Abstract
Increased homocysteine (Hcy) level has been implicated as an independent risk factor for various neurological disorders, including Parkinson’s disease (PD). Hcy has been reported to cause dopaminergic neuronal loss in rodents and causes the behavioral abnormalities. This study is an attempt to investigate molecular mechanisms underlying Hcy-induced dopaminergic neurotoxicity after its chronic systemic administration. Male Swiss albino mice were injected with different doses of Hcy (100 and 250 mg/kg; intraperitoneal) for 60 days. Animals subjected to higher doses of Hcy, but not the lower dose, produces motor behavioral abnormalities with significant dopamine depletion in the striatum. Significant inhibition of mitochondrial complex-I activity in nigra with enhanced activity of antioxidant enzymes in the nigrostriatum have highlighted the involvement of Hcy-induced oxidative stress. While, chronic exposure to Hcy neither significantly alters the nigrostriatal glutathione level nor it causes any visible change in tyrosine hydroxylase-immunoreactivity of dopaminergic neurons. The finding set us to hypothesize that the mild oxidative stress due to prolonged Hcy exposure to mice is conducive to striatal dopamine depletion leading to behavioral abnormalities similar to that observed in PD.
Keywords: Parkinson’s disease, Dopamine, Neurodegeneration, Motor behavior, Mitochondrial complex-I, Oxidative stress
Highlights
-
•
Chronic intraperitoneal Hcy injection causes parkinsonian like motor abnormalities.
-
•
Hcy injection caused complex-I inhibition in nigra and striatal dopamine depletion.
-
•
Hcy injection caused enhanced activity of antioxidant enzymes in nigrostriatum.
-
•
Hcy-induced mild oxidative stress is not sufficient to alter GSH and TH.
1. Introduction
Homocysteine (Hcy) - a non-proteogenic sulphur containing amino acid, is known to increase in plasma of Parkinson’s disease (PD) patients under treatment with the gold standard drug, L-DOPA (L-3,4-dihydroxyphenylalanine) [1], [2], [3], [4]. The methylation of L-DOPA by catechol-O-methyl transferase is the leading cause of elevated level of plasma Hcy in PD patients [5], [6]. Several in vitro studies have highlighted the neurotoxic potency of Hcy to various neuronal types, including dopaminergic neurons [7], [8], [9], [10]. The toxic potency of Hcy to dopaminergic neurons is also reported from animal models of PD [8], [10], [11], [12]. Intraperitoneal injection of Hcy at a high dose (500 mg/kg) for a long time (36 days) caused a reduction of tyrosine hydroxylase (TH)-positive neurons, while dopamine and its metabolite remained unchanged [13]. Chandra et al. [11] have shown for the first time the direct effect of Hcy on dopaminergic neurons by intranigral administration of Hcy. Unilateral, intranigral injection of Hcy caused a significant decrease in striatal dopamine levels and loss of striatal dopaminergic neurons [11]. However, the underlying mechanism of Hcy-induced neurotoxicity to nigrostriatal dopaminergic neurons remains unexplained. However, Hcy-induces excitotoxicity, oxidative stress and mitochondrial alterations in cellular neuronal model leading to apoptosis and neuronal cell death [14], [15], [16]. Also, Hcy itself can serve as a pro-oxidant that contributes to oxidative stress in neuronal cells [17]. Hoffman [18] suggested that Hcy is a putative marker of cellular oxidant status.
Although the exact molecular mechanism of death of midbrain dopamine containing neurons in PD is not known clearly, however, oxidative stress has been postulated as the foremost event in PD pathogenesis [19]. Human postmortem studies indicated down regulation of antioxidant protective mechanisms in PD brain [20], [21]. The present study examined the involvement of oxidative stress mechanisms in Hcy-induced dopaminergic neurotoxicity in mice. The effect of prolonged (60 days) systemic administration of Hcy on motor behavior, striatal dopamine levels and nigrostriatal enzymatic (SOD, superoxide dismutase and catalase) as well as non-enzymatic (reduced glutathione) oxidative stress parameters were investigated in mice. We also investigated the status of nigrostriatal mitochondrial complex-I after chronic Hcy treatment in mice. The findings may have important implications for understanding the pathogenesis of PD.
2. Materials and methods
2.1. Animals
Eight-weeks-old male Swiss albino mice (23–26 g) were used in the present study. The animals were maintained under standard conditions of 12 h light/dark cycles, 24±2 °C temperatures and 60±5% humidity. They were provided with food and water ad libitum. The experimental protocols met the National Guidelines and were approved by the Animal Ethics Committee of the University.
2.2. Materials
d,l-homocysteine (Sigma-Aldrich Cat# H4628), reduced glutathione (GSH), hydrogen peroxide (H2O2), ortho-phthalaldehyde, phosphoric acid (H3PO4), Triton X-100, bovine serum albumin (BSA), and ethylenediaminetetraacetic acid disodium salt (EDTA) were purchased from Sigma-Aldrich Co (St. Louis, MO, USA). Rabbit anti-tyrosine hydroxylase (TH) primary antibody and anti-rabbit goat secondary antibody were purchased from Millipore Co. (USA). Pyrogallol and other reagents were procured from Sisco Research Laboratories Pvt. Ltd. Maharashtra, India.
2.3. Experimental design
Two groups of mice containing 6 animals were treated with Hcy (100 or 250 mg/kg daily, i.p.) dissolved in vehicle (0.9% Sodium Chloride) daily for 60 days and another group received the vehicle. The volume of Hcy and vehicle injected as per body weight of the animals. In the lower dose group, Hcy was given once a day at a dose 100 mg/kg (74 mM). In the highest dose group, Hcy was given twice a day at a dose 125 mg/kg (92 mM), so cumulative dose was 250 mg/kg per day. Motor behavioral tests (akinesia and catalepsy) were conducted on 14th, 28th, 42nd and 56th day and swim test on the 56th day of daily Hcy or vehicle administration. After the last dose of Hcy or vehicle administration, animals were sacrificed for analysis of striatal dopamine, oxidative stress parameters and mitochondrial complex activity from substantia nigra (SN) and striatum (NCP). Animals that received Hcy alone or vehicle for 60 days were perfused for TH-immunohistochemistry of SN and NCP. Each assay was repeated at least twice on separate days.
2.4. Behavioral test
2.4.1. Akinesia
Akinesia was measured by noting the latency of animals in seconds (s) to move all the fore and hind limbs on an elevated wooden platform (40 cm×40 cm×30 cm). The test terminated if the animal remains latent upto 180 s [22].
2.4.2. Catalepsy
Catalepsy can be defined as the inability of an animal to correct an externally imposed posture. Animals were placed on a flat horizontal surface with both hind limbs placed on a 3 cm high square wooden block and the time period (in seconds) that the animal took to move from the block to horizontal surface was noted down [22].
2.4.3. Swim test
Swimming ability test [23] was carried out in tubs with 40 cm length, 25 cm width and 16 cm height. The level of water was maintained upto 12 cm at 27–28 °C. Animals were placed in water and the swimming ability was scored every min for a period of 10 min. The scoring scale is as follows: 3-continuous swimming, 2-swimming with occasional floating, 1-more floating with occasional swimming with hind limbs and 0-hind part sinks with only the head floating. Total swim score is the sum of scores obtained in each minute for the entire test period of 10 min.
2.5. Dopamine analysis
Striatum was dissected out from Hcy or vehicle treated animals after 60 daily injections and fresh 50 μl (1 μg/μl) striatal lysates (in STEN buffer) were detected with 50 μl primary antibody (1 h) and 100 μl anti-rabbit secondary antibody (30 min) at room temperature according to the manufacturer’s protocols (Abnova, Taiwan) for dopamine [24].
2.6. Estimation of brain Hcy levels
For brain Hcy estimation, the animals were killed at 2 h after the last dose of Hcy or vehicle on 60th day of treatment. The right and left NCP were dissected out, whereas the right and left SN were micropunched from 1 mm frozen brain sections. The right and left hemisphere were processed together for Hcy estimation. The tissues were weighed and sonicated in PBS and stored overnight. After two freeze–thaw cycles, the homogenates were centrifuged at 5000 g for 5 min and the supernatant was analyzed for Hcy content. The total Hcy content was analyzed in brain homogenates using the Hcy ELISA kit (KA1242; Abnova, Taiwan) as per the manufacturer’s protocol.
2.7. Mitochondrial complex-I activity
Complex-I activity was assayed as described earlier [25]. Mice were sacrificed after the last dose of Hcy or vehicle and complex-I activity was analyzed from nigral and striatal area. The micropunctured SN and NCP from fresh frozen sections were sonicated in 0.1 M potassium phosphate buffer, pH 7.8, centrifuge at 600 g for 20 s and complex-I activity as assessed from the supernatant. The assay was carried out in the presence and absence of 5 μmol/L rotenone in order to derive the rotenone sensitive complex-I activity. The specific enzyme activity is expressed as nmol NADH oxidized/min/mg protein (e340=6.23×10−3 M).
2.8. Estimation of reduced glutathione levels
The reduced glutathione (GSH) content in SN and NCP of mice treated with Hcy or vehicle was estimated after last dose of treatment by employing ortho-phthalaldehyde condensation reaction following the method described earlier [26], [27]. The micropunched tissues were homogenized separately in 0.1 M potassium phosphate buffer, pH 7.8. After centrifugation the cytosolic fractions were deprotonated with ice-cold 0.1 M H3PO4 (10%, v/v). The supernatant treated with o-pthaldialdehyde (0.1%) and incubated for 20 min at room temperature, which yield a fluorescent product due to ortho-phthalaldehyde condensation reaction with GSH. Readings of the fluorescent product were taken at activation/emission wavelengths of 337/423 nm. A standard curve was prepared by using commercially obtained GSH and results were expressed as nanograms per milligram fresh tissue.
2.9. Determination of SOD activity
Mice treated with Hcy or vehicle were sacrificed at the end of 60th day of treatment period following last dose of Hcy. SOD activity was analyzed in the cytosolic fractions of NCP and SN by employing the method of Marklund and Marklund [28]. The assay mixture (3 ml) contained 0.2 mM of pyrogallol, 1 mM of EDTA and 50 mM of Tris–HCl buffer, pH 8.2. Auto oxidation of pyrogallol was measured spectrophotometrically at 420 nm for 3 min with or without the enzyme. The inhibition of pyrogallol oxidation was linear with the activity of the enzyme present. Fifty percent inhibition in pyrogallol auto oxidation/mg protein/min is taken as one unit of the enzyme activity.
2.10. Determination of catalase activity
Catalase activity was assayed based on the method of Aebi H [29]. Mice treated with Hcy or vehicle for 60 days were sacrificed after the last dose and catalase activity was analyzed in the cytosolic fractions of NCP and SN. An assay mixture of 500 µL contained suitably diluted enzyme protein (75–100 µg), in 50 mM of phosphate buffer, pH 7.0. The reaction was started by the addition of hydrogen peroxide (30 mM) and decomposition of hydrogen peroxide was measured at 240 nm for 30 s in presence of the enzyme in spectrophotometer. The specific activity is represented as change in absorbance/min/mg protein.
2.11. TH-immunohistochemistry
The Hcy or vehicle treated mice were anesthetized with chloral hydrate (350 mg/kg; i.p.) and perfused intracardially with phosphate buffer saline (PBS, pH 7.4) followed by 4% para-formaldehyde. Brains were removed and kept in 4% para-formaldehyde, transferred to 30% sucrose and coronal sections (20 µm thickness) passing through SN or NCP was taken by using Cryotome (0620E Cryostat, Thermo Shandon, United Kingdom). The sections were rinsed three times with 0.1 M PBS (pH 7.4), incubated in 1% H2O2 in PBS, permeabilized with 0.4% Triton X-100, blocked with 8% BSA containing 0.1% Triton X-100. The sections were incubated with the primary antibody (1:100) in PBS, containing 4% BSA for overnight at 4 °C and then incubated with secondary anti-rabbit IgG-conjugated horseradish peroxidase antibody (1:500) in PBS containing 2% BSA for 1 h at room temperature. Visualization was performed by incubation in 3, 3-diaminobenzidine for 5 min and the sections were photographed by using a Trinocular microscope (Eclipse, Ci, Nikon, Japan).
2.12. Statistics
Statistical analysis was performed using the SigmaStat version 3.5 software. The data were analyzed employing Student’s t-test. Results are given as mean±S.E.M. Values of p≤0.05 were considered significant.
3. Results
3.1. Effect of chronic Hcy administration on motor behaviors
3.1.1. Akinesia
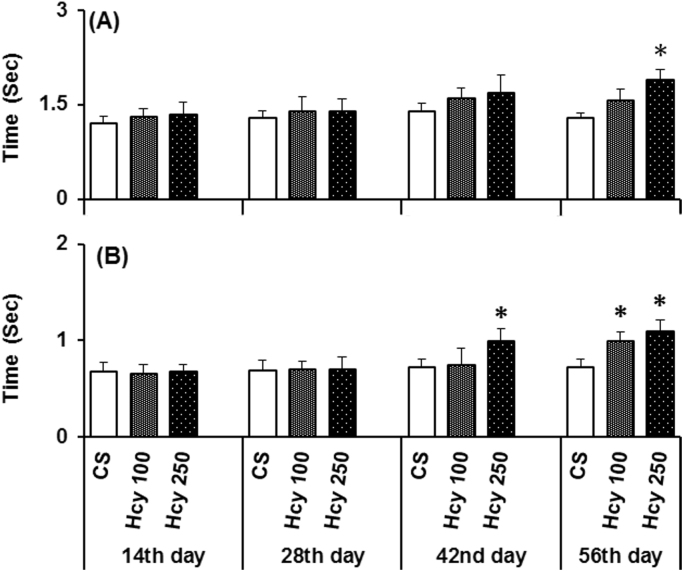
The animals that received the higher doses of Hcy (250 mg/kg) were significantly akinetic as compared to control (Fig. 1A) when examined on 56th day, but not at other time points used (Fig. 1A). The lower dose of Hcy (100 mg/kg) did not show any behavioral deficits at any time points in any animals examined (Fig. 1A).
Fig. 1.
Effect of chronic Hcy administration on (A) Akinesia and (B) Catalepsy. Akinesia and catalepsy were measured after daily administration of Hcy (100 mg/kg or 250 mg/kg) or vehicle on 14th, 28th, 42nd and 56th day. The effect of Hcy administration on the latency to move all four limbs (akinesia) as compared to the control group were examined (measured in sec). The effects of Hcy administration to correct an externally imposed posture (catalepsy) as compared to the control group were examined (measured in sec). The data represented are mean±SEM. *P≤0.05 as compared to control (n=6).
3.1.2. Catalepsy
Chronic administration of Hcy (250 mg/kg; i.p., daily) caused significant cataleptic deficits in animals when examined on 42nd and 56th day of Hcy administration (Fig. 1B). The lower dose of Hcy produces the deficits on 56th day, when compared to control (Fig. 1B). Hcy administration did not show any effect on catalepsy on other time points examined.
3.1.3. Swim test
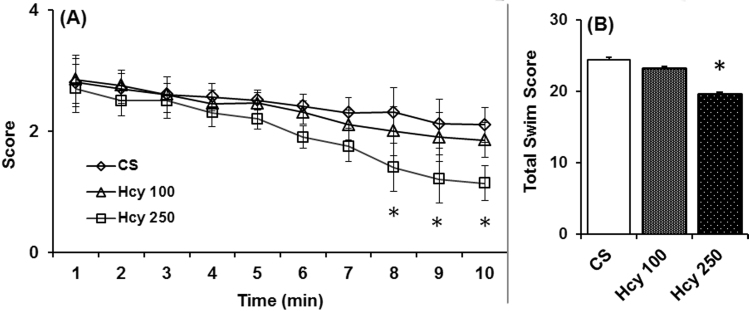
In swim ability test, where general motor activity is revealed, animals treated with higher dose of Hcy (250 mg/kg) displayed significantly poorer swimming ability on 56th day as compared to the control (Fig. 2A). However, the lower dose of Hcy (100 mg/kg) did not show any effect on the swimming ability when examined on 56th day (Fig. 3A). The swim scores at the 1st min was 2.8 and 2.7 for control and Hcy (250 mg/kg) group, whereas from 2nd min to 10th min there were progressive decrease in swimming score of Hcy (250 mg/kg) group compared to control group and at the 10th min score was 2.1 and 1.14 for control and Hcy (250 mg/kg) group respectively. Also, total swims score observed in the test period of 10 min of animals that received highest dose of Hcy significantly lesser which is 19.6 as compared to the vehicle treated animals (24.41) (Fig. 2B).
Fig. 2.
Effect of chronic Hcy administration on swimming ability. Swim test was performed after daily administration of Hcy (100 mg/kg or 250 mg/kg) or vehicle on 56th day. Animals were placed in water and the swimming ability was scored every min for a period of 10 min (2A). Total swim score (2B) significantly reduced in animals those received highest dose of Hcy. The data represented are mean±SEM. *P≤0.05 as compared to control (n=6).
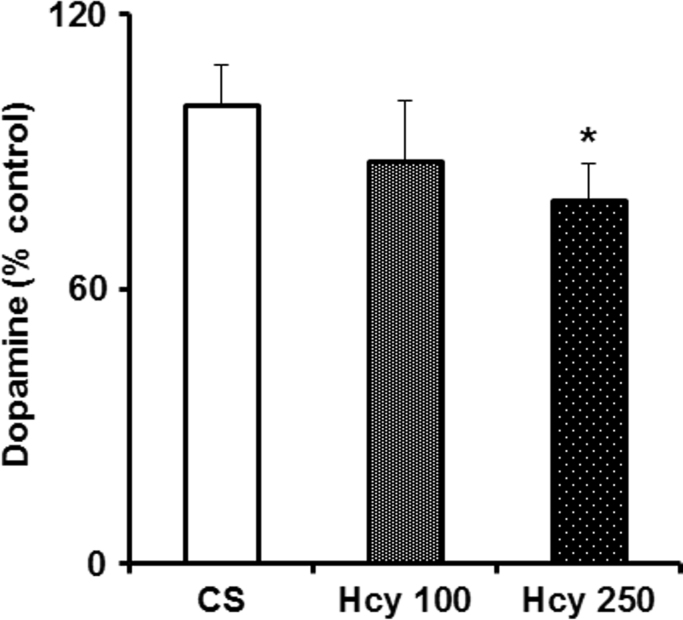
Fig. 3.
Effect of chronic Hcy administration on striatal dopamine (DA) level. Mice were administered Hcy (100 mg/kg or 250 mg/kg i.p) or vehicle for 60 days and were sacrificed. Striatal DA level was analyzed by using ELISA kit. Results are expressed as mean±SEM. *P≤0.05 as compared to control (n=6).
3.2. Effect of chronic Hcy administration on striatal dopamine levels
A significant decrease in striatal dopamine level was observed in animals treated with higher dose of Hcy (250 mg/kg) but not with the lower dose (100 mg/kg) after the 60th day of treatment (Fig. 3). The striatal dopamine level was decreased by 11% and 21% respectively in animals treated with lower and higher doses of Hcy.
3.3. Effect of chronic Hcy administration on nigrostriatal Hcy levels
A significant increase in the nigrostriatal Hcy level was observed in animals treated with higher dose of Hcy (250 mg/kg; i.p., daily) but not with the lower dose (100 mg/kg; i.p., daily) as compared to vehicle treated group. The value of Hcy in NCP and SN in vehicle treated group was 0.46±0.06 and 0.49±0.05 pmol/mg tissue, while in Hcy (250 mg/kg) treated group was 0.7±0.07 and 0.75±0.06 pmol/mg tissue respectively.
3.4. Effect of chronic Hcy administration on complex-I activity
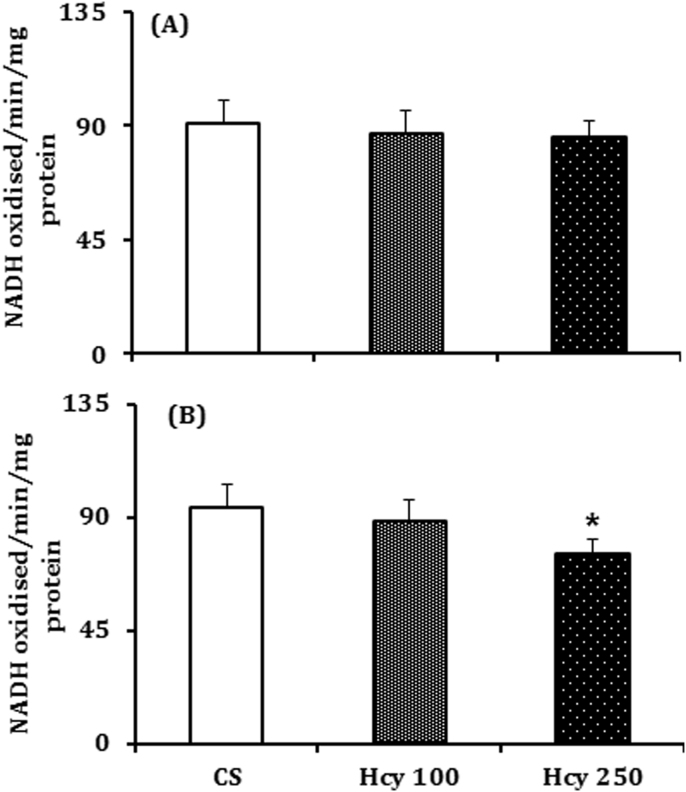
Mitochondrial complex-I activity was significantly inhibited by 20% in the SN of animals treated with higher dose of Hcy (250 mg/kg) but not with the lower dose (6%), as compared to control (Fig. 4B). However, in NCP the mitochondrial complex-I activity was inhibited by 4% and 5.5% by lower and higher doses of Hcy respectively as compared to control, which is not statistically significant (Fig. 4A).
Fig. 4.
Effect of chronic Hcy administration on complex-I activity. Mice were treated with Hcy (100 mg/kg or 250 mg/kg) or vehicle daily were sacrificed on 60th day after the last dose and complex-I activity was measured in the (A) nucleus caudatus putamen (NCP) and (B) substantia nigra (SN) region by employing a spectrophotometric procedure using NADH as substrate. Results given are mean±SEM of nmol of NADH oxidized/min/mg protein. *P≤0.05 as compared to control (n=6).
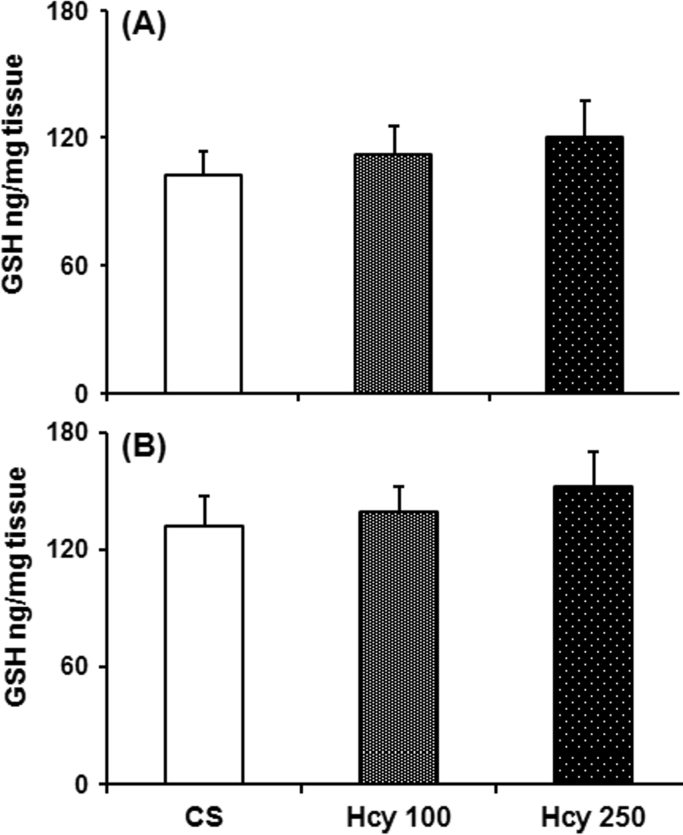
3.5. Effect of chronic Hcy administration on glutathione level
Daily treatment of Hcy (100 mg/kg and 250 mg/kg) caused no significant differences in the SN or striatal GSH contents, when compared with vehicle treated animals (Fig. 5).
Fig. 5.
Effect of chronic Hcy administration on nigrostriatal GSH. Mice were treated with Hcy (100 mg/kg or 250 mg/kg) or vehicle daily for 60 days. Animals were sacrificed after the last dose of Hcy or vehicle administration and GSH was measured in the (A) nucleus caudatus putamen (NCP) and (B) substantia nigra (SN) region by employing a sensitive spectrofluorimetric procedure. Results given are mean±SEM in ng/mg tissue. *P≤0.05 as compared to control (n=6).
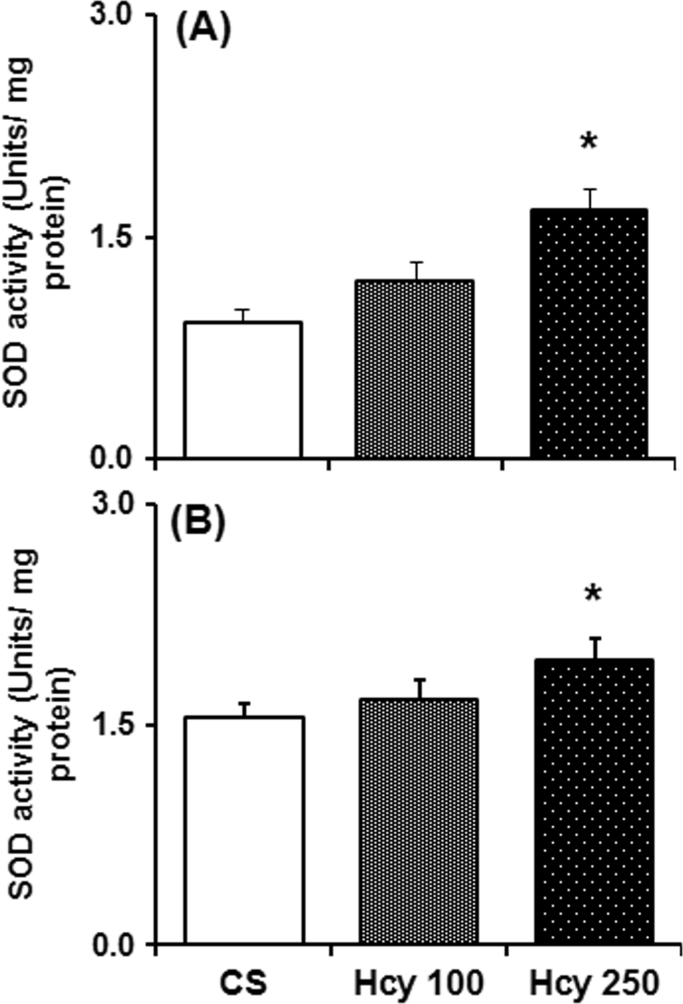
3.6. Effect of chronic Hcy administration on SOD activity
The brain antioxidant enzyme, superoxide dismutase (SOD) activity in the SN and NCP of mice treated with Hcy for 60 days was significantly increased as compared to vehicle treated mice (Fig. 6). In NCP and SN, higher dose of Hcy (250 mg/kg) significantly increases SOD activity by 81% and 25% respectively as compared to control. While the lower dose of Hcy (100 mg/kg) increases SOD activity in NCP by 30% and in SN by 7%, which are not significant statistically as compared to control.
Fig. 6.
Effect of chronic Hcy administration on superoxide dismutase (SOD) activity. Mice were sacrificed after 60 daily administration of Hcy (100 mg/kg or 250 mg/kg) or vehicle. SOD activity measured from the cytosolic fraction of (A) nucleus caudatus putamen (NCP) and (B) substantia nigra (SN) region by employing pyrogallol oxidation method. Results given are mean±SEM. *P≤0.05 as compared to control (n=6).
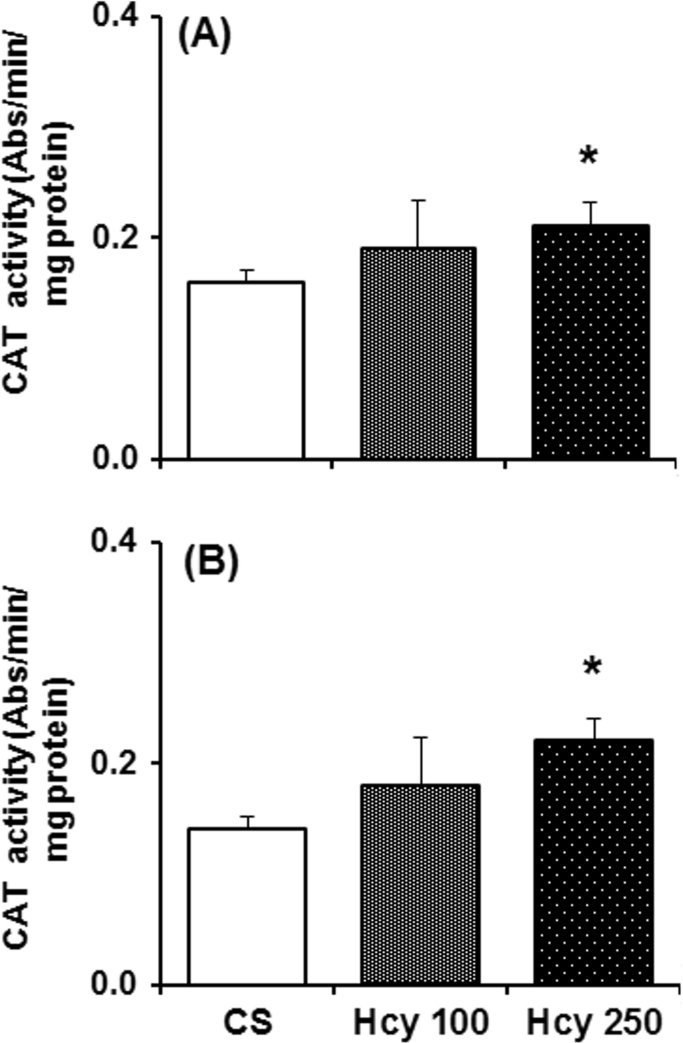
3.7. Effect of chronic Hcy administration on catalase activity
Catalase activity in the SN and NCP of mice treated with Hcy for 60 days was increased significantly as compared to vehicle treated mice (Fig. 7). In NCP and SN, higher dose of Hcy (250 mg/kg) significantly increases catalase activity by 31% and 57% respectively, as compared to control. The lower dose of Hcy (100 mg/kg) increased the catalase activity in SN by 29% and by 18% in NCP, which are not significant statistically as compared to control.
Fig. 7.
Effect of chronic Hcy administration on catalase (CAT) activity. Mice were sacrificed after 60 daily administration of Hcy (100 mg/kg or 250 mg/kg) or vehicle. CAT activity measured from the cytosolic fraction of (A) nucleus caudatus putamen (NCP) and (B) substantia nigra (SN) region. Results given are mean±SEM in pmol/mg tissue. *P≤0.05 as compared to control (n=6).
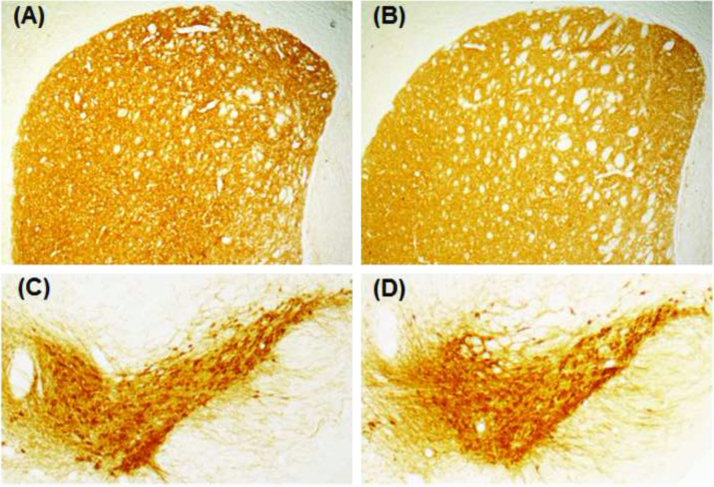
3.8. Effect of chronic exposure of Hcy on TH-immunoreactivity in SN and NCP of mice
Positive staining for TH was obtained as dark brown colour in the NCP and SN of mice that received vehicle for 60 days (Fig. 8A, C). No visible changes in the TH-immunoreactivity in NCP (Fig. 8B) as well as in SN (Fig. 8D) were observed on 60th day following daily Hcy (250 mg/kg) treatment, when compared to control.
Fig. 8.
Tyrosine hydroxylase-immunoreactivity (TH-IR) of dopaminergic neurons. Mice injected with Hcy (250 mg/kg; i.p.) or vehicle daily for 60 days were sacrificed after the last dose and perfused transcardially. Cryocut coronal sections (20 µm) passing through the striatum (A, B) and substantia nigra (C, D) were processed for TH-IR. (A) and (C) are sections from vehicle treated animals; (B) and (D) are sections from Hcy treated (250 mg/kg, i.p.) animals. No visible changes in TH-IR were observable in the neurons from treated sections.
4. Discussion
The present study demonstrates the involvement of oxidative stress mechanism in Hcy-induced dopaminergic neurotoxicity. The major findings come out of the present study that prolonged intraperitoneal administration of Hcy in mice leads to (i) motor behavioral deficits similar to PD (ii) depletion of dopamine in the striatum (iii) decrease in nigral mitochondrial complex-I activity and oxidative stress by modulating the levels of antioxidant enzymes in nigrostriatal regions.
One of most important finding of the present study is the loss of significant motor activity in Hcy exposed animals which is similar to that found in experimental models of PD. The display of increased latency in akinesia and catalepsy (Fig. 1) and decrease in swimming ability (Fig. 2) are the indications of reduced motor activity. Hcy infusion directly into the SN reported to exacerbate motor dysfunction in animal models of PD as evidenced from Rotarod test [8] and drug-induced rotational bias [12]. Intranigral infusion of Hcy caused dopaminergic drug-induced stereotypic rotational bias in rats [11]. When injected intraperitoneal route for 60 days, Hcy (50 mg/kg and 100 mg/kg) caused a significant decrease in locomotor activities in mice [13]. However, our result that chronic systemic exposure to Hcy decreased motor activity significantly by increasing latency in akinesia, catalepsy and decreasing swimming score is the first report of such. It has been demonstrated that motor deficits are correlated to depleted levels of striatal dopamine in experimental models of PD [30], [31]. Animals were sacrificed for striatal dopamine analysis and we observed a 21% decrease in striatal dopamine in animals that received higher dose of Hcy (250 mg/kg) (Fig. 3). Therefore, our data clearly demonstrate that higher concentration of Hcy administration at a long-time is detrimental to the motor abilities of animals, which could be due to significant striatal dopamine depletion (Fig. 3).
In the present study, prolonged Hcy exposure to mice inhibits nigral complex-I activity, which is an important and interesting finding (Fig. 4). Defects in mitochondrial complexes activity, particularly complex-I have been found in PD brain [32], [33] as well as in toxins-induced animal models of PD [34], [35]. Long-term Hcy treatment induced inhibition of complex-I activity could be resulting from the enhanced production of free radicals in the nigra [22], which in turn contributes to oxidative stress [36]. Yet another important observation of the present study is the significant increase in activity of antioxidant enzymes, SOD and catalase in nigrostriatum of mice by chronic intraperitoneal administration of Hcy (Fig. 6, Fig. 7). The parkinsonian neurotoxins-induced oxidative stress in nigral neurons also reported to elevate the activity of SOD and catalase [25], [37], [38]. Dopaminergic neurons are particularly vulnerable to oxidative stress due to the presence of low level of antioxidant defense system, including antioxidant enzymes [39].
GSH, a major cellular antioxidant defense system of brain, has been reported to be decreased in nigra of PD brain [40]. The diminished level of GSH is regarded as the pioneer of all the deleterious events involved in dopaminergic neurodegeneration [41], [42]. However, in the present study, we did not find a significant difference in the level of GSH in the nigrostriatum as a result of chronic Hcy exposure to mice (Fig. 5). Also, we could not find a marked visible change in TH-immunoreactivity in SN as well as NCP after prolonged intraperitoneal administration of Hcy (Fig. 8). Whereas, Chandra et al. [11] have shown previously Hcy-induced specific nigral neuronal loss and striatal dopamine depletion (50%) only after intranigral infusion of Hcy. In animal models of PD, intranigral infusion of Hcy exaggerates TH-positive neuronal loss, however, Hcy alone did not produce any effect [8], [12]. Thus, our result that the chronic systemic administration of Hcy caused mild oxidative stress in nigrostriatum, which is not sufficient to cause a significant dopaminergic neuronal loss.
5. Conclusion
The result indicates that Hcy has the ability to generate oxidative stress in nigrostriatum, which may cause conditions conducive to striatal dopamine depletion leading to behavioral abnormalities. Also, L-DOPA treatment is known to increase Hcy levels in plasma as well as in cerebro spinal fluid of PD patients [2], [4]. Thus, it can be concluded that elevated level of this molecule in parkinsonian patients may contribute to the progression of the disease.
Acknowledgement
We sincerely acknowledge the funding (under Rapid Grant for Young Investigator; Sanction Order no. BT/PR6806/GBD/27/480/2012, dated August 5, 2013) and support provided by Department of Biotechnology (DBT), Govt. of India. NB is the recipient of Assam University, Silchar, India Non-NET fellowship under University Grant Commission, Govt. of India. We also acknowledge Assam University-Biotech Hub funded by DBT, Govt. of India for providing necessary instrumentation facilities.
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.02.013.
Appendix A. Transparency document
Transparency Document
.
References
- 1.Selhub J. Homocysteine metabolism. Annu Rev. Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Zoccolella S., Lamberti S.V., Iliceto G., Santamato A., Lamberti P., Logroscino G. Hyperhomocysteinemia in L-dopa treated patients with Parkinson’s disease: potential implications in cognitive dysfunction and dementia? Curr. Med. Chem. 2010;17(28):3253–3261. doi: 10.2174/092986710792232012. [DOI] [PubMed] [Google Scholar]
- 3.Isobe C., Abe T., Terayama Y. L-dopa therapy increases homocysteine concentration in cerebrospinal fluid from patients with Parkinson’s disease. J. Clin. Neurosci. 2010;17(6):717–721. doi: 10.1016/j.jocn.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Hu X.W., Qin S.M., Li D., Hu L.F., Liu C.F. Elevated homocysteine levels in levodopa-treated idiopathic Parkinson’s disease: a meta-analysis. Acta Neurol. Scand. 2013;128(2):73–82. doi: 10.1111/ane.12106. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H., Gomes-Trolin C., Aquilonius S.M., Steinberg A., Lofberg C., Ekblom J. Levels of l-methionine S-adenosyltransferase activity in erythrocytes and concentrations of S-adenosylmethionine and S-adenosylhomocysteine in whole blood of patients with Parkinson’s disease. Exp. Neurol. 1997;145:580–585. doi: 10.1006/exnr.1997.6466. [DOI] [PubMed] [Google Scholar]
- 6.Zhao W.Q., Latinwo L., Liu X.X., Lee E.S., Lamango N., Charlton C.G. L-dopa upregulates the expression and activities of methionine adenosyl transferase and catechol-O-methyltransferase. Exp. Neurol. 2001;171:127–138. doi: 10.1006/exnr.2001.7726. [DOI] [PubMed] [Google Scholar]
- 7.Kruman I.I., Kumaravel T.S., Lohani A., Pedersen W.A., Cutler R.G. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J. Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan W., Ladenheim B., Cutler R.G., Kruman I.I., Cadet J.L., Mattson M.P. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 9.Heider I., Lehmensiek V., Lenk T., Müller T., Storch A. Dopaminergic neurotoxicity of homocysteine and its derivatives in primary mesencephalic cultures. J. Neural Transm. Suppl. 2004;68:1–13. doi: 10.1007/978-3-7091-0579-5_1. [DOI] [PubMed] [Google Scholar]
- 10.Imamura K., Takeshima T., Nakaso K., Nakashima K. Homocysteine Is toxic for dopaminergic neurons in primary mesencephalic culture. Neuroreport. 2007 27;18(13):1319–1322. doi: 10.1097/WNR.0b013e3282aaa0b4. [DOI] [PubMed] [Google Scholar]
- 11.Chandra G., Gangopadhyay P.K., Senthil Kumar K.S., Mohanakumar K.P. Acute intranigral homocysteine administration produces stereotypic behavioral changes and striatal dopamine depletion in Sprague-Dawley rats. Brain Res. 2006;1075:81–92. doi: 10.1016/j.brainres.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 12.Xing H., Peng H., Xuebing C., Sun S. Effect and mechanism of homocysteine on Parkinson’s disease induced by 6-OHDA. J. Nanjing Med. Univ. 2008;22(1):12–17. [Google Scholar]
- 13.Lee E.S., Chen H., Soliman K.F., Charlton C.G. Effects of homocysteine on the dopaminergic system and behavior in rodents. Neurotoxicology. 2005;26(3):361–371. doi: 10.1016/j.neuro.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Kruman I.I., Culmsee C., Chan S.L., Kruman Y., Guo Z., Penix L., Mattson M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zieminska E., Lazarewicz J.W. Excitotoxic neuronal injury in chronic homocysteine neurotoxicity studied in vitro: the role of NMDA and group I metabotropic glutamate receptors. Acta Neurobiol. Exp. 2006;66:301–309. doi: 10.55782/ane-2006-1619. [DOI] [PubMed] [Google Scholar]
- 16.Curro M., Condello S., Caccamo D., Ferlazzo N., Parisi G., Ientile R. Homocysteine-induced toxicity increases TG2 expression in Neuro2a cells. Amino Acids. 2008;36:725–730. doi: 10.1007/s00726-008-0122-x. [DOI] [PubMed] [Google Scholar]
- 17.Paul R., Borah A. The potential physiological crosstalk and interrelationship between two sovereign endogenous amines, melatonin and homocysteine. Life Sci. 2015;139:97–107. doi: 10.1016/j.lfs.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med. Hypotheses. 2011;77:1088–1093. doi: 10.1016/j.mehy.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Dawson T.M., Dawson V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 20.Ambani L.M., Van Woert M.H., Murphy S. Brain peroxidase and catalase in Parkinson disease. Arch. Neurol. 1975;32(2):114–118. doi: 10.1001/archneur.1975.00490440064010. [DOI] [PubMed] [Google Scholar]
- 21.Jenner P., Olanow C.W. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47(6 Suppl. 3):S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 22.Sengupta T., Mohanakumar K.P. 2-Phenylethylamine, a constituent of chocolate and wine, causes mitochondrial complex-I inhibition, generation of hydroxyl radicals and depletion of striatal biogenic amines leading to psycho-motor dysfunctions in Balb/c mice. Neurochem Int. 2010;57(6):637–646. doi: 10.1016/j.neuint.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Haobam R., Sindhu K.M., Chandra G., Mohanakumar K.P. Swim-test as a function of motor impairment in MPTP model of Parkinson’s disease: a comparative study in two mouse strains. Behav. Brain Res. 2005;163(2):159–167. doi: 10.1016/j.bbr.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Hebron M.L., Lonskaya L., Moussa C. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of a-synuclein in Parkinson’s disease models. Hum. Mol. Genet. 2013;22(16):3315–3328. doi: 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saravanan K.S., Sindhu K.M., Mohanakumar K.P. Acute intranigral infusion of rotenone in rats causes progressive bio- chemical lesions in the striatum similar to Parkinson’s disease. Brain Res. 2005;1049:147–155. doi: 10.1016/j.brainres.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 26.Cohn V.H., Lyle J. A fluorimetric assay for glutathione. Anal. Biochem. 1966;14:434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- 27.Thomas B., Mohanakumar K.P. Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J. Pineal Res. 2003;36:25–32. doi: 10.1046/j.1600-079x.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 28.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 29.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 30.Mitra N., Mohanakumar K.P., Ganguly D.K. Dissociation of serotoninergic and dopaminergic components in acute effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Brain Res. Bull. 1992;28(3):355–364. doi: 10.1016/0361-9230(92)90035-v. [DOI] [PubMed] [Google Scholar]
- 31.Muralikrishnan D., Mohanakumar K.P. Neuroprotection by bromocriptine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. FASEB J. 1998;12(10):905–912. doi: 10.1096/fasebj.12.10.905. [DOI] [PubMed] [Google Scholar]
- 32.Parker W.D., Jr., Parks J.K., Swerdlow R.H. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schapira A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 34.Betarbet R., Sherer T.B., MacKenzie G., Garcia-Osuna M., Panov A.V., Greenamyre J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 35.Blesa J., Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acuña Castroviejo D., López L.C., Escames G., López A., García J.A., Reiter R.J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 2011;11:221–240. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- 37.Thomas B., Mohanakumar K.P. Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J. Pineal Res. 2004;36:25–32. doi: 10.1046/j.1600-079x.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 38.Madathil S.K., Karuppagounder S.S., Mohanakumar K.P. Sodium salicylate protects against rotenone-induced Parkinsonism in rats. Synapse. 2013;67:502–514. doi: 10.1002/syn.21658. [DOI] [PubMed] [Google Scholar]
- 39.Maguire-Zeiss K.A., Short D.W., Federoff H.J. Synuclein, dopamine and oxidative stress: co-conspirators in Parkinson’s disease? Brain Res. Mol. Brain Res. 2005;;134(1):18–23. doi: 10.1016/j.molbrainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Smeyne M., Smeyne R.J. Glutathione metabolism and Parkinson’s disease. Free Radic. Biol. Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin H.L., Teismann P. Glutathione – a review on its role and significance in Parkinson’s disease. FASEB J. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Garcia A., Zavala-Flores L., Rodriguez-Rocha H., Franco R. Thiol-redox signaling, dopaminergic cell death, and Parkinson’s disease. Antioxid. Redox Signal. 2012;15:1764–1784. doi: 10.1089/ars.2011.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency Document