Abstract
Background
These studies investigated the serum pharmacokinetic (PK) profile of racemic (3,4)-methylenedioxypyrovalerone [(R,S)-MDPV)] and its (R)- and (S)-enantiomers in female and male Sprague Dawley rats.
Methods
Intravenous (R,S)-MDPV (3 and 5.6 mg/kg) and single enantiomer of (R)- and (S)-MDPV (1.5 mg/kg) were administered to both sexes for PK studies. Intraperitoneal (ip) bioavailability was determined at 3 mg/kg (R,S)-MDPV. Locomotor activity studies were conducted after ip treatment with saline and 0.3–5.6 mg/kg of (R,S)-MDPV.
Results
PK values after iv (R,S)-MDPV showed a significant (p<0.05) sex-dependent differences in the volume of distribution at steady state (Vdss) for (R)- and (R,S)-MDPV at both (R,S)-MDPV doses. The female S/R enantiomeric ratios for area under the concentration time curve (AUCinf) and clearance were significantly lower and higher, respectively, than values determined in males. Importantly, there was no evidence of in vivo inversion of (R)-MDPV or (S)-MDPV to its antipode. There were, however, significant sex-dependent differences in volume of distribution after administration of the (R)-enantiomer. Bioavailability studies of ip (R,S)-MDPV showed greater variability and significantly greater bioavailability in male rats. Accordingly, there was a significantly greater maximal distance traveled measurement in male rats at a 3.0 mg/kg dose.
Conclusion
PK sex differences in (R,S)-MDPV and enantiomers were most apparent in volume of distribution, which could be caused by differences in drug blood and tissue protein binding. The increased magnitude and variance in ip bioavailability in male compared to female rats could lead to sex-dependent differences in the pharmacological action caused by active enantiomer (S)-MDPV.
Keywords: (3,4)-methylenedioxypyrovalerone; chirality; pharmacokinetics; bioavailability; behavior; rat sex
1. Introduction
Racemic 3,4-methylenedioxypyrovalerone [(R,S)-MDPV] is a commonly detected synthetic psychoactive cathinone in forensic (Uralets et al., 2014) and emergency department drug screens (Borek and Holstege, 2012; Marinetti and Antonides, 2013; Murray et al., 2012; Spiller et al., 2011). (R,S)-MDPV and its analogs are potent reuptake inhibitors at dopamine and norepinephrine transporters (Marusich et al., 2014; Meltzer et al., 2006). Low doses of these agents produce typical psychostimulant effects, but abnormal behaviors emerge at higher doses, including profound ataxia and convulsions (Marusich et al., 2014). Because of their potent actions as catecholamine-selective reuptake blockers, these drugs pose a risk for addiction and other serious adverse effects in humans (Simmler et al., 2013). For example, abuse of (R,S)-MDPV results in serious physiological reactions (i.e., tachycardia, hypertension, rhabdomyolysis, acidosis), severe neuropsychiatric reactions (e.g., psychosis, hallucinations, agitation) (Beck et al., 2015; Froberg et al., 2015), and death (Froberg et al., 2015; Kesha et al., 2013; Murray et al., 2012; Wyman et al., 2013).
The pharmacokinetic (PK) profile of drugs is an important component of in vivo drug action (Lin and Lu, 1997). Novellas et al. (2015) report PK and pharmacodynamic evidence that after dosing male Sprague-Dawley (SD) rats with racemic MDPV, there is a strong correlation between psychostimulant effects and striatal levels of (R,S)-MDPV. Anizan et al. (2016) report the first plasma PK values for (R,S)-MDPV following subcutaneous (sc) administration of 0.5–2.0 mg/kg (R,S)-MDPV to male SD rats. They also include analysis of two primary metabolites 3,4-dihydroxypyrovalerone and 4-hydroxy-3-methoxypyrovalerone. These PK reports, however, are missing important elements of a more complete profile including analysis of in vivo clearance, volume of distribution, studies of male and female animals, liver first-pass effects on bioavailability, and studies of (R)- and (S)-MDPV enantiomers using a stereoselective assay for quantitating individual enantiomers of (R,S)-MDPV.
(R,S)-MDPV is commonly self-administered as the racemic mixture comprising two enantiomers. (S)-MDPV has profound pharmacological activity, while (R)-MDPV is less potent in mice and rats (Gannon et al., 2016; Kolanos et al., 2015). In human users, (R,S)-MDPV is often self-administered for a rapid onset of action by intravenous, inhalation, or insufflation routes, or by the oral route which has a slower onset of action and susceptibility to gut and first pass metabolism (Froberg et al., 2015).
Drugs of abuse can have sex differences in both pharmacokinetics and pharmacodynamics. For example, SD rat studies of (+)-methamphetamine (METH), a structural analog of (S)-MDPV, in male and female rats revealed significant sex- and dose-dependent differences in METH PK, metabolism, and locomotor effects (Milesi-Hallé et al., 2005). These sex-dependent PK properties also contribute to differences in METH self-administration behaviors in male and female rats (Roth and Carroll, 2004).
The premise for these studies was that a lack of knowledge about the effects of animal sex on PK properties of (R,S)-MDPV and the use of non-enantioselective assays for quantitating individual enantiomers of a racemic compound like (R,S)-MDPV could lead to less rigorous studies and difficulties in scaling the findings from rat to human. Therefore, a chiral liquid chromatography tandem mass spectrometry (LC-MS/MS) method was used to selectively quantitate serum concentrations of (S)-, (R)- and (R,S)-MDPV (Hambuchen et al., 2017) in male and female rats. We studied potential sex-, dose-, and enantiomer-dependent differences in the PK profile of (S)- (R)- and (R,S)-MDPV after ip or iv administration of (R,S)-MDPV. IV doses of (S)-MDPV and (R)-MDPV in male and female rats were also tested to determine PK properties of individual enantiomers, and if there was in vivo inversion of (R)-MDPV or (S)-MDPV to its antipode.
2. Materials and Methods
2.1. Drugs and Chemicals
(R,S)-, (S)-, and (R)-MDPV (purity ≥95%) for rat experiments were obtained from the National Institute on Drug Abuse (Bethesda, MD) drug supply program. (R,S)-MDPV HCl (1 mg/ml free base in methanol; Cerilliant, Round Rock, TX) was used to make 2, 6, 20, 60, 200, and 2000 ng/ml calibration standards in non-hemolyzed normal rat serum (NRS; Pel-Freeze Biologicals, Rogers, AR) or 3, 100, and 1600 ng/ml quality control standards in SD rat serum from Innovative Research (Novi, MI) for LC-MS/MS. (R,S)-MDPV-D8 (0.1 mg/ml free base in methanol; Cerilliant) in acetonitrile at 30 ng/ml was used as an internal standard. All MDPV doses and concentrations were calculated as the free base. All other chemicals were purchased from Sigma Chemical Company (St. Louis, MO) or Thermo Fisher Scientific Inc. (Waltham, MA) unless otherwise noted. (R,S)-MDPV doses for administration to rats were prepared in administration buffer containing 15 mM phosphate and 150 mM sodium chloride, pH 7.3.
2.2. Animals
Male (300–325 g) and female (250–275 g) adult rats were purchased from Charles Rivers Laboratories International Inc. (Wilmington, MA) just prior to use in various studies. Male and female rats were approximately two and three months old, respectively, at the start of each experiment and were kept ≤6 weeks to complete specific experiments. The rats were individually-housed for PK studies to protect the cannula or pair-housed for locomotor studies with a 14/10-hour light/dark cycle, 22°C environment. Lights were turned on and off at 6:00 am and 8:00 pm, respectively. Rats had free access to water and were fed approximately 20 g of food pellets on a daily basis that maintained their body weights between 250–300 g (females) and 290–370 g (males). Rats were acclimated to the new environment for 1–2 weeks before the studies.
Rats for the PK studies were surgically implanted with femoral and jugular vein polyurethane catheters at the vendor. Catheters were exposed one week after arrival under 2% inhaled isoflurane anesthesia. This was one day prior to experimental procedures. The patency of the catheters was maintained using glycerol containing 500 U of heparin (50 μl) as a locking solution. Studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the NIH. All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
2.3. Locomotor Activity
The locomotor activity studies of ip (R,S)-MDPV were performed in male and female SD rats (n=4/sex) similar to previous studies (Hambuchen et al., 2014). Females were tested first, followed by males. Briefly, overhead video cameras recorded horizontal motion and rearing behaviors for 6 h (1 h before and 5 h after treatments) in open-top polyethylene chambers. Ethovision 8 software (Noldus Information Technology, Inc., Sterling, VA) recorded the locomotor activity in 4 min bins. Rats were habituated in the behavior chambers for 2 h on the day prior to saline treatment and each of the (R,S)-MDPV doses. Saline, 0.3, 1, 3, and 5.6 mg/kg (R,S)-MDPV were administered in the same group of animals with 3–4 days between treatments. Since sensitization is known to occur with some daily MDPV multiple dosing schedules (Berquist et al., 2016; Buenrostro-Jáuregui et al., 2016; Watterson et al., 2016), it is possible that subsequent doses in our studies could have produced greater responses due to a previous exposure to the drug. However, we did not conduct studies of sensitization. The duration of action of horizontal distance traveled and rearing behavior was determined as the time required for animals to return to their baseline activity after each (R,S)-MDPV treatment. Baseline activity for each animal was considered to occur when two consecutive 4-min bins were below the animal’s averaged saline-induced horizontal distance traveled or rearing behavior bins plus one standard deviation. The maximal distance traveled and maximal number of rearing events occurring within a 4 min bin over the course of the testing session were determined for each rat.
2.4. PK Experiments
Prior to experimentation, rats were anesthetized with 2% isoflurane and placed in a tethered, infusion harness with a polyurethane extension (I.D. 0.38mm, O.D. 1.09m) connected to the jugular vein catheter for blood collection. An initial blood sample (50 μl) was taken to monitor hematocrit and to serve as a pre-drug administration sample. Rats were placed in metabolism cages (Nalgene Supply, Rochester, NY) and the tether attached to a swivel to allow free movement during blood sampling. Rats were removed from their cage immediately prior to (R,S)-MDPV injection and then returned to their metabolism cage for the remainder of the study.
Groups of male and female rats (n=5–6/sex) were administered doses of either 1, 3, or 5.6 mg/kg of (R,S)-MDPV through an iv femoral catheter. Male and female rats in the 3 mg/kg iv groups were administered an additional 3 mg/kg ip (R,S)-MDPV dose one week after iv dosing to determine (R,S)-MDPV bioavailability (see below). For ip doses, the syringe plunger was pulled back before injection to assure that the needle was not in a blood vessel.
A separate study with individual (S)-MDPV and (R)-MDPV enantiomers was conducted in male (n=4) and female rats (n=5). A 1.5 mg/kg iv dose of (S)-MDPV, the amount of (S)-MDPV present in a 3 mg/kg (R,S)-MDPV dose, was administered one week prior to a 1.5 mg/kg iv dose of (R)-MDPV.
After the 3 and 5.6 mg/kg (R,S)-MDPV and 1.5 mg/kg (S)- and (R)-MDPV studies, blood samples were collected from the jugular catheter at 5, 20, 60, 90, 120, 180, 240, and 360 min. After the 1 mg/kg dose, a 300-min time point was used instead of a 360-min time point to improve the chances of measuring concentrations above the lower limit of quantitation. The total blood volume collected during each experiment was <1% of the rat’s total body weight. We collected less blood volume at early time points (50 μl) when drug concentrations were highest and more blood volume when concentrations were lower (100–200 μl). Sterile saline was used to replace blood volume loss after each blood draw. Blood samples were maintained at 4°C for >1 hr to allow for clotting, centrifuged 7 min at 20,817 rcf (at 4°C) for serum collection, and stored at −80°C.
2.5. Serum MDPV Quantitation
Serum (S)-, (R)-, and (R,S)-MDPV concentrations were determined using a previously-validated liquid phase extraction and chiral LC-MS/MS method (Hambuchen et al., 2017). Briefly, separation was performed using an Acquity UPLC Chromatographic system with a Lux 5 μm Amylose-2 100 × 4.6 mm chiral analytical column (Phenomenex, Torrance, CA) at 19°C and a Quattro Premier XE mass spectrometer (Waters Corp, Milford, MA) with an electrospray ionization probe in positive ion mode. Since reporting this method, chiral separation was increased by slowing the flow rate from 1 ml/min to 0.5 ml/min. The lower and upper limits of quantitation were 1 and 1000 ng/ml, respectively, for each enantiomer. The limits for racemic MDPV were 2 and 2000 ng/ml. Five analytical runs conducted over 25 days showed all quality control concentrations for within day and between days were within 20% of the predicted values. The coefficient of variation percentage was less than 20% of the actual values.
2.6. PK Analysis of Serum (S)-MDPV, (R)-MDPV, and (R,S)-MDPV Concentration-Time Data
Following iv drug administration of 1.0, 3.0 and 5.6 mg/kg of (R,S)-MDPV or 1.5 mg/kg of enantiomer, the log concentration vs time curves of (S)-MDPV, (R)-MDPV, and (R,S)-MDPV from each rat were characterized by model-dependent PK methods using Phoenix WinNonlin (V6.4, Certara USA, Princeton, NJ). The concentration of (R,S)-MDPV at each time point after (R,S)-MDPV administration was calculated by summing the two enantiomer concentrations. Bi- and tri-exponential curves with first-order input functions were fit sequentially to each individual concentration–time data set using 1/y and 1/y2 weighting schemes, where y is the predicted concentration. An F-test was performed to choose the best-fit model between the multiple compartmental analyses (Boxenbaum et al., 1974). The best-fit line to each data set was selected after considering the visual inspection of the fit of each curve to the data, analysis of the residuals, and assessment of the magnitude of the coefficients of variation for each PK parameter. The PK parameters calculated by the WinNonlin software were area under the concentration-time curve from time 0 to infinity (AUCinf), total clearance (ClT), terminal elimination half-life (t1/2λz), and volume of distribution at steady-state (Vdss). The apparent volume of distribution (Vd) was calculated by ClT/λz. The average macroconstants (not reported) derived from the best-fit lines for the 2-comparment (A, B and λ1, λz) and 3-compartment models (A, B, C and λ1, λ2, λz) were also calculated. λ1 and λ2 are the calculated distribution rate constants, and λz is the terminal elimination rate constant. A-C are the y-intercepts.
To determine in vivo bioavailability (F) of (S)-, (R)- and (R,S)-MDPV after (R,S)-MDPV administration, we used the concentration-time data sets from the administration of an iv 3.0 mg/kg (R,S)-MDPV dose followed one week later by a 3.0 mg/kg ip dose in the same male and female animals. Using noncompartmental PK analysis of the log serum concentration–time curves, Cmax, t1/2λz, and AUCinf values were determined for each enantiomer and racemic drug after each route of administration. To calculate F, the AUCinf value after ip dosing was divided by the AUCinf value after iv dosing in the same animal.
The (S)-MDPV and (R)-MDPV drugs were provided by NIDA with a purity of >95%. Using our chiral selective LC-MS/MS method, we determined that the (S)-MDPV stock contained 1.2% (R)-MDPV as an impurity, and the (R)-MDPV stock contained 0.5% (S)-MDPV as an impurity. To calculate the apparent in vivo serum (R)-MDPV impurity following iv administration of (S)-MDPV, we divided the apparent serum AUC value of (R)-MDPV by the sum of the serum (R)-MDPV AUC plus the (S)-MDPV AUCinf, times 100%. The same calculation was made for the serum (S)-MDPV impurity following iv administration of (R)-MDPV.
The S/R PK enantiomer ratios were determined by dividing each (S)-MDPV PK value by the (R)-MDPV PK parameter (Brocks, 2006; Fitzgerald et al., 1990).
2.7. Statistics
All data were reported as an arithmetic mean ± standard deviation (SD) except for half-life values which are reported as a harmonic mean ± a pseudo SD as described by Lam et al. (1985). Results from ambulatory activity experiments were analyzed using a two-factor mixed-model analysis of variance (ANOVA) with sex as the between-subjects factor and dose as the within-subjects factor. Following a statistically significant ANOVA, Holm-Sidak multiple comparisons tests (or simple main effects tests in the case of a statistically significant interaction) were performed for all pairwise comparisons. Results from iv racemic PK data were analyzed using a two-way ANOVA with sex and dose as the between-subjects factors, followed by Holm-Sidak multiple comparisons tests (or simple main effects tests) for all pairwise comparisons. Results of ip bioavailability, Cmax, and t1/2λz between sexes, and comparisons of PK parameters and enantiomeric impurity between enantiomers in the single enantiomer iv PK studies in female rats, were first analyzed for equal variances using an F-test. If an F-test revealed a statistically significant inequality between variances, an unpaired Welch’s t-test was performed. In cases of equal variances, data were analyzed using an unpaired t-test. Results from iv single enantiomer PK data were analyzed using a two-factor mixed-model ANOVA with sex as the between-subjects factor and enantiomer as the within-subjects factor, followed by Holm-Sidak multiple comparisons tests (or simple main effects tests) for all pairwise comparisons. Statistical analysis and creation of figures was performed with GraphPad Prism software (V7, La Jolla, CA, USA). Statistical significance was declared at p<0.052-tail.
3. Results
3.1. Locomotor Effects of (R,S)-MDPV
Fig.1 A–D depicts the horizontal locomotion and rearing events produced in female and male rats by ip saline (control) and increasing (R,S)-MDPV doses over time. The 1, 3, and 5.6 mg/kg ip doses produced detectable activity in female and male rats, while 0.3 mg/kg did not. Fig. 2 A–B depicts the maximal activity measurements of both distance traveled and rearing. Fig. 2 C–D depicts the duration of activity measurement of both distance traveled and rearing. More details of all statistical comparisons are available in the supplement section (S.1)1.
Fig. 1.
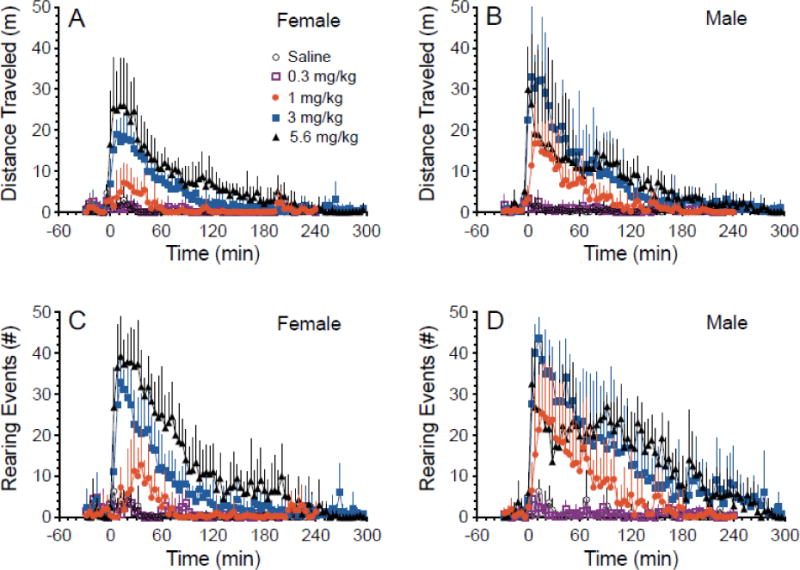
Distance traveled (Panel A–B) and rearing events (C–D) in female (A and C) and male (B and D) rats after ip doses of saline or (R,S)-MDPV. All values are mean + SD with n = 4 per sex. See Figure 2 for further data analysis.
Fig. 2.
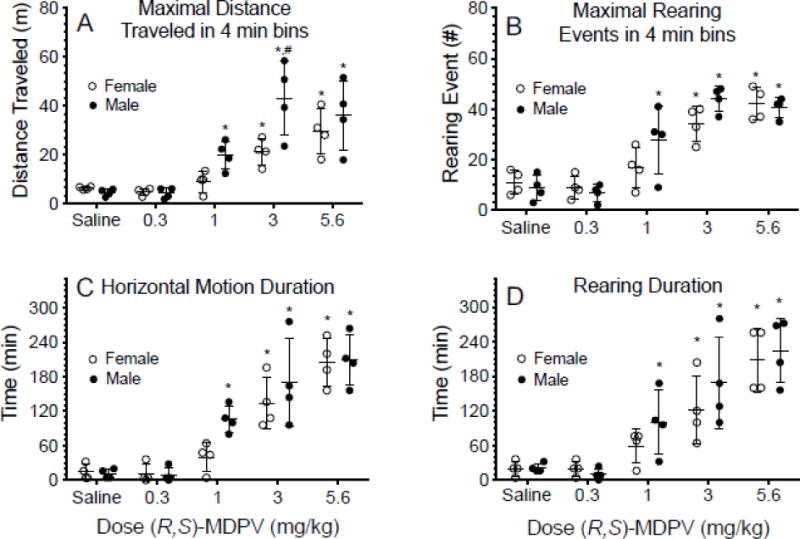
The maximal distance traveled (m) (Panel A) and maximal number of rearing events (Panel B) in the 4 min bins after ip (R,S)-MDPV dosing in female and male rats based on data from Figure 1. Panels C and D show the duration of action of horizontal motion and rearing events after ip (R,S)-MDPV dosing. All values are mean + SD with n = 4 per sex. The * denotes statistical differences (p < 0.05) between (R,S)-MDPV dose and saline within sex, and the # denotes a statistical difference (p < 0.05) between female and male rats.
3.2. PK Studies
Fig. 3 shows a representative average rat serum concentration-time plots of (S)-, (R)-, and (R,S)-MDPV after iv dosing of 1, 3 and 5.6 mg/kg of (R,S)-MDPV. Table 1 summarizes the PK parameters of (S)-, (R)-, and (R,S)-MDPV calculated from these concentration-time data. Due to a lower limit of quantitation of 1 ng/ml for (S)- and (R)-MDPV, and 2 ng/ml for (R,S)-MDPV, we could only accurately quantitate concentrations after the 1 mg/kg dose up to the 120 to 240 min time points. Based on PK analysis of the 3 and 5.6 mg/kg doses, there were not sufficient concentration-time data points in the terminal elimination phase after the 1 mg/kg dose of (R,S)-MDPV to fully characterize the PK profile in this dose group. While the estimated PK values for the 1 mg/kg dose are included for the reader’s evaluation, the data were not judged as sufficiently complete for the statistical comparisons with the PK values of 3 and 5.6 mg/kg.
Fig. 3.
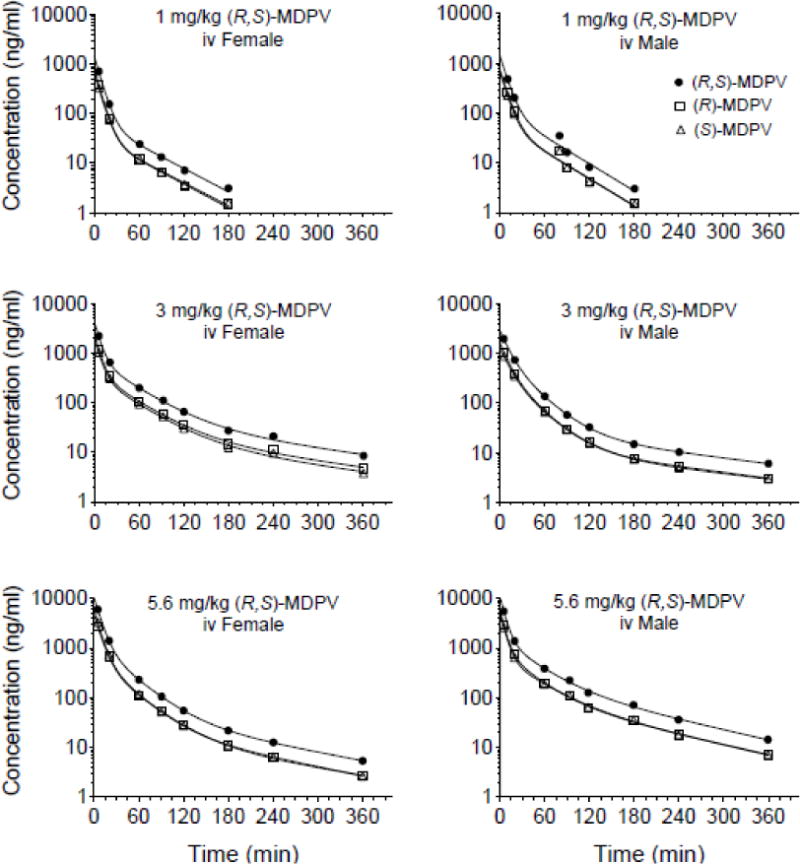
Serum concentration versus time curves of (S)-, (R)-, and (R,S)-MDPV from one animal representative of the average pharmacokinetics values after an iv bolus dose of 1, 3, or 5.6 mg/kg (R,S)-MDPV in female and male SD rats (n = 5–6 per group). Symbols denote observed concentrations. The best-fit solid lines in each plot were determined by model-dependent pharmacokinetic analysis. See Table 1 for comparison of pharmacokinetic values after the iv dosing.
Table 1.
Pharmacokinetic parameters for (S)-, (R)-, and (R,S)-MDPV after 1, 3, and 5.6 mg/kg iv administration of (R,S)-MDPV in male and female rats. Results from the 1 mg/kg dosing lacked sufficient concentration-time points in the terminal elimination phase to permit an accurate calculation of a complete pharmacokinetic profile (see Figure 3). These data were not statistically compared to the other two dose groups. See supplemental files for more details of the statistical analysis.
| (S)-MDPV | (R)-MDPV | (R,S)-MDPV | ||||
|---|---|---|---|---|---|---|
| Dose and PK Parameter | Female | Male | Female | Male | Female | Male |
| 1 mg/kg (R,S)-MDPV | n = 6 | n = 6 | n = 6 | n = 6 | n = 6 | n = 6 |
| AUCInf (ng min/ml)/dosea | 12740 ± 2771 | 15592 ± 3677 | 14219 ± 3134 | 17539 ± 4356 | 13471 ± 2944 | 16564 ± 4015 |
| t1/2λz (min)b | 38 ± 8 | 30 ± 3 | 38 ± 8 | 28 ± 4 | 38 ± 8 | 29 ± 3 |
| ClT (ml/min/kg) | 82 ± 18 | 67 ± 14 | 73 ± 16 | 60 ± 13 | 77 ± 17 | 63 ± 14 |
| Vdss (l/kg) | 2.2 ± 1.0 | 1.5 ± 0.4 | 1.8 ±0.8 | 1.2 ±0.3 | 2.0 ± 0.9 | 1.4 ± 0.3 |
| Vd (l/kg) | 4.9 ± 2.3 | 2.9 ± 0.4 | 4.4 ± 2.0 | 2.4 ± 0.3 | 4.6 ± 2.1 | 2.6 ± 0.4 |
| 3 mg/kg (R,S)-MDPV | n = 5 | n = 6 | n = 5 | n = 6 | n = 5 | n = 6 |
| AUCInf (ng min/ml)/dosea | 19291 ± 3272 | 16715 ± 3359 | 23038 ± 3825 | 18447 ± 3389 | 20673 ± 3400 | 17321 ± 3015 |
| t1/2λz (min)b | 94 ± 29 | 118 ± 48 | 91 ± 33 | 117 ± 47 | 104 ± 29 | 119 ± 47 |
| ClT (ml/min/kg) | 53 ± 9 | 62 ± 12 | 44 ± 7 | 56 ± 10 | 48 ± 8 | 59 ± 10 |
| Vdss (l/kg) | 2.2 ± 0.8 | 2.8 ± 1.0 | 1.8 ± 0.7c | 2.3 ± 0.7 | 2.0 ± 0.7c | 2.6 ± 0.8 |
| Vd (l/kg) | 7.9 ± 3.4 | 11.9 ± 4.5 | 6.4 ± 2.3 | 10.5 ± 3.7 | 8.1 ± 2.9 | 11.2 ± 3.7 |
| 5.6 mg/kg (R,S)-MDPV | n = 6 | n = 5 | n = 6 | n = 5 | n = 6 | n = 5 |
| AUCInf (ng min/ml)/dosea | 19535 ± 5124 | 20040 ± 4456 | 22981 ± 6109 | 21989 ± 5092 | 21255 ± 5604 | 21022 ± 4774 |
| t1/2λz (min)b | 106 ± 21 | 88 ± 22 | 107 ± 19 | 89 ± 20 | 106 ± 19 | 88 ± 20 |
| ClT (ml/min/kg) | 54 ± 13 | 53 ± 15 | 46 ± 11 | 48 ± 14 | 50 ± 12 | 50 ± 15 |
| Vdss (l/kg) | 1.7 ± 0.4 | 2.4 ± 0.9 | 1.3 ± 0.3c | 2.1 ± 0.8 | 1.5 ± 0.4c | 2.3 ± 0.8 |
| Vd (l/kg) | 8.3 ± 1.6 | 7.2 ± 3.4 | 7.1 ± 1.4 | 6.6 ± 3.0 | 7.6 ± 1.4 | 6.9 ± 3.2 |
Calculated by dividing AUCInf by enantiomer dose for (S)- and (R)-MDPV (i.e., 0.5, 1.5, 2.8 mg/kg) or by total dose for (R,S)-MDPV (i.e., 1, 3, 5.6 mg/kg) dose administered.
Harmonic mean and pseudo standard deviation (Lam et al., 1985).
Significantly different from males (p<0.05).
Bioavailability parameters and statistical analysis after ip administration of 3 mg/kg (R,S)-MDPV are shown in Table 2. Representative average rat serum concentration-time plots for a female and male subject are presented in Fig. 4.
Table 2.
Pharmacokinetic parameters for (S)-, (R)-, and (R,S)-MDPV after 3 mg/kg ip administration of (R,S)-MDPV in female (n = 5) and male (n = 6) rats. The Tmax was 5 min in all cases. This was also the first measured time point after dosing. Figure 4 shows a representative concentration-time plot after the 3.0 mg/kg ip dose in a male and female rat. See supplemental files for more details of the statistical analysis.
| 3 mg/kg (R,S)-MDPV | (S)-MDPV | (R)-MDPV | (R,S)-MDPV | |||
|---|---|---|---|---|---|---|
| PK Parameter | Female | Male | Female | Male | Female | Male |
| t1/2λz (min)a | 110 ± 51 | 78 ± 25 | 114 ± 45 | 80 ± 23 | 111 ± 48 | 74 ± 29 |
| Cmax (ng/ml) | 265 ± 82 | 496 ± 218 | 284 ± 90 | 527 ± 249 | 549 ± 159 | 1024 ± 463 |
| Bioavailability (F) | 0.36 ± 0.02b | 0.60 ± 0.22 | 0.32 ± 0.03 | 0.55 ± 0.23 | 0.34 ± 0.03b | 0.57 ± 0.22 |
Harmonic mean and pseudo standard deviation.
Significantly different from male rats (p < 0.05).
Fig. 4.
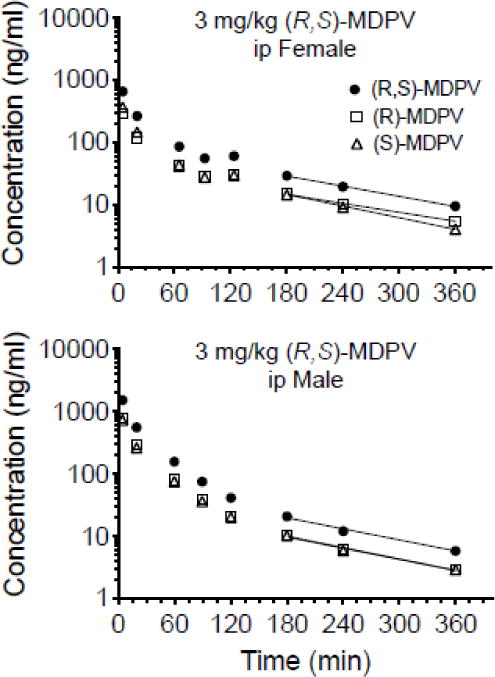
Serum concentration versus time curves of (S)-, (R)-, and (R,S)-MDPV from one animal representative of the average pharmacokinetic values after an ip dose of 3 mg/kg (R,S)-MDPV in female and male SD rats (n = 5–6 per group). Symbols denote observed concentrations. The terminal best-fit solid lines in each plot were determined by noncompartmental pharmacokinetic analysis. See Table 2 for the analysis of pharmacokinetic values after the iv and ip dosing.
Fig. 5 shows a representative average female and male rat serum concentration-time plot for (S)- and (R)-MDPV after iv dosing of 1.5 mg/kg of each enantiomer. Analytical sensitivity was not a problem for these studies of enantiomers at 1.5 mg/kg because this dose was three times greater than the concentration of each enantiomer following 1 mg/kg of (R,S)-MDPV (i.e., 0.5 mg/kg of each enantiomer). Individual PK parameters and statistical analysis are shown in Table 3. The in vivo antipode impurity of (S)- and (R)-MDPV was 3.2 ± 0.6 and 0.33 ± 0.27% in females and 2.6 ± 0.2 and 1.0 ± 0.1% in males, respectively. Table 4 shows the ratio of (S)- to (R)-MDPV PK values and results of statistical analysis in the rats administered iv (R,S)-MDPV.
Fig. 5.
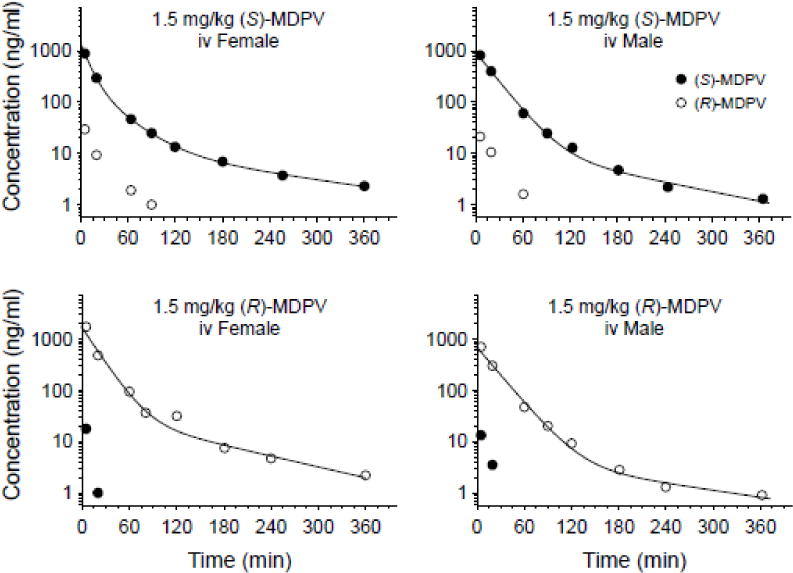
Serum concentration versus time curves from one animal representative of the average pharmacokinetic values after an iv bolus dose of 1.5 mg/kg iv (S)-MDPV (upper panels) and (R)-MDPV (lower panels) administration in female (left panels) and male (right panels) rats (n = 4–5 per group). Symbols denote observed concentrations. The best-fit solid lines were determined by model-dependent pharmacokinetic analysis. See Table 3 for the average PK values and statistical comparisons.
Table 3.
Serum pharmacokinetic parameters after a 1.5 mg/kg iv dose of (S)-MDPV or (R)-MDPV in female and male rats. Also, see the representative concentration-time plots in Figure 3. Note that the single enantiomer dose of 1.5 mg/kg is equivalent to its respective enantiomer in a 3 mg/kg (R,S)-MDPV dose. See supplemental files for more details of the statistical analysis.
| (S)-MDPV | (R)-MDPV | S/R ratio | ||||
|---|---|---|---|---|---|---|
| Dose and PK Parameter | Female | Male | Female | Male | Female | Male |
| 1.5 mg/kg (R,S)-MDPV | n = 5 | n = 4 | n = 5 | n = 4 | n = 5 | n = 4 |
| AUCInf (ng min/ml)/dosea | 14989 ± 1831 | 20108 ± 10088 | 20935 ± 5079 | 16492 ± 7447 | 0.74 ± 0.17 b | 1.30 ± 0.45 |
| t1/2λz (min)c | 84 ± 20 | 95 ± 29 | 91 ± 31 | 121 ± 66 | 0.97 ± 0.39 | 0.76 ± 0.21 |
| ClT (ml/min/kg) | 67 ± 8 | 57 ± 19 | 50 ± 12 | 72 ± 33 | 1.40 ± 0.34 | 0.87 ± 0.40 |
| Vdss (l/kg) | 3.4 ± 1.6d | 1.9 ± 0.7 | 1.6 ± 0.6 | 2.7 ± 1.1 | 2.06 ± 0.52 b | 0.74 ± 0.25 |
| Vd (l/kg) | 8.8 ± 2.9 | 8.9 ± 4.3e | 7.0 ± 2.7f | 15.4 ± 8.3 | 1.38 ± 0.70 | 0.60 ± 0.07 |
Calculated by dividing AUCInf by the 1.5 mg/kg (S)- or (R)-MDPV dose.
Significantly different (p < 0.05) from the male S/R ratio value.
Harmonic mean and pseudo standard deviation.
Significantly different (p < 0.05) from the (R)-MDPV Vdss values within females.
Significantly different (p < 0.05) from the (R)-MDPV Vd values within males.
Significantly different (p < 0.05) from male (R)-MDPV Vd values.
Table 4.
S/R enantiomer ratios in female and male rats after administration of 3 and 5.6 mg/kg doses of (R,S)-MDPV. PK data for this analysis was derived from Table 1. See supplemental files for more details of the statistical analysis.
| Pharmacokinetic Parameter | Female | Male |
|---|---|---|
| 3 mg/kg (R,S)-MDPV |
S/R Ratio n = 5 |
S/R Ratio n = 6 |
| AUCinf | 0.84 ± 0.05a | 0.90 ± 0.05 |
| t1/2λz | 1.12 ± 0.40 | 1.03 ± 0.23 |
| ClT | 1.20 ± 0.08a | 1.11 ± 0.05 |
| Vdss | 1.25 ± 0.16 | 1.17 ± 0.15 |
| Vd | 1.28 ± 0.45 | 1.15 ± 0.28 |
| 5.6 mg/kg (R,S)-MDPV | n = 6 | n = 5 |
| AUCinf | 0.85 ± 0.03a | 0.91 ± 0.02 |
| t1/2λz | 0.99 ± 0.11 | 1.00 ± 0.06 |
| ClT | 1.18 ± 0.04a | 1.10 ± 0.02 |
| Vdss | 1.27 ± 0.08 | 1.16 ± 0.05 |
| Vd | 1.17 ± 0.12 | 1.09 ± 0.07 |
Significantly different (p < 0.05) from male values.
4. Discussion
This is the first report of sex- and enantiomer-dependent differences in PK properties of MDPV in rodents. Human users of MDPV report self-administration of between 1 to 20 mg of (R,S)-MDPV by insufflation or 2 to 25 mg orally according to a website about the use of psychoactive drugs in humans (Erowid, 2013). This corresponds to a range of 0.014 to 0.29 mg/kg dose in a 70 kg individual. The range of racemic MDPV serum concentrations found in human samples from forensic and intoxicated patients is about 1–1500 ng/ml (Beck et al., 2015; Grapp et al., 2017; Spiller et al., 2011). The concentrations found in our rats after 1 – 5.6 mg/kg iv doses ranged from 2–6932 ng/ml.
Most of our PK studies were performed with iv (R,S)-MDPV, since human users often self-administer this drug by routes producing a rapid onset of action and because iv concentration-time data offer the most accurate measure of the major PK parameters. After 3 and 5.6 mg/kg iv doses of (R,S)-MDPV, there were no significant dose-dependent differences in the PK values but there were significant sex differences in Vdss for (R)- and (R,S)-MDPV (Table 1). The female S/R enantiomeric ratios for AUCinf and ClT were significantly lower and higher, respectively, than values determined in males (Table 4).
The distribution half-life values for (S)-, (R)- and (R,S)-MDPV after iv dosing of 3 and 5.6 mg/kg (R,S)-MDPV ranged from 17 to 23 min (data not shown). Since PK processes are usually considered complete after four half-lives, the MDPV enantiomers on average required 80 min (i.e., 4 × 20 min) to fully distribute. This long distribution phase does not necessarily indicate slow brain equilibration for drugs, but in a study of striatal (R,S)-MDPV concentrations in rats after sc dosing, peak concentrations of (R,S)-MDPV are not reached until 20–30 min (Novellas et al., 2015). With iv (R,S)-MDPV, however, peak brain concentrations will likely occur at a much earlier time point as is the case with phencyclidine and (+)-METH (Proksch et al., 2000; Rivière et al., 2000).
While Anizan et al. (2016) could not distinguish the (R)- and (S)-MDPV enantiomers, their LC-MS/MS quantitation method was sufficiently sensitive to characterize PK values after sc administration in male rats at lower doses of (R,S)-MDPV. Anizan et al. report the t1/2λz values for plasma (R,S)-MDPV after sc doses of (R,S)-MDPV at 0.5, 1.0, and 2.0 mg/kg in male SD rats are 97.9 ± 29.1, 77.8 ± 11.6, and 83.8 ± 32.2 min, respectively. Their half-life values, determined by a non-compartmental analysis, were reasonably similar to those determined in male SD rats after iv (R,S)-MDPV doses of 3 and 5.6 mg/kg in the present study (Table 1). Similar to the Anizan study of male SD rats using sc doses, dose-dependent differences in half-life or AUCinf values (normalized for dose) for our male rats following iv doses were not found, even though the 5.6 mg/kg dose of (R,S)-MDPV in the current studies was 2.8 times higher than their highest dose.
Studies of iv dosing with (S)- and (R)-MDPV enantiomers in male and female rats at 1.5 mg/kg doses were used to determine if either enantiomer substantially inverts to the antipode and to determine if the PK properties of the individual enantiomers are significantly different (Table 3, Fig. 5). Some enantiomeric drugs are known to convert in vivo from one enantiomer to another in an enzyme catalyzed unidirectional (e.g., ibuprofen) or chemical induced bidirectional (e.g., lorazepam or thalidomide) manner (Brocks, 2006; Nguyen et al., 2006). Based on in vivo AUCinf values from dosing with individual enantiomers, the average percentage of chiral antipode impurity was not substantively different from the enantiomer impurity of the stock dosing solutions for (S)-MDPV or (R)-MDPV. These data indicate the PK properties of MDPV enantiomers are not further complicated by in vivo racemization. Nevertheless, since the pharmacological effects are more potently due to (S)-MDPV (Gannon et al., 2016; Kolanos et al., 2015), it would be important for investigators to know the chiral purity of both (R)- and (S)-MDPV stock reagents before use in experiments.
A major determinate of drug distribution is protein binding to plasma proteins, red blood cells, and tissues (Brocks, 2006). These factors can result in enantioselective differences in volume of distribution, and sometimes ClT. Indeed, after the administration of single enantiomers, there were significant differences in (S)- and (R)-MDPV Vdss and Vd in female and male rats, respectively. Compared to males, females showed a significantly lower (R)-MDPV Vd, a significantly lower S/R ratio for AUCinf, and a significantly higher S/R ratio for Vdss. Further studies of protein binding and determination of PK values in critical organs like the brain, heart and kidney would help to explain these MDPV enantiomeric differences.
Previous reports show a sc (R,S)-MDPV dose of 0.3 mg/kg produces significant locomotor effects (Baumann et al., 2013; Novellas et al., 2015). After correcting for the average ip bioavailability (Table 2), the 0.3 mg/kg dose equaled approximately 0.1 and 0.17 mg/kg in female and male rats, respectively. Thus, the lack of locomotor effects of this dose in the current study was likely due to low ip bioavailability.
Male rats had an approximately 40% greater average bioavailability than females (Table 2). Upon closer examination of these data, the male values for bioavailability showed an apparent bimodal distribution of values that resulted in greater variance. In the ip (R,S)-MDPV locomotor studies, this may explain the apparent differences between sexes in distance traveled and rearing events after all doses and the significant elevation of maximal distance traveled (per 4 min bin) after the 3 mg/kg dose in male compared to female rats (Fig. 1 and 2). The reason(s) for this variability in males was not clear, but a previous PK study found high variability in ip bioavailability of cocaine in male rats (Ma et al., 1999).
5. Conclusions
In summary, following iv doses of (R)-, (S)-, and (R,S)-MDPV, the most significant PK differences were in the volume of distribution (Vdss or Vd) (Tables 1 and 3), which could be caused by differences in MDPV blood and tissue protein binding. Importantly, there was no evidence of in vivo inversion of (R)-MDPV or (S)-MDPV to its antipode. In the ip bioavailability study, there was significantly greater bioavailability and variability for (S)- and (R,S)-MDPV in male rats compared to female rats. The increased magnitude and variance in ip bioavailability in male compared to female rats could lead to sex-dependent differences in the pharmacological effects of (S)-MDPV.
Supplementary Material
Highlights.
First report of sex-dependent (R,S)-methylenedioxypyrovalerone (MDPV) and enantiomer differences in pharmacokinetics (PK).
Males had significantly greater (p<0.05) Intraperitoneal (ip) (S)- and (R,S)-MDPV bioavailability.
Most significant PK differences were in the volume of distribution (Vdss or Vd).
Sex differences in volume of distribution could be due to MDPV protein binding.
There was no evidence of in vivo inversion of (R)-MDPV or (S)-MDPV to its antipode.
Acknowledgments
Role of Funding Source
Funding for this work was provided by the NIH National Institute on Drug Abuse (Grant R01 DA039195); the National Center for Advancing Translational Sciences (Grant ULITR000039) and the Arkansas Biosciences Institute (the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000). The funding sources had no role in the study design; collection, analysis, and interpretation of data; in the writing of this paper; or the decision to submit this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MDH, HPH, MGG, and SMO participated in the research design. MDH, MGG, SJM, LEE, and DMG conducted experiments. MDH, HPH, MDB and SMO performed data analysis. MDH, SJM, MDB and SMO wrote the manuscript. All authors approved the final manuscript.
Conflict of Interest
No conflict declared.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
References
- Anizan S, Concheiro M, Lehner KR, Bukhari MO, Suzuki M, Rice KC, Baumann MH, Huestis MA. Linear pharmacokinetics of 3,4-methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: relationship to pharmacodynamic effects. Addict Biol. 2016;21:339–347. doi: 10.1111/adb.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck O, Franzen L, Bäckberg M, Signell P, Helander A. Intoxications involving MDPV in Sweden during 2010–2014: Results from the STRIDA project. Clin Toxicol (Phila) 2015;53:865–873. doi: 10.3109/15563650.2015.1089576. [DOI] [PubMed] [Google Scholar]
- Berquist MD, Traxler HK, Mahler AM, Baker LE. Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug Alcohol Depend. 2016;164:128–134. doi: 10.1016/j.drugalcdep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Boxenbaum HG, Riegelman S, Elashoff RM. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974;2:123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Brocks DR. Drug disposition in three dimensions: An update on stereoselectivity in pharmacokinetics. Biopharm Drug Dispos. 2006;27:387–406. doi: 10.1002/bdd.517. [DOI] [PubMed] [Google Scholar]
- Buenrostro-Jáuregui M, Ciudad-Roberts A, Moreno J, Muñoz-Villegas P, López-Arnau R, Pubill D, Escubedo E, Camarasa J. Changes in CREB and deltaFosB are associated with the behavioural sensitization induced by methylenedioxypyrovalerone. J Psychopharmacol (Oxford) 2016;30:707–712. doi: 10.1177/0269881116645300. [DOI] [PubMed] [Google Scholar]
- Erowid. MDPV Dose [WWW Document] 2013 Erowid.org. URL https://www.erowid.org/chemicals/mdpv/mdpv_dose.shtml (accessed 5.17.17)
- Fitzgerald RL, Blanke RV, Poklis A. Stereoselective pharmacokinetics of 3,4-methylenedioxymethamphetamine in the rat. Chirality. 1990;2:241–248. doi: 10.1002/chir.530020409. [DOI] [PubMed] [Google Scholar]
- Froberg BA, Levine M, Beuhler MC, Judge BS, Moore PW, Engebretsen KM, Mckeown NJ, Rosenbaum CD, Young AC, Rusyniak DE, ACMT Toxicology Investigators Consortium (ToxIC) Acute methylenedioxypyrovalerone toxicity. J Med Toxicol. 2015;11:185–194. doi: 10.1007/s13181-014-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: Drug Discrimination, Locomotor Activity, and Thermoregulation. J Pharmacol Exp Ther. 2016;356:615–623. doi: 10.1124/jpet.115.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapp M, Kaufmann C, Ebbecke M. Toxicological investigation of forensic cases related to the designer drug 3,4-methylenedioxypyrovalerone (MDPV): Detection, quantification and studies on human metabolism by GC-MS. Forensic Sci Int. 2017;273:1–9. doi: 10.1016/j.forsciint.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Hambuchen MD, Hendrickson HP, Owens SM. Chiral determination of 3, 4-methylenedioxypyrovalerone enantiomers in rat serum. Anal Methods. 2017;9:609–617. doi: 10.1039/C6AY03176E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambuchen MD, Rüedi-Bettschen D, Williams DK, Hendrickson H, Owens SM. Treatment of rats with an anti-(+)-methamphetamine monoclonal antibody shortens the duration of action of repeated (+)-methamphetamine challenges over a one month period. Vaccine. 2014;32:6213–6219. doi: 10.1016/j.vaccine.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. Methylenedioxypyrovalerone (“bath salts”), related death: Case report and review of the literature. J Forensic Sci. 2013;58:1654–1659. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- Kolanos R, Partilla JS, Baumann MH, Hutsell BA, Banks ML, Negus SS, Glennon RA. Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem Neurosci. 2015;6:771–777. doi: 10.1021/acschemneuro.5b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam FC, Hung CT, Perrier DG. Estimation of variance for harmonic mean half-lives. J Pharm Sci. 1985;74:229–231. doi: 10.1002/jps.2600740229. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lu AY. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev. 1997;49:403–449. [PubMed] [Google Scholar]
- Ma F, Falk JL, Lau CE. Within-subject variability in cocaine pharmacokinetics and pharmacodynamics after intraperitoneal compared with intravenous cocaine administration. Exp Clin Psychopharmacol. 1999;7:3–12. doi: 10.1037//1064-1297.7.1.3. [DOI] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: Method development, drug distribution and interpretation of results. J Anal Toxicol. 2013;37:135–146. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2- pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: A promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–213. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LA, He H, Pham-Huy C. Chiral drugs: An overview. Int J Biomed Sci. 2006;2:85–100. [PMC free article] [PubMed] [Google Scholar]
- Novellas J, López-Arnau R, Carbó ML, Pubill D, Camarasa J, Escubedo E. Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J Psychopharmacol (Oxford) 2015;29:1209–1218. doi: 10.1177/0269881115598415. [DOI] [PubMed] [Google Scholar]
- Proksch JW, Gentry WB, Owens SM. The effect of rate of drug administration on the extent and time course of phencyclidine distribution in rat brain, testis, and serum. Drug Metab Dispos. 2000;28:742–747. [PubMed] [Google Scholar]
- Rivière GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292:1042–1047. [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and ‘legal highs’ (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Uralets V, Rana S, Morgan S, Ross W. Testing for designer stimulants: Metabolic profiles of 16 synthetic cathinones excreted free in human urine. J Anal Toxicol. 2014;38:bku021–241. doi: 10.1093/jat/bku021. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, Olive MF. Sensitization to the motor stimulant effects of 3,4-methylenedioxypyrovalerone (MDPV) and cross-sensitization to methamphetamine in rats. J Drug Alcohol Res. 2016;5:1–10. doi: 10.4303/jdar/235967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP. Postmortem tissue distribution of MDPV following lethal intoxication by “bath salts”. J Anal Toxicol. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


