Abstract
Background:
Aliskiren was shown to increase adverse events in patients with diabetes and concomitant renin–angiotensin blockade. We aim to investigate the efficacy and safety of aliskiren in patients with diabetes and increased cardiovascular risk or established cardiovascular disease.
Methods:
MEDLINE and Embase were searched for prospective studies comparing addition of aliskiren to standard medical therapy in patients with diabetes and cardiovascular disease, or ⩾1 additional cardiovascular risk factor (hypertension, abnormal lipid profile, microalbuminuria/proteinuria, chronic kidney disease). Relative risk for efficacy (all-cause mortality, combined cardiovascular mortality and hospitalisation) and safety (hyperkalaemia, hypotension, renal impairment) outcomes was calculated.
Results:
Of 2151 studies identified in the search, seven studies enrolling 13,395 patients were included. Aliskiren had no effect on all-cause mortality (relative risk: 1.05, 95% confidence interval: 0.90 to 1.24, p = 0.53), or combined cardiovascular mortality or heart failure hospitalisation (relative risk: 1.07, 95% confidence interval: 0.81 to 1.40, p = 0.64). Patients receiving aliskiren had a greater risk of developing hyperkalaemia (relative risk: 1.32, 95% confidence interval: 1.14 to 1.53, p = 0.0003) and renal impairment (relative risk: 1.15, 95% confidence interval: 1.02 to 1.30, p = 0.03), but not hypotension.
Conclusion:
Patients with diabetes and cardiovascular disease or cardiovascular risk do not benefit from the addition of aliskiren to standard medical therapy. Detrimental safety profile in pooled analysis supports current warnings.
Keywords: Aliskiren, direct renin inhibitor, renin–angiotensin inhibition, diabetes, systematic review, meta-analysis
Background
The direct renin inhibitor (DRI) aliskiren has been proposed as an alternative to angiotensin II-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) therapy in the management of cardiovascular morbidity and mortality in diabetes mellitus,1 with similar antihypertensive efficacy to ACEi or ARB.2–5 However, large clinical trials evaluating cardiovascular clinical endpoints have failed to demonstrate non-inferiority with aliskiren compared to ACEi or ARB treatment and have identified potential safety concerns in diabetic patients when used in addition with ACEi or ARB.6,7
The aim of this meta-analysis is to investigate the efficacy and safety of aliskiren use in addition with background medical therapy in patients with diabetes and high cardiovascular risk or established cardiovascular disease.
Methods
This article is written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.8 No published protocol for this systematic review and meta-analysis exists.
Data sources and search strategy
A systematic search of MEDLINE and Embase databases was performed on 3 October 2016 using the terms Aliskiren, Tekturna, Rasilez, SPP100, renin inhibitor and Diabetes. Results were filtered from 1 January 2000. The reference lists of included studies and reviews were hand-searched for additional articles. The Novartis clinical trials database9 was searched for additional data of completed trials.
Study selection criteria
We included prospective studies that enrolled patients with diabetes and established or history of cardiovascular disease, or at least one cardiovascular risk factor (study-defined hypertension, raised low-density lipoprotein, reduced high-density lipoprotein, microalbuminuria, proteinuria or chronic kidney disease). Studies were required to compare aliskiren dual therapy with ACEi or ARB, with either placebo or ACEi/ARB monotherapy. Included studies were required to report all-cause mortality stratified by diabetes status. To assess the long-term efficacy, studies with follow-up (mean/median) of ⩾6 months were included.
Data extraction and quality assessment
Study selection, data extraction and quality assessment were performed independently by two authors (S.L.Z., A.R.). After removal of duplicates, title and abstracts were screened for relevance, with full texts of remaining results assessed for inclusion based on pre-determined inclusion criteria. Inclusion required agreement between reviewers (S.L.Z., A.R.). The data extracted from each report included general study characteristics (study name, primary investigator, year of publication, median or mean duration of follow-up, inclusion and exclusion criteria), participant characteristics (number, age, gender, cardiovascular co-morbidities – hypertension, heart failure, previous myocardial infarction, concomitant use of ACEi and/or ARB), outcome data [hazard risk, risk or odds ratios with 95% confidence intervals (CIs), and absolute numbers] and adverse events (study-defined hyperkalaemia, hypotension and renal impairment).
Study corresponding authors were contacted for additional information where required.
The Cochrane Collaboration risk of bias tool was used to assess risk of bias. All studies had low risk of bias, with no impact on data synthesis.
Statistical analysis
Primary efficacy outcome was all-cause mortality. Secondary efficacy outcome was combined cardiovascular mortality and heart failure hospitalisation. Primary safety outcomes were hyperkalaemia, hypotension and renal impairment. Relative risk (RR) was calculated from raw published study data. Review Manager 5.3 was used for statistical analysis.10 Mantel–Haenszel method was used to calculate estimates, CIs and p-values. Random-effects models were used to summarise data. p-values for heterogeneity were calculated using chi-square test.
Results
Study selection and baseline characteristics
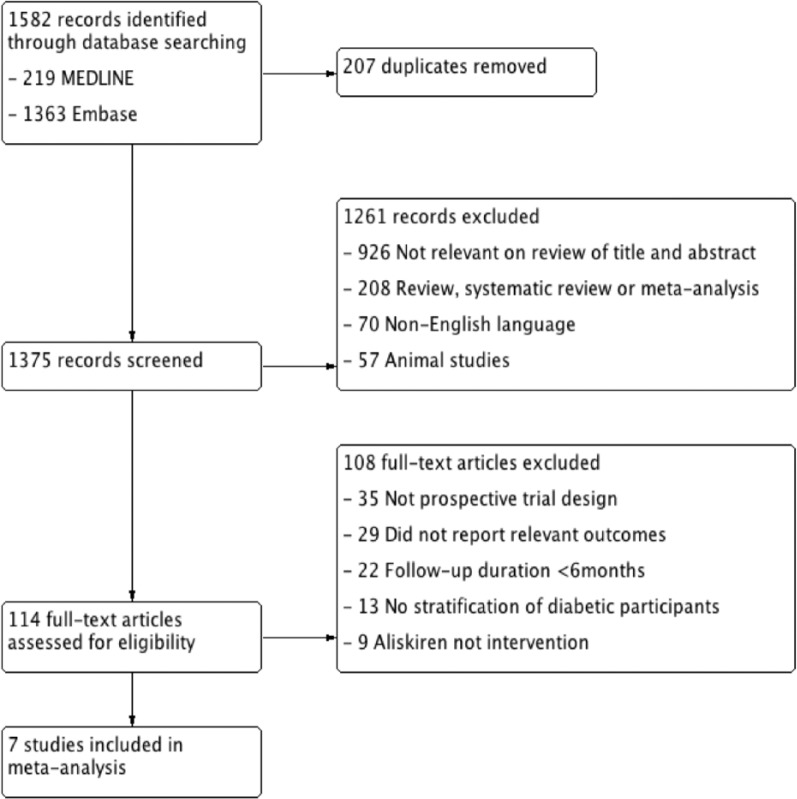
The search identified 2150 articles. Seven were included for meta-analysis (Figure 1 – study flow chart), enrolling 13,395 patients with diabetes (Table 1 – baseline characteristics). The participants were followed up for a mean of 24.8 months, totalling 27,683 patient-years follow-up. There were five randomised controlled trials (RCTs)11–16 and one observational study.17 The largest trials were Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE); (8552 participants),12 the 3A registry (1936)17 and Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure (ATMOSPHERE); (1317).16 Two RCTs enrolled participants with diabetes and high cardiovascular risk (Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID), ALTITUDE, n = 9151), four RCTs enrolled participants with diabetes and established cardiovascular disease [Aliskiren Study in Post-MI Patients to Reduce Remodelling (ASPIRE), Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT), Aliskiren Quantitative Atherosclerosis Regression Intravascular Ultrasound Study (AQUARIUS), ATMOSPHERE, n = 2935] and one observational study enrolled participants with hypertension (3A Registry, n = 3038).
Figure 1.
Study flow chart.
Table 1.
Study characteristics of included trials.
| Trial, author | Year | Trial type | Inclusion criteria | Primary outcomes | Intervention | Control | Follow-up (m) | Total | Intervention | Control | Male | Age | Htn | HF | Prior MI | ACEi | ARB | RAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVOID, Parving11 | 2008 | RCT | Hypertension, diabetes, nephropathy | Reduction in ACR | Aliskiren + Losartan | Losartan | 6 | 599 | 301 | 298 | 427 (71%) | 61 | 599 (100%) | NR | 34 (6%) | 0 (0%) | 599 (100%) | 599 (100%) |
| ALTITUDE, Parving12 | 2012 | RCT | Diabetes, micro or macroalbuminuria, CVD | CV mortality, cardiac arrest, nonfatal MI/stroke, HFH, renal outcomes | Aliskiren + SMT | Placebo, SMT | 32.9 | 8552 | 4274 | 4278 | 5826 (68%) | 65 | NR | 872 (10%) | 1424 (17%) | 3787 (44%) | 4796 (56%) | 8552 (100%) |
| ASPIRE, Shah13 | 2012 | RCT | Post-acute MI with HF | CV mortality, HFH, MI, stroke, cardiac arrest | Aliskiren + SMT | Placebo, SMT | 8.3 | 214 | 112 | 102 | 175 (82%) | 62 | 146 (68%) | 12 (6%) | 214 (100%) | 189 (88%) | 24 (11%) | 213 (100%) |
| ASTRONAUT, Maggioni14 | 2013 | RCT | HF, LVEF <40%, raised biomarkers | CV mortality, HFH | Aliskiren + SMT | Placebo, SMT | 12 | 662 | 319 | 343 | 508 (77%) | 66 | 542 (82%) | 662 (100%) | NR | 414 (61%) | 152 (23%) | 566 (84%) |
| AQUARIUS, Puri15 | 2015 | RCT | Coronary artery stenosis, CV risk factors | Change in % atheroma volume | Aliskiren + SMT | Placebo, SMT | 24 | 115 | 55 | 60 | 90 (78%) | 61 | 103 (90%) | NR | 33 (29%) | 71 (62%) | 29 (25%) | 100 (87%) |
| ATMOSPHERE, McMurray16 | 2016 | RCT | Symptomatic HF, LVEF <35%, raised biomarkers | CV mortality, HFH | Aliskiren | Enalapril | 24.1 | 1317 | 665 | 652 | 1027 (78%) | 64 | 988 (75%) | 856 (65%) | 632 (48%) | 1317 (100%) | 0 (0%) | 1317 (100%) |
| 3A Registry, Kistner17 | 2016 | POS | Hypertension | NA | Aliskiren + ACEi/ARB | ACEi/ARB | 12 | 1936 | 1381 | 555 | 1137 (59%) | 67 | 1936 (100%) | 447 (23%) | NR | – | – | 1936 (100%) |
ALTITUDE: Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints; ATMOSPHERE: Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure; ASPIRE: Aliskiren Study in Post-MI Patients to Reduce Remodelling; ASTRONAUT: Aliskiren Trial on Acute Heart Failure Outcomes; AQUARIUS: Aliskiren Quantitative Atherosclerosis Regression Intravascular Ultrasound Study; RCT: randomised controlled trial; POS: prospective observational study; CV: cardiovascular; HF: heart failure; LVEF: left ventricular ejection fraction; MI: myocardial infarction; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; RAS: renin–angiotensin system blocker; ACR: albumin-to-creatinine ratio; HFH: hospitalisation for heart failure; MI: myocardial infarction; CVD: cardiovascular disease; SMT: standard medical therapy (includes ACEi and ARB); NR: not recorded; Htn: hypertension.
Effect of aliskiren on outcomes
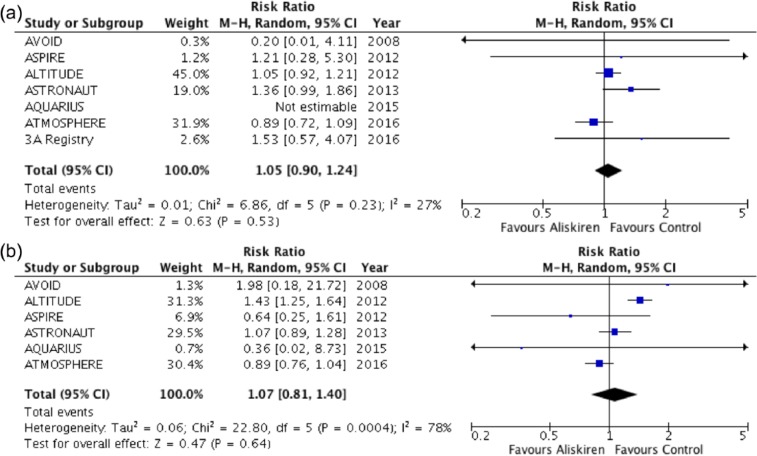
Aliskiren was associated with the same risk of death compared with controls (RR: 1.05, 95% CI: 0.90 to 1.24, p = 0.53, I2 = 27%; Figure 2(a)). There was no difference in combined cardiovascular mortality and heart failure hospitalisation between aliskiren and controls (RR: 1.07, 95% CI: 0.81 to 1.40, p = 0.64, I2 = 78%; Figure 2(b)).
Figure 2.
Forest plot of (a) all-cause mortality and (b) combined cardiovascular mortality and hospitalisation for heart failure.
Among 2308 patients with established cardiovascular disease, aliskiren was not associated with reductions in all-cause mortality (RR: 1.08, 95% CI: 0.76 to 1.54, p = 0.66) or combined cardiovascular mortality and heart failure hospitalisation (RR: 0.96, 95% CI: 0.83 to 1.09, p = 0.51) compared with controls (Supplementary Figure 1).
Effect of aliskiren on blood pressure and urinary albumin-to-creatinine ratio
Three trials reported change in blood pressure between aliskiren and control groups, all of which favoured the addition of aliskiren. Two trials reported on change in urinary albumin-to-creatinine ratio, again both in favour of aliskiren (Table 2).
Table 2.
Changes in urinary albumin-to-creatinine ratio, blood pressure (difference in change between aliskiren and control group) and discontinuations due to adverse events in randomised controlled trials.
| Trial | Baseline ACR (aliskiren group) | % change in urine ACR | Baseline sitting blood pressure (aliskiren group) | Change in blood pressure (aliskiren − control) | Discontinuations due to adverse event – aliskiren group | Discontinuations due to adverse event – control group |
|---|---|---|---|---|---|---|
| AVOID | 513 | −18% | 135/78 mmHg | −2/1 mmHg | 17 (5.6%) | 19 (6.4%) |
| ALTITUDE | 206 | −16% | 137/74 mmHg | −1.9/1 mmHg | 563 (13.2%) | 437 (10.2%) |
| ASPIRE | NR | NR | NR | NR | NR | NR |
| ASTRONAUT | NR | NR | NR | NR | NR | NR |
| AQUARIUS | NR | NR | 132/77 mmHg | −3/0.6 mmHg | NR | NR |
| ATMOSPHERE | NR | NR | 127 mmHg (SBP) | NR | NR | NR |
ALTITUDE: Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints; ATMOSPHERE: Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure; ASPIRE: Aliskiren Study in Post-MI Patients to Reduce Remodelling; ASTRONAUT: Aliskiren Trial on Acute Heart Failure Outcomes; AQUARIUS: Aliskiren Quantitative Atherosclerosis Regression Intravascular Ultrasound Study; ACR: albumin-to-creatinine ratio; NR: not reported; SBP: systolic blood pressure.
Adverse events
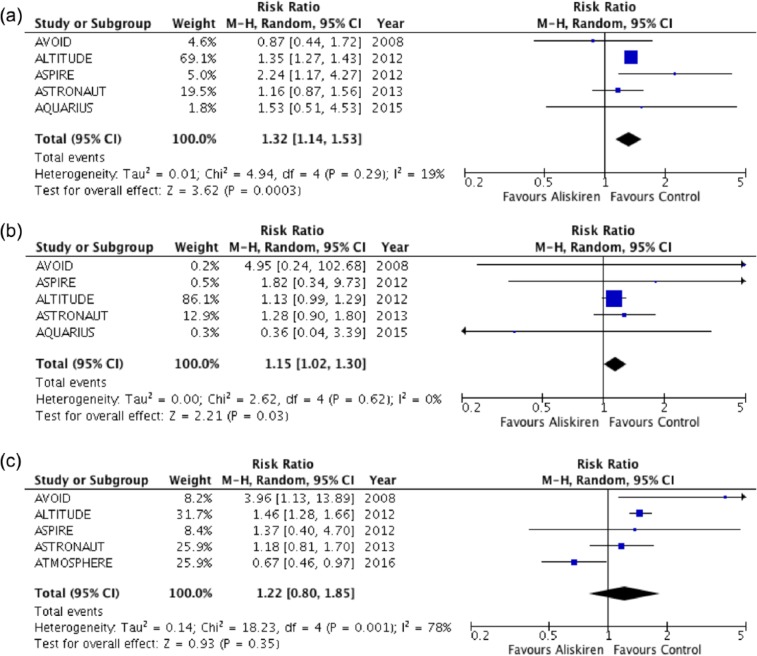
Rates of hyperkalaemia, renal impairment and hypotension were reported in five, five and four RCTs, respectively. Patients receiving aliskiren had greater risk of developing hyperkalaemia [RR: 1.32, 95% CI: 1.14 to 1.53, p = 0.0003, absolute risk reduction (ARR): 6%] and renal impairment (pooled RR: 1.15, 95% CI: 1.02 to 1.30, p = 0.03, ARR 1%) compared with controls (Figure 3(a) and (b)). There was no increase in risk of developing hypotension in patients with diabetes given aliskiren (RR: 1.22, 95% CI: 0.80 to 1.85, p = 0.035; Figure 3(c)). Study withdrawal in RCTs due to adverse events was only reported in two trials (Table 2).
Figure 3.
Forest plot of adverse events: (a) hyperkalaemia, (b) renal impairment and (c) hypotension.
Study quality and risk of publication bias
All RCTs had low overall risk of bias, and funnel plot showed little evidence of publication bias (Supplementary Figures 2 and 3).
Discussion
The findings of this meta-analysis demonstrate that aliskiren has no effect on all-cause mortality or combined cardiovascular mortality and heart failure hospitalisation in patients with diabetes and cardiovascular risk or established disease. Pooled analysis demonstrated an increased risk of hyperkalaemia and renal impairment with aliskiren.
This meta-analysis is the first to examine the efficacy and safety of aliskiren in trial patients with high-risk diabetes, a group where renin–angiotensin system (RAS) inhibition reduces cardiovascular outcomes.18 We compare use of aliskiren with placebo or single ACEi/ARB in patients with diabetes. There were high rates of concomitant ACEi or ARB use in trial participants (85–100% in individual trials, and 99.1% in the pooled trial population). Our findings can therefore be cautiously interpreted as an increased risk with dual RAS blockade using aliskiren for hyperkalaemia and renal impairment of 32% (population risk ranges: 14%–53%) and 15% (population risk ranges: 2%–30%) respectively. This supports the US Food and Drug Administration and European Medicines Agency safety announcements warning against the use of aliskiren in combination with ACEi or ARB in patients with diabetes.
Dual RAS blockade in diabetic patients with high cardiovascular risk does not improve outcomes and is associated with increased adverse events,19 a conclusion that is extended to the use of aliskiren and supported by this meta-analysis. It remains to be seen whether aliskiren as monotherapy is of benefit, and whether it serves as an alternative in patients intolerant of ACEi and ARB. In meta-analysis of blood pressure reduction, aliskiren showed superior efficacy over ACEi and equivalence with ARB.20 Current evidence suggests that in diabetic patients without specific indications for RAS blockade, RAS blockade was not superior in improving cardiovascular outcomes compared with alternative blood pressure–reducing therapies.21
Study limitations
Limitations of this study include those inherent in meta-analyses, which are driven primarily by availability and accessibility of data. Adverse events were trial defined, and so conclusions on increased risk of these are not with universal definitions. We are unable to comment on whether there may be benefit of aliskiren over other RAS blockers stratified by cardiovascular diagnosis (e.g. heart failure, post-myocardial infarction or hypertension) due to inability to extract relevant data. While subgroup data from ATMOSPHERE suggested benefit of aliskiren in patients with diabetes and heart failure (HR: 0.87, Upper confidence interval (UCI): 1.09), pooled data from the three studies that enrolled heart failure patients showed no effect (data not presented).
In conclusion, in patients with diabetes and cardiovascular disease or raised cardiovascular risk, the addition of aliskiren has no effect on all-cause mortality and combined cardiovascular mortality or heart failure hospitalisation compared with control treatment. There is an increased risk of hyperkalaemia and renal impairment with the use of aliskiren in addition to baseline ACEi and ARB therapy. This study supports current warnings against aliskiren use in patients with diabetes.
Supplementary Material
Acknowledgments
S.L.Z. designed, acquired, analysed and interpreted data, and drafted the manuscript. A.R. acquired and analysed data, and drafted the manuscript. S.A. analysed data and drafted the manuscript. All data generated or analysed during this study are included in this published article and its supplementary information files.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: S.A. was funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1. Brown MJ. Aliskiren. Circulation 2008; 118: 773–784. [DOI] [PubMed] [Google Scholar]
- 2. Teo KK, Pfeffer M, Mancia G, et al. Aliskiren alone or with other antihypertensives in the elderly with borderline and stage 1 hypertension: the APOLLO trial. Eur Heart J 2014; 35: 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oparil S, Yarows SA, Patel S, et al. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370: 221–229. [DOI] [PubMed] [Google Scholar]
- 4. Dusing R, Brunel P, Baek I, et al. Sustained decrease in blood pressure following missed doses of aliskiren or telmisartan: the ASSERTIVE double-blind, randomized study. J Hypertens 2012; 30: 1029–1040. [DOI] [PubMed] [Google Scholar]
- 5. Uresin Y, Taylor AA, Kilo C, et al. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. J Renin Angiotensin Aldosterone Syst 2007; 8: 190–198. [DOI] [PubMed] [Google Scholar]
- 6. Harel Z, Gilbert C, Wald R, et al. The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ 2012; 344: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gheorghiade M, Bohm M, Greene SJ, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA 2013; 309: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Novartis, http://www.novartisclinicaltrials.com
- 10. Centre CTNC. Review Manager (RevMan 5.3). London: The Cochrane Collaboration, 2014. [Google Scholar]
- 11. Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358: 2433–2446. [DOI] [PubMed] [Google Scholar]
- 12. Parving HH, Brenner BM, McMurray JJV, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204–2213. [DOI] [PubMed] [Google Scholar]
- 13. Shah AM, Shin SH, Takeuchi M, et al. Left ventricular systolic and diastolic function, remodelling, and clinical outcomes among patients with diabetes following myocardial infarction and the influence of direct renin inhibition with aliskiren. Eur J Heart Fail 2012; 14: 185–192. [DOI] [PubMed] [Google Scholar]
- 14. Maggioni AP, Greene SJ, Fonarow GC, et al. Effect of aliskiren on post-discharge outcomes among diabetic and non-diabetic patients hospitalized for heart failure: insights from the ASTRONAUT trial. Eur Heart J 2013; 34: 3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puri R, Nissen SE, Menon V, et al. Effects of aliskiren in diabetic and non-diabetic patients with coronary artery disease: insights from AQUARIUS. Atherosclerosis 2015; 243: 553–559. [DOI] [PubMed] [Google Scholar]
- 16. McMurray JJ, Krum H, Abraham WT, et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med 2016; 374: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 17. Kistner I, Zeymer U, Dechend R, et al. Benefits and risks of aliskiren treatment in patients with type 2 diabetes: analyses of the 3A Registry. J Clin Hypertens 2016; 18: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med 2014; 174: 773–785. [DOI] [PubMed] [Google Scholar]
- 19. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559. [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Meng L, Shao H, et al. Aliskiren vs. other antihypertensive drugs in the treatment of hypertension: a meta-analysis. Hypertens Res 2013; 36: 252–261. [DOI] [PubMed] [Google Scholar]
- 21. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ 2016; 352: i1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.