Abstract
The Hargreaves test is specifically designed to assess thermal pain sensation in rodents such as rats and mice. This test has been used in experiments involving pain sensitization or recovery of thermal pain response following neural injury and regeneration. We present here a step-by-step protocol highlighted with important notes to guide first-time users through the learning process. Additionally, we have also included representative data from a rat model of sensory denervation showing how the data can be analysed to obtain meaningful results. We hope that this protocol can also assist potential users in deciding whether the Hargreaves test is a suitable test for their experiment.
Keywords: Thermal pain, Hargreaves, Rats, Mice, Behavioral testing
Background
The somatosensory system is responsible for processing sensory stimuli received from the environment. These sensory stimuli include pain, touch, pressure and vibration. To study how these stimuli are processed with the ultimate goal of repairing the system in the event of injury, neuroscientists have used a plethora of animal models and behavioral tests. Some of these experiments include administration of a noxious substance into the nervous system to investigate increased pain sensitivity ( Zurowski et al., 2012 ), inducing a central or peripheral injury to the nervous system and then observing whether particular treatments promote neural regeneration ( Andrews et al., 2009 ; Tan et al., 2012 ; Cheah et al., 2016 ), or developing in vivo neurodegenerative models with pathology in sensory neurons ( Mellone et al., 2013 ). Whichever the case, having a suitable test to study the behavioral response of the animals is key.
Of all the sensations, pain is perhaps one of the most studied. Transmitted via different nociceptors, pain can be further categorized into different modalities such as thermal, mechanical and chemical. Depending on the experimental goal, all of these modalities can be assessed with a specific behavioral test. We present here our approach to the Hargreaves test which is a behavioral test designed for assessing response to thermal pain in rodents such as rats and mice ( Hargreaves et al., 1988 ). Based on our experience, the Hargreaves test is relatively straight-forward and novel users can master it in a short period of time. With the aid of this step-by-step protocol, we hope to assist potential users in deciding if the Hargreaves test is a suitable test for them and also guide them through the learning process in an efficient manner.
Materials and Reagents
-
Tissue paper
Note: The animals may urinate or defecate in the enclosure. Any fluid found on the framed glass panel should be cleaned immediately as this can affect the thermal conductance of the glass panel.
-
Disinfectant cleaning solution (e.g., 70% ethanol)
Use a disinfectant cleaning solution to remove animal odor, urine and/or feces after each session
-
Animals
Note: The activity level of each animal strain can affect the test tremendously as proper plantar placement of the paws is required for the test to be performed with reliable results. For whichever strain is chosen, habituation to the enclosure should be performed in the week prior to the start of experiment in order to acclimatize the animals to the setup as well as to take baseline readings.
For rats, the more docile Lewis strain may be ideal for the test because they have a tendency to move about their enclosures minimally compared to other strains, however they may fall asleep during the test resulting in delayed responsiveness. On the other hand, the more active Lister-Hooded strain may move too much, hence making the test difficult and/or potentially invalidating the results. Sprague Dawley rats have a tendency to have moderate activity level and are thus another ideal strain for this test.
For mice, the C57BL/6 strain is generally more active than other strains such as BALB/c. The more active strains may make progressing with the test difficult although with sufficient acclimatization to the testing apparatus, any strain can be used.
-
Sucrose/food pellets
Optional: Feeding animal a small handful (~5-10) of sucrose/food pellets may be helpful in reducing the movement and calming the animals in their enclosure.
Equipment
-
Hargreaves test (Ugo Basile, catalog number: 37370 or Harvard Apparatus, catalog number: 72-6692). A complete set of the Hargreaves test should comprise the following components (Figures 1-3):
-
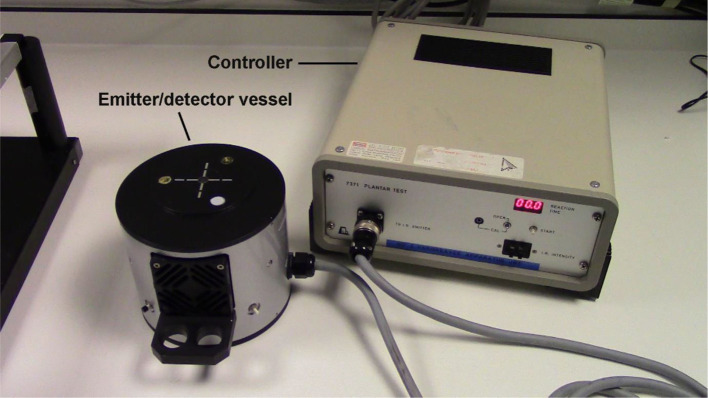
Controller
For manipulation of test settings such as infrared intensity, display of reaction time, and power input port
-
Emitter/detector vessel
With a fibre optic cable connecting to the controller for infrared emission and paw movement detection
-
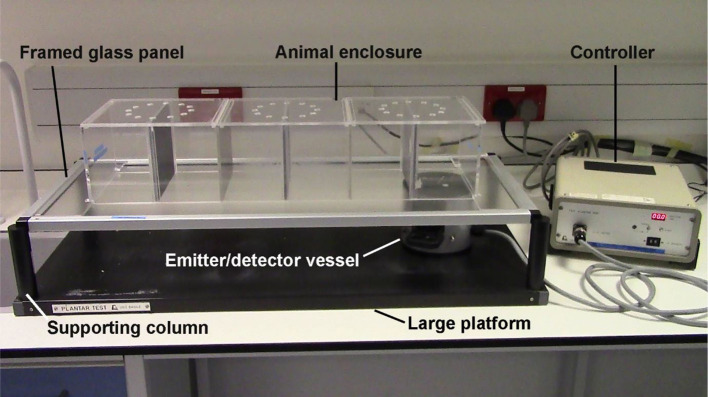
Framed glass panel
For optimal thermal conductance, the glass panel should be kept clean and protected from damage
-
Animal enclosure
Large: holds up to 6 rats at a time
Small: holds up to 12 mice at a time
Large platform
Supporting columns
Figure 2. The Hargreaves test controller and emitter/detector vessel.
Figure 3. Animal enclosure.
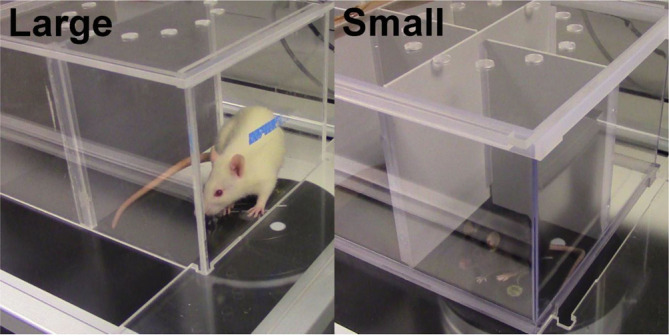 The large enclosure can hold up to 6 rats, one animal in each compartment, while the small enclosure can hold up to 12 mice.
The large enclosure can hold up to 6 rats, one animal in each compartment, while the small enclosure can hold up to 12 mice. -
Figure 1. A complete setup of the Hargreaves test.
Procedure
Note: All of the following animal procedures were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. Please check with local/national regulations before starting the protocol.
-
Choose a suitable room for behavioral assessment.
Note: External stimuli such as noise and vibration in the environment can cause anxiety in the animals. They may exhibit excessive movements if they are anxious or freeze if they are frightened. In either case, this would make progressing with the test difficult. It is recommended to choose a calm and quiet room for the test, and preferably somewhere without any disruptions during the test.
Set up the equipment as shown in Figure 1.
-
Habituate the animals to the testing chamber.
Transfer the animals from the holding room to the behavioral room in a calm manner.
Gently place each animal into its own compartment of the enclosure.
-
Acclimatize the animals to the enclosure for 30-60 min.
Note: The animals may show curiosity to the new environment by moving around in the enclosure for the first 10-15 min, after which they should calm down. Placing sucrose/food pellets in the enclosure may be helpful in calming them down.
Return the animals back to the holding room and repeat the habituation step for at least 2 days before performing the actual Hargreaves test.
Thoroughly clean the enclosure and framed glass panel after each use.
-
Perform the Hargreaves test.
It is recommended that a baseline reading be obtained before starting the experiment. This step also allows the experimenter to optimize the test for each particular experiment and animal strain.
Transfer the animals from the holding room to the enclosure as before.
-
Acclimatize the animals in the enclosure for 15-20 min or until they are calm.
Note: The animals may fall asleep while being acclimatized or during the test. Gently tap the enclosure to wake them up before performing the test if necessary.
-
Set the desired infrared intensity (Figure 4).
Note: This may require optimization before starting the experiment. 48 units was used for our experiment with adult male Lewis rats.
-
Position the infrared emitter/detector on the vessel to directly underneath the center of the paw being tested (Figures 5-6).
Note: The guiding lines on the vessel should be very useful for this step and there must be proper plantar placement of the paw for the test to be valid.
Figure 6. The correct positioning of the paw.
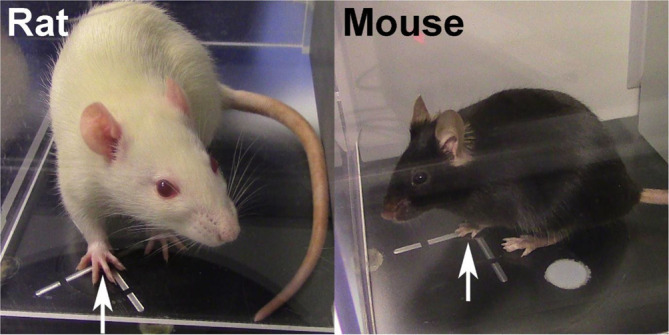 The infrared emitter/detector should be positioned directly underneath the center of the paw (white arrows). There must be proper plantar placement of the paw for the test to be valid.
The infrared emitter/detector should be positioned directly underneath the center of the paw (white arrows). There must be proper plantar placement of the paw for the test to be valid. -
Start the test by pressing the START button.
Note: The timer should start automatically at the same time as the infrared light is being switched on.
-
Observe the animal’s paw until the animal elicits a withdrawal response due to sensing of thermal heat.
Note: Movement of the paw will be detected by the vessel, and the controller will automatically switch off the infrared light and stop the timer altogether. If the animal moves the paw voluntarily (not as a reaction to the heat), this is not a withdrawal response and the particular trial should not be included. Typical withdrawal response due to the heat should be accompanied by the animal checking/observing or licking their paw. (Video 1)
-
Record the reaction time.
Note: Depending on local/national regulations, it is recommended that the trial should be terminated if the animal fails to respond within 20 sec to avoid potential burn injury.
-
Repeat the test for at least 3 trials* to obtain an average reaction time.
Notes:
*If time permits, 5 trials are recommended. In this case, the lowest and highest values can then be discounted to remove outlying values.
Allow for approximately 5 min of interval time before repeating the test on the same animal. The experimenter can test other animals in the enclosure during this interval.
Carefully return the animals back to the holding room.
Thoroughly clean the enclosure and framed glass panel after each use.
Repeat steps 4a-4j at the next experimental data point if required.
Figure 4. Controller.

The controller contains a display for reaction time in seconds, the START button and adjustable infrared intensity.
Figure 5. Emitter/detector vessel.
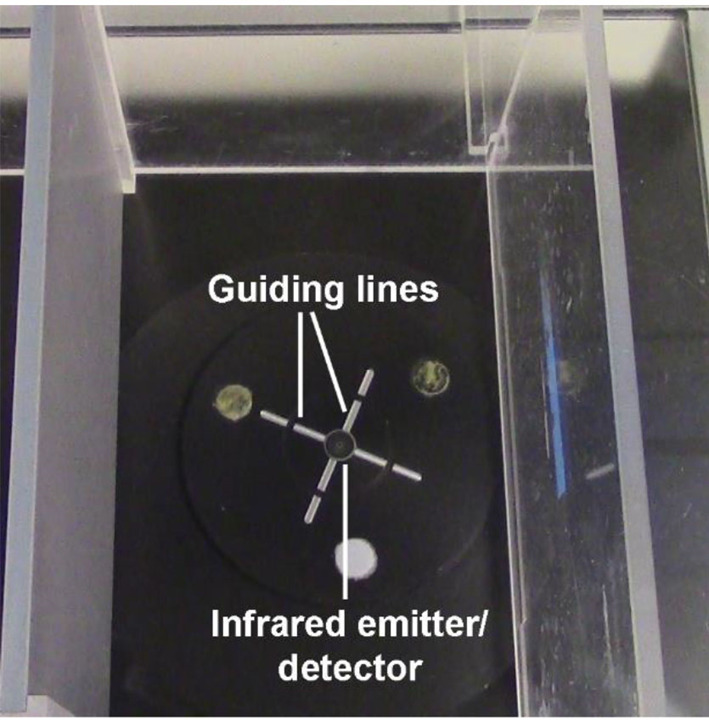
The guiding lines on the vessel are useful in positioning the infrared emitter/detector directly underneath the center of the paw.
Video 1. Typical withdrawal response in rats.

This video clip shows two different withdrawal scenarios of a rat that can occur whilst the animal is in the testing apparatus. The first example shows a proper withdrawal response in which the animal removes its paw from the heat and immediately licks its paw as a reflex. The second example shows removal of the paw from the heat source which is not due to the heat but rather is voluntary movement of the paw. In the second example there is no licking of the paw or acknowledgement of the heat and should therefore not be included as a proper withdrawal response.
Data analysis
The amount of time required to elicit a withdrawal response is termed as withdrawal latency which is measured in seconds. A longer withdrawal latency signifies a slower withdrawal response and vice versa. To obtain the average reaction time for an animal, calculate the mean from at least 3 trials. If 5 trials have been performed, the lowest and the highest values can be discounted as outlying values. To obtain a data point for a group of animals with one animal represents n = 1, calculate the mean of the average reaction time of the animals in the group. If the same set of animals is being tested repeatedly over different experimental conditions or timepoints, a paired or repeated measures statistical test should be used to determine the statistical significance. Usually, a P value of less than 0.05 is deemed to be statistically significant. Representative data are shown below (Figure 7).
Figure 7. Representative Hargreaves test data for adult Lewis rats which underwent a quadruple (C5-C8) dorsal root crush injury of the left forepaw or sham surgery.
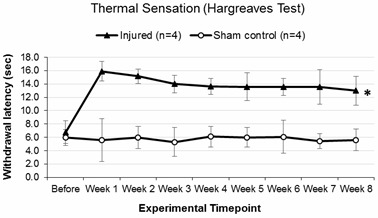
A baseline reading was recorded a week before surgery and experimental readings were recorded weekly during 1-8 weeks after surgery. As opposed to the sham control group, a longer time (in seconds) was required to elicit a withdrawal response from the injured group a week after surgery and this was sustained throughout the duration of the experiment signifying chronic thermal pain sensory deficit. The data were analyzed by repeated measures ANOVA and expressed as mean ± SEM, *P < 0.05 was statistically significant, n = 4.
Notes
It is important that the experimenter watch the animals closely during the test to make sure they do not remove their paw because they are moving it voluntarily, as opposed to being a reaction to the heat. If this occurs, the particular trial should be voided as the withdrawal response observed was likely not in response to the heat. The Hargreaves device itself cannot make the distinction whether the movement is due to the heat or is voluntary. Typical withdrawal response due to the heat should be accompanied with the animals checking or licking their paw.
In terms of animal welfare, please handle the animals very gently. Animals subject to the Hargreaves test may have hypersensitivity to thermal pain due to a procedure such as injection of a hyperalgesic substance as part of the experiment, or reduced pain sensation due to receiving a peripheral or central nervous system injury previously. In the event of reduced pain sensation, a cut-off point (recommended: 20 sec) should be set for the test so the animals do not suffer from potential burn injury.
Acknowledgments
M.R.A. is supported by a research grant from the Biotechnology and Biological Sciences Research Council (BBSRC). J.W.F. is supported by a grant from the European Union/Czech Ministry of Education–Operation Programme Research (CZ.02.1.01/0.0/0.0/15_003/0000419).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Andrews M. R., Czvitkovich S., Dassie E., Vogelaar C. F., Faissner A., Blits B., Gage F. H., C. ffrench-Constant and Fawcett J. W.(2009). α9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci 29(17): 5546-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheah M., Andrews M. R., Chew D. J., Moloney E. B., Verhaagen J., Fassler R. and Fawcett J. W.(2016). Expression of an activated integrin promotes long-distance sensory axon regeneration in the spinal cord. J Neurosci 36(27): 7283-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargreaves K. M., Dubner R., Brown F., Flores C. and Joris J.(1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77-88. [DOI] [PubMed] [Google Scholar]
- 4.Mellone M., Kestoras D., Andrews M.R., Dassie E., Crowther R.A., Stokin G.B., Tinsley J., Horne G., Goedert M., Tolkovsky A. M. and Spillantini M. G.(2013). Tau pathology is present in vivo and develops in vitro in sensory neurons from human P301S tau transgenic mice: a system for screening drugs against tauopathies . J Neurosci 33(46): 18175-18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan C. L., Andrews M. R., Kwok J. C., Heintz T. G., Gumy L. F., Fassler R. and Fawcett J. W.(2012). Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J Neurosci 32(21): 7325-7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zurowski D., Nowak L., Machowska A., Wordliczek J. and Thor P. J.(2012). Exogenous melatonin abolishes mechanical allodynia but not thermal hyperalgesia in neuropathic pain. The role of the opioid system and benzodiazepine-gabaergic mechanism. J Physiol Pharmacol 63(6): 641-647. [PubMed] [Google Scholar]