Abstract
Objective
To define the amount of opioid analgesics prescribed and consumed after discharge after cesarean delivery.
Methods
We conducted a survey at six academic medical centers in the United States from 9/2014 to 3/2016. Women who had undergone a cesarean delivery were contacted by phone two weeks after discharge and participated in a structured interview about the opioid prescription they received upon discharge and their oral opioid intake while at home.
Results
A total of 720 women were enrolled; of these, 615 (85.4%) filled an opioid prescription. The median number of dispensed opioid tablets was 40 (interquartile range (IQR) 30 to 40), the median number consumed was 20 (IQR 8 to 30), and leftover was 15 (IQR 3 to 26). Of those with leftover opioids, 95.3% had not disposed of the excess medication at the time of the interview. There was an association between a larger number of tablets dispensed and the number consumed independent of patient characteristics. The amount of opioids dispensed did not correlate with patient satisfaction, pain control, or the need to refill the opioid prescription.
Conclusion
The amount of opioid prescribed after cesarean delivery generally exceeds the amount consumed by a significant margin, leading to substantial amounts of leftover opioid medication. Lower opioid prescription correlates with lower consumption without a concomitant increase in pain scores or satisfaction.
Introduction
Prescription opioid abuse has reached epidemic proportions in the United States.1,2 Over the past decade and a half, the rates of substance abuse admissions and deaths attributable to prescription opioid misuse and abuse have markedly increased. 2,3 Additionally, prescription opioid abuse has been identified as a strong risk factor for subsequent heroin abuse.4
Leftover medication is an important source of opioids that are diverted or misused.5,6 A recent nationwide survey found that among those with leftover medication, nearly two-thirds did not dispose of the excess and about one-fifth of respondents reported having shared an opioid with another person.7 Additionally, leftover opioids create the risk of accidental ingestion, particularly in homes where young children reside.8 Consequently, there is an urgent need for clinicians to prescribe opioids in a manner that will meet patient’s analgesic requirement and minimize the amount of medication leftover.
Currently, there is a lack of information regarding normative patterns of outpatient opioid use after cesarean delivery. Such information is necessary to assist obstetricians in prescribing these medications in a responsible and targeted manner, ensuring a supply adequate for effective analgesia without overprescribing in a way that leads to large amounts of excess medication. Defining the optimal amount of prescription opioids after cesarean delivery is especially important as it is the most common inpatient surgical procedure performed in the United States, with approximately 1.3 million cases performed annually.9.
To address this gap in evidence, we undertook a survey study of women who underwent a cesarean delivery at one of six academic medical centers in the United States. Patients were contacted by phone two weeks after discharge and subjected to structured interviews in which they were asked questions about their use of oral opioids and other aspects of their experience after cesarean delivery.
Materials and Methods
This survey study was performed at the Massachusetts General Hospital (Boston, MA), Brigham and Women’s Hospital (Boston, MA), the University of Michigan (Ann Arbor, MI), Columbia University Medical Center (New York, NY), Wake Forest Health Science Center (Winston-Salem, NC) and Stanford University Medical Center (Palo Alto, CA) from September, 2014 to March, 2016. Institutional review board approval was obtained at all participating institutions.
At each institution, study teams identified a convenience sample of women who had undergone elective or unplanned cesarean delivery. Subjects were identified approximately 24 hours after delivery using notifications from the managing obstetric anesthesia team, electronic medical record systems, or daily rounding. All women who underwent a cesarean delivery were considered for inclusion unless they met one of the following exclusion criteria: limited English proficiency such that the women would have required an interpreter, lack of capacity to provide full consent, age <18 years, and hospital stays of greater than seven days after cesarean delivery (which may represent complex cases with altered postpartum analgesic requirements).
Consent procedures differed between participating institutions based on the requirements imposed by the centers’ Institutional Review Boards. At the Massachusetts General Hospital, Brigham and Women’s Hospital, and the University of Michigan, clinical research coordinators, research assistants, study staff or members of the patient’s care team approached study subjects and provided them with general information about the study. The women were informed that they would be contacted in approximately two weeks by a study team member to participate in interviews regarding their post-delivery experience. They were given the option to opt out at that time or at any subsequent time via phone or email. When they were contacted two weeks after discharge, verbal consent to participate in the study was obtained. At Stanford University Medical Center, Wake Forest Health Sciences, and Columbia University Medical Center, women who agreed to participate in the study provided written informed consent prior to hospital discharge.
Women enrolled in the study were contacted by phone approximately two weeks after hospital discharge. Those who did not answer the first phone call attempt were called 1–3 times daily for up to five days; at that time, if no contact was made, they were deemed lost to follow-up. Women who were still taking opioids at the time of the initial telephone conversation were called back approximately one week later.
Once reached, women completed a structured interview with a member of the study team in which they answered questions about their experience (maximal pain score on an 11-point numeric rating scale at 3 time points: immediately after discharge, the first week after discharge, and the second week after discharge), use of opioids and other analgesics, medication related side-effects at any timepoint (including drowsiness, nausea/vomiting, abdominal discomfort, constipation, dizziness, confusion, insomnia/sleeping issues, itching, difficult urination, mood swings), and overall satisfaction with their pain management (rated as very satisfied, satisfied, slightly satisfied, slightly dissatisfied, dissatisfied, or very dissatisfied).. After the interview was completed, with patient consent, a chart review was performed to gather additional information on patient demographics, indication for cesarean delivery, length of stay, smoking status, use of psychiatric medications, and anesthesia type.
The type of opioid dispensed, the strength, and the number of tablets were defined by asking women to read the label of the bottle of opioids (n=411). If the bottle was not available, this information was abstracted from the woman’s medical record (n=187). Finally, if neither of these sources were available, the patient was asked to recall these characteristics of the opioid prescription (n=7). For 10 women, information on the prescription was missing. Women also were asked about whether they required a refill of their opioid medication.
The number of leftover tablets after the patient finished taking opioids was then defined. If the patient reported taking all of the tablets dispensed from the initial prescription, the number of leftover tablets was defined as zero (n=101). If the patient had leftover medication from the initial prescription and the bottle was still available, the patient was asked to count the number of pills left in the bottle (n=373). If the bottle with leftover medications was not available, we asked the patient to estimate the number of leftover tablets (n=96). If the patient did not feel able to accurately estimate this number, we set the value to missing (n=45). The number of tablets consumed was defined by subtracting the number of tablets leftover from the number of tablets dispensed.
Collected survey data were transcribed into Research Electronic Data Capture database (REDCap). REDCap is a secure, web-based application designed to support data capture for research studies 10 The code book for the data elements collected in the survey is shown as Appendix 1, available online at http://links.lww.com/xxx. The data represent a convenience sample, with the number of subjects contributed by each center determined by logistical factors.
All analyses were conducted using R statistical software (RStudio, version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics are presented as means (standard deviations, SD) and medians [interquartile range, IQR] or frequency counts (%) as appropriate. Categorical variables were compared with Fisher’s exact tests and continuous variables by ANOVA tests or Kruskal-Wallis tests, as appropriate. Histograms were generated showing the distribution of pain scores at various time points. To identify the association between the number of tablets dispensed and the number consumed independent of other patient characteristics, we utilized a negative binomial model given the overdispersed nature of the count data.
Results
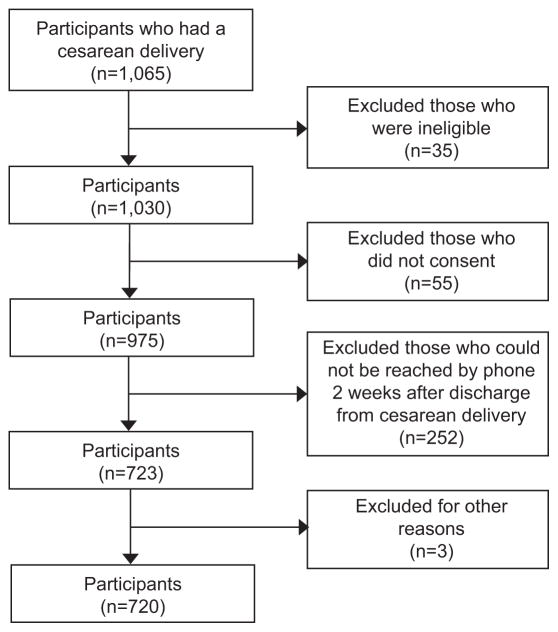
There were 1,065 women who underwent cesarean delivery and were considered for inclusion in the study. Failure to consent (n=55), meeting one or more of the exclusion criteria (n=35), and inability to reach by phone two weeks after the cesarean delivery (n=252), resulted in a final sample of participants of 720 women (Figure 1, additional details are in Appendix 2 [Appendix 2 available online at http://links.lww.com/xxx).
Figure 1.
Patient flow chart.
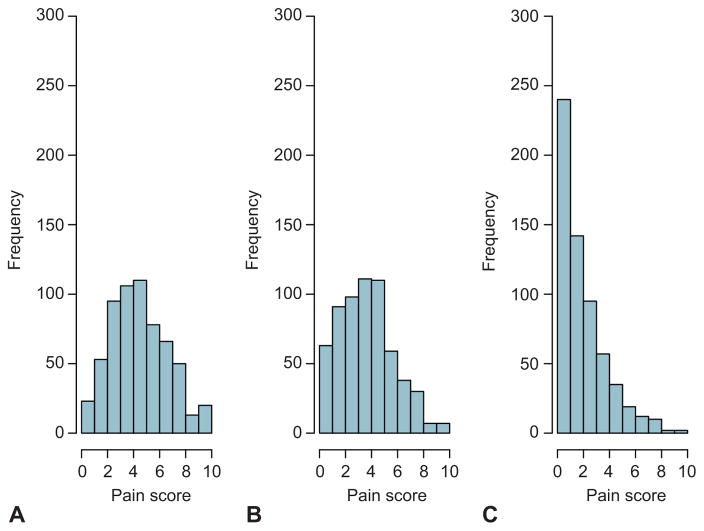
The mean age of participants was 32.7 years old; 59.3% of women were white, 77.2% were privately insured, 46% labored prior to cesarean delivery, 37.5% had a repeat cesarean delivery, 13.6% were smokers and less than 5% had a history of alcohol or other substance abuse issues. Nearly all (98.3%) of the cesarean deliveries were performed with neuraxial anesthesia (Table 1). The median maximal pain score at hospital discharge was 5 (IQR 3 to 6), during the first week the median was 4 (IQR 2 to 5), and during the second week the median was 2 (IQR 1 to 3, Figure 2).
Table 1.
Characteristics of the post-cesarean patients who participated in the survey.
| N (%), mean (SD), or median [IQR] | |
|---|---|
| Total | 720 |
| Age in years (mean (sd)) | 32.7 (5.4) |
| Race* | |
| White | 426 (59.3) |
| Black | 109 (15.2) |
| Hispanic | 79 (11.0) |
| Asian/Pacific Islander | 54 (7.5) |
| Other | 8 (1.1) |
| Unknown | 42 (5.8) |
| Insurance type* | |
| Medicaid | 146 (20.3) |
| Private | 554 (77.2) |
| Unknown | 7 (1.0) |
| Other | 7 (1.0) |
| None | 4 (0.6) |
| Labor prior to delivery | 328 (45.7) |
| Repeat cesarean | 270 (37.5) |
| Indications for cesarean delivery** | |
| Multiple gestation | 48 (6.7) |
| Elective cesarean delivery | 218 (30.3) |
| Failure to progess/Dystocia | 174 (24.2) |
| Malpresentation | 110 (15.3) |
| Fetal distress | 120 (16.7) |
| Other indication | 171 (23.8) |
| Total length of hospital stay (median [IQR]) | 4 [3 to 5] |
| Smokers | 98 (13.6) |
| History of alcohol abuse | 29 (4.0) |
| History of other substance abuse | 20 (2.8) |
| Anesthesia type** | |
| Spinal | 367 (51.0) |
| Epidural | 187 (26.0) |
| Combined spinal and epidural | 158 (21.9) |
| General | 12 (1.7) |
| Study Site | |
| Brigham and Women’s Hospital | 199 (27.6) |
| Columbia University Medical Center | 172 (23.9) |
| Massachusetts General Hospital | 196 (27.2) |
| Stanford University Medical Center | 7 (1.0) |
| University of Michigan Medical Center | 52 (7.2) |
| Wake Forest University Medical Center | 94 (13.1) |
Race and insurance type were missing for 2 patients.
Patients can be included in more than one category for indications for cesarean delivery and anesthesia type.
Figure 2.
Participants’ maximal pain score (on a scale from 0–10) at three time points: at hospital discharge (A), during the first week after discharge (B), and during the second week after discharge (C).
Of the 720 participants, 615 (85.4%) reported filling the opioid prescription. For the 105 women who did not fill the prescription, the most common reasons cited were not needing or wanting opioids (87%), not liking the way the opioids made them feel (11%), and negative side effects during prior exposure to opioids (9%) Appendix 3, available online at http://links.lww.com/xxx). The median reported pain scores were lower in women who did not fill the opioid prescription than those who did both during the hospitalization for delivery and after discharge (Appendix 4, available online at http://links.lww.com/xxx). Women who did not fill a prescription were not included in the subsequent analyses.
Nearly all prescriptions were for either oxycodone 5 mg (82.1%) or hydrocodone 5 mg (8.7%). The median number of tablets dispensed was 40 (IQR 30 to 40), the number consumed was 20 (IQR 8 to 30), and leftover was 15 (IQR 3 to 26). Of those with leftover tablets (n=514, 83.6%), 490 or 95.3% had not disposed of the excess at the time of the survey (Table 2).
Table 2.
The number of tablets dispensed, consumed, and leftover for women who filled a prescription for an opioid analgesic after cesarean delivery.
| Median | 25th–75th percentiles | 10th–90th percentiles | Range | |
|---|---|---|---|---|
| Tablets dispensed | 40 | 30 to 40 | 24 to 45 | 5 to 80 |
| Tablets consumed | 20 | 8 to 30 | 2 to 40 | 0 to 60 |
| Tablets leftover | 15 | 3 to 26 | 0 to 36 | 0 to 59 |
N=605; 10 patients who were dispensed an opioid were excluded from this analysis for missing data.
The sample was divided into tertiles based on the number of tablets of opioids dispensed, which corresponded to ≤30, 31 to 40, and >40 tablets, and patient reported outcomes were assessed in each tertile (Table 3). The proportion who reported being satisfied or very satisfied with their pain relief was similarly high (>80%) across each of the three groups. Pain scores were also similar across the three groups during the first two weeks after the discharge. The proportion requiring a refill was also similar across the three groups (around 5%). A higher proportion of women in the lowest tertile compared with the other two groups reported that the opioid quantity was too low (14.8%) and a higher proportion in the top tertile reported that the amount was too much (36.2%; p=0.032). Opioid-related side effects were reported more frequently with increasing tertile, from 47% in the first tertile to 71% in the top tertile (p<0.001). Details on the distribution of side effects stratified by tertile of opioid dispensed are shown in Appendix 5, available online at http://links.lww.com/xxx.
Table 3.
Patient outcomes stratified by tertiles of the number of opioid analgesic tablets dispensed.
| Tertile Dispensed | ||||
|---|---|---|---|---|
| ≤ 30 Tablets (n=237) | 31–40 Tablets (n=299) | >40 Tablets (n=69) | P-Value | |
| Satisfied or very satisfied with pain relief, n (%) | 200 (84.4) | 252 (84.3) | 56 (81.2) | 0.501 |
| Patient’s perception of opioid quantity dispensed, n (%) | 0.032 | |||
| Too Little | 35 (14.8) | 29 (9.7) | 6 (8.7) | |
| Just Right | 134 (56.5) | 179 (59.9) | 33 (47.8) | |
| Too Much | 49 (20.7) | 62 (20.7) | 25 (36.2) | |
| Experienced an opioid-related side-effect, n (%) | 111 (46.8) | 185 (61.9) | 49 (71.0) | <0.001 |
| Required a refill of opioid, n (%) | 14 (5.9) | 15 (5.0) | 4 (5.8) | 0.873 |
| Pain score at week 1, median (IQR)* | 4 [3, 5] | 4 [2, 5] | 4 [2, 5] | 0.034 |
| Pain score at week 2, median (IQR)* | 2 [1, 3] | 2 [1, 3] | 2 [1, 3] | 0.630 |
| Number of tablets consumed, median [IQR] | 15 [5 to 24] | 20 [10 to 32] | 32 [14 to 50] | <0.001 |
Pain score (0–10)
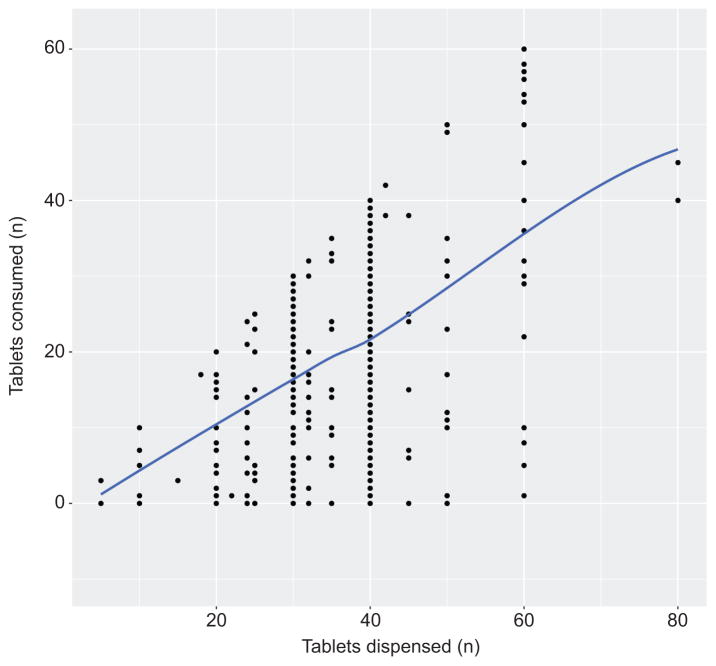
Increasing tertile of number of tablets dispensed also correlated with the number of tablets consumed, from a median of 15 in the first tertile to 32 in the top tertile (Table 3). A larger number of tablets dispensed associated with a larger number of tablets consumed (Figure 3). To test whether this association was independent of patient characteristics, we used a negative binomial model to examine the association between tertile of number of tablets dispensed and the number of tablets consumed, while adjusting for pain score at discharge, maternal age, labor prior to cesarean delivery, history of smoking, antidepressant use, benzodiazepine use, anesthesia type, length of stay after delivery, opioid type, non-steroidal anti-inflammatory (NSAID) use, and center where delivery occurred. Compared to the group dispensed ≤30 tablets, those dispensed 31 to 40 and >40 tablets consumed significantly more tablets independent of these potential confounders (incidence rate ratio [IRR] 1.35, 95% CI 1.10 to 1.65 and 2.01, 95% CI 1.48 to 2.76, respectively [Appendix 6, available online at http://links.lww.com/xxx]).
Figure 3.
Plot of the relationship between the number of tablets of opioid analgesic dispensed after discharge from cesarean delivery and the number of tablets consumed.
Discussion
In this survey sample of women who underwent cesarean delivery at six academic medical centers from across the United States, the amount of prescribed opioids significantly exceeded the amount consumed, leading to a large quantity of leftover opioids. We found that most women who had leftover opioids at the time of follow-up had not disposed of the excess tablets. Our data also suggest that the amount of opioid medication dispensed was not associated with patient satisfaction with their pain control regimen or with the need to receive a refill of the medication.
There is a lack of information on normative opioid consumption after cesarean delivery, which represents a challenge for obstetricians attempting to prescribe an appropriate amount upon patient discharge. In our survey, the median number of tablets consumed was half (n=20) of the median number of tablets prescribed (n=40), suggesting that obstetricians are routinely overprescribing opioids after this procedure. The mean number of leftover tablets in our sample was 16. Thus, if our results generalize to the United States as a whole, there are approximately 20 million opioid tablets introduced into communities from leftover medication after the treatment of pain after cesarean delivery each year which are potentially available for diversion or misuse.
Our results also demonstrate that women who were dispensed larger numbers of opioid tablets consumed a higher number, independent of pain severity at discharge and other patient characteristics. This suggests that the number of tablets prescribed may set women’s expectations regarding the appropriate amount of opioid medication to use during recovery from the cesarean delivery. A reasonable strategy to reduce opioid consumption may be simply to prescribe less. Our data suggest that this could be done without compromising effective pain control---satisfaction with pain control and pain scores were similar across each of the tertiles of amount dispensed. Potential benefits of reducing opioid consumption during recovery from cesarean delivery include a diminution of opioid-related side effects and opioid transfer in breast milk.11 Additionally, studies suggest that exposure to opioids in the context of cesarean delivery may be a triggering event for persistent use.12,13 Minimizing exposure to these addictive medications may help mitigate this risk.14
Overprescribing of opioid analgesics is problematic as it leads to leftover medication being introduced into communities where it is available for diversion, misuse, or accidental ingestion. Limiting initial opioid prescriptions has been the focus of several recent state laws, intended to address this problem.15 For example, the Commonwealth of Massachusetts recently enacted legislation mandating that initial opioid prescriptions (with some exceptions) be limited to no more than a seven days’ supply.16 While limiting the amount of opioids that can be prescribed may be an important policy approach to this issue, individual providers can also play an important role through judicious prescribing. Surgery specific data regarding normative opioid requirements, such as those presented here, can assist clinicians in this effort.
In our survey, the vast majority of women had not disposed of the leftover opioid medication at the time that they were interviewed. This is consistent with reports from other clinical settings, which have indicated that nearly three-quarters of patients with leftover opioids do not dispose of these medications. 7,17 Our data suggest that there is a need for providers to educate post-cesarean patients about the importance of disposing of leftover opioid analgesic pills. The Food and Drug Administration (FDA) provides guidance on the safe disposal of unused medications, including opioid analgesics. Recommended alternatives include returning medications to authorized take-back locations such as local law enforcement agencies or pharmacies, disposing of the medications in household trash after mixture with unpalatable substances and placement in a sealed plastic bag, or flushing medications down the sink or toilet (opioid analgesics are specifically mentioned in the FDA list of medications recommended for disposal by flushing).18 We believe providers should use postpartum encounters to ask patients who were prescribed opioids whether they have disposed of leftover medication and, if not, again educate them about options for safe disposal.
Our study must be interpreted in the context of the limitations inherent in its design. Survey participants represent a convenience sample of women who underwent cesarean delivery at these academic medical centers. As such, prescribing patterns and consumption patterns may not be fully representative of all cesarean deliveries in the United States and future studies will need to evaluate whether the patterns we describe also reflect those in community hospitals. Another limitation is that we were unable to contact about a quarter of women who were eligible for the study. It is possible that these women differ from study participants in their opioid consumption patterns. For some participants, we relied on their memory for the characteristics of the initial prescription and the number of tablets of opioids consumed. Given the small number of women for whom data was collected in this manner, it is unlikely that potential inaccuracies in recall would impact on our estimates. A small fraction of women required refills of the initial opioid prescription (n=33). For these women, the calculation of the number of tablets consumed was based on the initial prescription, given that the focus of the analysis was on the characteristics of the initial prescription. We do not have detailed information on why refills were obtained. Finally, since we asked about leftover opioid disposal at a time point shortly after women discontinued taking these medications, it is possible that our results underestimated the overall proportion disposing of the leftovers if women continued to dispose of the medication in the days or weeks that followed.
In conclusion, our data suggest that clinicians routinely prescribe excessive quantities of opioids after cesarean delivery and that patients consume greater opioid amounts when more are prescribed. These excess amounts may predispose patients to higher consumption without improved pain control, as well as to having large amounts of leftover medication. Given the frequency of cesarean deliveries in the United States, obstetricians can play an important role in decreasing the supply of opioid medication introduced into communities and should adopt more judicious prescribing patterns and counsel women about the importance of safe leftover medication disposal.
Supplementary Material
Acknowledgments
Financial support: BTB is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH (Bethesda, Maryland, United States) under Award Number K08HD075831. KFH was supported by a career development grant K01MH099141 from the National Institute of Mental Health.
The authors thank Sara Dejene for her editorial assistance.
Footnotes
Presented at the 32nd International Conference on Pharmacoepidemiology & Therapeutic Risk Management, The Convention Centre Dublin, Dublin, Ireland August 25–28, 2016, and the Society for Obstetrical Anesthesia and Perinatology, 48th Annual Meeting, Boston Massachusetts, May 18–22, 2016.
Financial Disclosure
Brian T. Bateman is an investigator on grants to his institution from Lilly, Pfizer, Baxalta, GSK, and Pacira.
Krista F. Huybrechts is an investigator on grants to her institution from Lilly and Pfizer. The other authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Contributor Information
Brian T. Bateman, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital and Harvard Medical School; Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 1620 Tremont Street, Suite 3030, Boston, Massachusetts 02120.
Naida M. Cole, Department of Anesthesiology, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Ayumi Maeda, Department of Anesthesiology, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Sara M. Burns, Department of Anesthesiology, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Timothy T. Houle, Department of Anesthesiology, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Krista F. Huybrechts, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 1620 Tremont Street, Suite 3030, Boston, Massachusetts 02120.
Caitlin R. Clancy, Department of Anesthesiology, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Stephanie B. Hopp, Department of Anesthesiology, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Jeffery L. Ecker, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Holly Ende, Department of Anesthesiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St. Boston, Massachusetts 02120.
Kasey Grewe, Department of Anesthesiology, Columbia University Medical Center, 622 W 168th St, New York, NY 10032.
Beatriz Raposo Corradini, Department of Anesthesiology, Columbia University Medical Center, 622 W 168th St, New York, NY 10032.
Robert E Schoenfeld, Division of Obstetric Anesthesiology, University of Michigan Health System, 1500 E Medical Center Dr, Ann Arbor, MI 48109.
Keerthana Sankar, Division of Obstetric Anesthesiology, University of Michigan Health System, 1500 E Medical Center Dr, Ann Arbor, MI 48109.
Lori J. Day, Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, University of Michigan Health System, 1500 E Medical Center Dr, Ann Arbor, MI 48109.
Lynne C. Harris, Department of Anesthesiology, Wake Forest School of Medicine, Medical Center Blvd., Winston-Salem, NC 27157-1009.
Jessica L. Booth, Department of Anesthesiology, Wake Forest School of Medicine, Medical Center Blvd., Winston-Salem, NC 27157-1009.
Pamela Flood, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University, Stanford, CA 94304.
Melissa E Bauer, Department of Anesthesiology, Division of Obstetric Anesthesiology, University of Michigan Health System, 1500 E Medical Center Dr, Ann Arbor, MI 48109.
Lawrence C. Tsen, Department of Anesthesiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St. Boston, Massachusetts 02120.
Lisa R. Leffert, Department of Anesthesiology, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02114.
Ruth Landau, Department of Anesthesiology, Columbia University Medical Center, 622 W 168th St, New York, NY 10032.
References
- 1.Florence CS, Zhou C, Luo F, Xu L. The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Medical care. 2016 Oct;54(10):901–906. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR. Morbidity and mortality weekly report. 2016 Dec 30;65(5051):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999–2008. MMWR. Morbidity and mortality weekly report. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 4.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. The New England journal of medicine. 2016 Jan 14;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inciardi JA, Surratt HL, Cicero TJ, Beard RA. Prescription opioid abuse and diversion in an urban community: the results of an ultrarapid assessment. Pain medicine. 2009 Apr;10(3):537–548. doi: 10.1111/j.1526-4637.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCabe SE, West BT, Teter CJ, Boyd CJ. Medical and nonmedical use of prescription opioids among high school seniors in the United States. Archives of pediatrics & adolescent medicine. 2012 Sep 1;166(9):797–802. doi: 10.1001/archpediatrics.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy-Hendricks A, Gielen A, McDonald E, McGinty EE, Shields W, Barry CL. Medication Sharing, Storage, and Disposal Practices for Opioid Medications Among US Adults. JAMA internal medicine. 2016 Jul 1;176(7):1027–1029. doi: 10.1001/jamainternmed.2016.2543. [DOI] [PubMed] [Google Scholar]
- 8.Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National Trends in Hospitalizations for Opioid Poisonings Among Children and Adolescents, 1997 to 2012. JAMA pediatrics. 2016 Oct 31;170(12):1195–1201. doi: 10.1001/jamapediatrics.2016.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2017 Jan;66(1):1. [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrickson RG, McKeown NJ. Is maternal opioid use hazardous to breast-fed infants? Clinical toxicology. 2012 Jan;50(1):1–14. doi: 10.3109/15563650.2011.635147. [DOI] [PubMed] [Google Scholar]
- 12.Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. American journal of obstetrics and gynecology. 2016 Sep;215(3):353e351–353 e318. doi: 10.1016/j.ajog.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA internal medicine. 2016 Sep 1;176(9):1286–1293. doi: 10.1001/jamainternmed.2016.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. Jama. 2016 Apr 19;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman BT, Choudhry NK. Limiting the Duration of Opioid Prescriptions: Balancing Excessive Prescribing and the Effective Treatment of Pain. JAMA internal medicine. 2016 May 1;176(5):583–584. doi: 10.1001/jamainternmed.2016.0544. [DOI] [PubMed] [Google Scholar]
- 16.An Act Relative to Substance Use Treatment, Education, and Prevention. HR 4056. [Accessed November 4, 2016];189th Leg (Ma 2016) https://malegislature.gov/Bills/189/House/H4056.
- 17.Centers for Disease, Control Prevention, Adult use of prescription opioid pain medications - Utah, 2008. MMWR. Morbidity and mortality weekly report. 2010 Feb 19;59(6):153–157. [PubMed] [Google Scholar]
- 18.http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.