functional) fallopian tube damage. We selected controls from among infertile women attending the same clinics as the TFI cases in order to increase comparability and minimize bias from unmeasured confounders; however, determination of C. trachomatis seropositivity in fertile controls drawn from the same population would be helpful to assess which of these explanations is most accurate.
Lower C. trachomatis seroprevalence in non-black case-patients, with PAF point estimates ranging from 8% to 26%, suggests that, while chlamydia likely plays a role in TFI in this population, other infectious or non-infectious sources of TFI are also prevalent. It is possible that chlamydia screening programs have resulted in decreased rates of chlamydia-associated PID in this population, or that early effective management of PID has resulted in decreased progression to TFI. This hypothesis is supported by declining rates of PID diagnoses and declining prevalence of TFI diagnosis among women using assisted reproductive technology (ART)15 in the United States, reported for all races combined. In addition, in a 2002 report, C. trachomatis was identified in the cervix or endometrium of less than one-quarter of women with acute PID.16
The fact that C. trachomatis seropositivity was substantially more common than reported or documented history of chlamydia underscores the need for a biomarker of prior chlamydia. Among nine studies published during 2000–2015 that assessed C. trachomatis seropositivity as a marker of prior chlamydia in TFI cases and infertile controls using IgG MOMP assays, seropositivity ranged from 23% to 74% among cases, and 9% to 33% among controls.17–25 The results we obtained using the Medac IgG MOMP assay were within this range, except that IgG MOMP seropositivity was lower in our non-black case-patients (22% [15%, 29%]), and higher in our black controls (48% [35%, 62%]). None of these studies presented PAF of TFI due to chlamydia, none were performed in the United States or a location with chlamydia screening recommendations in place, and all were either restricted to white participants or did not present participant race. Furthermore, in contrast to previous studies, we added a newer EB ELISA demonstrated to detect C. trachomatis seropositivity in 90% of individuals with current genitalchlamydia, compared to 73% detected with the Medac IgG MOMP assay.10 Not surprisingly, seropositivity was higher when based on the more sensitive EB ELISA, particularly in black participants. The magnitude of the EB ELISA response has been shown to differ by race, with stronger responses detected in black versus white persons with current genital chlamydia or unknown infection status.10 Because magnitude of antibody response (especially IgG3) may decline over time following resolution of infection, the ability of this assay to detect seropositivity in persons with a more remote history of infection may be greater in blacks. More frequent re-exposure and repeat infection among black women could also contribute to a more sustained antibody response.
A notable strength of our study was the use of a C. trachomatis serologic assay with improved sensitivity compared to assays used in previously published studies. In addition, our study provides new insights into the association between chlamydia and infertility in an era when chlamydia screening and treatment recommendations have been implemented, and attempts to assess the impact of race on this association, providing important data that will inform future studies. Our study also had limitations. Although we aimed to enroll all eligible women, some might have been missed. Our results might not be generalizable to all infertile women. We lacked statistical power to detect the hypothesized effect size among blacks. We lacked biomarkers to evaluate the contribution of other infections to TFI. Finally, laparoscopy, which is considered the reference standard for diagnosing TFI, was not routinely performed in all patients at our study sites; therefore, HSG was used for primary assessment of tubal occlusion. A recent meta-analysis of seven studies26 found that HSG had an overall 53% sensitivity and 87% specificity compared to laparoscopy for detecting unilateral tubal occlusion. Low sensitivity of HSG in detecting tubal occlusion might have resulted in misclassification of some cases as controls and biased our results towards the null. When we included as cases women with bilateral patent tubes but other tubal abnormalities on HSG or with historical evidence of tubal damage (i.e., the expanded case definition), we consistently detected a significant association between chlamydia and TFI among non-blacks. However, the authors of the above meta-analysis reported that HSG sensitivity compared to laparoscopy was significantly lower in women without as compared to with risk factors for tubal pathology (e.g., history of PID), suggesting that laparoscopy is not a perfect reference standard. Furthermore, neither HSG nor laparoscopy can reliably detect functional damage in patent tubes, highlighting the need for more reliable, non-invasive markers of tubal damage.
In addition to our primary outcome, key findings of our study include an extraordinarily high C. trachomatis seroprevalence among infertile black controls and variability in magnitude and statistical significance of OR and PAF estimates based on choice of C. trachomatis seropositivity measure and TFI case definition. Improved understanding of immune responses to chlamydia in this population and additional evaluation of tests used to measure these responses may allow refinement of estimates in future studies. Attention should also be directed towards exploring the contribution of other infectious and non-infectious exposures to TFI.
Supplementary Material
Table A.1: Description of Study Participants by Race and Case Status, Expanded Case Definition
Table A.2. Description of Study Participants by Race and Case Status, Laparoscopy Case Definition
Table A.3. Fraction of Tubal Factor Infertility (TFI) Attributable to Chlamydia, Adjusted for Selected Covariates, by Race
Acknowledgments
We wish to acknowledge the following individuals for their contribution to this project:
Deborah Bass, University of Pittsburgh: data management
Alissa Bernholc, FHI360: data analysis
Heather Bocan, Magee-Womens Research Institute: data collection
Jim Braxton, CDC: data management
LaDraka Brown, University of Alabama at Birmingham: laboratory support
Carolyn Deal, NIAID: study administration
Kelly Dunn, Magee-Womens Research Institute: data review/quality assurance
Jamie Haggerty, Magee-Womens Research Institute: regulatory support
Barbara Hahn, NIAID: study administration
Kathleen Hutchins, CDC: data management
Marga Jones, University of Alabama at Birmingham: data management
Ingrid Macio, Magee-Womens Research Institute: study coordination
Linda McNeil, FHI360: study administration
Richard Morrison, University of Arkansas: provision of C. trachomatis EBs for EB-ELISA
Charlotte Perlowski, FHI360: administrative support
Etta Volk, Magee-Womens Research Institute: study recruitment
Funding: Centers for Disease Control and Prevention, Prevention Research Centers (Grant# 5U48DP001915 to WMG and Grant# 5U48DP001918 to HCW); National Institutes of Health, Sexually Transmitted Infections Clinical Trials Group (Contract #HHSN272201300012I to EWH). Study sponsors were involved in study design (CDC), analysis and interpretation of data (CDC, NIH), writing of report (CDC, NIH), and decision to submit the article for publication (CDC, NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: HCW has received laboratory supplies from Hologic, Inc. MWG has received research funding to evaluate T cell responses in women with uncomplicated chlamydial infection and a consulting fee related to a chlamydia vaccine design meeting from Genocea Biosciences, Inc, and has received research funding from Moderna Therapeutics, Inc to investigate immune responses to different chlamydia proteins in women with uncomplicated chlamydial infection. All other authors have nothing to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
Conference Presentation: Preliminary results of this study were presented at the 2016 STD Prevention Conference held in Atlanta, GA, September 20–23, 2016.
Condensation: Attributable fraction of tubal factor infertility associated with chlamydia is low, but varies by race, C. trachomatis antibody measure, and definition of tubal factor infertility.
References
- 1.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Report. 2013 Aug;14(67):1–18. [PubMed] [Google Scholar]
- 2.Evers JL. Female subfertility. Lancet. 2002;360(9327):151–9. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 3.Torrone E, Papp J, Weinstock H, Centers for Disease Control and Prevention Prevalence of Chlamydia trachomatis genital infection among persons aged 14–39 years-United States, 2007–2012. MMWR Morb Mortal Wkly Rep. 2014 Sep 26;63(38):834–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Westrom L, Joesoef R, Reynolds G, Hadgu A, Thompson SE. Pelvic inflammatory disease and fertility: A cohort study of 1,844 women with laparoscopically verified disease and 657 women with normal laparoscopy. Sexually Transmitted Diseases. 1992;19:185–92. [PubMed] [Google Scholar]
- 5.Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol. 2012 Jul;120(1):37–43. doi: 10.1097/AOG.0b013e31825a6bc9. [DOI] [PubMed] [Google Scholar]
- 6.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2014. Atlanta: U.S. Department of Health and Human Services; 2015. [Google Scholar]
- 8.Green JA, Robins JC, Scheiber M, Awadalla S, Thomas MA. Racial and economic demographics of couples seeking infertility treatment. American Journal of Obstetrics and Gynecology. 2001;184(6):1080–2. doi: 10.1067/mob.2001.115222. [DOI] [PubMed] [Google Scholar]
- 9.Jain T. Socioeconomic and racial disparities among infertility patients seeking care. Fertility and Sterility. 2006;85(4):876–81. doi: 10.1016/j.fertnstert.2005.07.1338. [DOI] [PubMed] [Google Scholar]
- 10.Geisler WM, Morrison SG, Doemland ML, et al. Immunoglobulin-specific responses to Chlamydia elementary bodies in individuals with and at risk for genital chlamydial infection. J Infect Dis. 2012 Dec 15;206(12):1836–43. doi: 10.1093/infdis/jis621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steiner AZ, Diamond E, Legros RS, et al. Chlamydia trachomatis serostatus is an independent predictor of pregnancy and pregnancy outcome. Fertil Steril. 2015;104:1522–6. doi: 10.1016/j.fertnstert.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesenfeld HC, Cates W., Jr . Sexually Transmitted Diseases and Infertility. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, et al., editors. Sexually Transmitted Diseases. New York: McGraw-Hill; 2008. pp. 1511–27. [Google Scholar]
- 13.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. American Journal of Epidemiology. 1985;122(5):904–14. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 14.Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 15.Kawwass JF, Crawford S, Kissin DM, Session DR, Boulet S, Jamieson DJ. Tubal factor infertility and perinatal risk after assisted reproductive technology. Obstet Gynecol. 2013 Jun;121(6):1263–71. doi: 10.1097/AOG.0b013e31829006d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. American Journal of Obstetrics and Gynecology. 2002;186(5):929–37. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 17.Clausen HF, Fedder J, Drasbek M, et al. Serological investigation of Mycoplasma genitalium in infertile women. HumReprod. 2001;16(9):1866–74. doi: 10.1093/humrep/16.9.1866. [DOI] [PubMed] [Google Scholar]
- 18.Hjelholt A, Christiansen G, Johannesson TG, Ingerslev HJ, Birkelund S. Tubal factor infertility is associated with antibodies against Chlamydia trachomatis heat shock protein 60 (HSP60) but not human HSP60. Hum Reprod. 2011 Aug;26(8):2069–76. doi: 10.1093/humrep/der167. [DOI] [PubMed] [Google Scholar]
- 19.Kinnunen A, Surcel HM, Halttunen M, Tiitinen A, Morrison RP, Morrison SG, et al. Chlamydia trachomatis heat shock protein-60 induced interferon-gamma and interleukin-10 production in infertile women. ClinExpImmunol. 2003;131(2):299–303. doi: 10.1046/j.1365-2249.2003.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan S, Gazvani R, McKenzie H, Templeton A, Bhattacharya S. Can history, ultrasound, or ELISA chlamydial antibodies, alone or in combination, predict tubal factor infertility in subfertile women? Human Reproduction. 2003;18(11):2350–6. doi: 10.1093/humrep/deg471. [DOI] [PubMed] [Google Scholar]
- 21.Mouton JW, Peeters MF, Rijssort-Vos JH, Verkooyen RP. Tubal factor pathology caused by Chlamydia trachomatis: the role of serology. InternationalJournal of STD & AIDS. 2002;13(Suppl-9) doi: 10.1258/095646202762226128. [DOI] [PubMed] [Google Scholar]
- 22.Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility–a prospective study. Fertility and Sterility. 2008;90(3):513–20. doi: 10.1016/j.fertnstert.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 23.Sziller I, Babula O, Ujhazy A, et al. Chlamydia trachomatis infection, fallopian tube damage and a mannose-binding lectin codon 54 gene polymorphism. HumReprod. 2007;22(7):1861–5. doi: 10.1093/humrep/dem107. [DOI] [PubMed] [Google Scholar]
- 24.Tiitinen A, Surcel HM, Halttunen M, et al. Chlamydia trachomatis and chlamydial heat shock protein 60-specific antibody and cell-mediated responses predict tubal factor infertility. Human Reproduction. 2006;21(6):1533–8. doi: 10.1093/humrep/del014. [DOI] [PubMed] [Google Scholar]
- 25.Verweij SP, Kebbi-Beghdadi C, Land JA, Ouburg S, Morre SA, Greub G. Waddlia chondrophila and Chlamydia trachomatis antibodies in screening infertile women for tubal pathology. Microbes Infect. 2015 Nov-Dec;17(11–12):745–8. doi: 10.1016/j.micinf.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Broeze KA, Opmeer BC, Van Geloven N, et al. Are patient characteristics associated with the accuracy of hysterosalpingography in diagnosing tubal pathology? An individual patient data meta-analysis. Hum Reprod Update. 2011 May-Jun;17(3):293–300. doi: 10.1093/humupd/dmq056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A.1: Description of Study Participants by Race and Case Status, Expanded Case Definition
Table A.2. Description of Study Participants by Race and Case Status, Laparoscopy Case Definition
Table A.3. Fraction of Tubal Factor Infertility (TFI) Attributable to Chlamydia, Adjusted for Selected Covariates, by Race


