Abstract
Background:
Methotrexate (MTX) treatment in rheumatoid arthritis (RA) has been associated with lower cardiovascular risk compared to other disease-modifying antirheumatic drugs (DMARDs). We sought to identify whether the MTX-associated cardioprotection involves changes in blood pressure (BP) and/or arterial function.
Methods:
Clinic and 24-hour peripheral and central systolic and diastolic BP (SBP and DBP), augmentation index (AIx), pulse wave velocity (PWV) and plasma asymmetric dimethylarginine (ADMA) were assessed in RA patients on stable treatment with either MTX ± other DMARDs (MTX group, n = 56, age 61 ± 13 years, 70% females) or other DMARDs (non-MTX group, n = 30, age 63 ± 12 years, 76% females). Measurements were performed at baseline and after 8 months.
Results:
After adjusting for visit, age, gender, body mass index, folic acid use and 28-joint disease activity score, the MTX group had significantly lower clinic peripheral SBP (−7.7 mmHg, 95% CI −13.2 to −2.3, p = 0.006) and DBP (−6.1 mmHg, 95% CI −9.8 to −2.4, p = 0.001) and clinic central SBP (−7.8 mmHg, 95% CI −13.1 to −2.6, p = 0.003) and DBP (−5.4 mmHg, 95% CI −9.1 to −1.6, p = 0.005) versus the non-MTX group. Furthermore, the MTX group had significantly lower 24-hour peripheral and central SBP and DBP and PWV versus the non-MTX group (p < 0.01 for all comparisons). By contrast, there were no significant between-group differences in AIx and ADMA.
Conclusions:
RA patients on MTX treatment had significantly lower clinic and 24-hour peripheral and central BP compared to those who did not take MTX. The lower BP with MTX may be related to differences in PWV, but not in AIx or ADMA concentrations. Further longitudinal studies including randomized controlled trials are warranted to confirm these findings, to identify other possible mechanisms responsible for the effects of MTX on BP and PWV, and to establish whether these effects might account for the reduced cardiovascular risk with MTX.
Keywords: asymmetric dimethylarginine, augmentation index, blood pressure, disease-modifying antirheumatic drugs, methotrexate, pulse wave velocity, rheumatoid arthritis
Introduction
Patients with rheumatoid arthritis (RA) are at increased risk of all-cause mortality when compared to the general population.1 The excess mortality in RA is primarily due to fatal cardiovascular events, suggesting a link between RA and cardiovascular disease.2 Although the exact mechanisms responsible for the increased cardiovascular risk in RA patients are unknown, it is postulated that the chronic systemic inflammatory state in RA favours the onset and progression of vascular damage, atherosclerosis and thrombosis.3 These processes are associated with the presence of functional alterations of the arterial wall, such as endothelial dysfunction, increased arterial wave reflection and arterial stiffness. These alterations, which have been previously reported in RA, are attributed to an impaired endothelial synthesis of nitric oxide (NO), which in turn leads to an increase in blood pressure (BP) and cardiac afterload, left ventricular hypertrophy and cardiac dysfunction.4–6 Furthermore, endothelial dysfunction, increased wave reflection and arterial stiffness are strong and independent predictors of adverse cardiovascular outcomes in several patient groups.7–10
Traditional risk factors are also involved in the pathophysiology of cardiovascular disease in RA. A recent systematic review and meta-analysis showed that, similarly to the general population, the presence of hypertension, diabetes, hypercholesterolaemia and obesity significantly increase the risk of myocardial infarction and stroke in this group.11 However, epidemiological studies in RA have shown that the prevalence of hypertension is significantly higher than that of other risk factors.12,13 Furthermore, hypertension is the leading risk factor for the global burden of chronic disease and disability worldwide.14
Methotrexate (MTX), an analogue of the B-vitamin folic acid, is a first-line disease-modifying antirheumatic drug (DMARD) that reduces inflammation, provides symptom control and increases survival in RA patients.15,16 Recent meta-analyses have shown that the use of MTX in RA and other chronic inflammatory states is associated with a significantly lower risk of cardiovascular events, including myocardial infarction.17–19 This suggests that MTX might exhibit specific protective cardiovascular effects.20 Although MTX targets the pro-inflammatory state in RA, it is unknown whether other mechanisms might also account for the reduced cardiovascular risk associated with its use.15,16 Observational studies have shown that MTX treatment is associated with a lower clinic BP and a reduced prevalence of hypertension in RA patients.20–23 However, in no previous study has a comprehensive assessment of BP (i.e. clinic peripheral, central and 24-hour BP) or markers of arterial function (i.e. endothelial function, arterial wave reflection and arterial stiffness) been performed in patient groups matched for clinical and demographic characteristics. This approach would facilitate the identification of the potential mechanisms involved in the protective cardiovascular effects of MTX.
We sought to address these issues by investigating the associations between MTX treatment, clinic and 24-hour peripheral and central BP, and markers of arterial function in a cohort of RA patients.
Methods
Study population and design
We studied a consecutive series of patients with stable RA, aged ⩾18 years, recruited from the outpatient clinics of the Rheumatology Department at Flinders Medical Centre and the Repatriation General Hospital, in the Southern Health Local Heath Network, Adelaide, Australia. RA was diagnosed according to the 1987 American College of Rheumatology or the 2010 American College of Rheumatology/European League Against Rheumatism criteria.24 Study participants were classified as currently treated with MTX for at least 8 weeks (MTX group), or not taking MTX for at least 1 year or being MTX-naïve and treated with other synthetic and/or biologic DMARDs (non-MTX group). Exclusion criteria were atrial fibrillation, active cancer or current treatment with anti-cancer drugs, heart failure and cognitive impairment. The study (registered in the Australian New Zealand Clinical Trials Registry with the registration number ACTRN12616001366448) was approved by the Southern Adelaide Clinical Human Research Ethics Committee (Ethics Approval Number: 76.14). Each participant gave written consent before entering the study.
This observational study of repeated cross-sectional measurements (at baseline and 8-month follow up) allows for the separation of measurement error from true measurement values, thereby effectively increasing sample size compared to a single cross-sectional assessment. Initially, clinical and demographic characteristics, BP and markers of arterial function were assessed in the complete cohort at baseline. Then, a repeat cross-sectional assessment of BP and arterial function was conducted after 8 months. Since both exposed (MTX) and non-exposed (non-MTX) study groups were identified from within the same RA patient cohort and then followed over time, any observed differences in outcome measures between the two groups are more likely to be a result of differences in MTX exposure than differences in other demographic and clinical characteristics. This repeat cross-sectional study design has been frequently used with other longitudinal cohorts including the British Doctors Study,25 the US Nurses’ Health Study26 and the Framingham Study.27
Assessments
Clinical and demographic characteristics
The following data were collected from patient interviews, medical questionnaires, clinical notes and hospital administrative databases: age, gender, medical and medication history, Stanford health assessment questionnaire (HAQ),28 pain visual analogue scale,29 global health score,30 weight, height, body mass index (BMI) and the 28-joint disease activity score (DAS28).31
Biochemical parameters
High-sensitivity C-reactive protein (CRP) was measured in serum by latex-enhanced immunoturbidimetry on an automated Modular PPE Analyzer using instrument conditions and reagents supplied by the manufacturer (Roche Diagnostics, Indianapolis, IN, USA).32 Erythrocyte sedimentation rate was measured using a closed automated method, VES Matic Cube 80 (DIESSE S.p.A., Siena, Italy).33
Measurement of red blood cells (RBCs) MTX polyglutamate concentrations (MTX-PGs), which mediate the intracellular pharmacological effects of MTX and correlate with treatment response,34 was performed using a validated LC-MS/MS assay. Whole blood samples were centrifuged, plasma removed and cells were washed twice with phosphate-buffered saline. Packed cells were then stored at −80°C prior to analysis. Stable isotope internal standard [13C5, 15N, MTX-PG1-5, (M+6); Pepscan, Lelystad, the Netherlands] was added to 0.25 ml packed RBC and proteins were precipitated via the addition of 0.25 ml of 30% perchloric acid. The supernatant was removed, the pH adjusted to 5.5 and MTX-PGs were purified by solid phase extraction (Strata-X Strong Cation Exchange). The eluent was evaporated to dryness and reconstituted in 400 µl of mobile phase A (75:25 acetonitrile:water with 10 mM ammonium bicarbonate) prior to LC separation via HILIC column (ZIC-HILIC Column, Merck Millipore, Billerica, MA, USA). Mobile phase A was as above, and mobile phase B consisted of 40:60 acetonitrile with 10 mM ammonium bicarbonate. The mobile phase was run at 0.4 ml/min, and mobile phase A changed on a linear gradient from 100% to 0% over 6 min, and then returned to 100% and held for 2 min prior to injection of the next sample. Detection was achieved with a Shimazdu 8060 triple quadrupole MS/MS which quantified the five MTX-PGs commonly seen in RA patients, each of which had a limit of detection of <0.2 nmol/l packed RBCs. The total RBC MTX-PG concentration was determined by adding the concentration of each of the five MTX-PGs.
Plasma asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide (NO) synthase,35 was measured using an Acquity UPLC (Waters, Sydney, Australia) coupled to a qToF Premier high-resolution mass spectrometer (Waters, Sydney, Australia). Briefly, 20 µl of sample plasma, calibrator or QC was mixed with 20 µl internal standard solution (containing 1 µM d6-ADMA) for 1 min at 400 rpm. Following the addition of 150 µl 0.1% formic acid in methanol, the sample was vortex mixed for 3 min at 2000 rpm to extract ADMA. Centrifugation at 18,000 × g for 5 min precipitated the proteins; 5 µL of the supernatant layer was injected onto an Atlantis HILIC column (2.1 × 150 mm, 3 µm; Waters, Sydney, Australia) for analysis. A gradient mobile phase consisting of (A) 0.1% v/v formic acid in acetonitrile and (B) 10% v/v acetonitrile, 0.1% v/v formic acid and 10 mM ammonium formate in water was used at a flow rate of 0.4 ml/min. The starting mobile phase was 95% A, 5% B, which was varied linearly over 16 min to 50% A, 50% B then returned to the initial conditions and equilibrated for 4 min prior to injection of the next sample. The qToF Premier mass spectrometer was run in positive ionization mode with data collected using a Waters proprietary MSE data acquisition method at low (CE = 3 V) and a high-energy ramp (CE = 8–14 V). Parent or selected fragment ions were used for detection and quantitation based on their monoisotopic mass.
Clinic peripheral blood pressure
Clinic peripheral systolic (SBP) and diastolic (DBP) BP were measured in the morning, in a quiet environment at room temperature, using the AND automatic BP monitor (model UA-767PC), clinically validated against the British Hypertension Society (BHS) protocols.36 Prior to BP measurement, patients abstained from alcohol for ⩾12 hours, from tobacco and caffeine for ⩾4 hours, and fasted for ⩾6 hours. As per current guidelines,37 participants were advised to sit and relax for at least 5 min before taking the first BP reading. BP was measured three times, with a 2-min interval between measurements. The average of the last two BP measurements was calculated and used in analyses.
Clinic central blood pressure and arterial wave reflection
Clinic central BP was measured non-invasively using Pulse Wave Analysis (PWA, SphygmoCor version 7.1; AtCor Medical, Sydney, Australia).38 This method, clinically validated against invasive techniques such as aortic catheterization,39 uses a tonometer to capture the radial pressure waveforms by flattening the radial artery without occluding it. Then, the radial pressure waveform is mathematically transformed into a central pressure waveform (ascending aortic) using a generalized transfer function, which provides central SBP and DBP values.38 The augmentation index (AIx), a measure of the additional load to which the left ventricle is subject as a result of wave reflection, was calculated as the increment in pressure from the first shoulder in the ascending aortic pressure wave to the peak of this wave, expressed as a percentage of the peak ascending aortic pressure wave.38
Twenty-four-hour peripheral and central blood pressure and arterial stiffness
Twenty-four-hour, day-time (between 08:00 a.m. and 12:00 a.m.), and night-time (between 12:00 a.m. and 08:00 a.m.)40 peripheral and central BP and PWV, the gold-standard marker of arterial stiffness,41 were measured non-invasively using an ambulatory oscillometric BP monitor (Mobil-O-Graph PWA monitor; IEM, Stolberg, Germany), clinically validated against the BHS and the European Society of Hypertension protocols.42–45 The central BP measured using the Mobil-O-Graph PWA monitor is similar to that measured using the SphygmoCor device.46 The measurement is based on the ARCSolver method, which determines aortic BP and PWV using the oscillometric BP technique.47,48 This method is based on a generalized transfer function and three levels of mathematical algorithm. Two separate cycles are necessary to obtain data for the algorithms. In the first cycle, SBP and DBP are measured using a conventional oscillometric method. After the first BP reading, the second cycle begins, where the pulse wave is recorded using a pressure sensor. The sensor measures the strength of the pulse signal during the continuous deflation of the brachial BP cuff from the SBP to zero. Then, a transfer function modifies the frequency range of the obtained signal to generate the aortic pressure wave. Aortic PWV, considered similar to the carotid–femoral PWV, is calculated as the time between the first and second peak of the systolic pressure measured.49
Study endpoints, power sample size and statistical analysis
Clinic peripheral SBP was the primary study endpoint. Clinic peripheral DBP, central BP, AIx, ADMA, 24-hour peripheral and central BP and PWV were secondary endpoints. Based on previous reports of a 3 mmHg SBP difference between patients treated with MTX versus other DMARDs in observational studies,22 the initial target sample size consisted of 400 patients in total, assuming a standard deviation of 14 mmHg and a correlation between repeat measures of r = 0.65. However, this was re-calculated after a preliminary analysis of the baseline BP data after approximately 30 patients showed larger differences in SBP. The revised sample size of 86 patients in total had more than 80% power (α = 0.05) to detect a difference of 7 mmHg in SBP between the MTX and non-MTX groups.
Clinical and demographic characteristics were tested for normality using the Kolmogorov–Smirnov test and then described using means ± SD or medians and interquartile ranges as appropriate. Categorical variables were described using frequencies and percentages. Between-group differences at baseline were assessed using a two-way ANOVA (for normally distributed variables) or Mann–Whitney U test (for non-normally distributed variables). For the repeated cross-sectional analysis, linear mixed models were used to evaluate the overall differences in BP, AIx, PWV and ADMA concentrations according to MTX exposure, as well as differences in the changes over time, which was assessed by including an MTX group X time interaction term in each model. Since eight patients changed treatment during the study, MTX exposure was included as a time-varying covariate. We also compared differences in the estimates obtained from within and between subjects for MTX effects on BP using the Hausman test. Unadjusted results are presented as well as adjusted results with adjustment for age, visit, gender and BMI (model 2) and additional adjustment for (model 3) treatment with folic acid, DAS28 and (for the 24-hour assessment of peripheral and central SBP and DBP and PWV) hour of measurement. Analyses were performed using Stata (version 14.1; StataCorp, Texas, USA). A two-sided p < 0.05 was used to indicate statistical significance.
Results
Clinical and demographic characteristics
Baseline characteristics of the MTX and the non-MTX groups are described in Table 1. The groups were well matched for clinical and demographic characteristics, barring the prevalence of cardiovascular disease and depression, use of the biologic DMARD tocilizumab and DAS28, which were all higher in the non-MTX group. The prevalence of hypertension and other cardiovascular risk factors, treatment with drugs potentially affecting BP and arterial function such as antihypertensive medications, non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, and their doses, and inflammatory markers (ESR and CRP concentration) were similar in the two groups. As expected, in view of the known anti-folate effects of MTX,50 the use of folic acid supplements was more frequent in the MTX group. Both the use and the daily dose of NSAIDs and corticosteroids did not significantly change between the two assessment visits (data not shown).
Table 1.
Baseline clinical and demographic characteristics of the MTX and the non-MTX groups.
| Variables | MTX group (n = 56) |
Non-MTX group (n = 30) |
p-value |
|---|---|---|---|
| Age (years) | 61 ± 13 | 63 ± 12 | 0.71 |
| Females (%) | 69.6 | 76.7 | 0.49 |
| Body mass index (Kg/m2) | 27 ± 6 | 29 ± 7 | 0.27 |
| Current smoking (%) | 16.1 | 10.0 | 0.26 |
| Hypertension (%) | 37.5 | 33.4 | 0.83 |
| Diabetes (%) | 5.3 | 16.7 | 0.09 |
| Dyslipidaemia (%) | 26.8 | 30.0 | 0.75 |
| Previous cardiovascular event (%) | 1.8 | 23.3 | 0.01 |
| Chronic kidney disease (%) | 1.8 | 6.7 | 0.24 |
| Liver disease (%) | 1.8 | 3.4 | 0.65 |
| Depression (%) | 12.5 | 36.7 | 0.009 |
| RA duration (years) | 9.0 [4.0, 20.5] | 15.0 [9.0, 25.5] | 0.06 |
| MTX treatment duration (months) | 75.0 [25.0, 122.0] | – | – |
| MTX dose (mg/week) | 15.0 [10.0, 20.0] | ||
| RBCs MTX-PGs (nmol/l) | 118.8 ± 64.5 | ||
| DAS28 score | 2.7 ± 1.1 | 3.7 ± 1.2 | <0.001 |
| C-reactive protein (mg/l) | 2.1 [0.7, 5.1] | 2.0 [0.7, 4.1] | 0.72 |
| Erythrocyte sedimentation rate (mm/h) | 10.5 [6.0, 20.5] | 10.0 [6.0, 18.5] | 0.60 |
| Anti-cyclic citrullinated peptide (units) | 69.0 [11.5, 100.0] | 100.0 [21.0, 100.0] | 0.34 |
| Stanford HAQ score | 0.31 [0.00, 1.13] | 0.81 [0.22, 1.81] | 0.06 |
| Pain visual analogue score | 0.65 [0.08, 1.29] | 0.61 [0.25, 1.49] | 0.29 |
| Global health score | 0.35 [0.06, 0.85] | 0.61 [0.28, 1.38] | 0.05 |
| Hydroxychloroquine (%) | 25.0 | 23.3 | 0.86 |
| Leflunomide (%) | 8.9 | 0.0 | 0.09 |
| Sulfasalazine (%) | 10.7 | 20.0 | 0.48 |
| Abatacept (%) | 1.8 | 6.7 | 0.60 |
| Rituximab (%) | 0.0 | 3.4 | 0.25 |
| Tocilizumab (%) | 1.8 | 23.3 | 0.01 |
| Adalimumab (%) | 7.1 | 6.7 | 0.93 |
| Etanercept (%) | 17.9 | 23.3 | 0.54 |
| Certolizumab pegol (%) | 1.8 | 0.0 | 0.74 |
| Golimumab (%) | 5.4 | 0.0 | 0.52 |
| Prednisolone (%) | 32.1 | 40.0 | 0.77 |
| Prednisolone daily dose (mg) | 5.00 [3.75, 10.00] | 5.00 [5.00, 13.75] | 0.28 |
| Ibuprofen (%) | 8.9 | 6.6 | 0.72 |
| Ibuprofen daily dose (mg) | 280 ± 110 | 300 ± 100 | 0.85 |
| Aspirin* (%) | 14.3 | 13.3 | 0.90 |
| Antihypertensive drugs (%) | 28.6 | 23.3 | 0.88 |
| Folic acid (%) | 75.0 | 10.0 | <0.001 |
| Fish oil (%) | 32.1 | 26.7 | 0.88 |
DAS, disease activity score 28; HAQ, health assessment questionnaire; MTX, methotrexate; RA, rheumatoid arthritis; RBCs MTX-PGs, red blood cell methotrexate polyglutamates.
Daily dose 100 mg in all patients.
Clinic peripheral and central BP, AIx and ADMA
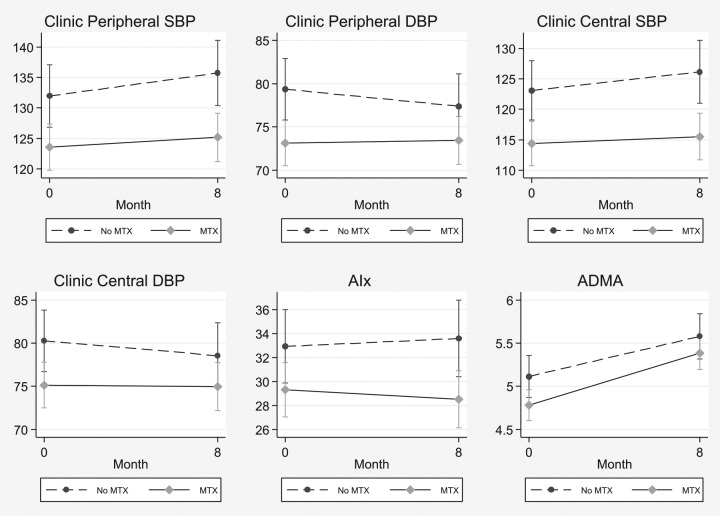
Age-adjusted means (±SE) and the p-value for differences in BP, AIx and ADMA concentrations between the MTX and the non-MTX groups at baseline and follow up are described in Figure 1 and Table 2, respectively. In the overall analysis, with full adjustment (Model 3), the MTX group had significantly lower clinic, but not 24-hour, peripheral and central SBP and DBP when compared to the non-MTX group. By contrast, there were no significant between-group differences in either AIx, PWV or plasma ADMA concentrations (Figure 1 and Table 2).
Figure 1.
Estimated marginal mean clinic peripheral systolic (SBP) and diastolic (DBP) blood pressure, central SBP and DBP, augmentation index (AIx) and plasma asymmetric dimethylarginine (ADMA) concentrations in the MTX and the non-MTX group at baseline and after 8 months.
Table 2.
Unadjusted and adjusted differences (95% CI) in blood pressure, augmentation index, pulse wave velocity and asymmetric dimethylarginine concentrations between the MTX and the non-MTX group.
| Variables | Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| Clinic peripheral SBP (mmHg) | −9.0 (−14.7, −3.3) | 0.002 | −8.1 (−13.4, −2.8) | 0.003 | −7.7 (−13.2, −2.3) | 0.006 |
| Clinic peripheral DBP (mmHg) | −6.0 (−9.8, −2.3) | 0.002 | −5.9 (−9.5, −2.3) | 0.001 | −6.1 (−9.8, −2.4) | 0.001 |
| Clinic central SBP (mmHg) | −9.8 (−15.3, −4.3) | <0.001 | −8.1 (−13.1, −3.0) | 0.002 | −7.8 (−13.1, −2.6) | 0.003 |
| Clinic central DBP (mmHg) | −5.3 (−9.0, −1.5) | 0.006 | −5.1 (−8.7, −1.5) | 0.006 | −5.4 (−9.1, −1.6) | 0.005 |
| 24-hour peripheral SBP (mmHg) | −1.4 (−6.7, 3.9) | 0.60 | −1.1 (−6.2, 4.1) | 0.69 | −2.0 (−7.4, 3.3) | 0.46 |
| 24-hour peripheral DBP (mmHg) | −0.4 (−3.4, 2.6) | 0.81 | −0.5 (−3.4, 2.5) | 0.76 | −1.3 (−4.4, 1.7) | 0.38 |
| 24-hour central SBP (mmHg) | −3.8 (−8.3, 0.6) | 0.09 | −4.0 (−8.4, 0.4) | 0.07 | −4.2 (−8.7, 0.4) | 0.07 |
| 24-hour central DBP (mmHg) | −1.9 (−4.6, 0.9) | 0.18 | −1.8 (−4.5, 0.9) | 0.18 | −1.8 (−4.5, 1.0) | 0.20 |
| Augmentation index (%) | −0.1 (−2.6, 2.4) | 0.93 | 0.4 (−1.9, 2.7) | 0.71 | 0.6 (−1.8, 3.0) | 0.65 |
| Pulse wave velocity (m/sec) | −0.3 (−0.9, 0.2) | 0.25 | −0.2 (−0.5, 0.1) | 0.14 | −0.2 (−0.5, 0.1) | 0.12 |
| Asymmetric dimethylarginine (µmol/l) | −0.21 (−0.47, 0.06) | 0.12 | −0.18 (−0.43, 0.07) | 0.15 | −0.19 (−0.45, 0.07) | 0.15 |
DBP, diastolic blood pressure; MTX, methotrexate; SBP, systolic blood pressure.
Model 1: unadjusted.
Model 2: adjusted for visit, age, gender and BMI.
Model 3: adjusted for visit, age, gender, BMI and disease activity score 28.
When considering the patients switching treatment between the baseline and the 8-month assessments (n = 8), a comparison of the within- versus between-subject effect for MTX exposure on BP showed that the within-subject effects, albeit not significantly different to the between-subject effects using the Hausman test, were generally larger and beneficial for those on MTX treatment (data not shown).
Twenty-four-hour peripheral and central BP and PWV
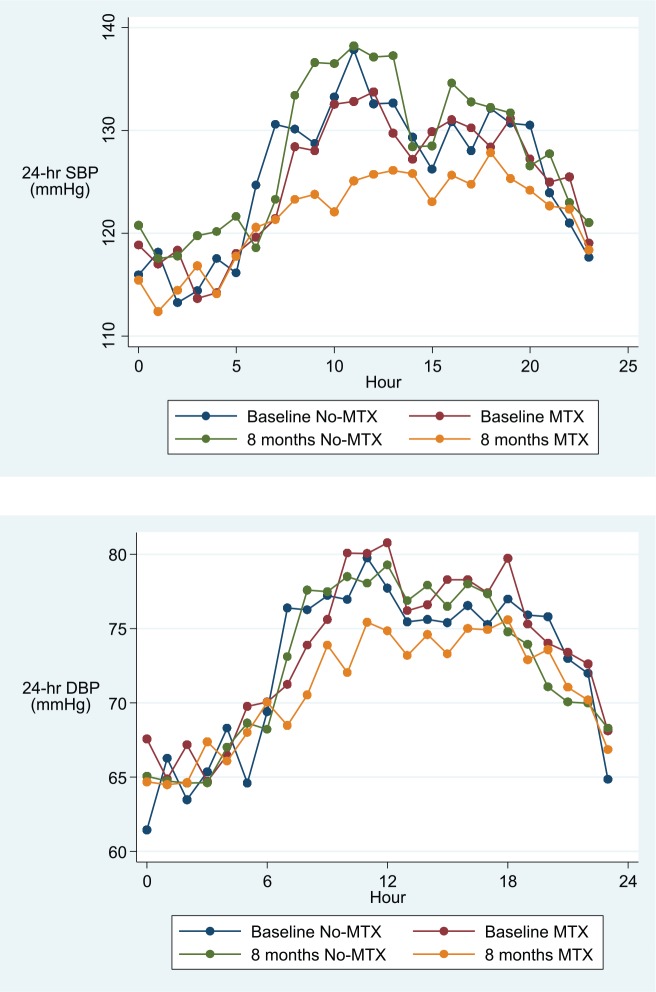
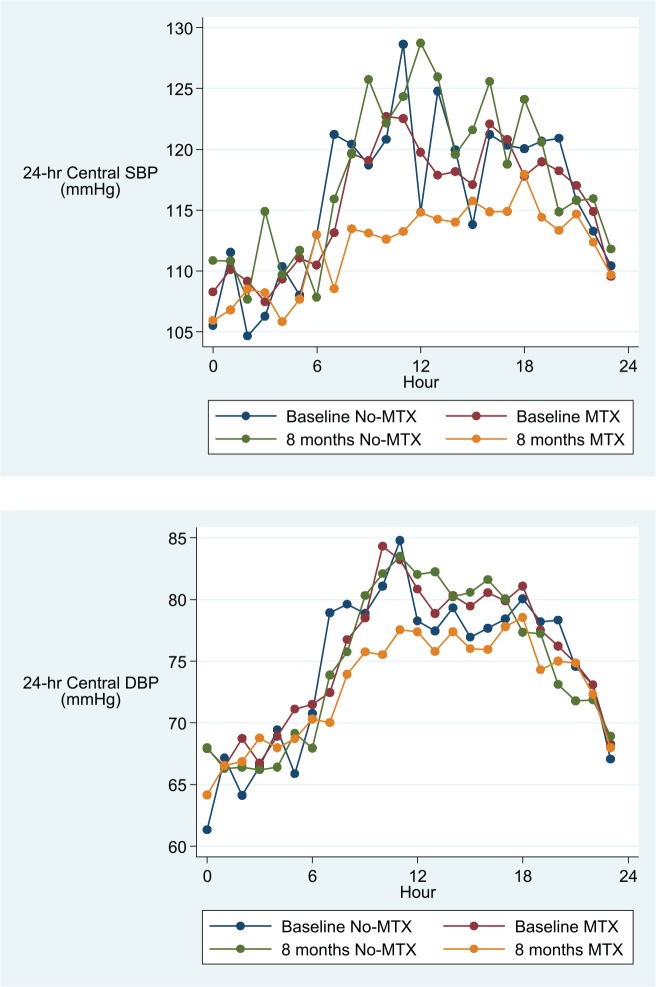
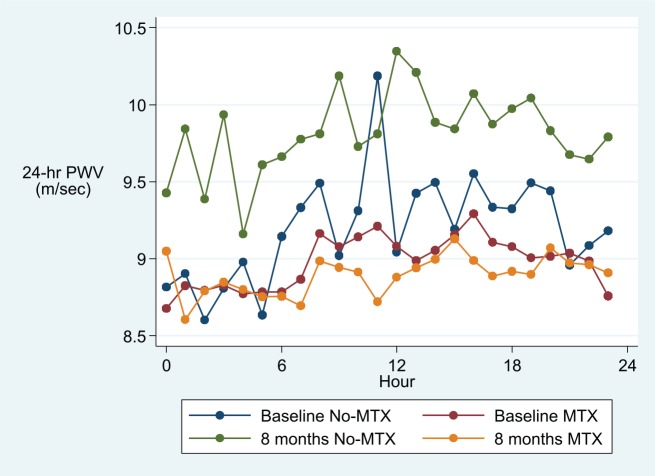
Adjusted 24-hour, day-time and night-time overall means (95% CI) for peripheral and central SBP and DBP and PWV are described in Table 3, and hourly means are shown in Figures 2–4. After adjusting for age, visit, gender, BMI, DAS28 and folic acid use, and hour of measurement, patients treated with MTX had significantly lower average 24-hour and day-time, but not night-time, peripheral and central SBP and DBP and PWV values when compared to patients in the non-MTX group (Table 3).
Table 3.
Adjusted1 mean (95% CI) 24-hour, day-time and night-time peripheral and central blood pressure and pulse wave velocity for MTX and non-MTX groups at baseline and after 8 months.
| Baseline |
p-value | 8 months |
p-value | MTX × visit interaction | p-value for MTX × visit interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No-MTX(n = 26) | MTX(n = 54) | Adjusted Δ | No-MTX(n = 26) | MTX(n = 54) | Adjusted Δ | |||||
| Peripheral SBP | ||||||||||
| Day | 132 (128, 136) | 127 (124, 130) | −6.8 (−11, −2.8) | <0.001 | 136 (132, 140) | 123 (120, 126) | −13 (−17, −9) | <0.001 | −6 (−8.7, −3.4) | <0.001 |
| Night | 121 (116, 126) | 117 (113, 121) | −4.3 (−9.9, 1.3) | 0.13 | 121 (116, 126) | 116 (112, 120) | −3.5 (−8.9, 2) | 0.21 | 0.8 (−3, 4.7) | 0.67 |
| 24-hour | 129 (125, 133) | 125 (122, 128) | −6.5 (−10, −3) | <0.001 | 133 (129, 137) | 121 (118, 124) | −11 (−15, −7.7) | <0.001 | −4.6 (−6.9, −2.3) | <0.001 |
| Peripheral DBP | ||||||||||
| Day | 77 (75, 79) | 76 (74, 77) | −2.9 (−5.5, −0.3) | 0.03 | 78 (75, 80) | 72 (70, 74) | −5.7 (−8.2, −3.2) | <0.001 | −2.8 (−4.6, −1.0) | 0.003 |
| Night | 68 (65, 71) | 68 (66, 70) | −1.9 (−5.6, 1.7) | 0.30 | 69 (66, 72) | 67 (65, 69) | −1.6 (−5.2, 2.1) | 0.40 | 0.4 (−2.5, 3.2) | 0.81 |
| 24-hour | 75 (73, 77) | 74 (72, 75) | −2.8 (−5.2, −0.5) | 0.02 | 76 (73, 78) | 71 (69, 73) | −5.1 (−7.3, −2.8) | <0.001 | −2.1 (−3.8,−0.6) | 0.006 |
| Central SBP | ||||||||||
| Day | 120 (117, 124) | 117 (115, 120) | −4.2 (−7.8, −0.6) | 0.02 | 124 (120, 127) | 113 (110, 116) | −10 (−14, −6.7) | <0.001 | −6.1 (−8.5, −3.7) | <0.001 |
| Night | 112 (107, 117) | 110 (106, 113) | −2.7 (−8.1, 2.7) | 0.33 | 113 (108, 118) | 108 (104, 112) | −3.7 (−9, 1.6) | 0.18 | −1.0 (−4.9, 3.0) | 0.64 |
| 24-hour | 118 (115, 122) | 116 (113, 118) | −3.7 (−7, −0.4) | 0.03 | 121, (118, 125) | 112 (109, 115) | −8.8 (−12, −5.5) | <0.001 | −5.1 (−7.2, −2.9) | <0.001 |
| Central DBP | ||||||||||
| Day | 79 (77, 82) | 77 (76, 79) | −3.4 (−5.9, −0.8) | 0.01 | 80 (78, 83) | 74 (72, 76) | −6.2 (−8.7, −3.7) | <0.001 | −2.8 (−4.6, −1.1) | 0.002 |
| Night | 69 (66, 72) | 69 (67, 71) | −1.5 (−5.1, 2.2) | 0.43 | 70 (67, 73) | 68 (66, 70) | −1.7 (−5.3, 1.9) | 0.35 | −0.25 (−3.2, 2.7) | 0.87 |
| 24-hour | 77 (75, 79) | 75 (74, 77) | −3 (−5.3, −0.8) | 0.009 | 78 (76, 80) | 73 (71, 74) | −5.4 (−7.7, −3.1) | <0.001 | −2.4 (−3.9, −0.8) | 0.003 |
| PWV | ||||||||||
| Day | 9.3 (9.1, 9.4) | 9.0 (9.0, 9.1) | −0.2 (−0.3, −0.1) | 0.003 | 10.0 (9.8, 10.2) | 8.8 (8.7, 8.9) | −0.4 (−0.6, −0.3) | <0.001 | −0.2 (−0.3, −0.1) | <0.001 |
| Night | 8.9 (8.7, 9.2) | 8.8 (8.6, 8.9) | −0.1 (−0.3, 0.1) | 0.16 | 9.7 (9.5, 9.9) | 8.6 (8.5, 8.8) | −0.2 (−0.4, 0.01) | 0.06 | −0.1 (−0.2, 0.1) | 0.57 |
| 24-hour | 9.2 (9.0, 9.4) | 9.0 (8.8, 9.1) | −0.2 (−0.3, −0.1) | 0.002 | 9.9 (9.8, 10.0) | 8.8 (8.6, 8.9) | −0.4 (−0.5, −0.2) | <0.001 | −0.2 (−0.3, −0.1) | <0.001 |
Adjusted for visit, methotrexate use, hour, age, gender, BMI, DAS28 and folic acid use.
DBP, diastolic blood pressure; MTX, methotrexate; PWV, pulse wave velocity; SBP, systolic blood pressure.
Figure 2.
Hourly mean peripheral systolic (SBP) and diastolic (DBP) blood pressure in the MTX and the non-MTX group at baseline and after 8 months.
Figure 3.
Hourly mean central peripheral systolic (SBP) and diastolic (DBP) blood pressure in the MTX and the non-MTX group at baseline and after 8 months.
Figure 4.
Hourly mean pulse wave velocity (PWV) in the MTX and the non-MTX group at baseline and after 8 months.
MTX dose, MTX-PGs, folic acid use, BP and markers of arterial function
Within the MTX group alone there were no independent associations between the MTX weekly dose, MTX-PGs concentrations, and clinic or 24-hour peripheral and central SBP and DBP values, AIx, PWV and ADMA concentrations. Similarly, there were no independent associations between the use of folic acid and the outcomes of interest (data not shown).
Discussion
In a repeated cross-sectional study of RA patients, those who were treated with MTX demonstrated significantly lower clinic and 24-hour peripheral and central SBP and DBP and PWV, a marker of arterial stiffness, when compared to those who did not receive treatment with MTX. The association between MTX treatment and lower 24-hour BP and PWV values was driven by significant day-time, but not night-time, differences in these parameters. By contrast, there were no significant between-group differences in AIx, a marker of arterial wave reflection, and ADMA, a marker of endothelial function and cardiovascular risk. The lower BP with MTX may be related to differences in arterial stiffness (as measured by PWV), but not in AIx or ADMA concentrations.
The significant differences in BP and PWV observed in our study are unlikely to be accounted for by differences in the concentrations of markers of inflammation, cardiovascular risk factors or treatment with other medications likely to affect BP and PWV – for example, antihypertensive drugs, NSAIDs and corticosteroids – as these parameters were similar between the two groups. Furthermore, the use of folic acid, which has been shown to reduce BP and improve endothelial function in other patient groups,51–53 was not associated with BP parameters or markers of arterial function. In the MTX group, we did not observe an independent association between the dose of MTX nor the MTX-PGs concentrations and the BP and arterial outcomes of interest. The significantly higher prevalence of cardiovascular disease and depression, the higher DAS28 and the more frequent use of tocilizumab in the non-MTX group when compared to the MTX group might have affected the BP and PWV values in our study. The relationship between a positive history of cardiovascular disease, BP and PWV is controversial. This is primarily due to the lack of data from large epidemiological studies investigating BP and PWV in patients with versus patients without previous cardiovascular events, matched for other clinical, demographic, biochemical and pharmacological characteristics. Theoretically, a history of cardiovascular disease would suggest the presence of more severe and/or advanced atherosclerotic disease, with a consequent increase in PWV and, possibly, BP.54 However, patients with previous cardiovascular events might also undertake salutary lifestyle changes, e.g. low-salt and low-fat diet, and physical exercise, and receive several vasoactive medications with BP- and PWV-lowering effects.55 A positive association between the presence of depressive disorders and higher PWV and BP has been reported in epidemiological studies, particularly in the older population.56,57 This might be explained by the increased sympathetic activity, and related neuro-hormonal changes, observed in patients with depression.58 Furthermore, measures of increased disease activity in RA, including the DAS28, have shown positive associations with arterial stiffness.59 By contrast, the treatment with the biological DMARD tocilizumab has been recently reported to significantly reduce PWV and BP in RA patients. Notably, treatment with either rituximab or abatacept was not associated with significant changes in these parameters.60 The more frequent use of tocilizumab in the non-MTX group in our study might have led to lower, not higher, BP and PWV values when compared with the MTX group.
Meta-analyses have shown that MTX treatment is associated with a lower risk of cardiovascular events in patients with chronic inflammatory states, including RA. For example, Micha and colleagues reported that MTX treatment was associated with a reduced risk of total cardiovascular disease events (RR 0.79, 95% CI 0.73–0.87, p < 0.001) and myocardial infarction (RR 0.82, 95% CI 0.71–0.96, p < 0.01) when compared with other DMARDs.17 Although no mechanism has been identified to explain the distinct protective cardiovascular effects of MTX versus other DMARDs, observational studies have reported a possible effect on BP, a major cardiovascular risk factor both in RA patients and in the general population.11,14 Rho and colleagues assessed cardiovascular risk factors in RA patients treated with either MTX (n = 120) or other DMARDs (n = 49).23 MTX treatment was associated with a trend towards a lower SBP (131.6 ± 21.0 versus 137.5 ± 18 mmHg, p = 0.09) and a significantly lower DBP (73.7 ± 11.1 versus 77.9 ± 9.5 mmHg, p = 0.02) versus treatment with other DMARDs. However, the BP differences were no longer significant after adjusting for age, sex, ethnicity, DAS28, history of hypertension and diabetes, smoking status and use of statins.23 Cuchacovich and colleagues investigated associations between specific DMARDs, BP, lipid profile and insulin resistance in RA patients without history of hypertension, diabetes and dyslipidaemia.22 When compared to treatment-naïve RA patients, MTX treatment was associated with lower SBP (120.1 ± 15.5 versus 123.6 ± 12.2 mmHg) and DBP (75.6 ± 10.5 versus 81.6 ± 11.3 mmHg) values, although no formal statistical analysis was performed.22 Furthermore, van Halm and colleagues observed a relatively lower prevalence of hypertension in RA patients treated with MTX (12%) when compared to sulfasalazine (26%), hydroxychloroquine (17%), or to patients never treated with any of these agents (24%).20 However, no information was provided in these studies regarding the methods and protocols used to assess BP. The latter can significantly affect BP measurement.37 Furthermore, no assessment of central BP, 24-hour BP or markers of arterial function was performed. Notably, the assessment of central and 24-hour BP is significantly superior to clinic peripheral BP in predicting cardiovascular events, with important clinical implications.61,62
A number of mechanisms might account for the differences in clinic and 24-hour peripheral and central BP and PWV between the MTX and the non-MTX group observed in our study. MTX-PGs, the intracellular form of MTX, are known to inhibit the enzyme aminoimidazole carboxamide ribonucleotide (AICAR) transformylase (ATIC).63 The consequent accumulation of the substrate AICAR, and its metabolites, inhibits adenosine deaminase and adenosine monophosphate deaminase.64 By reducing the catabolism of adenosine and adenine nucleotides, adenosine concentrations increase both directly and indirectly from AMP dephosphorylation. There is good evidence that adenosine reduces BP through direct vasodilation, increased NO synthesis or central mechanisms.65 Furthermore, the pharmacological inhibition of adenosine receptors causes an increase in BP, AIx and PWV, supporting the key role of adenosine in modulating BP and arterial function.66 Another possible mechanism mediating the effects of MTX on BP is related to the chemical similarities (pteridine nucleus) between MTX, folic acid, and the eNOS co-factor tetrahydrobiopterin (BH4). The active form of folic acid, 5-methyltetrahydrofolate (5-MTHF), has been shown to restore eNOS-mediated NO synthesis and endothelial function in conditions, such as RA, characterized by increased oxidative stress and inflammation, leading to reduced intracellular availability of BH4.67,68 Studies using molecular computer modelling have also shown that 5-MTHF binds the active site of eNOS, mimicking the orientation and interactions of BH4 with the enzyme.67 Whether MTX exerts similar effects on eNOS activity remains to be established, however. The consequent increase in NO synthesis would translate into a reduction in BP and PWV.69
No significant between-group differences in AIx or plasma ADMA concentrations were observed in our study. The main determinants of AIx in physiological and pathophysiological states, albeit not fully identified, include both cardiac factors (e.g. cardiac contractility and flow) and the arterial vasculature (e.g. regional compliance and wave reflection).70 It is therefore possible that specific factors influencing the AIx might have counterbalanced the MTX-mediated effects of BP on this parameter. The trend towards reduced ADMA concentrations in patients treated with MTX might be secondary to an increased activity of dimethylarginine dimethylaminohydrolase (DDAH), the enzyme involved in the metabolism of ADMA to citrulline and dimethylamine.71 DDAH activity is inversely associated with the production of reactive oxygen species.71,72 Although MTX has been associated with increased oxidative stress,73 studies have also reported a protective effect of this drug towards interleukin-6-mediated generation of reactive oxygen species in RA patients.74 Further experimental studies are warranted to test the hypothesis that MTX-mediated reduction in reactive oxygen species synthesis leads to increased DDAH activity and, consequently, reduced ADMA concentrations.
Pending further confirmation of our findings in randomized controlled studies, the observed clinic and 24-hour peripheral and central differences in SBP and DBP between the MTX and the non-MTX group are likely to be clinically significant, in terms of cardiovascular risk reduction, even if the average baseline BP in our study was within the ‘normotensive’ range (<140/90 mmHg). A recent systematic review and meta-analysis has shown that a similar reduction, 6–7 mmHg, in clinic peripheral SBP in patients with mild, grade 1, hypertension and low-to-moderate cardiovascular risk was associated with a significant reduction in stroke (RR 0.58, 95% CI 0.34–0.99), coronary heart disease (RR 0.75, 95% CI 0.58–0.96) and all-cause mortality (RR 0.67, 95% CI 0.51–0.87).75 The observed differences in central BP are also likely to be clinically significant. For example, in a large randomized controlled trial a reduction in central SBP of 4.3 mmHg was associated with a significant reduction in adverse cardiovascular outcomes.76 The observed differences in PWV values between the MTX and the non-MTX group might also be clinically significant. A recent systematic review and meta-analysis reported that a reduction in PWV by 1 m/s would translate into a 15% reduction in age-, sex-, and risk factor-adjusted risk of cardiovascular and all-cause mortality.7,8 Therefore, the smaller difference in PWV observed in our study (~0.2 m/s) might still correspond to a 3% reduction in cardiovascular risk.
Strengths of our study include the recruitment of a single cohort of RA patients treated with either MTX or other DMARDs, well matched for clinical, demographic, biochemical and pharmacological characteristics. In particular, key factors potentially affecting the outcomes of interest, such as prevalence of cardiovascular risk factors, inflammatory markers and treatment with other drugs such as antihypertensive agents, NSAIDs and corticosteroids, were similar between the two groups. The measurement of clinic and 24-hour peripheral and central BP, and markers of endothelial function, arterial wave reflection and arterial stiffness allowed a comprehensive assessment of established parameters with a significant clinical application. Furthermore, the repeated cross-sectional assessment provided additional power to confirm, or refute, the presence of statistically significant differences between the two groups.
The limitations of our study include its cross-sectional nature in regards to MTX exposure since differences in BP may already have been present at baseline when we defined MTX exposure and we can therefore not infer causality. However, the differences remained similar across time, and there were also further significant falls in BP and PWV at 8 months. There is also the possibility that other, unknown or unmeasured, confounders as well as the previously discussed differences in the prevalence of cardiovascular disease and depression, and DAS28, might have explained the observed differences in BP between the MTX and the non-MTX groups.
In conclusion, our study provides the first evidence of an association between MTX treatment and lower clinic and 24-hour peripheral and central SBP and DBP values and PWV values in RA patients. The lower PWV in patients treated with MTX might be responsible for the lower BP observed in this group. Further randomized controlled studies are warranted to confirm these findings, to identify the mechanisms responsible for the effects of MTX on BP, and to establish whether these effects account for the reduced cardiovascular risk reported with MTX treatment.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Arduino A. Mangoni, Department of Clinical Pharmacology, School of Medicine, Flinders University and Flinders Medical Centre, Bedford Park, SA 5042, Australia.
Leena R. Baghdadi, Department of Clinical Pharmacology, School of Medicine, Flinders University and Flinders Medical Centre, Adelaide, Australia; Centre for Epidemiology and Biostatistics, School of Medicine, Flinders University, Adelaide, Australia. Department of Family and Community Medicine, King Saud University, Riyadh, Saudi Arabia.
E. Michael Shanahan, Department of Rheumatology, Flinders University and Southern Adelaide Local Health Network, Adelaide, Australia.
Michael D. Wiese, School of Pharmacy and Medical Sciences and Sansom Institute for Health Research, University of South Australia, Adelaide, Australia
Sara Tommasi, Department of Clinical Pharmacology, School of Medicine, Flinders University and Flinders Medical Centre, Adelaide, Australia.
David Elliot, Department of Clinical Pharmacology, School of Medicine, Flinders University and Flinders Medical Centre, Adelaide, Australia.
Richard J. Woodman, Centre for Epidemiology and Biostatistics, School of Medicine, Flinders University, Adelaide, Australia
References
- 1. Dadoun S, Zeboulon-Ktorza N, Combescure C, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine 2013; 80: 29–33. [DOI] [PubMed] [Google Scholar]
- 2. Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol 1997; 24: 445–451. [PubMed] [Google Scholar]
- 3. Murdaca G, Colombo BM, Cagnati P, et al. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 2012; 224: 309–317. [DOI] [PubMed] [Google Scholar]
- 4. Maki-Petaja KM, Hall FC, Booth AD, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumour necrosis factor-alpha therapy. Circulation 2006; 114: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 5. Avalos I, Chung CP, Oeser A, et al. Increased augmentation index in rheumatoid arthritis and its relationship to coronary artery atherosclerosis. J Rheumatol 2007; 34: 2388–2394. [PubMed] [Google Scholar]
- 6. Di Minno MN, Ambrosino P, Lupoli R, et al. Clinical assessment of endothelial function in patients with rheumatoid arthritis: a meta-analysis of literature studies. Eur J Intern Med 2015; 26: 835–842. [DOI] [PubMed] [Google Scholar]
- 7. Vlachopoulos C, Aznaouridis K, O’Rourke MF, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010; 31: 1865–1871. [DOI] [PubMed] [Google Scholar]
- 8. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55: 1318–1327. [DOI] [PubMed] [Google Scholar]
- 9. Yannoutsos A, Levy BI, Safar ME, et al. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. J Hypertens 2014; 32: 216–224. [DOI] [PubMed] [Google Scholar]
- 10. Matsuzawa Y, Kwon TG, Lennon RJ, et al. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc 2015; 4: pii: e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baghdadi LR, Woodman RJ, Shanahan EM, et al. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 2015; 10: e0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radner H, Lesperance T, Accortt NA, et al. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res (Hoboken). Epub ahead of print 20 December 2016. DOI: 10.1002/acr.23171. [DOI] [PubMed] [Google Scholar]
- 13. Jafri K, Bartels CM, Shin D, et al. Incidence and management of cardiovascular risk factors in psoriatic arthritis and rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) 2017; 69: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinblatt ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc 2013; 124: 16–25. [PMC free article] [PubMed] [Google Scholar]
- 16. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: still an ‘Anchor’ drug? Autoimmun Rev 2014; 13: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 17. Micha R, Imamura F, Wyler Von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011; 108: 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almalag HM, Mangoni AA, Crilly MA. Methotrexate and risk of cardiovascular disease. Am J Cardiol 2012; 109: 1383–1384. [DOI] [PubMed] [Google Scholar]
- 19. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Halm VP, Nurmohamed MT, Twisk JW, et al. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther 2006; 8: R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007; 46: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 22. Cuchacovich R, Espinoza LR. Does Tnf-Alpha blockade play any role in cardiovascular risk among rheumatoid arthritis (RA) patients? Clin Rheumatol 2009; 28: 1217–1220. [DOI] [PubMed] [Google Scholar]
- 23. Rho YH, Oeser A, Chung CP, et al. Drugs used in the treatment of rheumatoid arthritis: relationship between current use and cardiovascular risk factors. Arch Drug Inf 2009; 2: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 25. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004; 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belanger CF, Hennekens CH, Rosner B, et al. The nurses’ health study. Am J Nurs 1978; 78: 1039–1040. [PubMed] [Google Scholar]
- 27. Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham heart study: a cohort study. Lancet 2001; 358: 1682–1686. [DOI] [PubMed] [Google Scholar]
- 28. Pincus T, Summey JA, Soraci SA, Jr, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford health assessment questionnaire. Arthritis Rheum 1983; 26: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 29. Mccormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med 1988; 18: 1007–1019. [DOI] [PubMed] [Google Scholar]
- 30. Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012; 64: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prevoo ML, Van‘T Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44–48. [DOI] [PubMed] [Google Scholar]
- 32. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111: 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cerutti H, Muzzi C, Leoncini R, et al. Erythrocyte sedimentation rate measurement by VES Matic Cube 80 in relation to inflammation plasma proteins. J Clin Lab Anal 2011; 25: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Rotte MC, Den Boer E, De Jong PH, et al. Methotrexate polyglutamates in erythrocytes are associated with lower disease activity in patients with rheumatoid arthritis. Ann Rheum Dis 2015; 74: 408–414. [DOI] [PubMed] [Google Scholar]
- 35. Tousoulis D, Georgakis MK, Oikonomou E, et al. Asymmetric dimethylarginine: clinical significance and novel therapeutic approaches. Curr Med Chem 2015; 22: 2871–2901. [DOI] [PubMed] [Google Scholar]
- 36. Palatini P, Frigo G, Bertolo O, et al. Validation of the a&D Tm-2430 device for ambulatory blood pressure monitoring and evaluation of performance according to subjects’ characteristics. Blood Press Monit 1998; 3: 255–260. [PubMed] [Google Scholar]
- 37. Gabb GM, Mangoni AA, Anderson CS, et al. Guideline for the diagnosis and management of hypertension in adults – 2016. Med J Aust 2016; 205: 85–89. [DOI] [PubMed] [Google Scholar]
- 38. O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol 2001; 51: 507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38: 932–937. [DOI] [PubMed] [Google Scholar]
- 40. Fagard R, Brguljan J, Thijs L, et al. Prediction of the actual awake and asleep blood pressures by various methods of 24 H pressure analysis. J Hypertens 1996; 14: 557–563. [DOI] [PubMed] [Google Scholar]
- 41. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605. [DOI] [PubMed] [Google Scholar]
- 42. Jones CR, Taylor K, Chowienczyk P, Poston L, et al. A validation of the Mobil O Graph (Version 12) ambulatory blood pressure monitor. Blood Press Monit 2000; 5: 233–238. [DOI] [PubMed] [Google Scholar]
- 43. Westhoff TH, Straub-Hohenbleicher H, Schmidt S, et al. Convenience of ambulatory blood pressure monitoring: comparison of different devices. Blood Press Monit 2005; 10: 239–242. [DOI] [PubMed] [Google Scholar]
- 44. Franssen PM, Imholz BP. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit 2010; 15: 229–231. [DOI] [PubMed] [Google Scholar]
- 45. Wei W, Tolle M, Zidek W, et al. Validation of the Mobil-O-Graph: 24 H-blood pressure measurement device. Blood Press Monit 2010; 15: 225–228. [DOI] [PubMed] [Google Scholar]
- 46. Weiss W, Gohlisch C, Harsch-Gladisch C, et al. Oscillometric estimation of central blood pressure: validation of the Mobil-O-Graph in comparison with the SphygmoCor device. Blood Press Monit 2012; 17: 128–131. [DOI] [PubMed] [Google Scholar]
- 47. Wassertheurer S, Kropf J, Weber T, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens 2010; 24: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weber T, Wassertheurer S, Rammer M, et al. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension 2011; 58: 825–832. [DOI] [PubMed] [Google Scholar]
- 49. Hametner B, Wassertheurer S, Kropf J, et al. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit 2013; 18: 173–176. [DOI] [PubMed] [Google Scholar]
- 50. Hellman S, Iannotti AT, Bertino JR. Determinations of the levels of serum folate in patients with carcinoma of the head and neck treated with methotrexate. Cancer Res 1964; 24: 105–113. [PubMed] [Google Scholar]
- 51. Mangoni AA, Sherwood RA, Swift CG, et al. Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. J Intern Med 2002; 252: 497–503. [DOI] [PubMed] [Google Scholar]
- 52. Mangoni AA, Sherwood RA, Asonganyi B, et al. Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens 2005; 18: 220–226. [DOI] [PubMed] [Google Scholar]
- 53. Paul B, Whiting MJ, De Pasquale CG, et al. Acute effects of 5-methyltetrahydrofolate on endothelial function and asymmetric dimethylarginine in patients with chronic heart failure. Nutr Metab Cardiovasc Dis 2010; 20: 341–349. [DOI] [PubMed] [Google Scholar]
- 54. Van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam study. Stroke 2001; 32: 454–460. [DOI] [PubMed] [Google Scholar]
- 55. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25: 932–943. [DOI] [PubMed] [Google Scholar]
- 56. Tiemeier H, Breteler MM, Van Popele NM, et al. Late-life depression is associated with arterial stiffness: a population-based study. J Am Geriatr Soc 2003; 51: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 57. Meng L, Chen D, Yang Y, et al. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens 2012; 30: 842–851. [DOI] [PubMed] [Google Scholar]
- 58. Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the aetiology of depression. Curr Neuropharmacol 2016; 14: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Botta E, Merono T, Saucedo C, et al. Associations between disease activity, markers of HDL functionality and arterial stiffness in patients with rheumatoid arthritis. Atherosclerosis 2016; 251: 438–444. [DOI] [PubMed] [Google Scholar]
- 60. Provan SA, Berg IJ, Hammer HB, et al. The impact of newer biological disease modifying anti-rheumatic drugs on cardiovascular risk factors: a 12-month longitudinal study in rheumatoid arthritis patients treated with rituximab, abatacept and tociliziumab. PLoS One 2015; 10: e0130709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Head GA, McGrath BP, Mihailidou AS, et al. Ambulatory blood pressure monitoring in Australia: 2011 consensus position statement. J Hypertens 2012; 30: 253–266. [DOI] [PubMed] [Google Scholar]
- 62. McEniery CM, Cockcroft JR, Roman MJ, et al. Central blood pressure: current evidence and clinical importance. Eur Heart J 2014; 35: 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cutolo M, Sulli A, Pizzorni C, et al. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis 2001; 60: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chan ES, Cronstein BN. Mechanisms of action of methotrexate. Bull Hosp Jt Dis 2013; 71(Suppl. 1): S5–S8. [PubMed] [Google Scholar]
- 65. Koupenova M, Johnston-Cox H, Ravid K. Regulation of atherosclerosis and associated risk factors by adenosine and adenosine receptors. Curr Atheroscler Rep 2012; 14: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mahmud A, Feely J. Acute effect of caffeine on arterial stiffness and aortic pressure waveform. Hypertension 2001; 38: 227–231. [DOI] [PubMed] [Google Scholar]
- 67. Hyndman ME, Verma S, Rosenfeld RJ, et al. Interaction of 5-methyltetrahydrofolate and tetrahydrobiopterin on endothelial function. Am J Physiol Heart Circ Physiol 2002; 282: H2167–H2172. [DOI] [PubMed] [Google Scholar]
- 68. Haruna Y, Morita Y, Komai N, et al. Endothelial dysfunction in rat adjuvant-induced arthritis: vascular superoxide production by Nad(P)H oxidase and uncoupled endothelial nitric oxide synthase. Arthritis Rheum 2006; 54: 1847–1855. [DOI] [PubMed] [Google Scholar]
- 69. Wilkinson IB, MacCallum H, Cockcroft JR, et al. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol 2002; 53: 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Avolio A, Butlin M, Xu K. Reflections on determinants of augmentation index. J Hypertens 2012; 30: 267–268. [DOI] [PubMed] [Google Scholar]
- 71. Wadham C, Mangoni AA. Dimethylarginine dimethylaminohydrolase regulation: a novel therapeutic target in cardiovascular disease. Expert Opin Drug Metab Toxicol 2009; 5: 303–319. [DOI] [PubMed] [Google Scholar]
- 72. Palm F, Onozato ML, Luo Z, et al. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 2007; 293: H3227–H3245. [DOI] [PubMed] [Google Scholar]
- 73. Herman S, Zurgil N, Deutsch M. Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 2005; 54: 273–280. [DOI] [PubMed] [Google Scholar]
- 74. Sung JY, Hong JH, Kang HS, et al. Methotrexate suppresses the interleukin-6 induced generation of reactive oxygen species in the synoviocytes of rheumatoid arthritis. Immunopharmacology 2000; 47: 35–44. [DOI] [PubMed] [Google Scholar]
- 75. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circ Res 2015; 116: 1058–1073. [DOI] [PubMed] [Google Scholar]
- 76. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (Cafe) Study. Circulation 2006; 113: 1213–1225. [DOI] [PubMed] [Google Scholar]