Abstract
Pentameric ligand-gated ion channels (pLGICs) are the targets of several clinical and endogenous allosteric modulators including anesthetics and neurosteroids. Molecular mechanisms underlying allosteric drug modulation are poorly understood. Here, we constructed a chimeric pLGIC by fusing the extracellular domain (ECD) of the proton-activated, cation-selective bacterial channel GLIC to the transmembrane domain (TMD) of the human ρ1 chloride-selective GABAAR, and tested the hypothesis that drug actions are regulated locally in the domain that houses its binding site. The chimeric channels were proton-gated and chloride-selective demonstrating the GLIC ECD was functionally coupled to the GABAρ TMD. Channels were blocked by picrotoxin and inhibited by pentobarbital, etomidate and propofol. The point mutation, ρ TMD W328M, conferred positive modulation and direct gating by pentobarbital. The data suggest that the structural machinery mediating general anesthetic modulation resides in the TMD. Proton-activation and neurosteroid modulation of the GLIC-ρ chimeric channels, however, did not simply mimic their respective actions on GLIC and GABAρ revealing that across domain interactions between the ECD and TMD play important roles in determining their actions. Proton-induced current responses were biphasic suggesting that the chimeric channels contain an additional proton sensor. Neurosteroid modulation of the GLIC-ρ chimeric channels by the stereoisomers, 5α-THDOC and 5β-THDOC, were swapped compared to their actions on GABAρ indicating that positive versus negative neurosteroid modulation is not encoded solely in the TMD nor by neurosteroid isomer structure but is dependent on specific interdomain connections between the ECD and TMD. Our data reveal a new mechanism for shaping neurosteroid modulation.
Keywords: Pentameric ligand-gated ion channel, GLIC, GABAA receptor, chimera, anesthetics, neurosteroid, propofol, etomidate, pentobarbital, THDOC, allosteric drug modulation
1. Introduction
Many clinically important drugs such as anesthetics, barbiturates and neurosteroids exert their CNS effects by binding to gamma-aminobutyric acid type-A receptors (GABAARs). These drugs bind to distinct sites located far from the orthosteric GABA binding sites, and allosterically modulate GABAAR function (Forman and Miller, 2011; Miller and Smart, 2010). The structural mechanisms by which these different classes of drugs either enhance or inhibit GABA-activated currents remain poorly understood and represent a major challenge in developing novel therapeutics that target GABAARs.
GABAARs are members of the pentameric ligand gated ion channel superfamily, which include nicotinic acetylcholine receptors (nAChR), serotonin type 3 receptors (5HT3R) and glycine receptors (GlyR), Pentameric ligand-gated ion channels (pLGICs) mediate fast synaptic neurotransmission, and signaling in the brain depends on their activity. For these receptors, neurotransmitter binding promotes opening of an integral membrane-spanning ion channel, which allows ions to flow across the membrane and change the cell’s activity (Miller and Smart, 2010). In the last decade, prokaryotic pLGIC homologs GLIC (Gloeobacter ligand-gated ion channel) (Bocquet et al., 2009; Bocquet et al., 2007; Hilf and Dutzler, 2009) and ELIC (Erwinia ligand-gated ion channel) (Hilf and Dutzler, 2008) have been identified.
With the goal of dissecting molecular mechanisms underlying how different classes of allosteric drugs modulate GABAAR function, we constructed a chimeric pLGIC by fusing the extracellular domain (ECD) of the prokaryotic proton-gated ion channel GLIC with the transmembrane domain (TMD) of GABAAR ρ subunit. We examined how anesthetics, barbiturates and neurosteroids modulate chimeric channel function and tested the hypothesis that drug actions are regulated locally in the domain that houses its binding site.
Based on high-resolution structures of prokaryotic (Bocquet et al., 2009; Cecchini and Changeux, 2015; Hilf and Dutzler, 2008; Sauguet et al., 2014) and eukaryotic pLGICs (Althoff et al., 2014; Du et al., 2015; Hassaine et al., 2014; Hibbs and Gouaux, 2011; Miller and Aricescu, 2014), pLGICs have a modular architecture. The N-terminal extracellular domain (ECD) consists mostly of beta sheets and houses the neurotransmitter binding site (Brejc et al., 2001; Miller and Smart, 2010). The transmembrane domain (TMD) consists of alpha helices that span the lipid bilayer, and contains the ion-conducting channel as well as the binding sites for various drugs including anesthetics, barbiturates and neurosteroids (Baenziger and Corringer, 2011; Cecchini and Changeux, 2015; Du et al., 2015; Fourati et al., 2017; Hibbs and Gouaux, 2011; Nemecz et al., 2016; Nury et al., 2011; Spurny et al., 2013). At the ECD-TMD interface, connections between flexible loops in the extracellular binding domain (loops 2, 7, 9) with the transmembrane channel domain (M2–M3 loop) structurally link the two domains and are essential for coupling ligand binding to channel gating (Miller and Smart, 2010). Agonist-mediated closed to open channel gating transitions are accompanied by substantial rearrangements of this interface (Bertozzi et al., 2016; Dellisanti et al., 2013; Gupta et al., 2017; Lee and Sine, 2005; Velisetty et al., 2014; Xiu et al., 2005). In chimeric channels assembled by combining the ECD and TMD of two distinct pLGICs, substantial loop substitutions are required to maintain complementarity and ensure normal channel function (Bouzat et al., 2008; Bouzat et al., 2004; Eisele et al., 1993).
In this study, we were interested in determining whether allosteric drug modulators, especially those that bind to the TMD, rely on the ECD-TMD interface for coupling their binding to modulation of channel activity. Previous studies using chimeric pLGICs, constructed from different eukaryotic ECDs and TMDs (Eisele et al., 1993; Mihic et al., 1997; Serafini et al., 2000) as well as prokaryotic-eukaryotic (Duret et al., 2011; Moraga-Cid et al., 2015) and prokaryotic-prokaryotic (Alqazzaz et al., 2017) domains, have shown that the pharmacological and functional properties of each domain are retained suggesting that a drug’s actions on channel activity are regulated locally in the domain that houses its binding site. However, in an ELIC(ECD)-nAChR(TMD) chimera, only when the ECD-TMD interfacial loops were identical to those of nAChR did nAChR-specific drugs modulate chimeric currents (Tillman et al., 2014), suggesting that across-domain interactions may play important roles in mediating the actions of some drugs..
Crystal structures of prokaryotic pLGICs homologs, GLIC (Gloeobacter ligand-gated ion channel) and ELIC (Erwinia ligand-gated ion channel), in different conformational states and in the presence of various therapeutic drugs (Bocquet et al., 2009; Fourati et al., 2017; Hilf and Dutzler, 2008, 2009; Nury et al., 2011; Pan et al., 2012a; Pan et al., 2012b; Spurny et al., 2012) have been solved making them attractive models to study pLGIC structure and function (Sauguet et al., 2015). However, common GABAAR ligands bind with low affinity and have modest effects on GLIC and ELIC (Alqazzaz et al., 2011; Chen et al., 2010; Thompson et al., 2012; Weng et al., 2010). Recently, Moraga-Cid et al. showed that the chimera approach can be used to great advantage to study the structural properties of the glycine receptor, and understanding its physiological role in hyperexplexia (Moraga-Cid et al., 2015). Here, we report the construction and characterization of a chimeric pLGIC consisting of the ECD from the proton-activated bacterial channel GLIC and the TMD from the chloride-selective GABAAR ρ subunit. As expected, this chimeric subunit formed functional Cl− conducting, proton-gated channels demonstrating that the ECD of GLIC was functionally coupled to the GABAAR TMD. However, proton-activation and neurosteroid modulation of the chimeric GLIC-rho receptor did not simply mimic their respective actions on GLIC and GABAAR ρ revealing that across domain interactions between the ECD and TMD play important roles in determining a ligand’s actions.
2. Methods
2.1 Generation of chimeric receptors
GLIC-GABAρI chimeric subunits were constructed by fusing the ECD of GLIC ending at pre-M1 R191 with the human GABAA receptor ρ subunit TMD beginning at H259 (Fig. 1). GLIC was previously cloned into the pUNIV expression vector (Ghosh et al., 2013; Laha et al., 2013; Venkatachalan et al., 2007). To remove the TMD of GLIC, a unique enzyme restriction site XmaI was introduced after Arg191 in pUNIV GLIC by site directed mutagenesis (Quickchange, Strategene). A MluI restriction site was already present at the 3’ end of GLIC. GABAρ TMD cDNA was PCR amplified between Arg 258 and the C-terminus of the human GABAρ1 subunit using primers with overhanging ends containing XmaI and MluI restriction sites at the 5’ and 3’end, respectively. pUNIV GLIC vector and the amplified GABAρ TMD were digested with the XmaI and MluI enzymes and then ligated overnight using T4 DNA Ligase (Promega). After transformation into E.coli, positive colonies containing GLIC-ρ were identified by colony PCR. The resulting GLIC-ρ chimeric construct was then mutated to remove the introduced restriction site in GLIC ECD and restore wild-type GLIC coding sequence upstream of the chimeric junction. This construct was called GLIC-ρ1 (Fig. 1 A).
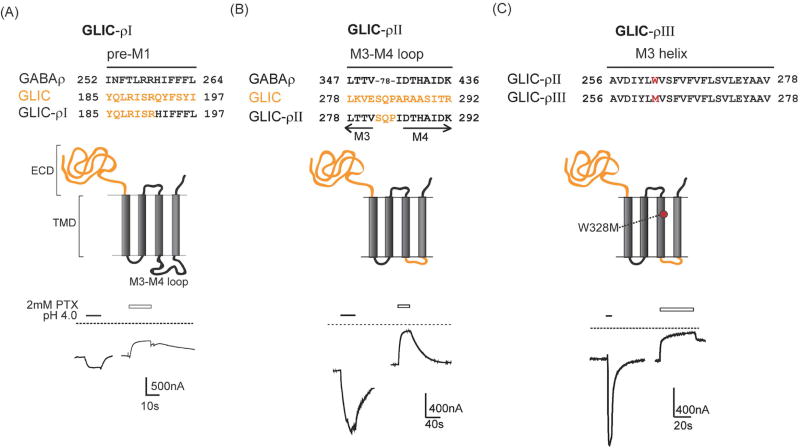
Figure 1. GLIC-GABAρ chimeric subunits form functional proton-gated channels.
(A) Top: Sequence alignments of pre-M1 regions of GABAρ, GLIC and GLIC-ρI chimera illustrating where ECD of GLIC was attached to TMD of GABAρ subunit; Middle: Schematic of GLIC–ρI chimeric subunit with GLIC protein in orange and GABAρ in black; Bottom: Representative pH 4.0 induced currents from an oocyte expressing GLIC–ρI chimeric channels. Dotted line represents zero current level highlighting resting leak current that is blocked by 2mM picrotoxin (PTX). (B) Top: Partial sequence alignments of M3, cytoplasmic M3–M4 loop and M4 of GABAρ, GLIC and GLIC-GABAρII chimera. Middle: The GLIC-GABAρII chimera was created by replacing the GABAρ M3–M4 cytoplasmic loop (78 residues) in GLIC–ρI with GLIC M3–M4 tri-peptide SQP. Bottom: Representative pH 4.0 and PTX induced current traces from an oocyte expressing GLIC–ρII. (C) Top, Middle: Sequences of M3 helices from GLIC-ρII and GLIC–ρIII highlighting Trp to Met mutation in M3 (red); Bottom: Representative pH 4.0 and PTX induced current traces from an oocyte expressing GLIC–ρIII.
To remove the 78 residue GABAρ M3–M4 loop from GLIC-ρI and replace it with the tri-peptide (SQP) sequence of GLIC M3–M4 loop, AgeI and SacII restriction sites were introduced at the C-terminal end of M3 and N-terminal end of M4 respectively in pUNIV-GLIC-ρI. A double-stranded oligonucleotide, encoding the tri-peptide M3–M4 loop of GLIC, was custom synthesized with AgeI and SacII restriction sites on its 5’ and 3’ end, respectively. The oligonucleotide insert and the pUNIV-GLIC-ρI vector with the introduced restriction sites were double digested with AgeI and SacII restriction enzymes. The insert was ligated to the vector overnight to obtain a chimeric construct with the tri-peptide M3–M4 loop. This construct was called GLIC-ρII (Fig. 1 B). The chimeric construct, GLIC-ρIII, was made by introducing a point mutation, W328M (ρ numbering) in the M3 helix of GLIC-ρII using site-directed mutagenesis (Fig. 1 C). All of the constructs were verified by double stranded DNA sequencing.
2.2 GLIC-ρ electrophysiology
GLIC, GABAρ, GLIC-ρI, II and III chimeric ion channels were expressed in Xenopus laevis oocytes and functionally characterized using two-electrode voltage clamp. Heterologous expression of channel proteins in Xenopus laevis oocytes is a well-established and widely used approach for measuring drugs effects on ion channel function. The large size of the oocytes, their ability to express large numbers of channel proteins and the relative absence of endogenous channels that might complicate analysis of electrophysiological measurements make them an ideal model system. (Stühmer and Parekh, 1995). Capped cRNAs were made by transcribing linearized GLIC, GABAρ, GLIC-ρI, II and III chimeric subunits in pUNIV using the mMessage mMachine T7 kit (Life Technologies (Ambion), Carlsbad, CA). Oocytes were obtained from an in-house Xenopus colony and prepared as described previously (Boileau et al., 1998; Ghosh et al., 2013). Briefly, oocytes were injected 24 hours after harvest with 27 nl of cRNA at 100–500 ng/l concentration. Injected oocytes were incubated at 16° C in ND96 (5 mM HEPES pH 8.5, 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) supplemented with 100 µg/ml of gentamycin and 100 µg/ml of bovine serum albumin for 2–5 days before use for electrophysiological recordings.
Two electrode voltage clamp electrophysiology was performed as described previously (Boileau et al., 1998; Ghosh et al., 2013). Oocytes expressing the respective channels were voltage clamped at −60 mV (for GLIC) or −80 mV (for GABAρ and GLIC-ρI, II and III) and continuously perfused with ND96 at pH 8.5 at a flow rate of 5 ml/min in a bath volume of 200µl. Borosilicate glass electrodes (Warner Instruments, Hamden, CT) were filled with 3 M KCl and had resistances of 0.4 to 1.0 MΩ. Electrophysiological data were collected at room temperature using GeneClamp 500 (Axon Instruments, Foster City, CA) interfaced to a computer with a Digidata 1200 A/D device (Axon Instruments). Data acquisition and analysis was performed using the Whole Cell Program, version 4.0.2 (provided by J. Dempster, University of Strathclyde, Glasgow, UK).
Proton induced currents from GLIC and GLIC-ρI, II and III chimeric channels were measured by perfusing ND96 buffered at various pHs (pH 7.6 - 2.5). We used three different buffers to ensure optimal buffering capacity for the different pH solutions needed to activate GLIC and the chimeric channels. For pH > 6.5, 5 mM HEPES (pKa = 7.55, buffering range 6.8–8.2) was used. For pH6.5 - 6.0, 5mM MES (pKa = 6.16, buffering range 5.5–6.7) was used. For pH 5.0 - 2.5, 5mM Na Citrate (pKa = 6.4, buffering range 3.0–6.2) was used. GABA-induced currents from GABAρ receptors were measured by perfusing GABA dissolved in ND96 at pH 7.5.
2.3 Reversal potential and ion replacement
Reversal potential (Erev) of GLIC, GABAρ, GLIC-ρII and GLIC-ρIII channels were determined by measuring GABA- or proton-induced currents from oocytes clamped at voltages between −80mV and −20mV for GABAρ, GLIC-ρII and GLIC-ρIII and between −60mV and +20mV for GLIC. To test whether GLIC-ρ ion channel is selective for [Cl−], Erev was measured in extracellular solutions where NaCl was replaced with 96 mM NaGluconate. The relative permeability of GABAARs for Gluconate and Cl−, , is reported in different sources to be 0.05 and 0.2 (O'Toole and Jenkins, 2011; Zhang et al., 1991). Using these values, the expected shift in reversal potential, ΔErev, upon replacing extracellular Cl− with Gluconate, was calculated from the following equation, assuming the membrane was exclusively permeable to anions:
| (1) |
where Erev2 and Erev1 are reversal potentials in 96 mM NaGluconate and 96 mM NaCl respectively, [Gluconate]out2, [Cl−]out2, [Cl−]out1, are the extracellular [Gluconate] in 96 mM NaGluconate, extracellular [Cl−] in 96 mM NaGluconate and extracellular [Cl−] in 96 mM NaCl (ND96) respectively. The expected ΔErev was compared with the measured ΔErev to validate anion-selectivity of the chimeric channels.
2.4 Concentration response curves
Proton dose–response curves were obtained by applying successive pH buffer jumps from pH 8.5 to pH 2.5, separated by 3 – 7 min washes (Ghosh et al., 2013). Biphasic pH dose response data were fit using Prism software (GraphPad) to the equation:
| (2) |
where, I is the peak response at a given pH, Imax is the maximum amplitude of current, pH501 and pH502 are the pH inducing half maximal response for each component, and nH1 and nH2 are the Hill coefficients of each components, Fraction indicates the relative contribution of the 1st component to the response.
GLIC–ρIII, which contains the W328M substitution, was activated by pentobarbital (PB) in the absence of external acidic pH. PB concentration response curves were measured by applying 4–5 PB concentrations between 30 µM – 10 mM at room temperature, separated by 3–7 minute washes. At high µM concentrations and above, PB blocks current responses. The relief of channel block upon drug washout yields a rebound tail current. At high PB concentrations, PB tail current amplitudes were measured. PB dose responses were fit using Prism software (GraphPad) with the following one-site equation:
| (3) |
where I is the peak current elicited by a given [PB], Imax is the maximum PB elicited current amplitude, EC50 is the [PB] eliciting half maximal response and nH is the Hill coefficient.
2.5 Modulation of GLIC-ρII and III, GABAρ and GLIC by allosteric drugs
Drug modulation of the various ion channels were measured as described previously (Ghosh et al., 2013; Sancar and Czajkowski, 2011). We tested pentobarbital (PB) (Sigma, St.Louis, MO), 5-αTetrahydrodeoxycorticosterone (5α–THDOC) (Steraloids, Newport, RI), 5β–THDOC (Steraloids, Newport, RI), etomidate and propofol (Sigma, St.Louis, MO) modulation of GLIC-ρII, GLIC-ρIII, GLIC and GABAρ currents. We measured equilibrium agonist-mediated currents in the presence and absence of the drug tested. First a concentration of agonist that elicited 20–30% of the maximum current was applied alone. When agonist-elicited current was stable, the perfusion was switched to a solution containing the same concentration of agonist along with appropriate concentrations for the drug to be tested, until a new stable current level was recorded. For GABA-ρ channels, the agonist was first applied alone followed by a buffer wash and then the drug was co-applied with the same concentration of agonist. The continuous method was not used for GABA-ρ because GABA-ρ currents developed with slower kinetics, and it was difficult to accurately measure drug effects on the slowly developing agonist currents. Both methods record equilibrium agonist-elicited currents in the absence and presence of the drug. Modulation was defined as (I+drug/I − 1) × 100, where I is current elicited by agonist in the absence of drug and I+drug is the current elicited when agonist is co-applied with the drug. The concentrations of the various drugs used were: pentobarbital - 100 µM, etomidate - 100 µM, propofol - 100 µM, 5α–THDOC - 10 µM (for GABAρ) or 30 µM (for GLIC and GLIC-ρ chimeras), 5β–THDOC - 10 µM (for GABAρ) or 30 µM (for GLIC and GLIC-ρ chimeras). The concentrations used were based on maximum effective concentrations reported in previous studies on GABAA receptors (Amin, 1999; Bali and Akabas, 2012; Belelli et al., 1999; Hosie et al., 2006; Li et al., 2006; Rusch et al., 2004).
2.6 Statistics
Data visualization and statistical significance tests were performed using Prism 7 software (GraphPad Software Inc., San Diego, CA). All data sets were from ≥3 oocytes from at least 2 different frogs. Significant differences in reversal potential and drug modulation between GLIC, GABAρ and GLIC-ρ chimeras were determined by one-way analysis of variance followed by a post-hoc Tukey test. Modulation of the constructs by different drugs was determined to be significantly different from zero effect by one-sample t-test.
3. Results
3.1 GLIC-GABAρ chimeric subunits form functional channels
GLIC-GABAρ chimeric subunits were generated by fusing the ECD of GLIC with the TMD of GABAρ at a conserved arginine residue in the pre-M1 region (Fig. 1A, Arg 191 in GLIC and Arg 258 in GABAρ). GLIC-ρI contained the entire TMD of GABAρ. For GLIC-ρII, we replaced the ρ subunit M3–M4 loop (78 amino acids) with the 3 residue GLIC M3–M4 loop (SQP, Fig. 1B). For GLIC-ρIII, Trp328 (ρ numbering) in the M3 helix of GLIC-ρII was mutated to methionine (W328M, Fig. 1C). This single amino acid substitution confers barbiturate sensitivity to GABAρ receptors (Amin, 1999).
All of the GLIC-GABAρ chimeric subunits formed proton-activated channels when expressed in Xenopus laevis oocytes indicating that the GLIC ECD is functionally coupled to the GABAρ TMD (Fig. 1). Expression of the chimeric subunits gave rise to higher than normal resting leak currents. Picrotoxin (2 mM), an open channel blocker of GABAρ receptors (Wang et al., 1995), elicited outward currents indicating that a proportion of the chimeric channels were constitutively open at pH 8.5, which accounted for the high resting conductance (Fig. 1). The ratio of pH 3-induced currents to constitutive (leak) current amplitudes was 0.6 ± 0.1 for GLIC–ρI (8 oocytes) and 3 ± 1 for GLIC-ρII (5 oocytes) indicating that GLIC–ρII channels were less leaky than GLIC-ρI channels. In general, the proton-activated currents from GLIC-ρII and GLIC–ρIII were larger and, rose and decayed faster than GLIC-ρI channel currents (Fig. 1). Due to these enhanced functional properties, the rest of our analyses were focused on GLIC-ρII and GLIC–ρIII chimeric channels.
3.2 Ion selectivity of the GLIC-ρ chimeric channels
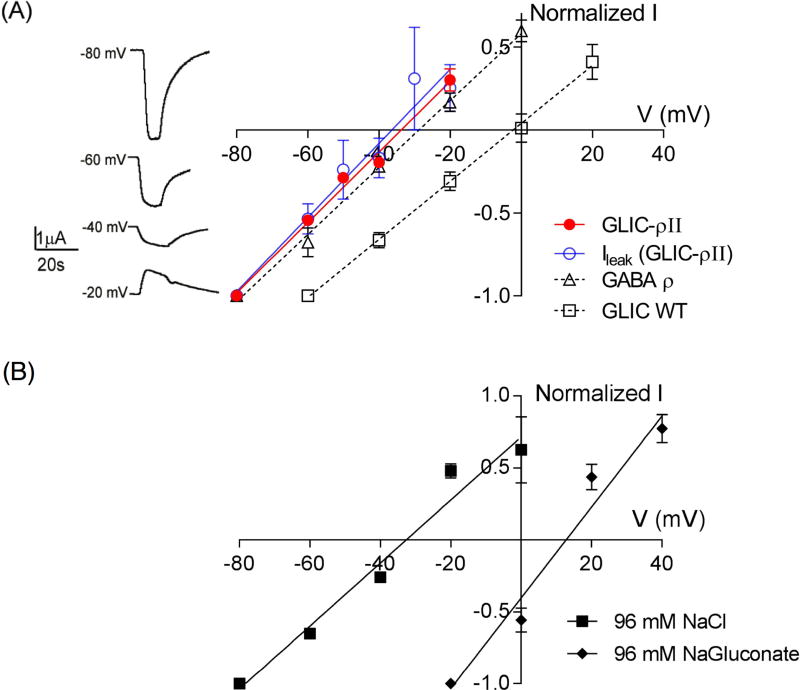
Homomeric GABAρ receptors are Cl− conducting channels, whereas GLIC is a mixed cationic channel (Na+/K+) activated by protons. Since the chimeric channels contain the GABAρ TMD, we expected them to be Cl− selective. pH 3-elicited currents from GLIC-ρII and GLIC–ρIII reversed at −34 ± 2 mV (n=5) and −32 ± 3mV (n=5), respectively, similar to the reversal potential of −28 ± 2 mV (n=4) measured for GABAρ receptors (Fig. 2A). GLIC-ρII spontaneous currents at pH 8.5 also reversed near the Cl− reversal potential at −32 ± 5 mV (n=6) (Fig 2A) indicating that the constitutively open chimeric channels were also Cl− selective. In contrast, proton-activated currents from GLIC reversed at −1.2 ± 4 mV (n=4) as expected for a cation-conducting ion channel (Fig. 2A). A one-way ANOVA with post hoc Tukey test showed that GLIC vs GABA-ρ reversal potentials and GLIC vs GLIC-ρ II values were significantly different from each other (p<0.01). GABA-ρ vs GLIC-ρ II values were not different (p=0.74).
Figure 2. GLIC-ρ chimeric channels conduct Cl−.
(A) Left, pH 3 induced currents from an oocyte expressing GLIC-ρII measured at different voltages; right, Current-voltage (I–V) plots for GLIC–ρII (●) GLIC WT (□), GABAρ (Δ), and GLIC-ρII leak currents (○). Reversal potentials measured were: GLIC-ρ II: −34 ± 2 (n=5), GLIC: −1.2 ± 4 mV (n=4); GABAρ: −28 ± 2 (n=4), and GLIC-ρII leak currents: −32 ± 5 mV, (n=6). Data are mean ± SEM. A one-way ANOVA with post hoc Tukey test showed that reversal potentials of GABAρ, GLIC-ρII and GLIC-ρII leak current were significantly different from GLIC (p<0.01) and that GABAρ, GLIC-ρII and GLIC-ρII leak current reversal potentials were not statistically different from each other (p>0.5). (B) Current-voltage (I–V) plots for GLIC–ρIII measured in presence of 96 mM NaCl (■) and 96 mM NaGluconate (◆). Replacing Cl− with the less permeable gluconate right shifted Erev to +13 mV as expected for a Cl−-conducting channel.
To further demonstrate that GLIC-ρ chimeric channels were Cl− selective, we measured the reversal potential of GLIC-ρ III channels in extracellular solution where NaCl (96 mM) was replaced with 96 mM NaGluconate. Relative gluconate vs Cl− permeability for GABAARs has been reported to range between 5% (Zhang et al., 1991) and 20% (O'Toole and Jenkins, 2011). Using these values and assuming exclusive permeability to anions, we calculated that the estimated shift in Erev for a GABAAR chloride channel on replacing Cl− with the less permeable gluconate anion should be between +35 to +55 mV (See Methods). As expected Erev for GLIC–ρIII currents shifted by +45mV to +13 ± 2 mV (n=3) (Fig. 2B). Our measured +45mV shift provides confirmation that the GLIC-ρ chimera is a Cl− selective ion channel.
3.3 pH responses of GLIC-ρ chimeric channels
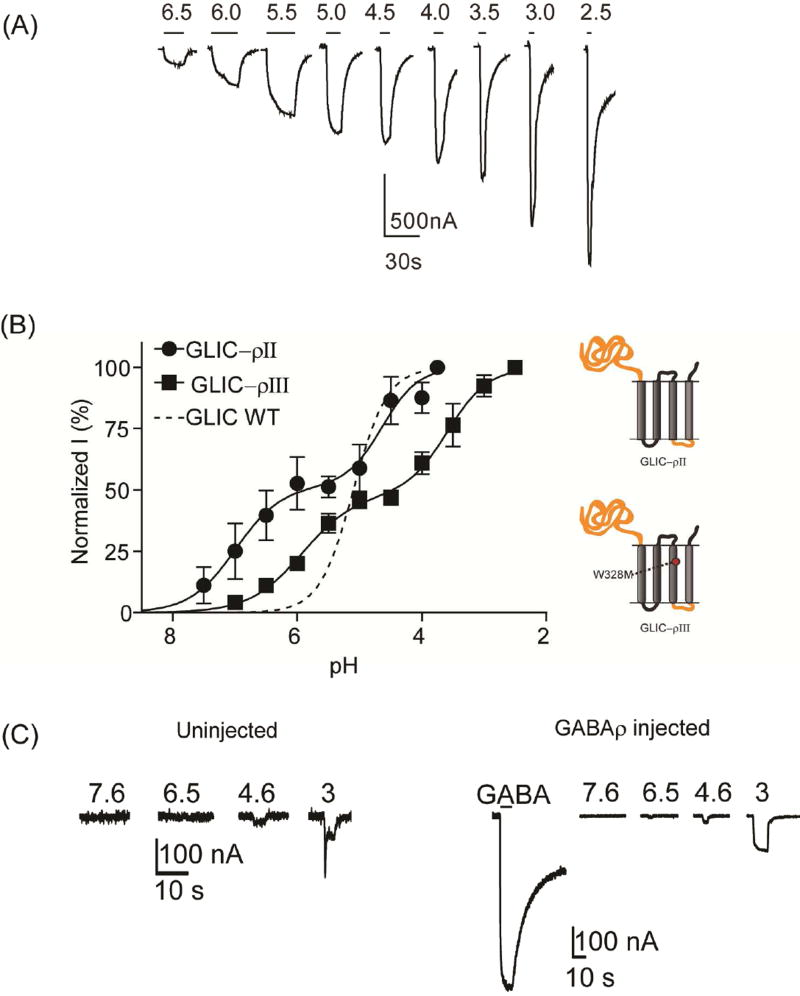
Others and we have shown that wild-type GLIC proton-dependent current responses have a pH50 of ~5 and are well fit with a single-site model (Fig. 3B), (Bocquet et al., 2007; Ghosh et al., 2013; Laha et al., 2013). Surprisingly, proton-induced current responses from oocytes expressing GLIC–ρII were biphasic and best fit with a two-site model with pH50 values of 6.9 ± 0.3 and 4.6 ± 0.3 (Fig. 3B) suggesting that the chimeric channel contains an additional proton sensor. For GLIC–ρIII, the W328M substitution right-shifted the sensitivity of both sites by ~1 pH unit to pH50 5.9 ± 0.1 and 3.5 ± 0.2 (Fig. 3B). pH 3 induced currents from GABAρ receptors were < 200 nA and comparable to currents elicited from uninjected oocytes (Fig. 3C) suggesting that extracellular proton activation of GLIC–ρII and GLIC–ρIII is due to proton sensors located in the GLIC-derived ECD. Determining the precise positions of the proton sensors in the chimeric channels will require further testing, especially since the location of proton binding site(s) in GLIC is still under debate (see Discussion).
Figure 3. Proton concentration response curves.
(A) Representative pH-induced currents from GLIC–ρIII. (B) Proton concentration response curves for GLIC (dashed line, data from Ghosh et al., 2013), GLIC-ρII (●) and GLIC-ρIII (■). GLIC–ρII and GLIC–ρIII concentration responses were biphasic. W328M right-shifted pH response to more acidic values. Data are mean±SEM from ≥ 3 oocytes. (C) pH-induced currents elicited from uninjected oocytes (left) and oocytes expressing GABAρ receptors (right) were significantly smaller (pH 3.0 elicited <200 nA currents) as compared to oocytes expressing GLIC, GLIC–ρII and GLIC–ρIII channels. For GABAρ injected oocytes, current elicited by 10 mM GABA is also shown confirming that the oocyte was expressing GABAρ receptors.
3.4 Pentobarbital modulation and activation of GLIC-ρ chimeric channels
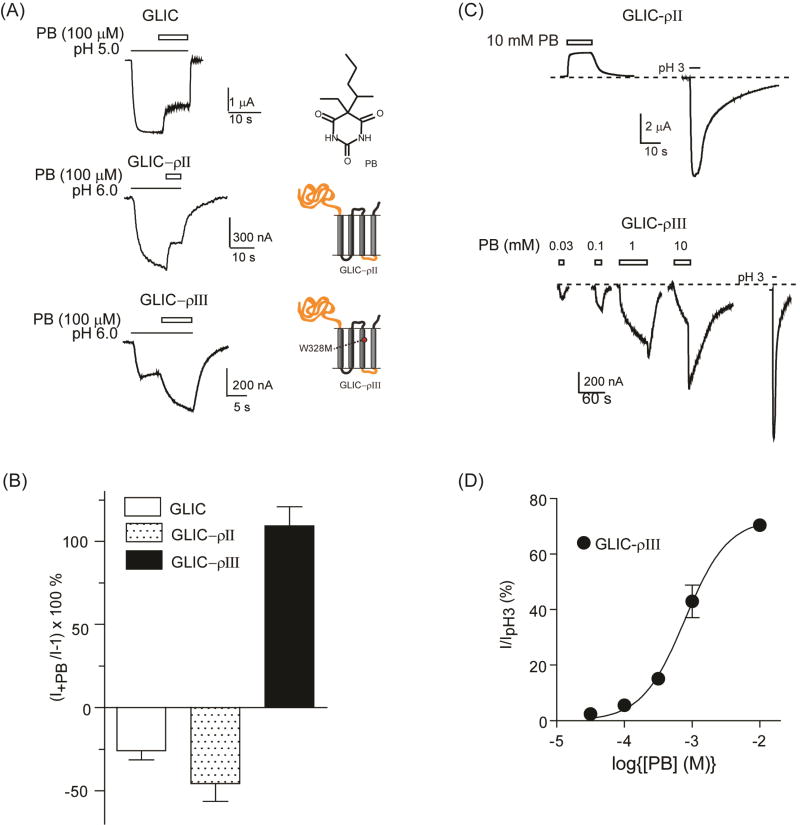
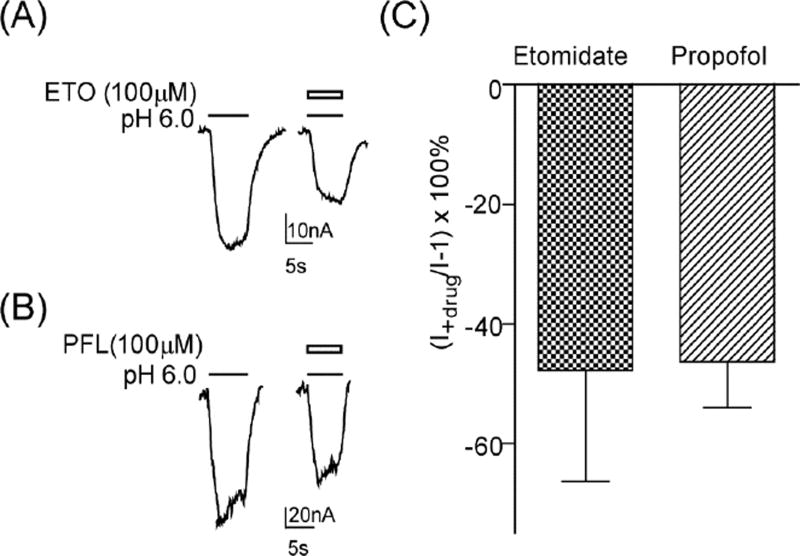
We examined the ability of pentobarbital to modulate and directly activate proton-mediated currents from GLIC and GLIC-ρ chimeric channels. Previous studies showed that homomeric GABAρ receptors are insensitive to pentobarbital except at high channel blocking concentrations (Amin, 1999; Belelli et al., 1999; Shimada et al., 1992), and that a single mutation in M3 (W328M) imparts positive modulation and direct activation by pentobarbital (Amin, 1999). Pentobarbital (100 µM) inhibited proton-mediated currents from GLIC by 26 ± 6 % (n=3, p=0.043 compared to no effect, one sample t-test) and GLIC-ρII by 46 ± 11 % (n=4, p= 0.023) (Fig. 4A, B). At high concentrations, pentobarbital (10 mM) applied alone blocked GLIC–ρII constitutive currents (Fig. 4C). Pentobarbital (100 µM) potentiated proton-induced currents from GLIC–ρIII, which contains the W328M point mutation, by 109 ± 12 % (n=4, p=0.0025) (Fig. 4A, B). Pentobarbital modulation of GLIC–ρIII was significantly different from that of GLIC and GLIC-ρII (p<0.01, one-way ANOVA with Tukey’s multiple comparisons test). We next examined the effect that W328M had on etomidate and propofol modulation of GLIC-ρ chimeric channels. Both etomidate (100 µM) and propofol (100 µM) inhibited proton elicited currents from GLIC-ρII (data not shown) and GLIC–ρIII (Fig. 5). These data indicate that W328M specifically confers pentobarbital positive modulation to the chimeric channels and does not influence the actions of other, structurally distinct, anesthetics.
Figure 4. Pentobarbital modulation and direct activation of GLIC-ρ chimeric channels.
(A) Representative proton induced (EC20–30) currents from oocytes expressing GLIC, GLIC–ρII and GLIC–ρIII in the absence and presence of 100 µM pentobarbital (PB). Black lines and open bars represent agonist (pH 5 for GLIC and pH 6 for GLIC–ρII and GLIC–ρIII) and PB application, respectively. Chemical structure of PB and schematics of chimeras are shown. (B) Summary of PB modulation of EC20–30 currents [(I+PB/I)−1]×100 (%) from GLIC, GLIC–ρII and GLIC–ρIII. PB potentiated GLIC–ρIII proton induced currents whereas PB inhibited GLIC and GLIC–ρII currents. Data are mean±SEM from ≥ 3 oocytes. (C) Top: PB (10 mM, open bars) blocked GLIC–ρII resting leak currents. Dotted line corresponds to resting leak current. pH 3 elicited current from the same oocyte is shown for comparison. Bottom: Representative PB elicited currents from an oocyte expressing GLIC–ρIII. pH 3 elicited current from the same oocyte is shown for comparison. (D) PB concentration response curve for GLIC–ρIII. Peak PB-elicited currents were measured after wash-out (tail currents). PB currents were normalized to maximal current elicited by pH 3.0. PB EC50 = 836 ± 115µM, nH = 1.4 ± 0.1. Data are mean±SEM from 3 oocytes.
Figure 5. Anesthetics inhibit GLIC–ρIII proton-mediated currents.
(A, B) Representative pH 6.0 elicited currents from oocytes expressing GLIC–ρIII in the absence and presence of 100 µM etomidate (ETO) or 100 µM propofol (PFL). (C) Summary of etomidate and propofol inhibition of pH 6.0 currents [(I+drug/I)−1]×100 (%) from GLIC–ρIII. Data are mean±SEM from ≥ 3 oocytes.
When applied alone, 30 µM to 10 mM pentobarbital directly activated GLIC–ρIII (Fig 4C). Following washout, pentobarbital at concentrations of 1 mM and above gave rise to a rebound current (i.e. transient increase in current, Fig. 4C) indicating that GLIC–ρIII channels were blocked by high mM concentrations of pentobarbital. Unlike proton-induced channel opening, pentobarbital activated currents were fit with a single site dose response curve with an EC50 of 836 ± 115 µM (n=3) (Fig. 4D) similar to the pentobarbital potency measured for GABAρ W328M receptors (Amin 1999). Overall, pentobarbital modulation and activation of the GLIC-ρ chimeric channels emulated GABAAR pharmacology suggesting that the structural machinery mediating pentobarbital actions is contained in the TMD.
3.5 Neurosteroid modulation of GLIC-ρ chimeric channels
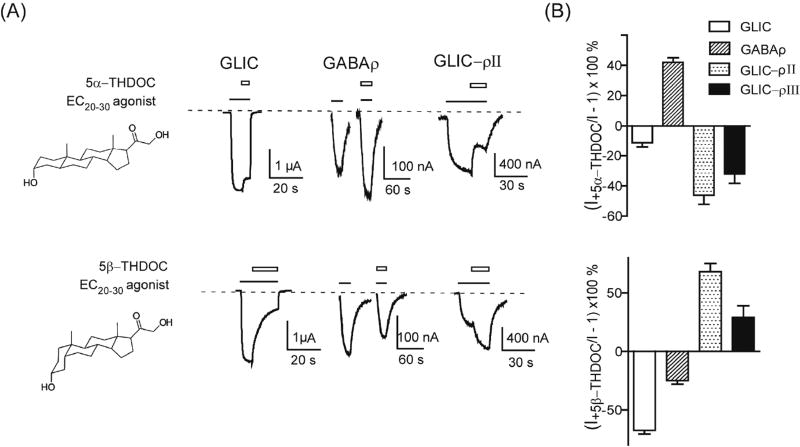
Neurosteroids also modulate pLGIC activity by binding in the GABAAR TMD (Akk et al., 2008; Bracamontes et al., 2012; Hosie et al., 2006). To test whether the GLIC-ρ chimeric channels retained GABAAR neurosteroid pharmacology, we measured and compared neurosteroid modulation of agonist-mediated currents from GLIC, GABAρ receptors and GLIC-ρ chimeric channels. At 30 µM, both 5α-THDOC and 5β-THDOC inhibited GLIC proton-mediated currents by 11 ± 2 % (n=4, p=0.01) and 67 ± 3 % (n=3, p= 0.002) respectively and acted as negative allosteric modulators (Fig. 6). For GABAρ receptors, 10 µM 5α-THDOC was a positive allosteric modulator and potentiated GABA currents by 42 ± 2 % (n=3, p=0.002) whereas 10 µM 5β-THDOC was a negative modulator and inhibited GABA currents by 25 ± 2 % (n=3, p=0.005) (Fig. 6). Surprisingly, even though GLIC–ρII and GLIC–ρIII chimeric channels contain the GABAρ receptor TMD, the effects of the neurosteroid isomers were opposite to their effects on GABAρ receptors. 5α-THDOC (30 µM) inhibited GLIC–ρII and GLIC–ρIII chimeric channels by 46 ± 6 % (n=5, p=0.002) and 32 ± 6 % (n=5, p=0.006) respectively whereas 5β-THDOC (30 µM) potentiated proton-induced currents from GLIC–ρII and GLIC–ρIII chimeric channels by 68 ± 7 % (n=4, p=0.002) and 29 ± 6 % (n=3, p=0.035) respectively (Fig. 6). At 30 µM, THDOC had no observable agonist-like actions from GABAρ receptors and GLIC-ρ chimeric channels. At concentrations higher than 30µM, solubility of THDOC decreased and thus precluded our testing THDOC for agonist-like and blocking actions. Overall, the data suggest that neurosteroid modulation of the chimeric GLIC-ρ channels do not simply mimic their effects on GABAρ channels and that across domain ECD-TMD interactions shape neurosteroid modulation.
Figure 6. Neurosteroid modulation of GLIC–ρ chimeras.
(A) Representative agonist induced (EC20–30) currents from oocytes expressing GLIC, GABAρ, and GLIC–ρII in the absence and presence of neurosteroid isomers 5α-THDOC (upper panel) and 5β-THDOC (lower panel). Black bars represent agonist application (pH 5 for GLIC, 1µM GABA for GABAρ and pH 6 for GLIC–ρII). Open bars represent neurosteroid application, 30µM for GLIC and GLIC–ρII and 10µM for GABAρ. (B) Summary of neurosteroid modulation of EC20–30 currents from GLIC, GABAρ, GLIC–ρII and GLIC–ρIII, [(I+DRUG/I)−1]×100 (%). 5α-THDOC potentiated EC20–30 agonist mediated GABAρ currents but inhibited GLIC, GLIC–ρII and GLIC–ρIII currents. 5β-THDOC inhibited EC20–30 agonist mediated GABAρ and GLIC currents but potentiated GLIC–ρII and GLIC–ρIII currents. Data are mean±SEM from ≥ 3 oocytes.
4. Discussion
Here, we report the construction and functional characterization of GLIC-GABAρ chimeric pLGICs. Consistent with previous studies (Alqazzaz et al., 2017; Bartos et al., 2009; Bouzat et al., 2008; Bouzat et al., 2004; Cooper et al., 1999; Duret et al., 2011; Eisele et al., 1993; Grutter et al., 2005a; Grutter et al., 2005b; Henault and Baenziger, 2017; Moraga-Cid et al., 2015; Schmandt et al., 2015; Tillman et al., 2014), our data underscore the modular nature of pLGICs. Several properties of the GABAρ TMD were preserved in our chimeric channels. First, the chimeric channels were Cl− selective the same as GABAρ receptors. Second, GLIC–ρ chimeric channel currents were blocked by picrotoxin and inhibited by the general anesthetics pentobarbital, etomidate and propofol similar to GABAρ receptors. Third, a point mutation, ρ TMD W328M, which confers positive modulation and direct gating by pentobarbital on GABAρ receptors (Amin, 1999), had the same effect on the chimeric channels. Notably, the pentobarbital EC50 we measured in our experiments (840 mM) was very similar to the EC50 for pentobarbital direct activation of GABAρ W328M (802 mM) reported by Amin (Amin, 1999). Since the chimeric channels have a high spontaneous open probability, the direct gating can be interpreted as positive modulation of spontaneously open channels. We envision that pentobarbital shifts the channel equilibrium towards an open state in GLIC-ρIII, which can explain both positive modulation and apparent direct activation. Consistent with this idea, the allosteric co-agonist model postulated by the Forman group posits that enhancement of GABAAR gating explains both positive modulation and direct activation by the allosteric activators etomidate (Rusch et al., 2004), propofol (Ruesch et al., 2012) and pentobarbital (Ziemba and Forman, 2016). Taken together, our data indicate that many of the functional and pharmacological properties of the GABAρ-TMD were preserved in the chimera.
However, some properties of the chimeric channels were distinct compared to GLIC and GABAρ receptors. The GLIC-GABAρ chimeric channels had unexpected biphasic proton concentration response curves (Fig. 3). Since GABAρ channels are not proton activated and GLIC proton concentration responses are well fit with a single-site model, these data suggest that the chimeric channels contain two proton sensors with different sensitivities. The lower affinity site (pH50 = 4.6) has similar proton sensitivity as GLIC (pH50 = 5) (Bocquet et al., 2007; Ghosh et al., 2013) suggesting that this site stems from the GLIC-derived ECD. The higher affinity site (pH50 = 6.9) may represent a novel sensor that is formed in the chimera. Alternatively, it is possible that GLIC has more than one pH-sensing site with similar proton sensitivities. In the GLIC-GABAρ chimeric channels, the affinity of one of the proton sensors may be left-shifted, which leads to the biphasic proton response. Regardless of whether a new proton sensor is created or proton sensitivity of an existing site is altered, the biphasic proton dose response curves stem from mismatched interactions between the GLIC ECD and GABAρ TMD. These data provide support for the idea that agonist-mediated pLGIC gating transitions are regulated by specific interdomain interactions between the ECD and TMD (Bertozzi et al., 2016; Gupta et al., 2017; Lee and Sine, 2005; Xiu et al., 2005).
The precise location of the proton sensor in GLIC is still not clear (see (Alqazzaz et al., 2017; Duret et al., 2011; Henault and Baenziger, 2017; Schmandt et al., 2015; Wang et al., 2012)). Chimeric receptors containing the ECD of GLIC and the TMD of proton-insensitive pLGICs are activated by protons (Alqazzaz et al., 2017; Duret et al., 2011; Henault and Baenziger, 2017) suggesting that a proton sensor is located in the GLIC ECD. However, chimeric channels formed by fusing the proton-insensitive ECD of ELIC and TMD of GLIC are also activated by protons (Henault and Baenziger, 2017; Schmandt et al., 2015) suggesting that there is a proton binding site(s) in the GLIC TMD. Protonation of a histidine residue in the TMD of GLIC has been shown to be crtical for GLIC activation suggesting it is a proton sensor in GLIC (Rienzo et al., 2014; Wang et al., 2012). We envision that multiple proton binding sites exist in GLIC with different sensitivities that are uncovered in different receptor configurations. Consistent with this idea, mutation of protonatable residues in the ECD had minimal effects on proton activation in GLIC but larger effects in a GLIC-ELIC chimera (Alqazzaz et al., 2017).
The biphasic proton concentration responses we observed in our GLIC-ρ chimeras have not been reported for other GLIC containing chimeras. For a GLIC-glycine receptor chimera (Duret et al., 2011) and a GLIC-ELIC chimera (Alqazzaz et al., 2017) the pH50’s of activation were reported to be 6.5 and 6.7, respectively; similar to the higher affinity site we measured for GLIC-ρII channels (pH50 = 6.9). A lower affinity proton site was not observed in these studies but might have been missed since current responses to pH changes more acidic than pH 5 were not measured. Henault and Baenziger (2017) also examined a GLIC-ELIC chimera (Henault and Baenziger, 2017) and reported a reduced pH sensitivity with a pH50 of 3.63. Taken together, these data indicate that proton sensitivity of GLIC channel activation is sensitive to perturbations of the ECD-TMD interface.
Effects of neurosteroid isomers on GLIC-ρ chimera channel activity were also different compared to their actions on GLIC and GABAρ receptors. 5α-THDOC is a positive allosteric modulator and 5β-THDOC is a negative modulator of GABA ρ receptors (Fig. 6 and (Li et al., 2007; Morris et al., 1999)). Surprisingly, in GLIC-ρII and GLIC-ρIII, current modulation by 5α–THDOC and a 5β–THDOC was switched to inhibiting and potentiating, respectively (Fig. 6) demonstrating that positive versus negative neurosteroid modulation is not encoded exclusively by neurosteroid isomer structure (Li et al., 2007; Morris et al., 1999) but is also dependent on specific interdomain connections between the ECD and TMD.
The underlying mechanisms of differential modulation of GABAρ receptor by neurosteroid isomers are not clear. While some studies posit that distinct sites on the GABAρ receptors are responsible for the potentiating and inhibitory actions of 5α-reduced and 5β-reduced neurosteroid isomers (Li et al., 2006), neurosteroid structure activity relationships point towards overlapping binding sites (Li et al., 2007). In a voltage clamp fluorimetry study, dependent on whether it acted as positive or negative modulator, 5β pregnanolone induced different fluorescence changes in GABAρ receptors suggesting that allosteric mechanisms underlying neurosteroid potentiation and inhibition are distinct (Eaton et al., 2014). We envision that, depending on their orientation in the binding site, structurally diverse neurosteroids can induce different downstream allosteric rearrangements, which result in positive or negative modulatory activity. Our data show that interdomain interactions between the ECD and TMD also regulate whether neurosteroids positively or negatively modulate receptor activity revealing a new role for the coupling interface in controlling neurosteroid modulation. In agreement with our findings, the pharmacological properties ELIC-α7 nAChR chimeric channels can be tuned to resemble either ELIC or α7 by exchanging loops in the ECD-TMD coupling interface (Tillman et al., 2014).
In summary, we constructed a chimeric GLIC-GABAρ receptor that was gated by acidic extracellular pH like GLIC and conducted Cl− like GABAρ channels. These data support the idea that pLGICs have a modular architecture and demonstrate that functional coupling between the ECD and TMD of pLGICs from evolutionarily distant species can be accomplished with minimal engineering of the interface. Agonist-mediated currents were inhibited by pentobarbital, etomidate and propofol suggesting that the structural machinery mediating general anesthetic modulation resides in the TMD. Proton-activation and neurosteroid THDOC modulation of the chimeric GLIC-ρ receptor, however, did not simply mimic their respective actions on GLIC and GABAρ receptors underscoring the importance of the ECD-TMD coupling interface in regulating not only agonist mediated gating but also neurosteroid allosteric modulation. Our results, which identify that across domain interactions between the ECD and TMD can govern whether a neurosteroid potentiates or inhibits channel function, provide new insights into the structural mechanisms underlying neurosteroid allosteric modulation. Moreover, our data indicate that chimeric channels may not always display the same properties as its parent component domains, and a case-by-case examination of function and pharmacology is necessary.
Highlights.
A chimeric GLIC/GABAρ pentameric ligand gated ion channel was made and studied.
Like GABAρ, chimeric channels were Cl− conducting and inhibited by anesthetics.
Anesthetic modulation of current is mediated locally in domain where it binds.
Neurosteroid modulation of chimeric channels was swapped compare to GABAρ.
Interactions between extracellular and channel domains shape neurosteroid actions.
Acknowledgments
This work was supported, in whole or in part, by National Institutes of Health Grant 34727 (NINDS; to C. C).
Abbreviations
- pLGIC
pentameric ligand-gated ion channel
- GABAAR
gamma amino butyric acid type A receptor
- GLIC
Gloeobacter ligand-gated ion channel
- ECD
extracellular domain
- TMD
transmembrane domain
- PB
pentobarbital
- THDOC
Tetrahydrodeoxycorticosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqazzaz M, Thompson AJ, Price KL, Breitinger HG, Lummis SC. Cys-loop receptor channel blockers also block GLIC. Biophys J. 2011;101:2912–2918. doi: 10.1016/j.bpj.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqazzaz MA, Price KL, Lummis SCR. The Proton Responsiveness in the Extracellular Domain of GLIC Differs in the Presence of the ELIC Transmembrane Domain. Biochemistry. 2017;56:2134–2138. doi: 10.1021/acs.biochem.6b00900. [DOI] [PubMed] [Google Scholar]
- Althoff T, Hibbs RE, Banerjee S, Gouaux E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature. 2014;512:333–337. doi: 10.1038/nature13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J. A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Mol Pharmacol. 1999;55:411–423. [PubMed] [Google Scholar]
- Baenziger JE, Corringer PJ. 3D structure and allosteric modulation of the transmembrane domain of pentameric ligand-gated ion channels. Neuropharmacology. 2011;60:116–125. doi: 10.1016/j.neuropharm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bali M, Akabas MH. Gating-induced conformational rearrangement of the gamma-aminobutyric acid type A receptor beta-alpha subunit interface in the membrane-spanning domain. J Biol Chem. 2012;287:27762–27770. doi: 10.1074/jbc.M112.363341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Corradi J, Bouzat C. Structural basis of activation of cys-loop receptors: the extracellular-transmembrane interface as a coupling region. Mol Neurobiol. 2009;40:236–252. doi: 10.1007/s12035-009-8084-x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Pau D, Cabras G, Peters JA, Lambert JJ. A single amino acid confers barbiturate sensitivity upon the GABA rho 1 receptor. Br J Pharmacol. 1999;127:601–604. doi: 10.1038/sj.bjp.0702611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi C, Zimmermann I, Engeler S, Hilf RJ, Dutzler R. Signal Transduction at the Domain Interface of Prokaryotic Pentameric Ligand-Gated Ion Channels. PLoS Biol. 2016;14:e1002393. doi: 10.1371/journal.pbio.1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux JP, Corringer PJ. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Kucken AM, Evers AR, Czajkowski C. Molecular dissection of benzodiazepine binding and allosteric coupling using chimeric gamma-aminobutyric acidA receptor subunits. Mol Pharmacol. 1998;53:295–303. doi: 10.1124/mol.53.2.295. [DOI] [PubMed] [Google Scholar]
- Bouzat C, Bartos M, Corradi J, Sine SM. The interface between extracellular and transmembrane domains of homomeric Cys-loop receptors governs open-channel lifetime and rate of desensitization. J Neurosci. 2008;28:7808–7819. doi: 10.1523/JNEUROSCI.0448-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, Taylor P, Sine SM. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature. 2004;430:896–900. doi: 10.1038/nature02753. [DOI] [PubMed] [Google Scholar]
- Bracamontes JR, Li P, Akk G, Steinbach JH. A neurosteroid potentiation site can be moved among GABAA receptor subunits. J Physiol. 2012;590:5739–5747. doi: 10.1113/jphysiol.2012.237255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Cecchini M, Changeux JP. The nicotinic acetylcholine receptor and its prokaryotic homologues: Structure, conformational transitions & allosteric modulation. Neuropharmacology. 2015;96:137–149. doi: 10.1016/j.neuropharm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Chen Q, Cheng MH, Xu Y, Tang P. Anesthetic binding in a pentameric ligand-gated ion channel: GLIC. Biophys J. 2010;99:1801–1809. doi: 10.1016/j.bpj.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ST, Harkness PC, Baker ER, Millar NS. Up-regulation of cell-surface alpha4beta2 neuronal nicotinic receptors by lower temperature and expression of chimeric subunits. J Biol Chem. 1999;274:27145–27152. doi: 10.1074/jbc.274.38.27145. [DOI] [PubMed] [Google Scholar]
- Dellisanti CD, Ghosh B, Hanson SM, Raspanti JM, Grant VA, Diarra GM, Schuh AM, Satyshur K, Klug CS, Czajkowski C. Site-directed spin labeling reveals pentameric ligand-gated ion channel gating motions. PLoS Biol. 2013;11:e1001714. doi: 10.1371/journal.pbio.1001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Lu W, Wu S, Cheng Y, Gouaux E. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature. 2015;526:224–229. doi: 10.1038/nature14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret G, Van Renterghem C, Weng Y, Prevost M, Moraga-Cid G, Huon C, Sonner JM, Corringer PJ. Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci U S A. 2011;108:12143–12148. doi: 10.1073/pnas.1104494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton MM, Lim YB, Covey DF, Akk G. Modulation of the human rho1 GABAA receptor by inhibitory steroids. Psychopharmacology (Berl) 2014;231:3467–3478. doi: 10.1007/s00213-013-3379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele JL, Bertrand S, Galzi JL, Devillers-Thiery A, Changeux JP, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- Fourati Z, Ruza RR, Laverty D, Drege E, Delarue-Cochin S, Joseph D, Koehl P, Smart T, Delarue M. Barbiturates Bind in the GLIC Ion Channel Pore and Cause Inhibition by Stabilizing a Closed State. J Biol Chem. 2017;292:1550–1558. doi: 10.1074/jbc.M116.766964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B, Satyshur KA, Czajkowski C. Propofol Binding to the Resting State of the Gloeobacter violaceus Ligand-gated Ion Channel (GLIC) Induces Structural Changes in the Inter- and Intrasubunit Transmembrane Domain (TMD) Cavities. J Biol Chem. 2013;288:17420–17431. doi: 10.1074/jbc.M113.464040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter T, de Carvalho LP, Dufresne V, Taly A, Edelstein SJ, Changeux JP. Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc Natl Acad Sci U S A. 2005a;102:18207–18212. doi: 10.1073/pnas.0509024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter T, Prado de Carvalho L, Virginie D, Taly A, Fischer M, Changeux JP. A chimera encoding the fusion of an acetylcholine-binding protein to an ion channel is stabilized in a state close to the desensitized form of ligand-gated ion channels. C R Biol. 2005b;328:223–234. doi: 10.1016/j.crvi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chakraborty S, Vij R, Auerbach A. A mechanism for acetylcholine receptor gating based on structure, coupling, phi, and flip. J Gen Physiol. 2017;149:85–103. doi: 10.1085/jgp.201611673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, Moreau C, Li XD, Poitevin F, Vogel H, Nury H. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature. 2014;512:276–281. doi: 10.1038/nature13552. [DOI] [PubMed] [Google Scholar]
- Henault CM, Baenziger JE. Functional characterization of two prokaryotic pentameric ligand-gated ion channel chimeras - role of the GLIC transmembrane domain in proton sensing. Biochim Biophys Acta. 2017;1859:218–227. doi: 10.1016/j.bbamem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Laha KT, Ghosh B, Czajkowski C. Macroscopic kinetics of pentameric ligand gated ion channels: comparisons between two prokaryotic channels and one eukaryotic channel. PLoS One. 2013;8:e80322. doi: 10.1371/journal.pone.0080322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- Li W, Covey DF, Alakoskela JM, Kinnunen PK, Steinbach JH. Enantiomers of neuroactive steroids support a specific interaction with the GABA-C receptor as the mechanism of steroid action. Mol Pharmacol. 2006;69:1779–1782. doi: 10.1124/mol.106.022863. [DOI] [PubMed] [Google Scholar]
- Li W, Jin X, Covey DF, Steinbach JH. Neuroactive steroids and human recombinant rho1 GABAC receptors. J Pharmacol Exp Ther. 2007;323:236–247. doi: 10.1124/jpet.107.127365. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014;512:270–275. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci. 2010;31:161–174. doi: 10.1016/j.tips.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Moraga-Cid G, Sauguet L, Huon C, Malherbe L, Girard-Blanc C, Petres S, Murail S, Taly A, Baaden M, Delarue M, Corringer PJ. Allosteric and hyperekplexic mutant phenotypes investigated on an alpha1 glycine receptor transmembrane structure. Proc Natl Acad Sci U S A. 2015;112:2865–2870. doi: 10.1073/pnas.1417864112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KD, Moorefield CN, Amin J. Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids. Mol Pharmacol. 1999;56:752–759. [PubMed] [Google Scholar]
- Nemecz A, Prevost MS, Menny A, Corringer PJ. Emerging Molecular Mechanisms of Signal Transduction in Pentameric Ligand-Gated Ion Channels. Neuron. 2016;90:452–470. doi: 10.1016/j.neuron.2016.03.032. [DOI] [PubMed] [Google Scholar]
- Nury H, Delarue M, Corringer PJ. X-ray structures of general anesthetics bound to their molecular targets. Med Sci (Paris) 2011;27:1056–1057. doi: 10.1051/medsci/20112712006. [DOI] [PubMed] [Google Scholar]
- O'Toole KK, Jenkins A. Discrete M3–M4 intracellular loop subdomains control specific aspects of gamma-aminobutyric acid type A receptor function. J Biol Chem. 2011;286:37990–37999. doi: 10.1074/jbc.M111.258012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Chen Q, Willenbring D, Mowrey D, Kong XP, Cohen A, Divito CB, Xu Y, Tang P. Structure of the pentameric ligand-gated ion channel GLIC bound with anesthetic ketamine. Structure. 2012a;20:1463–1469. doi: 10.1016/j.str.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Chen Q, Willenbring D, Yoshida K, Tillman T, Kashlan OB, Cohen A, Kong XP, Xu Y, Tang P. Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine. Nat Commun. 2012b;3:714. doi: 10.1038/ncomms1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzo M, Lummis SC, Dougherty DA. Structural requirements in the transmembrane domain of GLIC revealed by incorporation of noncanonical histidine analogs. Chem Biol. 2014;21:1700–1706. doi: 10.1016/j.chembiol.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesch D, Neumann E, Wulf H, Forman SA. An allosteric coagonist model for propofol effects on alpha1beta2gamma2L gamma-aminobutyric acid type A receptors. Anesthesiology. 2012;116:47–55. doi: 10.1097/ALN.0b013e31823d0c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA A receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–20992. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- Sancar F, Czajkowski C. Allosteric modulators induce distinct movements at the GABA-binding site interface of the GABA-A receptor. Neuropharmacology. 2011;60:520–528. doi: 10.1016/j.neuropharm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Shahsavar A, Delarue M. Crystallographic studies of pharmacological sites in pentameric ligand-gated ion channels. Biochim Biophys Acta. 2015;1850:511–523. doi: 10.1016/j.bbagen.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Sauguet L, Shahsavar A, Poitevin F, Huon C, Menny A, Nemecz A, Haouz A, Changeux JP, Corringer PJ, Delarue M. Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc Natl Acad Sci U S A. 2014;111:966–971. doi: 10.1073/pnas.1314997111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmandt N, Velisetty P, Chalamalasetti SV, Stein RA, Bonner R, Talley L, Parker MD, McHaourab HS, Yee VC, Lodowski DT, Chakrapani S. A chimeric prokaryotic pentameric ligand-gated channel reveals distinct pathways of activation. J Gen Physiol. 2015;146:323–340. doi: 10.1085/jgp.201511478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini R, Bracamontes J, Steinbach JH. Structural domains of the human GABAA receptor 3 subunit involved in the actions of pentobarbital. J Physiol. 2000;524(Pt 3):649–676. doi: 10.1111/j.1469-7793.2000.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Cutting G, Uhl GR. gamma-Aminobutyric acid A or C receptor? gamma-Aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol Pharmacol. 1992;41:683–687. [PubMed] [Google Scholar]
- Spurny R, Billen B, Howard RJ, Brams M, Debaveye S, Price KL, Weston DA, Strelkov SV, Tytgat J, Bertrand S, Bertrand D, Lummis SC, Ulens C. Multi-Site Binding Of A General Anesthetic To The Prokaryotic Pentameric Ligand-Gated Ion Channel ELIC. J Biol Chem. 2013 doi: 10.1074/jbc.M112.424507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurny R, Ramerstorfer J, Price K, Brams M, Ernst M, Nury H, Verheij M, Legrand P, Bertrand D, Bertrand S, Dougherty DA, de Esch IJ, Corringer PJ, Sieghart W, Lummis SC, Ulens C. Pentameric ligand-gated ion channel ELIC is activated by GABA and modulated by benzodiazepines. Proc Natl Acad Sci U S A. 2012;109:E3028–3034. doi: 10.1073/pnas.1208208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W, Parekh AB. Electrophysiological Recordings from Xenopus Oocytes. In: Sakmann B, Neher E, editors. Single-Channel Recording. Springer US; Boston, MA: 1995. pp. 341–356. [Google Scholar]
- Thompson AJ, Alqazzaz M, Ulens C, Lummis SC. The pharmacological profile of ELIC, a prokaryotic GABA-gated receptor. Neuropharmacology. 2012;63:761–767. doi: 10.1016/j.neuropharm.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman TS, Seyoum E, Mowrey DD, Xu Y, Tang P. ELIC-alpha7 Nicotinic acetylcholine receptor (alpha7nAChR) chimeras reveal a prominent role of the extracellular-transmembrane domain interface in allosteric modulation. J Biol Chem. 2014;289:13851–13857. doi: 10.1074/jbc.M113.524611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velisetty P, Chalamalasetti SV, Chakrapani S. Structural basis for allosteric coupling at the membrane-protein interface in Gloeobacter violaceus ligand-gated ion channel (GLIC) J Biol Chem. 2014;289:3013–3025. doi: 10.1074/jbc.M113.523050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalan SP, Bushman JD, Mercado JL, Sancar F, Christopherson KR, Boileau AJ. Optimized expression vector for ion channel studies in Xenopus oocytes and mammalian cells using alfalfa mosaic virus. Pflugers Arch. 2007;454:155–163. doi: 10.1007/s00424-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Cheng X, Sine SM. Intramembrane proton binding site linked to activation of bacterial pentameric ion channel. J Biol Chem. 2012;287:6482–6489. doi: 10.1074/jbc.M111.305839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TL, Hackam AS, Guggino WB, Cutting GR. A single amino acid in gamma-aminobutyric acid rho 1 receptors affects competitive and noncompetitive components of picrotoxin inhibition. Proc Natl Acad Sci U S A. 1995;92:11751–11755. doi: 10.1073/pnas.92.25.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y, Yang L, Corringer PJ, Sonner JM. Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesth Analg. 2010;110:59–63. doi: 10.1213/ANE.0b013e3181c4bc69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Hanek AP, Wang J, Lester HA, Dougherty DA. A unified view of the role of electrostatic interactions in modulating the gating of Cys loop receptors. J Biol Chem. 2005;280:41655–41666. doi: 10.1074/jbc.M508635200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Spigelman I, Carlen PL. Development of GABA-mediated, chloride-dependent inhibition in CA1 pyramidal neurones of immature rat hippocampal slices. J Physiol. 1991;444:25–49. doi: 10.1113/jphysiol.1991.sp018864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba AM, Forman SA. Correction for Inhibition Leads to an Allosteric Co-Agonist Model for Pentobarbital Modulation and Activation of alpha1beta3gamma2L GABAA Receptors. PLoS One. 2016;11:e0154031. doi: 10.1371/journal.pone.0154031. [DOI] [PMC free article] [PubMed] [Google Scholar]