Abstract
Objective
Marine polyunsaturated n-3 fatty acids (n-3 PUFA) may have cardioprotective effects and beneficial influence on the fibrotic process. We evaluated the associations between serum marine n-3 PUFA and selected biomarkers of fibrosis and cardiac remodeling in elderly patients with acute myocardial infarction.
Setting
From the ongoing OMega-3 fatty acids in Elderly patients with Myocardial Infarction (OMEMI) trial, 299 patients were investigated. Soluble ST2 (sST2), Galectin-3 (Gal-3) and the serum content of major marine n-3 and n-6 PUFA were analyzed 2–8 weeks after the index acute myocardial infarction.
Results
Gal-3 was inversely correlated to eicosapentaenoic acid (r = −.120, p = .039) and docosahexaenoic acid (r = −.125, p = .031) and positively correlated to the n-6/n-3 ratio (r = .131, p = .023). Gal-3 levels were significantly higher in diabetics vs non-diabetics (12.00 vs 9.61 ng/mL, p = .007) and in patients with NYHA class ≥III for dyspnea at inclusion (11.33 vs 9.75 ng/mL, p = .006).
Conclusions
The associations between the marine n-3 PUFA and levels of Gal-3 indicate beneficial effects of n-3 PUFA on cardiac remodeling in an elderly population with acute myocardial infarction.
Keywords: Elderly, heart failure, acute myocardial infarction, cardiac remodeling, marine n-3 fatty acids
Introduction
The tissue repair process after an acute myocardial infarction (AMI) involves scar formation, fibrosis, and structural and functional changes, leading to post-infarction ventricular remodeling and possibly to the progression of heart failure (HF).1
Galectin-3 (Gal-3) is a multifunctional protein that is widely expressed in different cell types. The macrophage is considered to be the main contributor to Gal-3 production,2 and the protein has previously been linked to fibrosis formation.3 It has been mostly studied in patients with HF, however with conflicting results.4 Several in vivo animal experiments have shown its importance in post-myocardial infarction fibrosis and remodeling, but only a few clinical studies, with limited cohort size, have reported on its effect on cardiac remodeling after AMI.5
The ST2 receptor has been identified as a member of the interleukin (IL)-1 receptor family and was initially found on type-2 helper T cells.6 IL-33/ST2 synthesized by cardiac fibroblasts has been suggested to comprise a cardioprotective paracrine system, reducing cardiomyocyte fibrosis and hypertrophy, with the biological effects of IL-33 downregulated by soluble ST2 (sST2).7 In this way, elevated levels of sST2 attenuates the positive effects of IL-33. sST2 is easily detectable in human serum and has been related to myocardial dysfunction, fibrosis, and remodeling,8 and shown to be a strong predictor of adverse outcomes in AMI, coronary heart disease (CHD), and HF.9–11
Observational studies and large-scale clinical trials indicate that marine omega-3 polyunsaturated fatty acids (n-3 PUFA) have beneficial influence on CHD, HF, and cardiac remodeling and fibrosis.12–16
The aim of the present study was therefore to evaluate the associations between major marine n-3 and n-6 PUFA and selected biomarkers of fibrosis and cardiac remodeling in elderly patients with AMI. Our hypothesis was that serum phospholipid levels of n-3 PUFA were beneficially associated with the pattern of these markers. Whether these markers were related to disease entities in this population and presumed to influence HF was further investigated.
Materials and methods
Study design
The present study was a substudy of the OMega-3 fatty acids in Elderly patients with Myocardial Infarction (OMEMI) trial, and its design has previously been described in detail.17 In short, the study is a Norwegian prospective randomized placebo-controlled multicenter trial evaluating the effect of a 2-year intervention with n-3 PUFA supplementation (1.8 g/d) on cardiovascular endpoints in elderly patients, aged 70–82 years, having suffered an AMI (types 1–4), and without co-morbidity thought to be incompatible with study drugs or a 2-year follow-up. Compliance is secured by measurement of fatty acids in serum phospholipids.
Diabetes mellitus was defined as insulin dependent or non-insulin dependent. HF was defined as treated for/or diagnosed with HF, either previously or during the index AMI. Atrial fibrillation (AF) was defined as a history of ECG-documented paroxystic, persistent or permanent AF and smokers were defined as current smokers. The study was carried out in compliance with the Helsinki Declaration and approved by the Regional Ethics Committee (2012/1422). All subjects gave their written informed consent to participate (ClinicalTrials.gov, NCT01841944). The present results are based on the participants included into the study from November 2012 to October 2014 (n = 299).
Methods
Baseline characteristics were obtained and blood samples for Gal-3, sST2, N-terminal brain natriuretic peptide (NT-proBNP), and serum fatty acids were collected at inclusion, i.e. 2–8 weeks after the AMI. Blood samples were collected in fasting state (>10 h) by standard venipuncture between 08:00 and 11:00 am, before daily intake of medication. Serum was prepared by centrifugation within 1 h at 2500 g for 10 min, and kept frozen (−80℃) until analyzed. Routine blood samples, including NT-proBNP, were determined with conventional methods.
Gal-3 levels were determined in serum using the Quantikine Human Galectin-3 immunoassay (R&D Systems, Minneapolis, US) with a coefficient of variation (CV) of 8.5%. Presage ST2 Assay (Critical Diagnostics, San Diego, US) was used to determine serum levels of sST2 with a CV of 10.9 %.
Fatty acid composition of serum phospholipids was analyzed by gas chromatography at the Lipid Research Laboratory, Aalborg University Hospital, Denmark, as previously described in detail.18 Briefly, serum lipids were extracted by the Folch procedure,19 and separation of phospholipids fatty acid fraction from total lipids was performed by the method of Burdge et al.20 The serum content of linoleic acid (LA) (18:2, n-6), arachidonic acid (AA) (20:4, n-6), eicosapentaenoic acid (EPA) (20:5, n-3), and docosahexaenoic acid (DHA) (22:6 n-3) was expressed as percent of total fatty acids (wt%) and the CVs were 0.4%, 0.6%, 1.1%, and 1.8%, respectively.
Statistics
As most data had a skewed distribution, the results are presented as median values (25, 75 percentiles) or as absolute numbers and percentages. Non-parametric statistics were used throughout. For group comparison, Mann-Whitney U test was used for continuous variables. Analyses of correlations were performed with Spearman's rho. Linear regression was performed on log transformed data. A two-tailed value of p ≤ 0.05 was considered statistically significant. The statistical analyses were performed with IBM SPSS Statistics, version 21.0.0.2 (IBM, New York, US)
Results
Characteristics of the study population at inclusion are shown in Table 1. The median age was 75 years, 70% were male, 23% had diabetes, 14% were current smokers, 12% had a history of HF, and 13% were in New York Heart Association (NYHA) class ≥3 for dyspnea. Previous intake of n-3 PUFA supplements prior to inclusion was reported in 45% of patients.
Table 1.
Characteristics of the study cohort; data presented as percentages or median values (25, 75 percentiles).
| Age (y) | 75 (72, 78) |
| Gender (male/female) (%) | 70.2/29.8 |
| Smoker (current/previous) (%) | 13.7/46.8 |
| Previous hypertension (%) | 182 (60.9) |
| Previous atrial fibrillation (%) | 65 (21.7) |
| Previous myocardial infarction (%) | 90 (30.1) |
| Previous heart failure (%) | 36 (12.0) |
| Previous diabetes (%) | 69 (23.1) |
| Body mass index (kg/m2) | 25.6 (23.8, 28.3) |
| S-total cholesterol (mmol/L) | 3.70 (3.20, 4.20) |
| S-LDL (mmol/L) | 2.00 (1.60, 2.40) |
| S-HDL (mmol/L) | 1.24 (1.00, 1.54) |
| S-triglycerides (mmol/L) | 1.15 (0.86, 1.54) |
| Creatinine (umol/L) | 90.0 (80.0, 108.0) |
| NT-proBNP (pmol/L) (n = 173) | 75.0 (33.0, 162.5) |
| STEMI (%) | 94 (31.4) |
| Troponin-T (peak level) (ng/L)a | 700 (153, 2500) |
| NYHA class ≥III for dyspnea (%) | 40 (13,4) |
| Galectin-3 (ng/mL) | 9.96 (7.95, 12.81) |
| sST2 (ng/mL) | 29.17 (23.81, 35.77) |
| Medication: | |
| Aspirin (%) | 93.6 |
| Clopidogrel (%) | 41.8 |
| Prasugrel (%) | 11.7 |
| Ticagrelor (%) | 36.5 |
| Anticoagulation (%) | 13.4 |
| Betablocker (%) | 91.3 |
| ACE-I/AT II blocker (%) | 59.9 |
| Calcium channel blocker (%) | 21.1 |
| Statin (%) | 96.6 |
| Diuretic (%) | 27.4 |
| Nitrates (%) | 12.0 |
| n-3 PUFA supplements (%) | 45.6 |
ACE-I/AT II: angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; S-LDL: low density lipoprotein; S-HDL: high-density lipoprotein; STEMI: ST-segment elevation myocardial infarction; LVEF: left ventricular ejection fraction.
aAt index infarction.
Serum fatty acids as related to markers of fibrosis and cardiac remodeling
The proportions of the n-6 PUFA, AA, and LA and the major marine n-3 PUFA, EPA, and DHA, and the AA + LA / EPA + DHA (n-6/n-3) ratio (Table 2) have previously been described in this population.18
Table 2.
Median values of the major marine n-3 and n-6 PUFA (wt%) in serum phospholipids at inclusion.
| Wt% | |
|---|---|
| Eicosapentaenoic acid (EPA) n-3 (20:5) | 2.4 |
| Docosahexaenoic acid (DHA) n-3 (22:6) | 5.6 |
| Linoleic acid (LA) n-6 (18:2) | 19.0 |
| Arachidonic acid (AA) n-6 (20:4) | 9.6 |
| n-6/n-3 ratio | 3.6 |
Wt%: percent of total fatty acids in serum phospholipids.
The main results are shown in Table 3. Gal-3 was inversely correlated to the content of EPA (p = .039) and DHA (p = .031) in serum phospholipids and positively correlated to the n-6/n-3 ratio (p = .023). There were no significant correlations between the levels of sST2 and any of the fatty acids or the n-6/n-3 ratio (p ≥ .098 for all). No significant differences were observed in levels of Gal-3 or sST2 between patients reporting intake of n-3 PUFA supplements (n = 135) or not prior to enrollment (data not shown). However, patients with NYHA class ≥III for dyspnea were shown to have significantly lower levels of EPA (2.08 vs 2.51, p = .008) and a higher n-6/n-3 ratio (4.08 vs 3.52, p = .034).
Table 3.
Coefficients of correlationsa between the major marine n-3 and n-6 PUFA (% of total fatty acids in serum phospholipids) and the measured markers of cardiac remodeling and fibrosis.
| Gal-3 | sST2 | ||
|---|---|---|---|
| Eicosapentaenoic acid (EPA) n-3 (20:5) | r = p = | −.120 .039 | −.096 .098 |
| Docosahexaenoic acid (DHA) n-3 (22:6) | r = p = | −.125 .031 | −.058 .318 |
| Linoleic acid (LA) n-6 (18:2) | r = p = | .026 .659 | .055 .341 |
| Arachidonic acid (AA) n-6 (20:4) | r = p = | .096 .098 | −.080 .166 |
| n-6/n-3 ratio | r = p = | .131 .023 | .079 .175 |
Statistically significant values in bold.
Spearmans Rho are given.
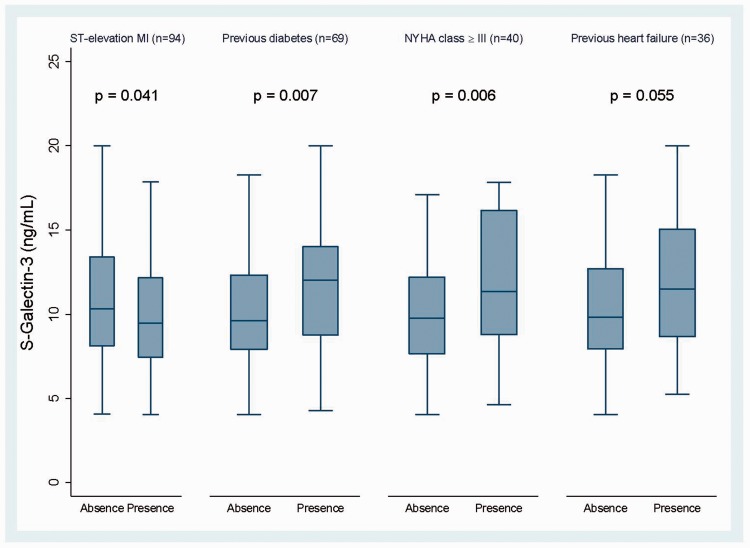
Gal-3 levels were significantly higher in patients with non-ST elevation myocardial infarction (NSTEMI) vs ST-segment elevation myocardial infarction (STEMI) (p = .041), in diabetics vs non-diabetics (p = .007), in patients with NYHA class ≥ III (p = .006) for dyspnea and borderline significantly higher in patients with previous HF (n = 36) (p = .055) (Figure 1, Table 4). NT-proBNP at inclusion correlated significantly with levels of sST2 (r = .158, p = .038), but not with Gal-3 (r = .018, p = .81). None of these markers associated with age, but sST2 levels were significantly lower in females (Table 4).
Figure 1.
Comparisons of Galectin-3 levels in relevant disease states. Standardized box-plot of galectin-3 levels according to presence or absence of the selected disease states. Values defined by the median line, and 25 and 75 percentile represented by the lower and upper border of the box, respectively. Outliers are excluded.
Table 4.
Serum levels of the measured biomarkers as related to relevant disease entities which may influence post-MI cardiac remodeling.
| Gal-3 (ng/mL) |
sST2 (ng/mL) |
|||||
|---|---|---|---|---|---|---|
| ÷ | + | p | ÷ | + | p | |
| Female gender (n = 89) | 9.79 | 10.67 | .318 | 30.56 | 25.49 | .000 |
| ST-segment elevation MI (index) (n = 94) | 10.29 | 9.46 | .041 | 29.17 | 28.95 | .496 |
| Previous heart failure (n = 36) | 9.83 | 11.50 | .055 | 28.74 | 31.44 | .066 |
| Previous diabetes mellitus (n = 69) | 9.61 | 12.00 | .007 | 28.86 | 29.71 | .320 |
| NYHA class ≥ III for dyspnea (baseline) (n = 40) | 9.75 | 11.33 | .006 | 28.34 | 31.51 | .050 |
| Previous n-3 PUFA supplementation (n = 135) | 9.89 | 9.99 | .949 | 29.76 | 27.99 | .134 |
Statistically significant values in bold. +: presence of risk factors or disease entities; ÷: absence of risk factors or disease entities.
When further exploring the relationship between Gal-3 and n-3 PUFAs, a multivariate linear regression model, adjusting for relevant covariates (diabetes and STEMI/NSTEMI) showed that Gal-3 was still significantly associated with DHA (p = .041) and the n-6/n-3 ratio (p = .030), but only borderline significantly to EPA (p = .059).
Discussion
The main finding in this cross-sectional study was a significant association between the levels of Gal-3 and the content of long-chained marine n-3 PUFA in serum phospholipids in a population of elderly patients with a recent AMI. This relationship was still significant after adjusting for type of AMI and diabetes.
To our knowledge, this is the first report showing that Gal-3 levels are inversely associated with marine n-3 PUFA levels and positively associated with the n-6/n-3 ratio in this setting. Ventricular remodeling may continue for weeks or months after an acute loss of myocardium and is considered to be an important factor in HF development.21 The underlying mechanisms of inflammation and fibrosis are crucial in this process. Gal-3 is produced predominantly by macrophages and has been shown to be involved in the activation of fibroblasts into myofibroblasts and secretion of matrix proteins, leading to cardiac fibrosis.5 Circulating Gal-3 has also been introduced as a predictor of reduced left ventricular ejection fraction (LVEF) after 4 months in patients with acute STEMI.22
Long-chain marine n-3 PUFA have been shown to improve outcome in patients with HF in a large clinical trial16 and one study also reported a significantly improved LVEF and functional capacity in dilated cardiomyopathy after 1-year treatment with 2 g/day of n-3 PUFA.23 In an experimental study in mice, dietary n-3 PUFAs were shown to prevent cardiac dysfunction and fibrosis after aortic banding.24 Results from the recent OMEGA-REMODEL trial15 showed a reduction in surrogate markers of cardiac remodeling and fibrosis in AMI patients after 4 -g/day of omega-3 supplementation. This could be discussed in line with our observation of significantly lower levels of EPA and a higher n-6/n-3 ratio in patients with NYHA class ≥III for dyspnea. Interestingly, 18-HEPE, an EPA metabolite, was shown to inhibit macrophage-mediated activation of cardiac fibroblasts in culture.25 Although n-3 PUFA ability to reduce fibrosis and cardiac remodeling is not clearly understood today, one could speculate that this beneficial effect is linked to reduced macrophage activation and inhibited Gal-3 secretion.
We found no significant correlation between any of the selected n-3 or n-6 PUFA and sST2 levels. The existing research on n-3 PUFA and ST2 is very limited. Our results are somewhat in contrast to the results from the OMEGA-REMODEL trial15 in which a reduction of ST2 levels were observed after 6 months’ intervention with high-dose n-3 PUFA.
Gal-3 levels were higher in patients with NYHA class ≥III and with a tendency of higher levels in patients with previous HF, which is in accordance with findings in other study populations.26,27 We could, however, not show any significant association between Gal-3 and NT-proBNP, which reflects impaired cardiac function and remodeling. This is to some degree in accordance with the study of De Boer et al.26 who found that the prognostic value of Gal-3 in HF patients was independent of NT-proBNP, and that serum Gal-3 levels were not significantly different between patients with an LVEF >40% vs those with a lower LVEF.26 According to the authors, natriuretic peptides like NT-proBNP respond readily to ventricular stress, in contrast to Gal-3 which is thought to have a role in interstitial fibrosis.
Interestingly, Gal-3 levels were significantly lower in STEMI compared to NSTEMI patients, although the numerical difference was small. Patients with NSTEMI are known to have worse prognosis and more extensive coronary disease at presentation,28 which could imply that these patients have increased inflammation and fibrosis formation. It should be considered that unlike NT-proBNP, Gal-3 is not limited to cardiac dysfunction as increased plasma levels have been observed in other diseases like restrictive lung disease.29 In addition, it should be emphasized that our results are obtained at 2–8 weeks after the AMI.
Gal-3 levels were also increased in patients with diabetes mellitus. Several studies have found increased levels of Gal-3 in subjects with type-2 diabetes mellitus. However, the possible effects on glucose homeostasis are not clarified.30
Finally, we observed that sST2 was positively correlated with NT-proBNP. These results are in accordance with Rehman et al.10 who found ST2 to be strongly correlated with the severity of HF, LVEF, and NT-proBNP in patients with acute HF.
Conclusions
In an elderly population with a recent AMI, significant inverse correlations were demonstrated between the content of marine n-3 PUFA in serum phospholipids and serum levels of Gal-3 indicating beneficial effects of n-3 PUFA on cardiac remodeling. The findings are to some degree supported by the association found between Gal-3, n-3 PUFA and myocardial function assessed by NYHA class.
Strengths and limitations
The strengths of the study are attributed to the rather large population and serum phospholipid analysis of n-3 PUFA as an objective marker of intake of seafood. Results should, however, be judged with caution considering the small number of patients in the different subgroups. Furthermore, the findings are of exploratory nature and provide no information on any possible causality.
Acknowledgements
We thank the staff at Center for Clinical Heart Research for excellent assistance.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by unrestricted grants from Stein Erik Hagen Foundation for Clinical Heart Research, Oslo, Norway and Olav Thons Foundation, Oslo, Norway.
Ethical approval
Study approved by the Regional Ethics Committee (2012/1422).
Guarantor
Kristian Laake
Contributorship
IS, HA and SS contributed substantially to the design of the study. KL drafted the main manuscript. KL and PM performed data acquisition and analysis. AT, JN and EBS was involved in data collection and lab analysis. IS and SS contributed to statistically analysis. All authors reviewed and revised the manuscript drafts and approved of the final version.
References
- 1.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014; 11: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaspyridonos M, McNeill E, de Bono JP, et al. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol 2008; 28: 433–440. [DOI] [PubMed] [Google Scholar]
- 3.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 2008; 172: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivatsan V, George M, Shanmugam E. Utility of galectin-3 as a prognostic biomarker in heart failure: where do we stand? Eur J Prev Cardiol 2015; 22: 1096–1110. [DOI] [PubMed] [Google Scholar]
- 5.Meijers WC, van der Velde AR, Pascual-Final DA, et al. Galectin-3 and post-myocardial infarction cardiac remodeling. Eur J Pharmacol 2015; 763: 115–121. [DOI] [PubMed] [Google Scholar]
- 6.Xu D, Chan WL, Leung BP, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med 1998; 187: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanada S, Hakuno D, Higgins LJ, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117: 1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol 2015; 115: 3B–7B. [DOI] [PubMed] [Google Scholar]
- 9.Kohli P, Bonaca MP, Kakkar R, et al. Role of ST2 in non-ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 trial. Clin Chem 2012; 58: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehman SU, Mueller T, Januzzi JL., Jr Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 2008; 52: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 11.Shimpo M, Morrow DA, Weinberg EO, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation 2004; 109: 2186–2190. [DOI] [PubMed] [Google Scholar]
- 12.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 1999; 354: 447–455. [PubMed] [Google Scholar]
- 13.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989; 2: 757–761. [DOI] [PubMed] [Google Scholar]
- 14.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med 2011; 364: 2439–2450. [DOI] [PubMed] [Google Scholar]
- 15.Heydari B, Abbasi SA, Shah R, et al. Effect of purified omega-3 fatty acids on reducing left ventricular remodeling after acute myocardial infarction (OMEGA-REMODEL study): a double-blind randomized clinical trial. J Cardiovasc Magn Reson 2015; 17: O7–O7. [Google Scholar]
- 16.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008; 372: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 17.Laake K, Myhre P, Nordby LM, et al. Effects of omega3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study. BMC Geriatr 2014; 14: 74–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laake K, Seljeflot Ir, Schmidt EB, et al. Serum fatty acids, traditional risk factors, and comorbidity as related to myocardial injury in an elderly population with acute myocardial infarction. J Lipid 2016; 2016 DOI: 10.1155/2016/4945720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497–509. [PubMed] [Google Scholar]
- 20.Burdge GC, Wright P, Jones AE, et al. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br J Nutr 2000; 84: 781–787. [PubMed] [Google Scholar]
- 21.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 2000; 101: 2981–2988. [DOI] [PubMed] [Google Scholar]
- 22.van der Velde AR, Lexis CP, Meijers WC, et al. Galectin-3 and sST2 in prediction of left ventricular ejection fraction after myocardial infarction. Clin Chim Acta 2015; 452: 50–57. [DOI] [PubMed] [Google Scholar]
- 23.Nodari S, Triggiani M, Campia U, et al. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol 2011; 57: 870–879. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Shearer GC, Chen Q, et al. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation 2011; 123: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo J, Sano M, Isobe Y, et al. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med 2014; 211: 1673–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 2011; 43: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felker GM, Fiuzat M, Shaw LK, et al. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ Heart Fail 2012; 5: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan MY, Sun JL, Newby LK, et al. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation 2009; 119: 3110–3117. [DOI] [PubMed] [Google Scholar]
- 29.Ho JE, Gao W, Levy D, et al. Galectin-3 is associated with restrictive lung disease and interstitial lung abnormalities. Am J Respir Crit Care Med 2016; 194: 77–83–77–83. DOI: 10.1164/rccm.201509-1753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menini S, Iacobini C, Blasetti FC, et al. Role of Galectin-3 in obesity and impaired glucose homeostasis. Oxid Med Cell Longev 2016; 2016: 9618092–9618092. [DOI] [PMC free article] [PubMed] [Google Scholar]