Abstract
Osteoarthritis (OA) is a chronic condition characterized by a loss of joint cartilage and is a major cause of disability in Canada, with an estimated CN$195 billion annual cost. Knee OA leads to persistent pain and loss of function, and treatment goals primarily focus on symptom relief and retention of function. Intra-articular hyaluronic acid (IAHA) has therapeutic benefits, and numerous recently published meta-analyses (MAs) and commentaries have highlighted new evidence on the role of IAHA therapy for knee OA. A diverse, multidisciplinary group of specialists met independently in closed sessions to review findings from eight MAs with literature search end dates no earlier than 2012 to address controversies surrounding IAHA therapy for mild-to-moderate knee OA within the Canadian treatment context. Outcomes from a total of eight MAs were reviewed, and consistent and statistically significant improvements in pain, function and stiffness up to 26 weeks were found with IAHA therapy compared with IA placebo or controls, regardless of MA size or trial quality. These findings are in line with those of a Cochrane review, another recent systematic review and patient satisfaction survey. Overall, three MAs reported outcomes based on molecular weight (MW), with the two reporting effect sizes showing significantly improved pain outcomes for higher compared with lower MW HAs. Recent evidence suggests that HA therapy is well tolerated with no increased risk of serious adverse events compared with placebo and the full therapeutic effect of IAHA therapy appears to have considerable clinical importance, consisting of the combined IA placebo and HA therapeutic effects. IAHA therapy is a well-tolerated and effective option for patients with mild-to-moderate knee OA failing first-line pharmacological therapy.
Keywords: gonarthrosis, intra-articular hyaluronic acid, knee, osteoarthritis, viscosupplementation
Introduction
Osteoarthritis (OA) affects approximately 4.6 million Canadians (13%) with an additional 400,000 new cases each year.1,2 OA is a chronic condition and major cause of disability in Canada, leading to a loss of joint cartilage, persistent pain and loss of function. The cumulative cost of OA in Canada from 2010-2015 was CN$195.2 billion, primarily attributed to loss of productivity, a burden expected to double by 2020 and again by 2030.1,2 The lifetime risk of symptomatic OA in at least one knee is 44.7%,3 representing one of the largest disease burdens in terms of disability-adjusted life years among nontransmittable diseases.4,5
Treatment for knee OA focuses on symptom relief and retention or improvement of function.6 In addition to weight loss, exercise and physiotherapy,7 initial pharmacological options include symptomatic slow-acting drugs for OA with topical analgesics as needed.8–10 If symptoms persist, oral nonsteroidal anti-inflammatory drugs (NSAIDs) should be considered.8 Following oral therapy, more invasive options such as intra-articular (IA) injections may be indicated,8 which include benefits such as increased bioavailability, reduced systemic exposure, and minimal adverse events (AEs).11
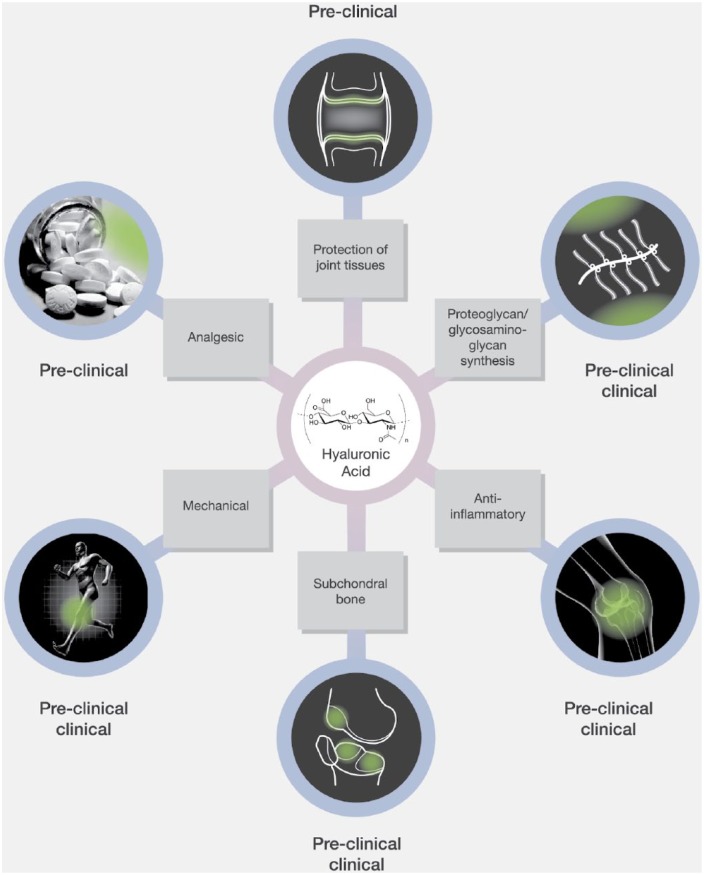
Hyaluronic acid (HA) is a natural long-chain polymer with repeating disaccharide units that naturally provides lubrication and elastic shock absorption.12–14 Reported benefits of supplemental HA injections include pain relief, improved function and reduced stiffness,5,15–21 although possible mechanisms of action for HA have not been fully elucidated22–31 (Figure 1).
Figure 1.
Possible mechanisms of action of IAHA therapy for knee osteoarthritis. A number of potential mechanisms for IAHA benefit have been proposed, including protection of joint tissue through reduced chondrocyte apoptosis and increased chondrocyte proliferation, enhanced proteoglycan/glycosaminoglycan synthesis which could delay OA progression, anti-inflammatory effects through suppression of IL-1β and other factors, limitation of subchondral bone changes characteristic of early OA through suppression of MMP-13 and IL-6 expression, mechanical benefits through direct lubrication of the joint capsule, and analgesic effects through reduction of mechanosensitive stretch-activated ion channel activity. From Altman and colleagues14, after 1989 with data cutoff of 4 May 2014.
IAHA, intra-articular hyaluronic acid; IL-1β, interleukin 1 beta; IL-6, interleukin 6; MMP-13, matrix metallopeptidase 13; OA, osteoarthritis.
Available HA preparations vary in their MW, cross-linkage and derivation source [avian (AD-HA) and bacterial fermentation (Bio-HA)].14
IAHA therapy was approved in Canada in 1992 for mild-to-moderate (Kellgren–Lawrence II-III) knee OA following NSAID failure.32,33 Many randomized trials have examined the efficacy and safety of IAHA for knee OA. Substantial variations in trial design, methodology and reported outcomes, however, have challenged data interpretation.17,20,34 Earlier recommendations indicated a benefit for IAHA in knee OA,10,35 although recent class-based MAs including Rutjes and colleagues20 and American Academy of Orthopaedic Surgeons (AAOS) guidelines15 have questioned its clinical importance and safety. Use of IAHA therapy therefore remains controversial, with many international guideline recommendations providing uncertain, partial, or no support for its use.9,10,15,36,37
Clinical need remains for effective treatment options.38,39 Numerous published MAs and associated commentaries were published in 2015, highlighting the importance of intrinsic HA properties and the IA placebo treatment effect, providing renewed perspectives on HA therapy clinical benefit.17,40–42 A diverse, multidisciplinary group of Canadian specialists met to independently discuss the clinical implications of this new evidence and assess whether there is a role for IAHA therapy in the treatment of mild-to-moderate knee OA.
Methods
To permit a more comprehensive analysis of IAHA class-based evidence, MAs published in the last 5 years were included in our analysis to ensure consideration of the majority of randomized controlled trials (RCTs) as well as topermit comparison of new MA findings with those of the AAOS and Rutjes and colleagues.15,20 PubMed was searched from Jan 2012 to Jan 29 2016 using the search terms viscosupplementation (OR aliases) AND knee osteoarthritis (OR aliases) to identify recently published English-only MAs of RCTs evaluating the class effects of IAHA therapy compared with IA placebo or noninterventional controls for knee OA. Findings were supplemented with a bibliographic search. MAs reporting data with a literature search end date of no earlier than January 2012 were considered for our analysis, and outcomes of interest were pain, stiffness, function and safety. Overall, two independent reviewers confirmed eligibility and extracted data of interest.
A multidisciplinary group of Canadian OA thought-leaders (three orthopaedic surgeons, two rheumatologists, two sports medicine experts, and one clinical scientist) gathered on 18 March 2016 in Toronto to independently review and consider evidence from the last 5 years in closed sessions devoid of commercial influence. An evidence-based medicine (EBM) methodologist, two EBM graduate students and a publications firm aided authors. The p-values were reported when available; alternatively, findings were considered statistically significant if the 95% confidence intervals (CIs) did not cross zero. The only independent analysis performed by our group was for the higher versus lower molecular weight (MW) comparisons of the Rutjes and colleagues and Altman and colleagues MW subgroup analyses.16,20 For this analysis, two-tailed p-values were estimated by applying an unpaired Student’s t test to the comparison of pain effect sizes of groups for comparison using GraphPad’s QuickCalcs.43 Standard error (or deviation) used in the t test was derived from the respective 95% CIs, following the procedure44 described in the Cochrane Handbook for Systematic Reviews of Interventions, and Gaussian distributions were assumed for both the unpaired t test and the derivation of standard deviation from 95% CIs.
Results
A total of eight published MAs were identified,5,15–21 with different numbers of included studies and participants based on varied methodological frameworks and timeframes. A total of six MAs15,16,18–21 used a traditional pair-wise design while two5,17 used network methodology. Excluded studies included Miller and colleagues45 (earlier report of Strand and colleagues21), Pai and colleagues because it addressed product-specific effects,46 and Colen and colleagues47 which only evaluated evidence with a literature search end date prior to January 2012.
Overall pain outcomes
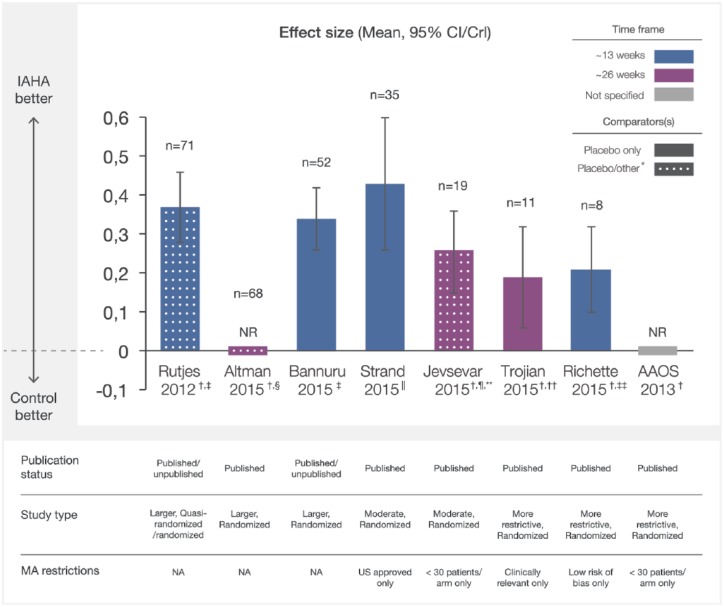
A total of seven MAs included overall pain outcomes.5,15,17–21 Overall, six reported overall pain effect sizes, showing consistent and statistically significant improvements favouring HA therapy over placebo or nonintervention controls regardless of methodology (Figure 2).5,17–21
Figure 2.
Overall pain effect sizes of IAHA therapy compared with IA placebo from recent meta-analyses. Effect sizes reported from recent MAs were plotted with CI/CrI intervals. Assessment time frames for each study are indicated. Solid bars indicate comparison of IAHA with placebo only and mottled bars indicate comparison with placebo and other interventions. CI/CrI ranges above zero indicate statistically significant outcomes.
CI, confidence interval; CrI, credible interval; IAHA, intra-articular hyaluronic acid; MA, meta-analysis; n, number of trials included in MA; NA, not applicable; NR, not reported
*other defined as nonintervention control, usual care, or IAHA added to another active treatment
† effect sizes reported as negative values at source, transformed to positive values for comparison with other studies
‡ examined outcomes at a timepoint nearest to 13 weeks
§ examined outcomes at a timepoint nearest to 26 weeks
|| reported outcomes between 4 and 13 weeks
¶ comparator was placebo (n = 14) or usual care (n = 2), or alternatively, IAHA added to an active treatment (n = 3) ~ 13 weeks
** most common endpoint at 26 weeks,
†† the time of best response over 8–26 weeks
‡‡ at 13 weeks follow up.
Similar outcomes were seen in two larger, more comprehensive MAs (n ⩾ 52 RCTs), which examined outcomes at a timepoint nearest to 13 weeks (~13 weeks).17,20 Rutjes and colleagues reported a statistically significant pain effect size [ES, ([mean of HA group]-[mean of control group])/standard deviation] of 0.37 (95% CI 0.28–0.46) among 71 randomized or quasi-randomized trials evaluating HA versus sham or nonintervention controls.20 A network MA (NMA) by Bannuru and colleagues compared relative effect estimates of many knee OA treatments at ~13 weeks,17 reporting a statistically significant relative pain ES of 0.34 [95% credible interval (CrI) 0.26–0.42] among 52 trials comparing HA with placebo. Another larger MA by Altman and colleagues identified 68 published randomized trials comparing HA with a variety of interventions at a timepoint nearest to 26 weeks (~26 weeks).16 Although subgroup pain outcomes for HA compared with placebo were analyzed (described below), overall outcomes were not reported.
Overall, two moderate size pair-wise MAs (52 > n > 8) using published RCTs also showed consistent outcomes.18,21 Strand and colleagues compared United States (US)-approved HAs with placebo, reporting a statistically significant pain ES of 0.43 (95% CI 0.26–0.60, p < 0.001) among 20 studies with endpoints ranging from 4 to 13 weeks (~13 weeks) and 0.38 (95% CI 0.21–0.55, p < 0.001) among 15 studies from 14 to 26 weeks (~26 weeks).21 Jevsevar and colleagues restricted eligibility to RCTs with ⩾30 patients per arm, comparing HA therapy with placebo (n = 14), usual care (n = 2), or HA added to an active treatment (n = 3).18 A statistically significant overall pain ES of 0.49 (95% CI 0.29–0.70) was reported among these 19 trials with the most common endpoint at approximately 26 weeks (~26 weeks).
Outcomes remained consistent among smaller MAs (n ⩽ 8 RCTs) with more restrictive placebo-controlled RCT eligibility.5,15,19 The Trojian and colleagues network MA evaluated only clinically relevant pain outcomes (OMERACT OARSI or WOMAC pain outcomes; n = 7), reporting a statistically significant pain ES of 0.19 at the time of best response over 8–26 weeks (~26 weeks, 95% CI 0.06–0.32).5 The pair-wise MA by Richette and colleagues evaluated eight trials with lowrisk of bias at ~13 weeks, showing a statistically significant pain ES of 0.21 (95% CI 0.10–0.32) for HA therapy compared with placebo.19 The AAOS MA reported a statistically significant group effect for pain of 0.32 (95% CI 0.11–0.52) as a minimal important difference (MID) ratiofor HA compared with placebo at an unspecified follow up among eight trials with ⩾30 patients per arm.15
Overall function and stiffness outcomes
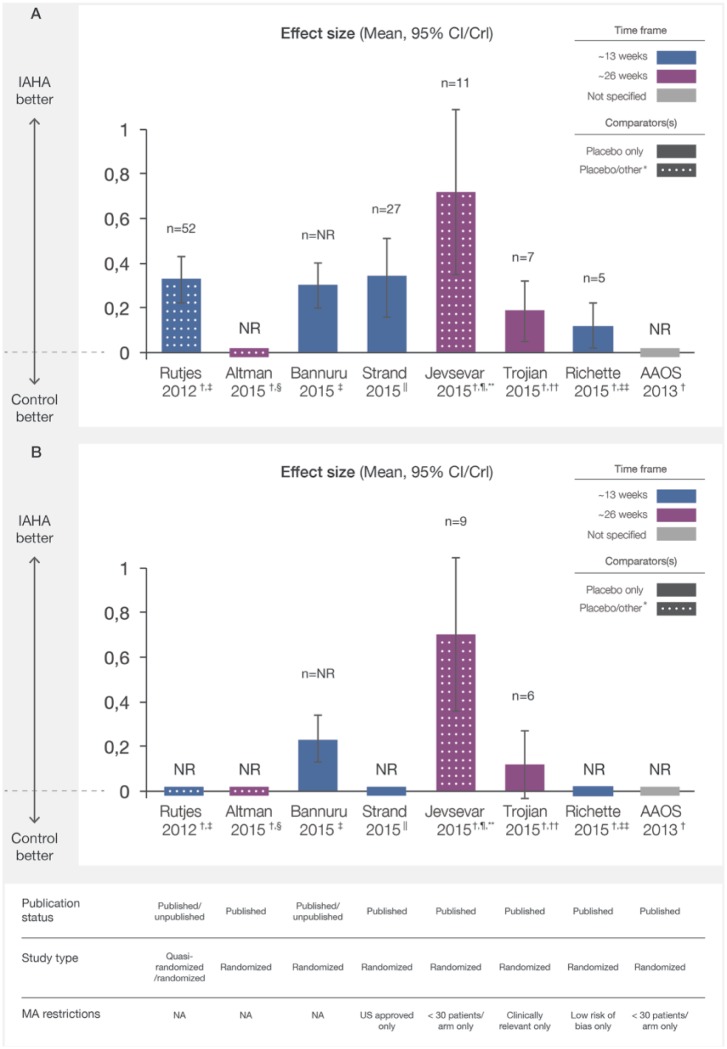
Function outcomes were available from seven MAs.5,15,17–21 Consistent and significant improvements favouring HA therapy compared with controls were observed in the six MAs reporting function ES regardless of size (Figure 3(a)).5,17–21
Figure 3.
Overall function (a) and stiffness (b) effect sizes of IAHA versus IA placebo from meta-analyses. Effect sizes of IAHA therapy reported from recent MAs were plotted with CI/CrI intervals. Assessment time frames for each study are indicated. Solid bars indicate comparison of IAHA with placebo only and mottled bars indicate comparison with placebo and other interventions. CI/CrI ranges above zero indicate statistically significant outcomes.
CI, confidence interval; CrI, credible interval; IAHA, intra-articular hyaluronic acid; MA, meta-analysis; n, number of trials included in MA; NA, not applicable; NR, not reported
* other defined as nonintervention control, usual care or IAHA added to another active treatment
† effect sizes reported as negative values at source, transformed to positive values for comparison with other studies
‡ examined outcomes at a timepoint nearest to 13 weeks
§ examined outcomes at a timepoint nearest to 26 weeks
|| reported outcomes between 4 and 13 weeks
¶ comparator was placebo (n = 14) or usual care (n = 2), or alternatively, IAHA added to an active treatment (n = 3) ~ 13 weeks
** most common endpoint at 26 weeks
†† the time of best response over 8–26 weeks; ‡‡ at 13 weeks follow up.
The AAOS MA reported an MID ratio for function of 0.49 (95% CI 0.11–0.86), indicating a statistically significant improvement for HA over placebo among five trials.15Stiffness outcomes were available from four MAs,5,15,17,18 showing statistically significant improvements for HA in all MAs reporting ES (Figure 3(b))5,17,18 except the Trojian and colleagues NMA, which restricted eligibility to six trials reporting clinically relevant outcomes.5 The AAOS MA reported a statistically significant improvement in stiffness for HA compared with placebo with a MID ratio of 0.39 (CI 0.12–0.67).15
Pain outcomes by intrinsic properties
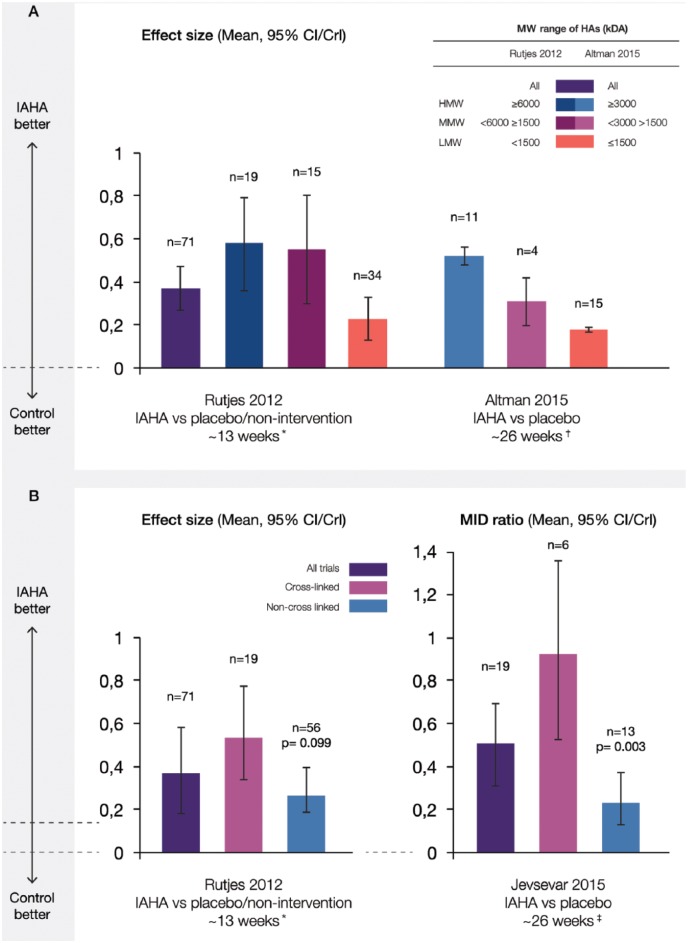
A total of four MAs evaluated pain outcomes based on intrinsic HA properties15,16,18,20; three evaluated MW15,16,20 and two assessed HA polymer chain covalent cross-linking.18,20 Among studies reporting ES, higher MW HA demonstrated a statistically significant improvement in pain outcomes compared with lower MW HA (Figure 4 (a)).16,20
Figure 4.
Pain effect sizes of IAHA versus IA placebo by (a) molecular weight and (b) cross-linking. Effect sizes reported from recent MAs were plotted with CI/CrI intervals. Various molecular weight cutoffs and cross-linked status of agents for each study are indicated. CI/CrI ranges above zero indicate statistically significant outcomes.
CI, confidence interval; CrI, credible interval; IAHA, intra-articular hyaluronic acid; kDa, kiloDalton; MA, meta-analysis; MID, minimum important difference; (H/M/L) MW, (high/moderate/low) molecular weight; n, number of trials included in MA
* if outcomes were reported at several timepoints, timepoint closest to 3 months after the end of treatment was extracted
† at the reported time frame nearest to 26 weeks
‡ follow-up durations in the included trials ranged from 6 to 52 weeks, with the most common endpoint being at approximately 26 weeks.
Rutjes and colleagues used a higher MW cutoff of ⩾6000 kDa, <6000– ⩾1500 kDa for moderate MW, and <1500 kDa for lower MW.20 At ~13 weeks, pain ES were 0.58 (95% CI 0.36–0.79, n = 18) for higher MW, 0.55 (95% CI 0.30–0.80, n = 15) for moderate MW, and 0.23 (95% CI 0.13–0.33, n = 34) for lower MW HA (interaction p-value = 0.110). Altman and colleagues observed a similar pattern of response despite a less conservative MW cutoff for higher MW (⩾3000 kDa) and moderate MW (<3000–>1500 kDa) and a later timepoint (~26 weeks).16 Mean pain scores were 0.52 (95% CI 0.48–0.56, n = 11) for higher MW, 0.31 (95% CI 0.20–0.42, n = 4) for moderate MW, and 0.18 (95% CI, 0.17–0.19, n = 15) for lower MW HA (interaction p-value not reported). A total of 8 of 12 pain outcomes (67%) from studies included in the AAOS MA favoured higher MW agents (⩾6000 kDa) over placebo and 9 out of the 12 RCTs showing statistically significant improvement in pain outcomes for HA compared with placebo evaluated moderate to higher MW agents (⩾2400 kDa).15
Cross-linked HAs showed numerically greater ES in two MAs (Figure 4(b)), with significant differences for cross-linking only apparent at a later timepoint.18,20 Rutjes and colleagues reported a pain ES of 0.53 (95% CI 0.34–0.73) at ~13 weeks among 19 studies using cross-linked and 0.29 (95% CI 0.20–0.39) among 56 studies using noncross-linked formulations (interaction p-value = 0.099).20 Jevsevar and colleagues however, observed a statistically significantly improved MID ratio of 0.93 (95% CI 0.52–1.33) among a subgroup of 6 studies evaluating cross-linked compared with 0.25 (95% CI 0.12–0.38) among 13 studies assessing noncross-linked HAs (p = 0.003, ~ 26 weeks).18
Safety outcomes
A total of six MAs reported safety outcomes,5,15–17,20,21 with four reporting any AEs, local AEs or serious AEs (SAEs, Table 1).16,17,20,21
Table 1.
Safety outcomes of IAHA therapy from recent meta-analyses. Any AEs, local reactions, serious AEs and withdrawal rates are reported, with additional data for derivation method and intrinsic HA properties when available.
| Rutjes 201220 | Bannuru 201517 | Altman 201616 | Strand 201521 | |
|---|---|---|---|---|
| Relative risk (95% CI), [trials] |
Median event rates (interquartile range), % [trials] IAHA vs IAP |
Pooled incidence % (95% CI) |
Absolute risk difference, % (95% CI), [trials] |
|
| Any AEs | 1.04 (0.99–1.09), p = 0.158 [n = 25] |
16 (54.6) vs 21.7 (56.0), [n = 35] | NR | NR |
|
Local AE/
injection site flare-ups* |
Local AE 1.34 (1.13–1.60) p = 0.001, [n = 31] Injection site flare up subgroup 1.51 (0.84–2.72) p = 0.165, [n = 6] |
Local AE 8.4 (14.4) vs 4.7 (16.1), [n = 39] |
Injection site flare-ups AD-HA > Bio-HA 13.19 (12.04–14.44) vs 3.04 (2.34–3.95), p ⩽ 0.001 HMW (⩾3000 kDa) > LMW (⩽1500 kDa) 13.73 (12.33–15.27) vs 10.73 (9.27–12.39), p = 0.007 |
NR |
| Overall SAEs | 1.41 (1.02–1.97) p = 0.039 [n = 14] |
0 (0.9) vs 0 (0), [n = 36] Septic joint 0 (0) vs 0 (0), [n = 18] |
NR | 0.7 (−0.2–1.5), p = 0.12 [n = 28] |
| Withdrawals due to AEs | 1.33 (1.01–1.74) p = 0.04 [n = 23] |
0.9 (3.9) vs 1.0 (2.6), [n = 36] | Withdrawals due to treatment-related AEs AD-HA vs BIO-HA 1.49 (1.05–2.12) vs 1.00 (0.73–1.37), p = 0.09 HMW (⩾3000 kDa) vs LMW (⩽1500 kDa) 0.77 (0.48–1.21) vs 2.20 (1.70–2.84), p = 0.004 |
0.2 (−0.4–0.8), p = 0.46 [n = 31] |
local flare-ups and other local reactions as reported by study authors, including pain, swelling, and arthralgia.
AD-HA, avian-derived hyaluronic acid; AE, adverse event; BIO-HA, bacterial fermentation-derived hyaluronic acid; CI, confidence interval, HMW, high molecular weight; IAHA, intra-articular hyaluronic acid, IAP, intra-articular placebo; LMW, low molecular weight; kDA, kilo Dalton; NR, not reported; SAE, serious adverse event; SMD, standard median deviation; vs, versus.
Most expressed concerns regarding safety data reporting, including low quality, heterogeneous or inadequate reporting,16,17,20 short trial duration,17 or potential bias.20 No statistically significant increases in any AEs were observed for HA in two MAs17,20; any AE event rates were lower for HA compared with placebo (16% versus 21.7%) among 35 RCTs in Bannuru and colleagues17 and the relative risk of any AE was 1.04 (95% CI 0.99–1.09) for HA compared with placebo or nonintervention controls among 25 studies in Rutjes and colleagues.20
Local AEs
A total of three MAs assessed local reactions16,17,20 including local AEs (n = 2),17,20 injection site flare-ups (n = 2)16,20 and septic reactions (n = 1).17 Modest increases in local AEs were observed for HA compared with controls in two MAs.17,20 Rutjes and colleagues defined local AEs as flare-ups or any other local AEs, reporting a significantly increased risk ratio of 1.34 (95% CI 1.13–1.60, p = 0.001) for HA compared with placebo or nonintervention among 31 studies.20 Bannuru and colleagues reported transient local AEs such as pain, swelling, and arthralgia, with a median event rate of 8.4% for HA compared with 4.7% for placebo among 39 trials.17 Overall, two MAs assessed injection site flare-ups (IS-FU).16,20 Rutjes and colleagues defined a typical IS-FU as a hot, painful, swollen knee 24–72 hours post-injection, reporting a nonsignificant risk increase for HA therapy versus placebo or nonintervention among six studies (RR 1.51, 95% CI 0.84–2.72, p = 0.165).20 Although IS-FU was not defined and overall results not reported by Altman and colleagues, significantly increased IS-FU was reported among 3070 patients receiving AD-HA compared with 1776 patients receiving Bio-HA (13.19% versus 3.04%, p ⩽ 0.001).16 Significantly higher IS-FU rates were also reported for higher MW (⩾3000 kDa, 13.73%) compared with either moderate MW (<3000 and >1500 kDa, 3.31%, p ⩽ 0.001) or lower MW (⩽1500 kDa, 10.73%, p = 0.007), and also for moderate MW compared with lower MW HA (p ⩽ 0.001; Table1). Bannuru and colleagues identified a single septic joint event (placebo) among 29 RCTs evaluating HA compared with placebo.17
Serious adverse events
A total of four MAs reported SAEs and withdrawals due to AEs (WAEs) (Table 1).16,17,20,21 A significant increase in SAEs was reported in one of three MAs.20 Rutjes and colleagues defined SAE as those resulting in inpatient hospitalization, prolongation of hospitalization, persistent or significant disability, congenital abnormality of offspring, life-threatening events, or death; reporting significantly increased risk for HA compared with controls among 14 studies (RR 1.41, 95% CI 1.02–1.97, p = 0.039).20 Reported SAEs included six cancer and five cardiovascular events for HA compared with none and two events for placebo, respectively. Strand and colleagues reported a nonsignificant absolute SAE risk difference of 0.7% (95% CI –0.2–1.5%, p = 0.12) for HA compared with placebo among 28 studies, although no SAEs were deemed related to treatment.21 Bannuru and colleagues reported median SAE rates of 0% in both arms (interquartile range 0.9 and 0 for HA and placebo, respectively) among 36 studies.17 WAEs were significantly increased in only one of three MAs. Rutjes and colleagues reported a significantly increased relative risk of 1.33 (95% CI 1.01–1.74, p = 0.04) for HA,20 although event rates were not significantly different in the Strand and colleagues (absolute risk difference 0.2%, 95% CI –0.4–0.8%, p = 0.46)21 or Bannuru and colleagues (median event rates, 0.9% versus 1.0%) MAs.17 WAEs based on intrinsic properties were reported in the Altman and colleagues MA, with significantly increased event rates for lower MW compared with higher MW HA (2.20% versus 0.77%, p = 0.004) and numerically increased rates for Bio-HA compared with AD-HA (1.49% versus 1.00%, p = 0.09).16
Discussion
Is IAHA therapy effective?
Pain from knee OA is a chief complaint, representing the primary reported outcome in most RCTs, and there is a need for effective treatments. Despite the highly variable data set and the range of methodological methods employed in the MAs reviewed, a statistically significant improvement in pain for HA therapy compared to placebo or noninterventional controls was observed, which remained consistent among larger,17,20 medium18,21 and smaller MAs5,15,19 at both earlier (~13 weeks)17,19–21 and later (~26 weeks) timepoints.18,21 Strand and colleagues demonstrated comparable pain outcomes for HA from 4 to 13 weeks (ES 0.43, 95% CI 0.26–0.60) and 14 to 26 weeks (ES 0.38, 95% CI 0.21–0.55),21 suggesting enduring HA benefit. An earlier analysis by Bannuru and colleagues comparing HA with placebo also showed statistically significant improved pain outcomes as early as 4 weeks with continued benefit up to 24 weeks.48
Statistically significant improvements in pain outcomes were also consistent regardless of trial quality. A subanalysis of Rutjes and colleagues20 considering only placebo-controlled trials, reported an identical pain ES to Bannuru and colleagues (0.34, 95% CI 0.24–0.44 versus 0.34, 95% CrI 0.26–0.42, respectively).17 Outcomes remained significantly improved when restricted to studies with adequate concealment (Rutjes and colleagues, 0.32, 95% CI −0.49 to −0.1420 and Richette and colleagues, 0.21, 95% CI 0.10–0.3219), in larger studies (>100 patients per arm),20 or medium sized trials (>30–50 patients).17,18 Significant improvements in function were also reported in six MAs5,17–21 and stiffness in three MAs.5,17,18 Our review of recent MAs shows consistent and statistically significant improvements in pain, function and stiffness outcomes for HA therapy compared with placebo or nonintervention up to 26 weeks for mild-to-moderate knee OA, regardless of study type.
Is IAHA therapy safe?
Most studies reviewed expressed concerns regarding safety data reporting, including low quality, heterogeneous or inadequate reporting,16,17,20 short trial duration,17 or potential bias.20 SAE rates for HA were very low (0, IQR 0.9).17 The significant increased relative risk of SAEs (1.41, p = 0.039) among 14 trials and withdrawals due to AEs (1.33, p = 0.04) among 23 studies analyzed by Rutjes and colleagues20 was not confirmed in subsequent MAs by Bannuru and colleagues17 and Strand and colleagues,21 which considered more than double the number of RCTs for SAEs (n = 28 and n = 36) and a comparable number for WAEs (n = 36 and n = 31). Additionally, the most concerning SAEs identified by Rutjes and colleagues were not reasonably attributable to IAHA therapy (cardiovascular and cancer)20,49 and Strand and colleagues did not identify a single HA-related SAE event.21 Finally, a recently published NMA conducted by Bannuru and colleagues on various HA agents concluded that HA therapy is well tolerated and SAEs rare.50
Modestly increased rates of local AEs/IS-FUs may be associated with IAHA compared with placebo. Bannuru and colleagues reported a 3.7% net increase in the median local AE rate among 39 trials17 and Rutjes and colleagues reported significantly increased local AE risk among 31 studies (RR 1.34, 95% CI 1.13–1.60, p = 0.001).20 Altman and colleagues explored the relationship between local AEs/IS-FUs and HA derivation source, reporting increased IS-FU rates for AD-HA versus Bio-HA (13.19% versus 3.04%, p ⩽ 0.001) among 4846 patients, and for higher MW versus lower MW HAs (13.73% versus 10.73%, p = 0.007).16 Although intriguing, low overall event rates and concerns about safety reporting quality raise questions regarding the accuracy of these findings. Additionally, confirmatory trends were not seen for withdrawals due to AEs, with comparable event rates for Bio-HA versus AD-HA and significantly lower rates for higher versus lower MW groups,16 suggesting that increased IS-FU rates may have marginal clinical relevance. Administration techniques may affect rates of local AEs, so proper techniques should be considered and events managed with rest, elevation, ice pack and anti-inflammatories as needed.51,52 Concerns regarding the quality of safety data reporting with IAHA therapy were noted and available data suggests that IAHA therapy is relatively well tolerated, with no significantly increased risk of any AEs compared with placebo.17,20
Are intrinsic qualities of HA therapy important?
Available HA preparations differ in their MW and degree of cross-linkage. A total of three MAs reported improved outcomes with higher MW compared with lower MW HAs.15,16,20 Differences in outcomes between the higher and lower MW groups of Rutjes and colleagues and Altman and colleagues MAs were considerable,16,20 although variations existed in the ranges of MW HAs compared, and p-values for the higher versus lower MW comparisons were not provided for either study. Calculation of a two-tailed p-value revealed statistically significant differences between the two groups (0.0018 and 0.0001 for Rutjes and colleagues and Altman and colleagues respectively). This trend toward incremental efficacy with increased MW was also observed in the AAOS guideline15 and in an earlier MA by Bannuru and colleagues,48 showing a considerable difference in pain ES for higher MW (⩾6000 kDA, ES 0.60, 95% CI 0.33–0.88) compared with lower MW HA (<1000 KDa, ES 0.29, 95% CI 0.14–0.44). Although the optimal MW cutoff remains unclear and more extensive comparative studies are needed, current data support a cutoff of ⩾6000 kDA for improved pain outcomes at ~13 and ~26 weeks16,20 and a cutoff of ⩾3000 kDA for improved pain outcomes at ~26 weeks.16
Overall, two MAs reported improved pain outcomes for cross-linked compared with noncross-linked HA at ~13 and ~26 weeks.18,20 Although nonsignificant at ~13 weeks,20 differences in treatment effects were statistically significant at ~26 weeks.18 It is unclear whether these differences were due to methodological variations between trials, the higher MW of most cross-linked agents, or possibly more enduring benefits for cross-linked agents. Consistent and significant differences in observed treatment effects for higher compared with lower MW agents are intriguing and could have important clinical implications.
What are the implications of an IA placebo treatment effect?
Research on knee OA therapies has traditionally assumed equivalence among various placebo options. A notable finding from Bannuru and colleagues17 evaluating numerous treatment options for knee OA at 3 months was that IA saline had a therapeutic effect on pain when compared with oral placebo (ES 0.29, 95% CrI 0.04–0.54), an effect which may have been enhanced in part by a placebo effect related to the invasive nature of the procedure. Regardless, this analysis showed that when IAHA therapy was compared with oral placebo, the full therapeutic effect of IAHA therapy on pain was ES 0.63 (95% CI 0.39–0.88); the sum of both the HA (ES 0.34, 95% CI 0.26–0.42) and IA (ES 0.29, 95% CI 0.04–0.54) treatment components.17,41 One might therefore conclude that the estimated full therapeutic effect ofhigher MW IAHA is likely even greater (~0.58 + ~0.29 = ~0.87).17,20
Are IAHA outcomes clinically important?
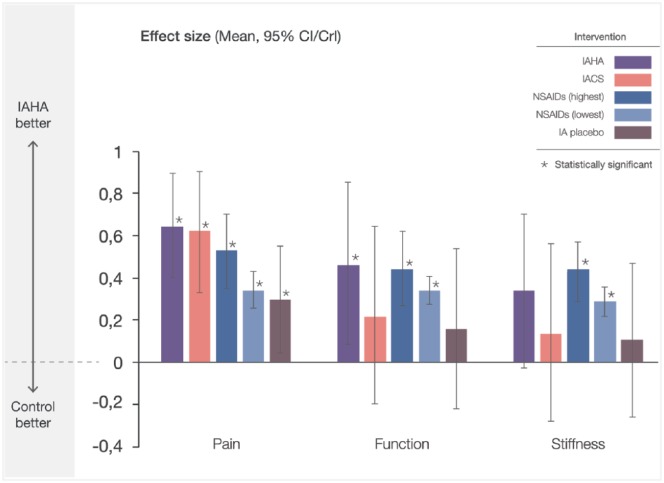
The relevance of statistically significant IAHA outcomes has been questioned due to failure reaching predesignated thresholds of clinical importance.15,16,18,20 Multiple metrics for establishing clinically important relevance were employed in the reviewed MAs. The baseline-derived minimum clinically important difference (MCID) threshold of 0.37 was used in four MAs15,16,18,20; OMERACT OARSI responder-based thresholds were used in two MAs.5,17 Bannuru and colleagues defined a minimum clinically important improvement as a 20-point improvement from baseline pain on a WOMAC 0–100 scale,17 while Trojian and colleagues pooled outcomes using OMERACT OARSI responder criteria outcomes.5 When HA effects alone were considered among trials assessing clinical importance,5,15,17,18,20 outcomes were deemed important in three studies5,17,20 and unimportant in two.15,18 However, when the full therapeutic effect of IAHA was considered (factoring in the IA placebo effect), all MAs reporting effect size met or exceeded even the most stringent MCID threshold of 0.37,5,17–21 with higher MW IAHA agents clearly surpassing thresholds of clinical importance.16,20 Finally, when Bannuru and colleagues17 compared the full therapeutic effect of IAHA with two established and clinically relevant OA treatments, IA corticosteroids (IACS) and oral NSAIDs, IAHA demonstrated comparable or improved outcomes for pain, function and stiffness, underscoring the clinical importance of this intervention (Figure 5).
Figure 5.
Relative pain, function and stiffness effect sizes for select knee OA treatments versus oral placebo. Effect sizes reported from Bannuru and colleagues17 were plotted with CI/CrI intervals. Interventions thatwere compared with IAHA therapy are indicated. CI/CrI ranges above zero indicate statistically significant outcomes.
CS, corticosteroid; HA, hyaluronic acid; IA, intra-articular; NSAID, nonsteroidal anti-inflammatory drug; OA, osteoarthritis.
What is the place in treatment of HA therapy?
IA therapy is indicated following first-line pharmacological therapy for mild-to-moderate knee OA.8 Comparable or improved effectiveness of IAHA therapy to IACS and NSAIDs confirms the role of IAHA therapy in this setting.17 Additionally, comparison of IAHA therapy with NSAIDs by Bannuru and colleagues showed a statistically significant increased risk of gastrointestinal AEs for NSAIDs compared with IAHA,53 pointing to a potential earlier role in patients who do not tolerate or have contraindications to NSAID therapy.54–59
What are the strengths and limitations of this work?
Our review of recent MAs draws together seemingly incongruent outcomes from a large number of MAs, providing new perspective on an ongoing controversy. Our review is limited to a review of MAs from the last 5 years and does not employ systematic methodology. Furthermore, significance indicators not explicitly reported have been estimated, including p-values for higher and lower MW comparisons and for higher MW HA full therapeutic effects. Although these estimates may be useful for exploring data patterns, they should be interpreted with caution and confirmed at source.
Despite these limitations, our findings are in line with other important works in this area, including a more comprehensive systematic MA review by Xing and colleagues60 using the Assessment of Multiple Systematic Reviews (AMSTAR) method61,62 and Jadad and colleagues’ decision algorithm63 to evaluate the methodological quality and identify sources of inconsistencies between MAs, respectively. This work identified a Cochrane systematic review as the highest quality MA on this subject,34 confirming pain, function and stiffness benefits for IAHA compared with placebo, conclusions which were also supported by another recent expert perspective on this seminal work.64 Furthermore, a prospective, internet-based, double-blind survey of adults with knee OA recently showed that IAHA was perceived by patients as the most effective treatment compared with narcotics and steroid injection.65 The proportion of patients perceiving IAHA as very effective (74.1%) was significantly higher than for all treatments taken together (53.0%, p = 0.0001), and the proportion of patients rating IAHA to be not effective (6.7%) was significantly lower than for all treatments taken together (12.5%, p = 0.0001).
Conclusion
Our independent review of recent evidenceindicates that IAHA therapy is well tolerated, with significantly improved pain, function and stiffness outcomes compared with placebo or noninterventional controls in patients with mild-to-moderate knee OA up to 26 weeks. These findings are supported by a Cochrane review by Bellamy and colleagues34 a systematic review by Xing and colleagues60 as well as a recent patient satisfaction survey by Posnett and colleagues65 Our findings suggest that the full therapeutic effect of IAHA therapy is clinically important and is likely greater for higher MW agents. Current evidence indicates a role for IAHA therapy after first-line pharmacological failure in mild-to-moderate knee OA.
Key messages
IAHA safely improves pain, function and stiffness for knee OA which has been recently confirmed by a more systematic analysis, and higher MW HA may be more effective.
Full therapeutic benefit results from combined IA placebo and HA treatment effects.
This review of recent MAs indicates a potential role for IAHA after first-line pharmacological failure in mild-to-moderate knee OA.
Acknowledgments
We would like to thank Ilidio Martins and Paul Card of Kaleidoscope Strategic Inc. for their research and editorial assistance preparing this manuscript as well as Sanofi Canada (Laval QC, Canada) for proving funding for this initiative. All data have been critically reviewed by authors and opinions presented represent those of the authors. The sponsor played no part in the design and development of this publication nor did they view the manuscript prior to submission. Although the sponsor was able to suggest clinicians for inclusion in the working group, final selection of group members was done independently by the lead author.
Footnotes
Funding: Kaleidoscope Strategic Inc., an independent publications firm, provided research and editorial support to authors in the preparation of this publication. Their services were funded in an independent fashion by Sanofi Canada (Laval QC, Canada).
Conflict of interest statement: MB - Consultancy/advisory from Sanofi (Laval QC, Canada), Pendopharm (Montreal QC, Canada), Ferring (North York, ON, Canada); honoraria from Sanofi (Laval QC, Canada), Pendopharm (Montreal QC, Canada); research funding from Ferring (North York, ON, Canada).
RRB - Consultancy/advisory from Fidia (New Jersey, USA); honoraria from Sanofi (Laval QC, Canada).
EB - Consultancy/advisory from Pendopharm (Montreal QC, Canada); honoraria from Pendopharm (Montreal QC, Canada), Sanofi (Laval QC, Canada).
JMP - Consultancy/advisory from Ferring (North York, ON, Canada); stock ownership from ArthroLab Inc. (Montreal QC, Canada); research funding from Ferring (North York, ON, Canada).
MK - None.
JPR - Honoraria from Amgen (Mississauga, ON, Canada), UCB (Oakville, ON, Canada), Roche (Mississauga, ON, Canada), Janssen Toronto, ON, Canada, BMS (Montreal QC, Canada), Lilly (Scarborough, ON, Canada), Novartis (Dorval, QC, Canada).
RF - Consultancy/advisory from Sanofi (Laval QC, Canada), Pendo-pharm (Montreal QC, Canada); Honoraria from Sanofi (Laval QC, Canada), Pendopharm (Montreal QC, Canada).
DM - Consultancy/advisory from Sanofi (Laval QC, Canada), research funding from Sanofi (Laval QC, Canada); payment for involvement in the preparation of this submission.
TD - None.
MP - None.
EHS - Consultancy/advisory from Sanofi, Bioventus.
JPP - Consultancy/advisory from Ferring; stock ownership from ArthroLab Inc.; research funding from Ferring.
Contributor Information
Mohit Bhandari, Division of Orthopaedics and Department of Clinical Epidemiology and Biostatistics, McMaster University, 293 Wellington Street, Hamilton, ON L8L2X2, Canada.
Raveendhara R. Bannuru, Center for Treatment Comparison and Integrative Analysis, Division of Rheumatology, Tufts Medical Center, Boston, MA, USA
Eric M. Babins, University of Calgary, Calgary, Alberta, Canada
Johanne Martel-Pelletier, Osteoarthritis Research Unit, University of Montréal Hospital Research Centre (CRCHUM), Montréal, Quebec, Canada.
Moin Khan, Division of Orthopaedics, McMaster University, Hamilton, Ontario, Canada.
Jean-Pierre Raynauld, University of Montréal Hospital Research Centre (CRCHUM), Montréal, Quebec, Canada.
Renata Frankovich, Department of Family Medicine, University of Ottawa, Ontario, Canada.
Deanna Mcleod, Kaleidoscope Strategic Inc. Toronto, Ontario, Canada.
Tahira Devji, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Ontario, Canada.
Mark Phillips, McMaster University, Hamilton, Ontario, Canada.
Emil H. Schemitsch, Department of Surgery, Western University, London, Ontario, Canada
Jean-Pierre Pelletier, Osteoarthritis Research Unit, University of Montréal Hospital Research Centre (CRCHUM), Montréal, Quebec, Canada.
References
- 1. Bombadier CHG, Mosher D. The impact of arthritis in Canada: today and over the next 30 years. The Arthritis Alliance of Canada, http://www.arthritisalliance.ca/en/initiativesen/impact-of-arthritis (2011, accessed 2 August 2016).
- 2. Petrella RJ, Wakeford C. Pain relief and improved physical function in knee osteoarthritis patients receiving ongoing hylan G-F 20, a high molecular weight hyaluronan, versus other treatment options: data from a large real-world longitudinal cohort in Canada. Drug Des Devel Ther 2015; 9: 5633–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 2008; 59: 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013; 21(9): 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Clin J Sport Med 2016; 26(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 6. Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-art management of knee osteoarthritis. World J Clin Cases 2015; 3(2): 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newberry SJ, Fitzgerald JD, Maglione MA, et al. Systematic review for effectiveness of hyaluronic acid in the treatment of severe degenerative joint disease (DJD) of the knee. Rockville, MD: AHRQ Technology Assessments, 2015. [PubMed] [Google Scholar]
- 8. Bruyere O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-from evidence-based medicine to the real-life setting. Semin Arthritis Rheum 2016; 45(Suppl. 4): S3–S11. [DOI] [PubMed] [Google Scholar]
- 9. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22(3): 363–388. [DOI] [PubMed] [Google Scholar]
- 10. Hochberg MC, Altman RD, April KT, et al. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012; 64(4): 465–474. [DOI] [PubMed] [Google Scholar]
- 11. Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol 2014; 10(1): 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li P, Raitcheva D, Hawes M, et al. Hylan G-F 20 maintains cartilage integrity and decreases osteophyte formation in osteoarthritis through both anabolic and anti-catabolic mechanisms. Osteoarthritis Cartilage 2012; 20(11): 1336–1346. [DOI] [PubMed] [Google Scholar]
- 13. Brandt KD, Smith GN, Jr, Simon LS. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum 2000; 43(6): 1192–1203. [DOI] [PubMed] [Google Scholar]
- 14. Altman RD, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord 2015; 16: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AAOS Clinical Practice Guideline. Treatment of osteoarthritis of the knee: evidence-based guideline. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons, http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf (2013, accessed 4 August 2016). [DOI] [PubMed] [Google Scholar]
- 16. Altman RD, Bedi A, Karlsson J, et al. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med 2016; 44(8): 2158–2165. [DOI] [PubMed] [Google Scholar]
- 17. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162(1): 46–54. [DOI] [PubMed] [Google Scholar]
- 18. Jevsevar D, Donnelly P, Brown GA, et al. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am 2015; 97(24): 2047–2060. [DOI] [PubMed] [Google Scholar]
- 19. Richette P, Chevalier X, Ea HK, et al. Hyaluronan for knee osteoarthritis: an updated meta-analysis of trials with low risk of bias. RMD Open 2015; 1(1): e000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutjes AW, Juni P, da Costa BR, et al. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012; 157(3): 180–191. [DOI] [PubMed] [Google Scholar]
- 21. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res 2015; 8: 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sasaki A, Sasaki K, Konttinen YT, et al. Hyaluronate inhibits the interleukin-1beta-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med 2004; 204(2): 99–107. [DOI] [PubMed] [Google Scholar]
- 23. Wang CT, Lin YT, Chiang BL, et al. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage 2006; 14(12): 1237–1247. [DOI] [PubMed] [Google Scholar]
- 24. Gomis A, Miralles A, Schmidt RF, et al. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthritis Cartilage 2009; 17(6): 798–804. [DOI] [PubMed] [Google Scholar]
- 25. Pena Ede L, Sala S, Rovira JC, et al. Elastoviscous substances with analgesic effects on joint pain reduce stretch-activated ion channel activity in vitro. Pain 2002; 99(3): 501–508. [DOI] [PubMed] [Google Scholar]
- 26. Williams JM, Zhang J, Kang H, et al. The effects of hyaluronic acid on fibronectin fragment mediated cartilage chondrolysis in skeletally mature rabbits. Osteoarthritis Cartilage 2003; 11(1): 44–49. [DOI] [PubMed] [Google Scholar]
- 27. Yatabe T, Mochizuki S, Takizawa M, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis 2009; 68(6): 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobayashi K, Matsuzaka S, Yoshida Y, et al. The effects of intraarticularly injected sodium hyaluronate on levels of intact aggrecan and nitric oxide in the joint fluid of patients with knee osteoarthritis. Osteoarthritis Cartilage 2004; 12(7): 536–542. [DOI] [PubMed] [Google Scholar]
- 29. Greenberg DD, Stoker A, Kane S, et al. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthritis Cartilage 2006; 14(8): 814–822. [DOI] [PubMed] [Google Scholar]
- 30. Brun P, Panfilo S, Daga Gordini D, et al. The effect of hyaluronan on CD44-mediated survival of normal and hydroxyl radical-damaged chondrocytes. Osteoarthritis Cartilage 2003; 11(3): 208–216. [DOI] [PubMed] [Google Scholar]
- 31. Brun P, Zavan B, Vindigni V, et al. In vitro response of osteoarthritic chondrocytes and fibroblast-like synoviocytes to a 500–730 kDa hyaluronan amide derivative. J Biomed Mater Res B Appl Biomater 2012; 100(8): 2073–2081. [DOI] [PubMed] [Google Scholar]
- 32. Agence d’évaluation des technologies et des modes d’intervention en santé (AETMIS). Viscosupplementation for the treatment of osteoarthritis of the knee. Report prepared for AETMIS by Pierre Dagenais and Alicia Framarin (AETMIS 07–06). Montréal: AETMIS, 2007. [Google Scholar]
- 33. Dagenais S. Intra-articular hyaluronic acid (viscosupplementation) for knee osteoarthritis. Issues Emerg Health Technol 2006; (94): 1–4. [PubMed] [Google Scholar]
- 34. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006; (2): CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000; 59(12): 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence-based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003; 62(12): 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Clinical Guideline Centre. Osteoarthritis: care and management in adults. London: National Institute for Health and Clinical Excellence: Guidance, 2014. [PubMed] [Google Scholar]
- 38. Buttgereit F, Burmester G, Bijlsma JW. Non-surgical management of knee osteoarthritis: where are we now and where do we need to go? RMD Open 2014; 1: e000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li CS, Karlsson J, Winemaker M, et al. Orthopedic surgeons feel that there is a treatment gap in management of early OA: international survey. Knee Surg Sports Traumatol Arthrosc 2014; 22(2): 363–378. [DOI] [PubMed] [Google Scholar]
- 40. Altman RD, Schemitsch E, Bedi A. Assessment of clinical practice guideline methodology for the treatment of knee osteoarthritis with intra-articular hyaluronic acid. Semin Arthritis Rheum 2015; 45(2): 132–139. [DOI] [PubMed] [Google Scholar]
- 41. Bannuru RR, McAlindon TE, Sullivan MC, et al. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta-analysis of osteoarthritis trials. Ann Intern Med 2015; 163(5): 365–372. [DOI] [PubMed] [Google Scholar]
- 42. Altman RD, Devji T, Bhandari M, et al. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum 2016; 46(2): 151–159. [DOI] [PubMed] [Google Scholar]
- 43. GraphPad. QuickCalcs (website), http://www.graphpad.com/quickcalcs/contMenu/ (2017, accessed 3 April 2017).
- 44. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- 45. Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord 2013; 6: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pai SK, Allgar V, Giannoudis PV. Are intra-articular injections of Hylan G-F 20 efficacious in painful osteoarthritis of the knee? A systematic review & meta-analysis. Int J Clin Pract 2014; 68(8): 1041–1047. [DOI] [PubMed] [Google Scholar]
- 47. Colen S, van den Bekerom MP, Mulier M, et al. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012; 26(4): 257–268. [DOI] [PubMed] [Google Scholar]
- 48. Bannuru RR, Natov NS, Dasi UR, et al. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis–meta-analysis. Osteoarthritis Cartilage 2011; 19(6): 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McAlindon TE, Bannuru RR. Osteoarthritis: is viscosupplementation really so unsafe for knee OA? Nat Rev Rheumatol 2012; 8(11): 635–636. [DOI] [PubMed] [Google Scholar]
- 50. Bannuru RR, Osani M, Vaysbrot EE, et al. Comparative safety profile of hyaluronic acid products for knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthritis Cartilage 2016; 24(12): 2022–2041. [DOI] [PubMed] [Google Scholar]
- 51. Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging 2007; 24(8): 629–642. [DOI] [PubMed] [Google Scholar]
- 52. Webber TA, Webber AE, Matzkin E. Rate of adverse reactions to more than 1 series of viscosupplementation. Orthopedics 2012; 35(4): e514–e519. [DOI] [PubMed] [Google Scholar]
- 53. Bannuru RR, Vaysbrot EE, Sullivan MC, et al. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2014; 43(5): 593–599. [DOI] [PubMed] [Google Scholar]
- 54. Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf 2012; 35(12): 1127–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Masso Gonzalez EL, Patrignani P, Tacconelli S, et al. Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheum 2010; 62(6): 1592–1601. [DOI] [PubMed] [Google Scholar]
- 56. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med 2009; 169(2): 141–149. [DOI] [PubMed] [Google Scholar]
- 57. Huerta C, Castellsague J, Varas-Lorenzo C, et al. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis 2005; 45(3): 531–539. [DOI] [PubMed] [Google Scholar]
- 58. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidalanti-inflammatory drugs: network meta-analysis. BMJ 2011; 342: c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coxib and traditional NSAID Trialists(CNT), Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effectsof non-steroidal anti-inflammatory drugs:meta-analyses of individual participant data from randomised trials. Lancet 2013; 382(9894): 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xing D, Wang B, Liu Q, et al. Intra-articular hyaluronic acid in treating knee osteoarthritis: a PRISMA-compliant systematic review of overlapping meta-analysis. Sci Rep 2016; 6: 32790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One 2007; 2(12): e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. CMAJ 1997; 156(10): 1411–1416. [PMC free article] [PubMed] [Google Scholar]
- 64. Evaniew N, Simunovic N, Karlsson J. Cochrane in CORR(R): viscosupplementation for the treatment of osteoarthritis of the knee. Clin Orthop Relat Res 2014; 472(7): 2028–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Posnett J, Dixit S, Oppenheimer B, et al. Patient preference and willingness to pay for knee osteoarthritis treatments. Patient Prefer Adherence 2015; 9: 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]