Abstract
Objective
Hospitalizations for acute bacterial skin and skin structure infection (ABSSSI) are common. Optimizing antibiotic use for ABSSSIs requires an understanding of current management. The objective of this study was to evaluate antibiotic prescribing practices and factors affecting prescribing in a diverse group of hospitals.
Design
Multicenter, retrospective cohort study
Setting
Seven community and academic hospitals
Methods
Children and adults hospitalized between June 2010 and May 2012 for cellulitis, wound infection, or cutaneous abscess were eligible. The primary endpoint was a composite of two prescribing practices representing potentially avoidable antibiotic exposure: 1) use of antibiotics with a broad spectrum of activity against gram-negative bacteria; or 2) treatment duration >10 days.
Results
533 cases were included: 320 with non-purulent cellulitis, 44 with wound infection or purulent cellulitis, and 169 with abscess. Of 492 cases with complete prescribing data, the primary endpoint occurred in 394 (80%) cases and varied significantly across hospitals (64 – 97%, p<.001). By logistic regression, independent predictors of the primary endpoint included wound infection or purulent cellulitis (odds ratio [OR] 5.12, 95% confidence interval [CI] 1.46 – 17.88), head or neck involvement (OR 2.83, 95%CI 1.17 – 6.82), adult cases (OR 2.20, 95%CI 1.18 – 4.11), and admission to a community hospital (OR 1.90, 95%CI 1.05 – 3.44).
Conclusions
Among patients hospitalized for ABSSSI, use of antibiotics with broad gram-negative activity or treatment courses longer than 10 days were common. There may be substantial opportunity to reduce antibiotic exposure through shorter courses of therapy targeting gram-positive bacteria.
Keywords: Acute bacterial skin and skin structure infection, skin and soft tissue infection, cellulitis, cutaneous abscess, antimicrobial stewardship
Introduction
The Centers for Disease Control and Prevention recently reported on the alarming morbidity and mortality associated with antibiotic-resistant microorganisms and Clostridium difficile infection in the United States [1]. Improving antibiotic use for common infections is a key strategy to slow the emergence and spread of antibiotic resistance and prevent antibiotic-related adverse events [2]. In the United States, acute bacterial skin and skin structure infections (ABSSSIs) such as cellulitis and cutaneous abscess result in nearly 900,000 hospitalizations per year, second only to pneumonia among infections leading to hospitalization [3]. Since the emergence of community-associated methicillin-resistant Staphylococcus aureus, hospitalizations for ABSSSI have markedly increased in both children and adults [3–6].
For patients hospitalized with ABSSSI, current Infectious Diseases Society of America (IDSA) guidelines recommend antibiotics targeted toward Staphylococcus aureus and streptococcal species, including vancomycin, linezolid, daptomycin, televancin, or clindamycin; anti-staphylococcal β-lactams such as cefazolin or nafcillin may be considered for non-purulent cellulitis [7]. Although the optimal treatment duration is not known, the IDSA guideline suggests 7 to 14 days of therapy, with individualization based on clinical response [7]. In clinical practice, however, single-center studies have demonstrated frequent use of antibiotics with a broad spectrum of gram-negative activity and longer treatment durations [8–11]. We aimed to provide more generalizable data on current ABSSSI management by evaluating antibiotic prescribing and factors affecting prescribing in a diverse group of hospitals.
Methods
Study Design
This was a multicenter, retrospective cohort study of patients hospitalized for ABSSSI between June 1, 2010 and May 31, 2012. Children and adults with a principal discharge diagnosis of cellulitis, wound infection, or cutaneous abscess were eligible for inclusion. Cases associated with the following complicating factors were excluded: deep tissue involvement (fascia, muscle, bone, joint, or organ space), odontogenic infection, infected ulcer, surgical site infection, periorbital or perineal infection, human or animal bite, hidradenitis suppurativa, or hospital onset of infection. We also excluded cases involving a co-existing infection requiring antibacterial therapy, transfer to or from an outside hospital, refusal of medical care, insufficient documentation to classify the case, age less than 30 days or greater than 89 years, and certain vulnerable populations (prisoners, pregnant women, decisionally-challenged) (Figure 1).
Figure 1.
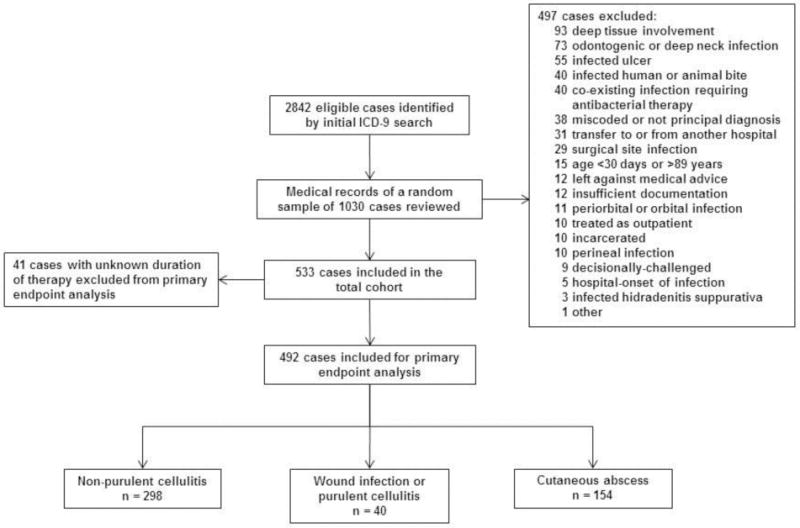
Study flow diagram
Study Setting and Population
Seven Colorado institutions were selected to provide a representative sample of regional acute care hospitals, including a public safety-net hospital, tertiary care referral center, free-standing children’s hospital, Veteran’s Affairs hospital, and three private community hospitals (for additional hospital details, see Appendix Table 1).
Cohort Identification and Data Collection
Within each hospital, potential cases were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes (680.*, 681.*, 682.*, 686, 035). Only the first hospitalization during the study period was eligible for inclusion. A site investigator with clinical experience managing ABSSSIs performed medical record abstraction. Several techniques were used to standardize data collection across sites [12]. First, prior to starting data collection, site investigators reviewed a set of mock cases in order to classify the type of ABSSSI and clinical outcomes. Second, site investigators reviewed ten actual cases from their own institution that were not included in the final dataset in order to locate data elements in the medical record and develop a structured sequence for review. Finally, questions regarding individual cases were adjudicated by the principal investigator and site investigators. At each hospital, cases were reviewed in a random sequence until the target number of cases meeting study entry criteria was reached. Data entry was performed and verified in duplicate at the central site. The study was approved by each hospital’s institutional review board.
Definitions and Study Outcomes
Cellulitis, wound infection, and major cutaneous abscess were defined according to Food and Drug Administration guidance [13], with the exception that a minimum surface area of erythema was not required since measurements of erythema are not consistently documented. Because of expected differences in treatment among specific types of ABSSSI, cases were categorized and analyzed within three groups: (1) non-purulent cellulitis; (2) wound infection or purulent cellulitis (i.e., non-drainable purulent infections); or (3) cutaneous abscess. The pre-specified primary endpoint was a composite of two prescribing practices representing potentially avoidable antibiotic exposure: (1) prescription of antibiotics with a broad spectrum of activity against aerobic gram-negative pathogens (β-lactam/β-lactamase inhibitor combinations, 2nd – 5th generation cephalosporins, fluoroquinolones, carbapenems, tigecycline, aminoglycosides, or colistin); or (2) treatment duration greater than 10 calendar days. We performed a sensitivity analysis of the primary endpoint by excluding cases with clinical factors that could reflect complicated, severe, or poorly responsive infection (see Table 4 footnote for definition). To assess drivers of prescribing practices, we performed multivariable logistic regression to identify factors associated with the primary endpoint and its individual components.
Table 4.
Primary and secondary analyses for the 492 patients with complete prescribing data
| Non-purulent cellulitis n=298 |
Wound infection or purulent cellulitis n=40 |
Cutaneous abscess n=154 |
Total n =492 |
|
|---|---|---|---|---|
| Primary endpoint | ||||
| Prescribed antibiotics with broad gram-negative activity or treatment duration >10 days | 240 (81) | 37 (93) | 117 (76) | 394 (80) |
| Prescribed antibiotics with broad gram-negative activity | 140 (47) | 18 (45) | 56 (36) | 214 (44) |
| Treatment duration >10 days | 206 (69) | 31 (78) | 107 (69) | 344 (70) |
| Sensitivity analysis excluding cases with factors that may reflect complicated, severe, or poorly responsive infection a | n=37 | n=3 | n=13 | n=53 |
| Broad gram-negative therapy or treatment duration >10 days | 26 (70) | 2 (67) | 11 (85) | 39 (74) |
| Prescribed antibiotics with broad gram-negative activity | 11 (30) | 0 | 2 (15) | 13 (25) |
| Treatment duration >10 days | 23 (62) | 2 (67) | 11 (85) | 36 (68) |
| Secondary endpoint based on IDSA guideline treatment recommendations | ||||
| Prescribed antibiotics with broad gram-negative activity or treatment duration >14 days | 172 (58) | 23 (58) | 78 (51) | 273 (55) |
| Prescribed antibiotics with broad gram-negative activity | 140 (47) | 18 (45) | 56 (36) | 214 (43) |
| Treatment duration >14 days | 78 (26) | 9 (23) | 49 (32) | 136 (28) |
Data presented as n (%) unless otherwise noted. IDSA, Infectious Diseases Society of America.
Note: Of the 41 cases not included in this analysis because the duration of therapy was unknown, 21 (51%) were prescribed antibiotics with broad gram-negative activity.
Analysis excluded cases involving diabetes mellitus, injection drug use, HIV infection, other immunosuppressing conditions, lower extremity vascular impairment, intensive care unit admission, temperature >39°C, serum white blood cell count >15,000 cells/mm3, positive blood cultures, laboratory risk indicator for necrotizing fasciitis (LRINEC) score ≥6, need for fascial biopsy, undrained abscess, multiple sites of infection, recurrence of a skin infection within the prior 30 days, and failure of initial inpatient therapy (treatment failure).
Of note, current IDSA treatment guidelines for ABSSSIs were published after the conception of this study [7]. Our primary endpoint included a threshold of greater than 10 days of therapy as a marker for potentially avoidable antibiotic exposure; whereas, the IDSA guideline suggests up to 14 days of therapy with individualization based on clinical response. Rather than alter our pre-specified primary endpoint, we added a secondary analysis to evaluate prescribing practices in the context of IDSA treatment recommendations. Prescribing discordant with IDSA guidance was therefore defined as use of antibiotics with a broad spectrum of gram-negative activity or treatment duration greater than 14 days [7].
All subsequent hospitalizations and ambulatory care visits within 45 days of the date of admission were reviewed. Clinical failure included any of the following within this follow-up period: (1) treatment failure, defined as a change in antibiotic therapy or unplanned drainage procedure due to inadequate clinical response more than 5 days after hospital admission; (2) recurrence, defined as re-initiation of antibiotics for skin infection after completion of the initial treatment course; or (3) re-hospitalization due to skin infection [8]. Changes in therapy due to allergies, medication intolerance, adverse drug effects, or conversion from parenteral to oral therapy were not classified as treatment failures.
Statistical Analysis
The primary endpoint and its individual components were stratified by type of ABSSSI and by hospital. Differences among hospitals were compared using the Cochran–Mantel–Haenszel test of association. Because the duration of antibiotic therapy prescribed at hospital discharge was not documented in some cases, the primary and secondary analyses were limited to cases with a known duration of therapy.
We used a pre-specified conceptual model for logistic regression (see Appendix Figure 1) and considered a number of factors that may be associated with complicated, severe, or poorly responsive infection. We performed bivariate analyses and retained variables with a P value ≤0.25 for inclusion in the regression models. Presence of diabetes mellitus, other immunosuppressing conditions, and lower extremity vascular impairment were retained irrespective of their bivariate association since it was felt these conditions may be particularly likely to affect prescribing. Multicollinearity was assessed by calculating the variance inflation factor for each variable included in the final model. The models were assessed for goodness-of-fit using the c-statistic and Hosmer-Lemeshow test. We hypothesized the composite primary endpoint would occur in 50% of cases and that up to 15 variables would be included in the final model. To assure adequate statistical power for multivariable analysis, our sample size goal was therefore 600 cases, or 85 per hospital. The target of 85 cases was reached at five of the seven hospitals. We used SAS Version 9.3 (SAS Institute, Cary, NC) for data analysis.
Results
The initial ICD-9-CM searches yielded 2842 potentially eligible cases. Of a random sample of 1030 cases that were manually reviewed, 497 were excluded for reasons detailed in Figure 1. The total study cohort therefore consisted of 533 cases; 492 cases with a documented duration of antibiotic therapy were included for the primary and secondary analyses. Demographic and clinical characteristics are presented in Table 1. 102 (19%) patients were ≤18 years old. Diabetes mellitus was documented in 107 (20%) cases, and fever or leukocytosis was present in 291, 55%.
Table 1.
Patient demographic and clinical characteristics
| Non-purulent cellulitis n=320 |
Wound infection or purulent cellulitis n=44 |
Cutaneous abscess n=169 |
Total n =533 |
|
|---|---|---|---|---|
| Age, mean (standard deviation) | 50.0 (24.3) | 32.2 (23.6) | 37.2 (22.4) | 44.5 (24.6) |
| Male | 176 (55) | 27 (61) | 114 (67) | 317 (59) |
| Diabetes mellitus | 72 (23) | 8 (18) | 27 (16) | 107 (20) |
| Current smoker | 56 (18) | 6 (14) | 54 (32) | 116 (22) |
| Alcohol abuse or dependence | 30 (9) | 4 (9) | 18 (11) | 52 (10) |
| Injection drug use | 8 (3) | 0 | 28 (17) | 36 (7) |
| Cirrhosis | 19 (6) | 1 (2) | 1 (1) | 21 (4) |
| HIV infection | 4 (1) | 0 | 8 (5) | 12 (2) |
| Dialysis dependence | 6 (2) | 1 (2) | 0 | 7 (1) |
| Lower extremity vascular impairment | 84 (26) | 3 (7) | 9 (5) | 96 (18) |
| Chronic venous stasis | 51 (16) | 2 (5) | 6 (4) | 59 (11) |
| Lymphedema | 42 (13) | 0 | 2 (1) | 44 (8) |
| Peripheral arterial disease | 9 (3) | 0 | 0 | 9 (2) |
| Saphenous vein harvest | 16 (5) | 1 (2) | 1 (1) | 18 (3) |
| Prior skin infection | 81 (25) | 13 (30) | 51 (30) | 145 (27) |
| Prior MRSA infection or colonization | 30 (9) | 7 (16) | 23 (14) | 60 (11) |
| Immunosuppressing condition a | 51 (16) | 6 (14) | 12 (7) | 69 (13) |
| Charlson age-comorbidity index, median (IQR) | 2 (0–4) | 0 (0–2) | 0 (0–2) | 1 (0–4) |
| Anatomical location | ||||
| Lower extremity | 232 (73) | 28 (64) | 65 (38) | 325 (61) |
| Upper extremity | 51 (16) | 5 (11) | 46 (27) | 102 (19) |
| Head and neck | 28 (9) | 6 (14) | 20 (12) | 54 (10) |
| Buttock or inguinal | 9 (3) | 2 (5) | 29 (17) | 40 (8) |
| Chest, abdomen, back, or axilla | 9 (3) | 4 (9) | 25 (15) | 38 (7) |
| Multiple distinct sites | 16 (5) | 3 (7) | 19 (11) | 38 (7) |
| Recurrent infection | 15 (5) | 2 (5) | 6 (4) | 23 (4) |
| Intensive care unit admission | 2 (1) | 0 | 3 (2) | 5 (1) |
| Medical primary service b | 289 (90) | 38 (86) | 114 (67) | 441 (83) |
| Surgical consultation c | 63 (20) | 13 (30) | 62 (37) | 138 (26) |
| Infectious Diseases consultation | 103 (32) | 7 (16) | 42 (25) | 152 (29) |
| Failed initial outpatient antibiotic therapy | 98 (31) | 21 (48) | 60 (36) | 179 (34) |
| Fever (temperature ≥38.0°C) | 74 (23) | 8 (18) | 41 (24) | 123 (23) |
| Leukocytosis (WBC >10,000 cells/mm3) | 125 (39) | 23 (52) | 93 (55) | 241 (45) |
Data presented as n (%) unless otherwise noted. MRSA, methicillin-resistant Staphylococcus aureus; IQR, interquartile range; WBC, white blood cells.
Immunosuppressing medication (42), connective tissue disease or vasculitis (20), solid organ malignancy undergoing chemotherapy (15), organ transplant (12), hematologic malignancy (11), neutropenia (5)
Internal or Family Medicine (353) or Pediatrics (88)
General Surgery (66), Orthopedics (46), Podiatry (13), Otolaryngology (11), Urology (2), Vascular Surgery (1), or Neurosurgery (1)
Of 202 (38%) cases with a positive culture, an aerobic gram-positive organism was identified in 190 (94%) (Table 2). Aerobic gram-negative organisms were isolated in 20 cases (13 when excluding superficial wound cultures). In 16 of these 20 cases, cultures were polymicrobial, with S. aureus or streptococci present in 13. Blood cultures were positive in 22 (4%) cases.
Table 2.
Microbiological data
| Non-purulent cellulitis n=320 |
Wound infection or purulent cellulitis n=44 |
Cutaneous abscess n=169 |
Total n =533 |
|
|---|---|---|---|---|
| Any microbiological culture obtained | 246 (77) | 39 (89) | 155 (92) | 440 (83) |
| Wound or other surface culture | 44 (14) | 22 (50) | 16 (9) | 82 (15) |
| Abscess material | 5 (2) | 3 (7) | 130 (77) | 138 (26) |
| Tissue or aspirate | 19 (6) | 9 (20) | 7 (4) | 35 (7) |
| Blood | 225 (70) | 24 (55) | 95 (56) | 344 (65) |
| Any microorganism identified a | 48 (15) | 26 (59) | 128 (76) | 202 (38) |
| Aerobic gram-positive organism | 46 (96) | 25 (96) | 119 (93) | 190 (94) |
| Staphylococcus aureus | 23 (48) | 21 (81) | 83 (65) | 127 (63) |
| Methicillin-susceptible | 13 (27) | 10 (38) | 35 (27) | 58 (29) |
| Methicillin-resistant | 9 (19) | 9 (35) | 46 (36) | 64 (32) |
| Susceptibility not performed | 1 (2) | 2 (8) | 2 (2) | 5 (2) |
| Streptococcal species | 15 (31) | 4 (15) | 40 (31) | 59 (29) |
| β-hemolytic streptococcus | 12 (25) | 3 (12) | 22 (17) | 37 (18) |
| S. anginosus-milleri group | 1 (2) | 0 | 14 (11) | 15 (7) |
| Other alpha-hemolytic streptococcus | 2 (4) | 1 (4) | 5 (4) | 8 (4) |
| Other streptococci | 0 | 0 | 2 (2) | 2 (1) |
| S. aureus or streptococci | 33 (69) | 24 (92) | 114 (89) | 171 (85) |
| Coagulase-negative staphylococcus | 15 (31) | 4 (15) | 14 (11) | 33 (16) |
| Enterococcus species | 2 (4) | 0 | 0 | 2 (1) |
| Aerobic gram-negative organism b,c | 6 (13) | 3 (12) | 11 (9) | 20 (10) |
| Only gram-negative organism(s) identified d | 1 (2) | 1 (4) | 2 (2) | 4 (2) |
| Anaerobic organism(s) | 3 (6) | 1 (4) | 13 (10) | 17 (8) |
| Other | 3 (6) | 1 (4) | 6 (5) | 10 (5) |
| Positive blood culture e | 15 (5) | 1 (2) | 6 (4) | 22 (4) |
Data presented as n (%) unless otherwise noted.
Denominator for the proportion reported for each individual organism is the number of cases where an organism was identified
When excluding wound or other surface cultures, aerobic gram-negative organisms were isolated in 13 (6%) cases
Isolates include Klebsiella species (3), Pseudomonas aeruginosa (2), Pasturella multocida (2), E. coli (1), Proteus mirabilis (1), Serratia marcescens (1), Acinetobacter species (1), Aeromonas hydrophila (1), Haemophilus parainfluenzae (1), mixed gram-negative flora (1), non-lactose fermenting gram-negative rod, unspecified (2), lactose fermenting gram-negative rod, unspecified (1), gram-negative rod, unspecified
Gram-positive or anaerobic organisms were also isolated in 16 of the 20 cases
12 (2%) were judged to represent true bacteremia, including MSSA (4), MRSA (3), beta-hemolytic streptococci (4), and polymicrobial with MRSA, Streptococcus milleri, and coagulase-negative staphylococcus (1)
During the hospitalization, the initial antibiotic regimen prescribed consisted of multiple agents in 190 (36%) cases. Vancomycin was the most commonly prescribed agent for all three types of ABSSSI (61 – 82% of cases) (Table 3). Use of cefazolin or nafcillin for non-purulent cellulitis was relatively uncommon. Antibiotic therapy was continued after hospital discharge in 519 (97%) cases and most frequently included an agent with activity against MRSA. Combination antibiotic regimens were prescribed at discharge in 92 (17%) cases, typically a β-lactam plus an MRSA-active agent (50, 9%) or a fluoroquinolone plus an MRSA-active agent (24, 5%). In 47 (9%) cases, discharge therapy included a parenteral antibiotic (range 0 to 20% across hospitals, p <0.001).
Table 3.
Antibiotics prescribed during the hospitalization and at discharge
| Non-purulent cellulitis n=320 |
Wound infection or purulent cellulitis n=44 |
Cutaneous abscess n=169 |
Total n =533 |
|
|---|---|---|---|---|
| Inpatient antibiotic therapy | ||||
| Antibiotic regimen ordered by admitting provider | ||||
| Single antibiotic | 202 (63) | 29 (66) | 111 (66) | 342 (64) |
| Combination antibiotic regimen | 117 (37) | 15 (34) | 58 (34) | 190 (36) |
| Individual antibiotics prescribed during the inpatient stay a, b | ||||
| Parenteral antibiotics | ||||
| Vancomycin | 226 (71) | 27 (61) | 138 (82) | 391 (73) |
| Clindamycin | 88 (28) | 21 (48) | 42 (25) | 151 (28) |
| β-lactam/β-lactamase inhibitor | 73 (23) | 11 (25) | 51 (30) | 135 (25) |
| 2nd generation or higher cephalosporin | 53 (17) | 5 (11) | 16 (9) | 74 (14) |
| Cefazolin | 46 (14) | 8 (18) | 17 (10) | 71 (13) |
| Carbapenem | 29 (9) | 2 (5) | 10 (6) | 41 (8) |
| Fluoroquinolone | 25 (8) | 3 (7) | 9 (5) | 37 (7) |
| Daptomycin | 24 (8) | 1 (2) | 7 (4) | 32 (6) |
| Linezolid | 6 (2) | 1 (2) | 1 (1) | 8 (2) |
| Other β-lactam | 13 (4) | 3 (7) | 3 (2) | 19 (4) |
| Oral antibiotics | ||||
| Clindamycin | 24 (8) | 6 (14) | 21 (12) | 51 (10) |
| Trimethoprim-sulfamethoxazole | 25 (8) | 4 (9) | 21 (12) | 50 (9) |
| Doxycycline | 18 (6) | 2 (5) | 10 (6) | 30 (6) |
| Cephalexin | 23 (7) | 0 | 5 (3) | 28 (5) |
| Fluoroquinolone | 17 (5) | 2 (5) | 8 (5) | 27 (5) |
| Amoxicillin-clavulanate | 9 (3) | 1 (2) | 10 (6) | 20 (4) |
| Other β-lactam | 2 (1) | 0 | 4 (2) | 6 (1) |
| Antibiotic with broad gram-negative activity | 136 (43) | 15 (34) | 58 (34) | 209 (39) |
| Antibiotic therapy prescribed at hospital discharge b | 309 (97) | 44 (100) | 166 (98) | 519 (97) |
| Single antibiotic | 251 (78) | 35 (80) | 140 (83) | 426 (80) |
| Combination antibiotic regimen | 58 (18) | 9 (20) | 25 (15) | 92 (17) |
| Parenteral antibiotics | 32 (10) | 6 (14) | 9 (5) | 47 (9) |
| Vancomycin | 12 (4) | 4 (9) | 4 (2) | 20 (4) |
| Daptomycin | 12 (4) | 1 (2) | 2 (1) | 15 (3) |
| Other parenteral antibiotic c | 10 (3) | 1 (2) | 4 (2) | 15 (3) |
| Oral antibiotics | 276 (86) | 40 (91) | 159 (94) | 475 (89) |
| Clindamycin | 75 (23) | 19 (43) | 48 (28) | 142 (27) |
| Trimethoprim-sulfamethoxazole | 63 (20) | 12 (27) | 43 (25) | 118 (22) |
| Doxycycline | 40 (13) | 2 (5) | 26 (15) | 68 (13) |
| Cephalexin | 46 (14) | 3 (7) | 17 (10) | 66 (12) |
| Amoxicillin-clavulanate | 39 (12) | 4 (9) | 21 (12) | 64 (12) |
| Fluoroquinolone | 27 (8) | 2 (5) | 12 (7) | 41 (8) |
| Linezolid | 23 (7) | 1 (2) | 3 (2) | 27 (5) |
| Other β-lactam | 15 (5) | 3 (7) | 8 (5) | 26 (5) |
| Other d | 1 (0.3) | 1 (2) | 2 (1) | 4 (1) |
| Antibiotic with broad gram-negative activity | 78 (24) | 8 (18) | 36 (21) | 122 (23) |
| Treatment duration e | ||||
| Total duration of therapy, median days (IQR) | 12 (10–15) | 12 (11–14) | 12 (10–15) | 12 (10–15) |
| Duration of inpatient therapy, median days (IQR) | 4 (3–5) | 3 (3–4) | 4 (3–5) | 4 (3–5) |
| Duration of therapy after discharge, median days (IQR) | 8 (7–10) | 10 (7–10) | 9 (7–10) | 8 (7–10) |
Data presented as n (%) unless otherwise noted. IQR, interquartile range.
Includes antibiotics initiated by the emergency department, urgent care, or clinic.
Patients could receive more than one antibiotic.
Includes carbapenem (6), ceftriaxone (4), ceftaroline (1), nafcillin (1), linezolid (1), rifampin (1), trimethoprim-sulfamethoxazole (1), levofloxacin (1).
Includes metronidazole (3), erythromycin (1)
Analyses limited to 492 cases with a known duration of therapy.
Either during the hospitalization and/or at discharge, 214 patients (43%) received antibiotics with broad gram-negative activity. Among these patients, broad gram-negative therapy accounted for 1799 (41%) of the 4404 total days of therapy prescribed. The median duration of therapy was similar among all three types of ABSSSI (Table 3). Total treatment durations of 7 days or less were infrequent (29, 6%), while durations of greater than 10 days (344, 70%) or greater than 14 days (136, 28%) were common. Treatment after hospital discharge accounted for the majority of the total duration for all three types of ABSSSI.
Of the 492 cases with a known duration of therapy that were included in the primary endpoint analysis, prescription of antibiotics with broad gram-negative activity or treatment for greater than 10 days occurred in 394 (80%) cases and was common among all three types of ABSSSI (Table 4). There was significant variation in the frequency of the primary endpoint among the seven hospitals (range 64 – 97%, p <.001) (Figure 2). When excluding cases with factors that could reflect complicated, severe, or poorly responsive infection, the primary endpoint occurred in 39 (74%) of 53 cases. Antibiotic prescribing was discordant with IDSA treatment recommendations in 273 (55%) cases (Table 4).
Figure 2.
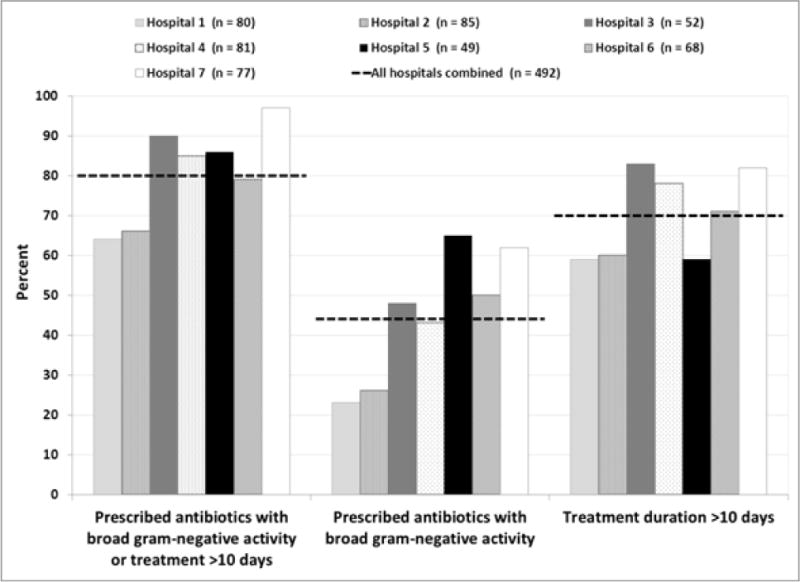
Primary endpoint for the 492 patients with complete prescribing data, by hospital. For all three analyses, the between-hospital comparison was statistically significant with p<0.001
By logistic regression, factors independently associated with the composite primary endpoint included wound infection or purulent cellulitis, head or neck involvement, adult (vs. pediatric) cases, and admission to a community (vs. academic) hospital (Figure 3). The presence of multiple distinct sites of infection was inversely associated. The logistic regression model to evaluate factors associated with discordance with IDSA treatment recommendations yielded similar results, except type of ABSSSI was not a predictor (Appendix Figure 2). When considering the components of the primary endpoint individually, independent predictors of use of antibiotics with broad gram-negative activity included diabetes mellitus, adult cases, admission to a community hospital, head or neck involvement, and initial treatment failure. Prior MRSA infection or colonization was inversely associated (Appendix Figure 3). Factors associated with treatment duration of more than 10 days included adult cases, medical (vs. surgical) primary service, Infectious Diseases consultation, and presence of leukocytosis (Appendix Figure 4).
Figure 3.
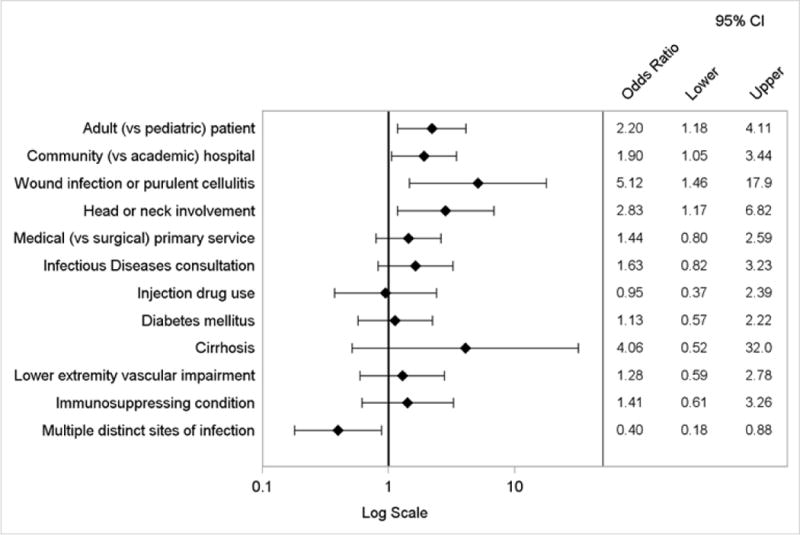
Logistic regression model of factors associated with the composite primary endpoint of prescription of antibiotics with broad gram-negative activity or treatment duration greater than 10 days
All but one patient survived the hospitalization (Appendix Table 2). The median length of hospital stay was 4 days (interquartile range 3 – 5). In total, clinical failure occurred in 59 (11%) cases.
Discussion
Prescription of antibiotics with a broad spectrum of gram-negative activity or treatment for longer than 10 days occurred in 80% of cases of ABSSSI. While there were differences between hospitals, potentially avoidable antibiotic exposure was common in all 7 hospitals. Treatment was discordant with current IDSA guidance in over half of cases. Adults, patients admitted to a community hospital, and those with non-drainable purulent infections or head or neck infections were at increased risk of exposure to broad gram-negative therapy or treatment courses longer than 10 days.
Understanding current clinical practice in the management of ABSSSIs is essential to develop interventions to improve antibiotic use and frame key questions for clinical trials. To our knowledge, this is the first multicenter description of antibiotic prescribing practices for ABSSSIs utilizing patient-level data. National administrative databases have been used to study skin infections [4, 14–16] but cannot reliably distinguish between types of ABSSSI [17] or provide detailed information on antibiotic treatment. Previous single-center studies utilizing patient-level data have shown that up to two-thirds of patients receive antibiotics with broad gram-negative activity [8, 10] with a median treatment duration of 13 days [8]. In the present study, we found similar results in a broader range of hospitals including community and academic institutions and pediatric and adult populations. This study also extends previous work by identifying factors associated with use of broad gram-negative therapy or extended treatment durations.
The optimal antibiotic choice and duration of therapy for patients hospitalized with ABSSSI have not been clearly delineated. Our composite primary endpoint – a surrogate marker for potentially avoidable antibiotic exposure – therefore warrants further discussion. First, ABSSSIs are predominantly caused by gram-positive pathogens [8, 14, 18]; numerous antibiotics with gram-positive activity are recommended as first-line therapy in IDSA treatment guidelines [7]. Discordant with IDSA guidance, we found that antibiotics with broad gram-negative activity were prescribed in over 40% of all cases and in 25% of the least complicated cases; when prescribed, these agents accounted for over 40% of the total days of therapy. We felt that an evaluation of the appropriateness of such therapy would have been imprecise and biased for the following reasons: (1) the infecting pathogen is not identified in most cases of ABSSSI; (2) provider rationale for choice of empiric therapy is not routinely documented; and (3) clinical features that may warrant a broader spectrum of therapy are not well-established. Furthermore, when gram-negative organisms are identified in polymicrobial skin infections, they may be of limited clinical relevance [19]. Of note, we found that over one-third of the gram-negative organisms were isolated from superficial wound cultures, and 80% were part of a polymicrobial milieu. We therefore cannot estimate the true proportion of cases where broad gram-negative therapy might have been appropriate; however, our data suggest that efforts to promote the routine use of antibiotics targeting gram-positive organisms, as recommended by the IDSA, are warranted.
Second, we considered treatment durations of greater than 10 days to represent potentially avoidable antibiotic exposure. Although the optimal duration of therapy for patients hospitalized with ABSSSI is not known, emerging evidence suggests short courses are adequate. First, drainage has been shown to be the mainstay of therapy for cutaneous abscess; as such, prolonged antibiotic therapy is not likely necessary [20, 21]. Second, cellulitis is an infection with a low burden of microorganisms [22, 23]. Finally, a recent randomized trial demonstrated that 6 days of tedizolid was as effective as 10 days of linezolid for patients hospitalized with various types of ABSSSI [18]. Although it is not known whether the results of this trial can be extrapolated to currently licensed antibiotics, it suggests shorter treatment courses are adequate. As discussed above, IDSA guidelines recommend 7 to 14 days of therapy, with individualization based on clinical response [7]. In the present study, extended treatment durations were similarly common among both the least complicated cases and the more complicated cases, suggesting that duration of therapy is not routinely individualized. Shortening treatment durations could therefore substantially reduce antibiotic exposure. As the majority of antibiotic therapy for ABSSSIs is completed after the hospitalization, interventions should focus on reducing the duration prescribed at discharge.
The factors associated with broad gram-negative therapy or treatment longer than 10 days by logistic regression provide several insights for the field of antimicrobial stewardship. First, it may be appropriate for stewardship programs to focus interventions to providers who care for adults with ABSSSI. Second, efforts to improve antibiotic use for ABSSSIs must involve community as well as academic hospitals. Finally, as stewardship interventions are developed, patients with non-drainable purulent infections, head or neck involvement, diabetes mellitus, and those not responding to initial therapy may warrant particular attention given their increased risk of exposure to broad-spectrum antibiotics or extended treatment durations.
This study has at least 6 limitations. First, the hospitals represent only one geographic region of the United States. We included a broad range of hospital types to maximize generalizability, but antibiotic prescribing patterns may differ in other regions [24]. Second, the retrospective nature of the study introduces the possibility of reviewer bias. We used an objective primary endpoint and undertook a number of measures to standardize medical record review (see Methods) to mitigate such bias. Third, the study design was not conducive to determine the appropriateness of antibiotic therapy prescribed. Fourth, the medical record systems differed among sites which led to differences in the ability to capture certain data elements such as clinical encounters after discharge. We therefore could not make inferences about the association between prescribing practices and clinical outcomes. Fifth, our data do not include antibiotic allergies. Since a number of antibiotics with gram-positive activity are available to treat ABSSSIs, allergies to β-lactams or other antibiotics should not have affected the frequency of the primary endpoint. Finally, the duration of therapy prescribed at hospital discharge was not documented in approximately 8% of cases. We therefore limited our primary and secondary analyses to cases with a known duration of therapy. Although we did not achieve our sample size goal of 600 cases, the logistic regression models were adequately powered since the primary endpoint was more common than we postulated.
In summary, use of antibiotics with broad gram-negative activity and treatment courses longer than 10 days were common among patients treated for ABSSSI in a diverse group of hospitals. Interventions to promote shorter courses of therapy targeting gram-positive pathogens may reduce unnecessary antibiotic exposure for such patients. Given the frequency of ABSSSIs and the prescribing practices described herein, these infections are important target conditions for hospital antimicrobial stewardship programs. Furthermore, these findings demonstrate the need for a large randomized trial to provide definitive data on the shortest effective treatment duration.
Acknowledgments
Financial Support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institute of Health (TCJ: K23 AI099082).
We are grateful to Brad Spellberg, David Gilbert, Jason Haukoos, David West, and Vance Fowler for input during the development of the study and Kaitlin Gorman and Kevin Silva for assistance with data entry.
Appendix
Appendix Figure 1.
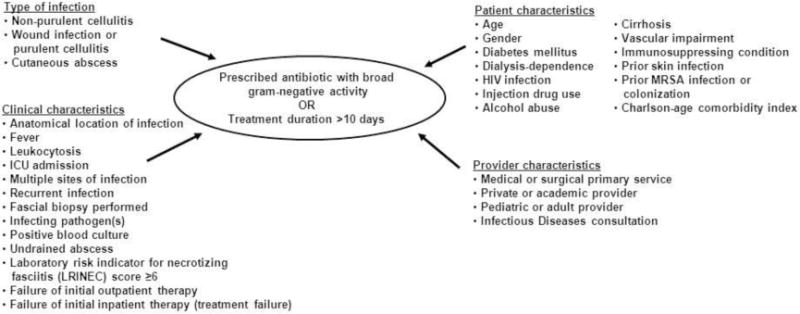
Conceptual model of factors associated with prescription of antibiotics with broad gram-negative activity or treatment duration greater than 10 days
Appendix Figure 2.
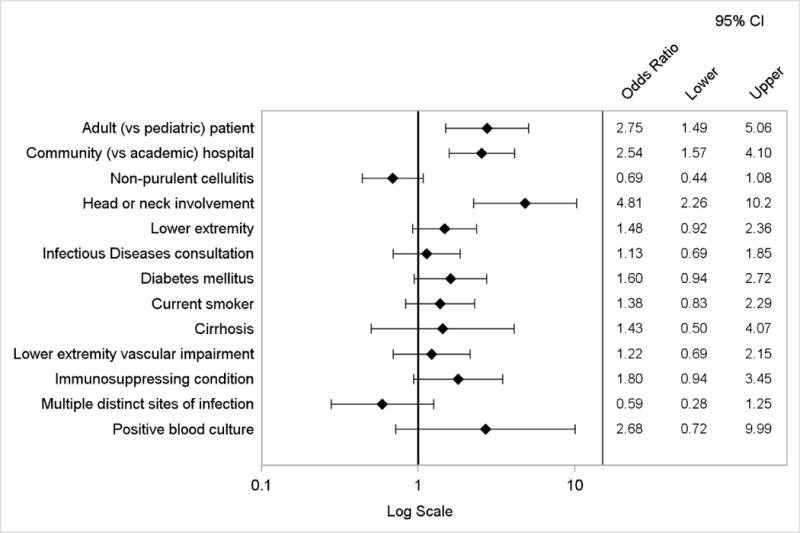
Logistic regression model of factors associated with discordance with IDSA treatment recommendations, prescription of antibiotics with broad gram-negative activity or treatment duration greater than 14 days
Appendix Figure 3.
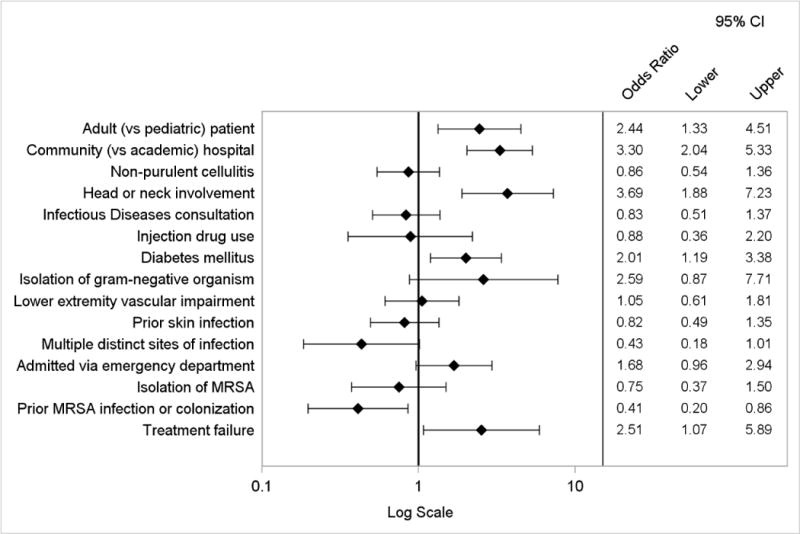
Logistic regression model of factors associated with prescription of antibiotics with broad gram-negative activity
Appendix Figure 4.
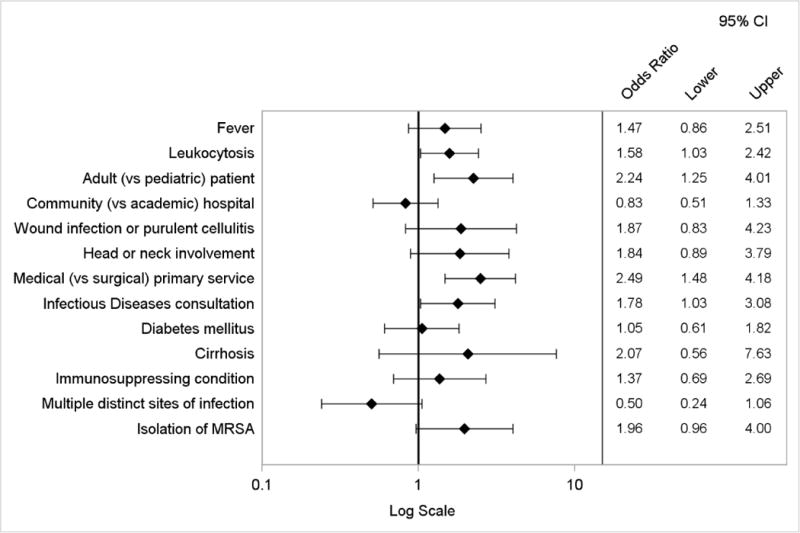
Logistic regression model of factors associated with treatment duration greater than 10 days
Appendix Table 1.
Characteristics of participating hospitals
| Academic/community | Type | Housestaff program | Patient population | Infectious Diseases consult service | Antimicrobial stewardship program | Hospital beds | Cases contributed |
|---|---|---|---|---|---|---|---|
| Academic | Children’s hospital | Yes | Pediatric | Yes | No | 536 | 85 |
| Academic | Tertiary care | Yes | Adult | Yes | Yes | 407 | 85 |
| Academic | Veteran’s Affairs | Yes | Adult | Yes | No | 123 | 52 |
| Academic | Safety-net | Yes | Adult and pediatric | Yes | Yes | 525 | 85 |
| Community | Private | No | Adult and pediatric | Yes | Yes | 58 | 56 |
| Community | Private | Yes a | Adult and pediatric | Yes | No | 368 | 85 |
| Community | Private | No | Adult | Yes | No | 368 | 85 |
Family Medicine residency program only
Appendix Table 2.
Clinical outcomes
| Non-purulent cellulitis n=320 |
Wound infection or purulent cellulitis n=44 |
Cutaneous abscess n=169 |
Total n =533 |
|
|---|---|---|---|---|
| Survived to discharge | 319 (99) | 44 (100) | 169 (100) | 532 (99) |
| Clinical failure | 38 (12) | 3 (7) | 18 (11) | 59 (11) |
| Treatment failure | 16 (5) | 3 (7) | 11 (7) | 30 (6) |
| Recurrence | 17 (5) | 1 (2) | 9 (5) | 27 (5) |
| Re-hospitalization due to skin infection | 23 (7) | 2 (5) | 7 (4) | 32 (6) |
| Re-hospitalization for reason other than skin infection | 24 (8) | 1 (2) | 6 (4) | 31 (6) |
| Length of stay, median days (IQR) | 4 (3 – 5) | 3 (3 – 4) | 4 (4 – 5) | 4 (3 – 5) |
IQR, interquartile range
Footnotes
This work was presented at IDWeek 2013, San Francisco, California.
Conflicts of interest. DMP reports potential conflicts of interests with Optimer, Cubist, and Forest Pharmaceuticals. All other authors, nothing to disclose.
References
- 1.US Department of Health and Human Services, Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2013 http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013–508.pdf. Accessed 1 October 2013.
- 2.Bartlett JG, Gilbert DN, Spellberg B. Seven ways to preserve the miracle of antibiotics. Clin Infect Dis. 2013;56(10):1445–50. doi: 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- 3.Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009;15(9):1516–8. doi: 10.3201/eid1509.081228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168(14):1585–91. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 5.Lopez MA, Cruz AT, Kowalkowski MA, Raphael JL. Trends in resource utilization for hospitalized children with skin and soft tissue infections. Pediatrics. 2013;131(3):e718–25. doi: 10.1542/peds.2012-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lautz TB, Raval MV, Barsness KA. Increasing national burden of hospitalizations for skin and soft tissue infections in children. J Pediatr Surg. 2011;46(10):1935–41. doi: 10.1016/j.jpedsurg.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins TC, Sabel AL, Sarcone EE, Price CS, Mehler PS, Burman WJ. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903. doi: 10.1086/656431. [DOI] [PubMed] [Google Scholar]
- 9.Carratala J, Roson B, Fernandez-Sabe N, et al. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur J Clin Microbiol Infect Dis. 2003;22(3):151–7. doi: 10.1007/s10096-003-0902-x. [DOI] [PubMed] [Google Scholar]
- 10.Amara S, Adamson RT, Lew I, Huang X. Clinical response at Day 3 of therapy and economic outcomes in hospitalized patients with acute bacterial skin and skin structure infection (ABSSSI) Curr Med Res Opin. 2013;29(7):869–77. doi: 10.1185/03007995.2013.803056. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072–9. doi: 10.1001/archinternmed.2011.29. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996;27(3):305–8. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Draft guidance for industry: acute bacterial skin and skin structure infections—developing drugs for treatment. 2010 Aug; Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed 6 February 2013.
- 14.Lipsky BA, Weigelt JA, Gupta V, Killian A, Peng MM. Skin, soft tissue, bone, and joint infections in hospitalized patients: epidemiology and microbiological, clinical, and economic outcomes. Infect Control Hosp Epidemiol. 2007;28(11):1290–8. doi: 10.1086/520743. [DOI] [PubMed] [Google Scholar]
- 15.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA., Jr Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–8. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Huttner B, Jones M, Huttner A, Rubin M, Samore MH. Antibiotic prescription practices for pneumonia, skin and soft tissue infections and urinary tract infections throughout the US Veterans Affairs system. J Antimicr Chemo. 2013;68(10):2393–9. doi: 10.1093/jac/dkt171. [DOI] [PubMed] [Google Scholar]
- 17.Qualls ML, Mooney MM, Camargo CA, Jr, Zucconi T, Hooper DC, Pallin DJ. Emergency department visit rates for abscess versus other skin infections during the emergence of community-associated methicillin-resistant Staphylococcus aureus, 1997–2007. Clin Infect Dis. 2012;55(1):103–5. doi: 10.1093/cid/cis342. [DOI] [PubMed] [Google Scholar]
- 18.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;309(6):559–69. doi: 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 19.Joseph WS, Lipsky BA. Medical therapy of diabetic foot infections. J Vasc Surg. 2010;52(3 Suppl):67S–71S. doi: 10.1016/j.jvs.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56(3):283–7. doi: 10.1016/j.annemergmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55(5):401–7. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Sigurdsson AF, Gudmundsson S. The etiology of bacterial cellulitis as determined by fine-needle aspiration. Scand J Infect Dis. 1989;21(5):537–42. doi: 10.3109/00365548909037882. [DOI] [PubMed] [Google Scholar]
- 23.Hook EW, 3rd, Hooton TM, Horton CA, Coyle MB, Ramsey PG, Turck M. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Int Med. 1986;146(2):295–7. [PubMed] [Google Scholar]
- 24.Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Arch Int Med. 2012;172(19):1465–71. doi: 10.1001/archinternmed.2012.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]


