Abstract
Diabetes mellitus (DM) is an important risk factor for adverse outcomes of coronary artery bypass grafting. The bypass grafts harvested from patients with DM tend to go into spasm after their implantation into the coronary circulation. To clarify the contribution of 5-hydroxytriptamine (5-HT) and angiotensin II (AngII) in the bypass graft spasm, we examined the contractile reactivity to 5-HT or AngII of isolated human endothelium-denuded saphenous vein (SV) harvested from DM and non-DM patients. The 5-HT-induced constriction of the SV was significantly augmented in the DM group than in the non-DM group, which is similar to our previous report. AngII-induced constriction of the SV was also significantly augmented in the DM group than the non-DM group. Especially in the non-DM group, the AngII-induced maximal vasoconstriction was markedly lower than the 5-HT-induced one. Meanwhile, the increasing rates of AngII-induced vasoconstriction in the DM group to the non-DM group were significantly greater than those of 5-HT-induced vasoconstriction. These results indicate that 5-HT is a potent inducer of SV graft spasm in both DM and non-DM patients, while AngII is a potent inducer of SV graft spasm only in patients with DM. Furthermore, the protein level of AngII AT1 receptor (AT1R), but not the protein level of 5-HT2A receptor, in the membrane fraction of the SV smooth muscle cells of DM patients was significantly increased as compared with that of the non-DM patients. These results suggest that the mechanism for hyperreactivity to AngII in the SV from DM patients is due to, at least in part, the increase in the amount of AT1R on membrane of the SV smooth muscle cells.
Keywords: 5-hydroxytryptamine, Angiotensin II, Coronary artery bypass grafting, Diabetes mellitus, Saphenous vein, Vasospasm
Highlights
-
•
The vasoconstrictive reactivity to 5-HT was significantly enhanced in the DM.
-
•
The vasoconstrictive reactivity to AngII was significantly enhanced in the DM.
-
•
In DM patients, the hyperreactivity to AngII was significantly higher than that to 5-HT.
-
•
The protein level AT1 R in membrane fraction of saphenous vein smooth muscle was significantly increased in the DM.
-
•
AngII could be a potent inducer of SV graft spasm only in DM patients.
1. Introduction
Coronary artery bypass grafting (CABG) remains the gold standard therapy for severe coronary artery disease involving three-vessel or left main coronary disease [1]. Especially among patients with diabetes mellitus (DM), CABG has a better prognosis than percutaneous coronary intervention [1], [2]. However, DM is an established risk factor for early and late adverse outcomes after CABG [3], with the most serious associated complication being the spasm of bypass grafts after their implantation into the coronary circulation, which can lead to premature occlusion and increased perioperative morbidity [4], [5]. The saphenous vein (SV) has been widely used as a conduit for CABG because of its ready availability and suppleness [6]. We previously reported that the constrictive responses induced by 5-hydroxytryptamine (5-HT) in the human endothelium-denuded SVs isolated from patients with DM were significantly augmented compared with those in veins isolated from patients without DM (non-DM) [7]. Based on these results, we hypothesized that increased reactivity to 5-HT may be responsible for vasospasm after CABG among diabetic patients.
Insulin resistance, a well-known risk factor for type 2 DM, upregulates the renin-angiotensin-aldosterone system (RAAS), which is involved in the pathogenesis of hypertension, arteriosclerosis, and ischemic heart failure [8], [9]. Angiotensin II (AngII) is part of the RAAS and plays important roles in regulating the vascular tone and the pathogenesis of cardiovascular diseases [10]. AngII acts primarily through the angiotensin AT1 receptor (AT1R) to exert the most of its biological effects, including the constriction of vascular smooth muscle. AngII-induced constrictive responses of the SVs harvested from DM patients may be augmented in a similar manner to 5-HT-induced responses, because AT1R, like the 5-HT2A receptor, is a Gq-coupled receptor. However, little is known about the influence of DM on AngII-induced vasoconstriction of human SVs. Hence, we examined the effect of DM on AngII-induced vasoconstriction, in comparison to 5-HT-induced vasoconstriction, of endothelium-denuded SV grafts harvested from both DM and non-DM patients. We further determined the levels of 5-HT2A receptor and AT1R on the SV smooth muscle membrane.
2. Materials and methods
2.1. Preparation of blood vessels and contractile studies
The human SVs were obtained from patients undergoing CABG at Miyazaki Prefectural Nobeoka Hospital (Nobeoka, Japan) or surgical varicose vein treatment at Kuwabara Clinic (Miyazaki, Japan). SV samples from 15 patients with DM (DM group) and 25 patients without DM (non-DM group) were used in this study. The diabetes status of patients was accepted as diagnosed from the medical records. The hemoglobin A1c (HbA1c) levels of the DM group were 6.6±0.1 (only 7 DM patients who were confirmed by us). We could not follow the HbA1c data of the other DM patients or non-DM patients. At Miyazaki Prefectural Nobeoka Hospital, portions of each great SV graft were sectioned to the desired lengths for bypassing the occluded coronary arteries, while the remainder was used for the experiments. At Kuwabara Clinic, portions of the SV were sectioned from each patient as a surgical treatment for varix of the lower extremity, and only the non-distended areas were used for the experiments. The small SV segments were transported and their constrictive responses were measured as described previously [11], [12]. In brief, isolated vessels were placed in modified Krebs buffer, which had been previously aerated with 95% O2 and 5% CO2, and transported promptly to our laboratory. The composition of modified Krebs buffer solution was as follows: 118.0 mM NaCl, 4.7 mM KC1, 25.0 mM NaHCO3, 1.2 mM MgSO4, 1.1 mM KH2PO4, 2.5 mM CaCl2, 0.01 mM EDTA, and 11.0 mM glucose, pH 7.4 at 37 °C. After removal of fat and connective tissue, the vessel was cut into 2-mm rings, and endothelium was removed to exclude the influences of it to contractile response. Each ring was suspended between stainless steel hooks in a 5-mL organ bath containing modified Krebs buffer maintained at 37 °C and continuously aerated with 95% O2 and 5% CO2. One hook was connected to a force transducer (Nihon-Kohden, Japan) to record the isometric tension in a computer system (PowerLab8/30; Bio Research Center Co., Ltd., Japan). The SV rings were stretched progressively to the optimal tension (2.0 g) and allowed to equilibrate for 1 h. The buffer was changed every 30 min. After the tension of rings had completely stabilized, they were preconstricted with 60 mM KCl. Once maximum vasoconstriction was reached at a plateau, the ring was washed three times with fresh modified Krebs buffer solution. Thereafter, cumulative concentration-response curves to 5-HT (Sigma-Aldrich Co.) or AngII (Sigma-Aldrich Co.) over concentration ranges of 1 nM to 10 μM were constructed. Finally, the ring was washed three times, and the contraction in response to a second exposure to 60 mM KCl was recorded as the control contraction. The contractile reactivity of vein rings was evaluated using the percentage of the second KCl-induced vasoconstriction as 100%.
2.2. Western blot analysis of the AT1R and 5-HT2A receptor in membrane fractions
Following the contractile studies, the SV rings were flash frozen in liquid nitrogen and stored at −80 °C for Western blot analysis. Frozen SV rings were crushed with manual mill SK-200 (Tokken, inc.) and suspended in transmembrane protein extraction buffer 1 (ProteoExtract Transmembrane Protein Extraction Kit; Calbiochem-Novabiochem). Preparation for the membrane fractions and Western blot analysis was performed as described previously [11]. Equal amounts of protein (5.5 μg per lane) were separated by SDS-10% polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane Hybond-P (GE Healthcare Japan). The membrane was preincubated with 1% skim milk in Tween-Tris-buffered saline (10 mM Tris–HCl [pH 7.4], 150 mM NaCl, and 0.1% Tween-20) and reacted with rabbit anti-5-HT2A receptor (1:2000, Santa Cruz Biotechnology), rabbit anti-β-actin (1:2000, Cell Signaling Technology) or mouse anti-AT1R (1:1000, abcam) overnight at 4 °C in Immuno-enhancer Reagent A (Wako). The immunoreactive bands were reacted with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies in Immuno-enhancer Reagent B (Wako), visualized using the Immuno-Star enhanced chemiluminescent detection system (Wako), and quantified using a luminoimage LAS-4000 analyzer (GE Healthcare Japan). Protein levels of 5-HT2A receptor and AT1R were normalized by the level of β-actin.
2.3. Ethics
The Ethics Committees at both Miyazaki Prefectural Nobeoka Hospital and Kyusyu University of Health and Welfare approved this study, with acceptance number 09-004 (study using human saphenous vein). All patients provided written consent to participate in the study. These experiments were conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for Experiments Involving Humans.
2.4. Statistical analysis
All the data were averaged for each patients. Thus, the numbers of subject indicated in figures are the number of patients. Statistical comparisons of mean value of 5-HT- or AngII-induced vasoconstriction were made using two-way analysis of variance (ANOVA). If significant differences in vasoconstriction between the DM and non-DM groups were found, the rank-sum test of the increasing effect of DM on the vasoconstriction induced by AngII or 5-HT was carried out using the Mann-Whitney U test. Statistical comparisons of 5-HT2A receptor and AT1R in the membrane fraction of the SV smooth muscle were made using Welch's t-test. The data are presented as mean±standard error of the mean (SEM). Significance was assumed at P<0.05. Statistical analyses were performed using SPSS 21.0 J for Windows.
3. Results
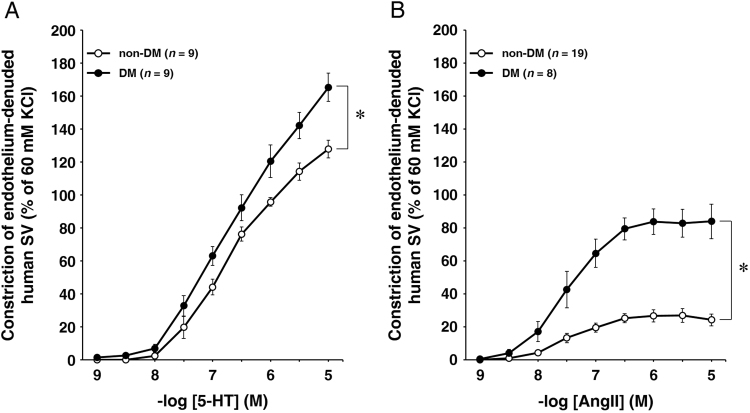
We examined the 5-HT- or AngII- induced constriction of isolated human endothelium-denuded SVs. The cumulative administration of 5-HT (1 nM–10 μM) induced the constriction of the SV in a concentration-dependent manner, however, the responses did not reach a plateau at less than 10 nM 5-HT in either the DM group (n=9) or the non-DM group (n=9) (Fig. 1A). Two-way ANOVA revealed that the 5-HT- induced vasoconstriction in the DM group was significantly greater than that in the non-DM group (P=0.011). The cumulative administration of AngII (1 nM–10 μM) induced the constriction of the SV in a concentration-dependent manner, and the responses reached a plateau at 300–1000 nM AngII in both the DM group (n=8) and the non-DM group (n=19) (Fig. 1B). The maximal constrictive degree was approximately 80% of 60 mM KCl-induced constriction in the DM group, but no more than 20% of 60 mM KCl-induced constriction in the non-DM group. Two-way ANOVA revealed that the AngII-induced vasoconstriction in the DM group was significantly greater than that in the non-DM group (P<0.0005).
Fig. 1.
(A) The contractile reactivity to 5-HT (A) of endothelium-denuded SVs harvested from non-DM (open circles, n=9) and DM patients (closed circles, n=9). The cumulative addition of 5-HT (1 nM to 10 μM) caused SV constriction in a concentration-dependent manner in non-DM and DM groups. (B) The contractile reactivity to AngII of endothelium-denuded SVs harvested from non-DM (open circles, n=19) and DM patients (closed circles, n=8). The cumulative addition of AngII (1 nM to 10 μM) caused SV constriction in a concentration-dependent manner in the non-DM and DM groups. The data are expressed as mean±SEM. The contractile reactivity of SV to 5-HT or AngII were evaluated as the percentage of 60 mM KCl-induced vasoconstriction as the control in each SV ring. *P<0.05 compared with the non-DM group.
To show the effect of DM on 5-HT- or AngII-induced vasoconstriction, we determined the increasing rate by dividing the value of contractile ligand-induced vasoconstriction at each concentration in the DM group by that at the same concentration in the non-DM group (Table). A rank-sum test revealed that the increasing rates of vasoconstriction elicited by AngII in the DM group to those in the non-DM group were significantly greater than those of vasoconstriction elicited by 5-HT. (P<0.005).Table 1.
Table 1.
The increasing rates of vasoconstriction found in the DM group.
| Ligand concentration (M) | 1.0×10−8 | 3.0×10−8 | 1.0×10−7 | 3.0×10−7 | 1.0×10−6 | 3.0×10−6 | 1.0×10−5 |
|---|---|---|---|---|---|---|---|
| 5-HT | 2.94 | 1.66 | 1.43 | 1.21 | 1.26 | 1.24 | 1.29 |
| AngII | 3.96 | 3.20 | 3.29 | 3.14 | 3.12 | 3.08 | 3.46 |
Indicated numerical numbers are calculated as dividing value of 5-HT- or AngII-induced vasoconstriction at each concentration in the DM group by those at same concentration in the non-DM group.
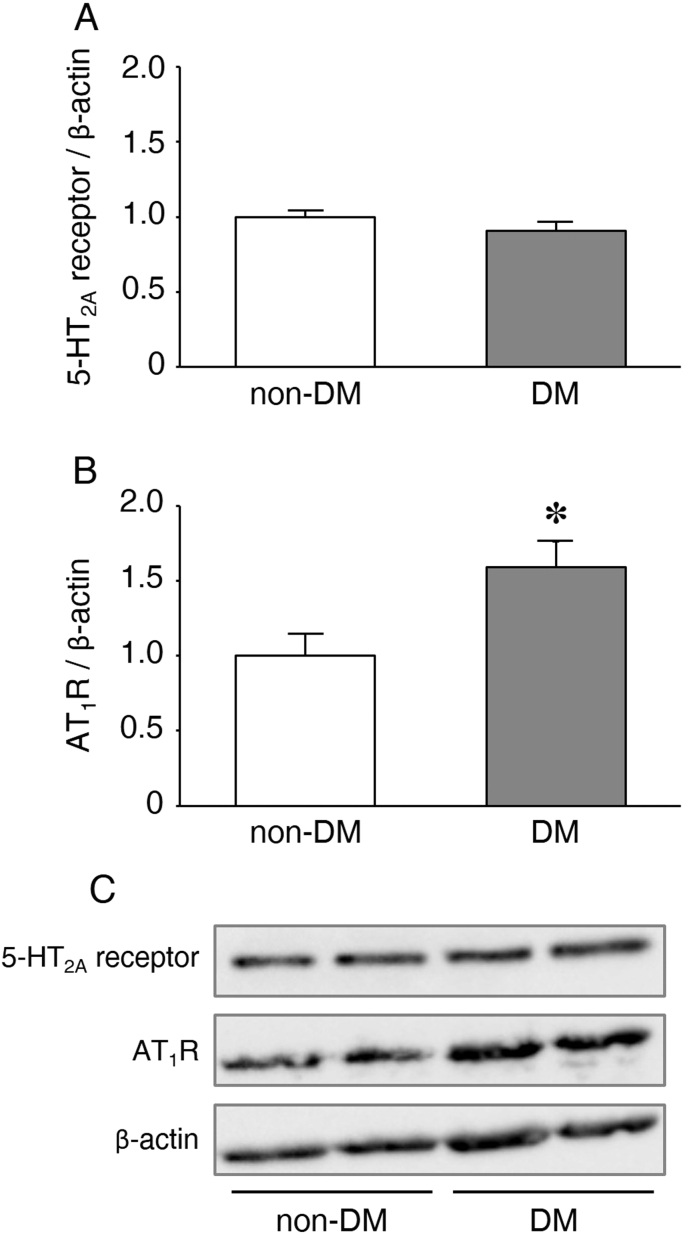
To clarify the reason why the constrictive responses of the SV, especially to AngII, were significantly enhanced in the DM group, we determined the protein levels of 5-HT2A receptor and AT1R in the membrane fraction prepared from the SVs harvested from the DM or non-DM patients. Fig. 2 shows the relative amounts of receptors/β-actin quantified using a bioimage analyzer. The level of AT1R in the membrane fraction of the DM group was significantly higher than that of the non-DM group (P=0.03). The level of 5-HT2A receptor in the membrane fraction did not differ between the DM and non-DM groups.
Fig. 2.
Protein levels of (A) 5-HT2A receptor and (B) AT1R, normalized by the β-actin, in the isolated membrane fraction of SV smooth muscle harvested from DM (n=6) or non-DM patients (n=6). The data are expressed as mean±SEM. *P<0.05 compared with the non-DM group. (C) Western blots of 5-HT2A receptor, AT1R and β-actin.
4. Discussion
We found that the 5-HT-induced constriction of isolated human endothelium-denuded SVs was significantly augmented in the DM group compared with the non-DM group, which was consistent with our previous report [4]. The AngII-induced constriction of SVs was also significantly augmented in the DM group than in the non-DM group. Especially in the non-DM group, AngII-induced maximal vasoconstriction was markedly lower than the 5-HT-induced one. Meanwhile, the increasing rates of AngII-induced vasoconstriction in the DM group to the non-DM group were significantly greater than those of 5-HT-induced vasoconstriction. These prominent differences shown between the DM and non-DM groups, in spite of the broad grouping, were unexpected. It has been reported that insulin resistance, a well-known risk factor for type 2 DM, upregulates the RAAS [8], [9]. Taken together, we demonstrated, for the first time, that not only 5-HT but also AngII is a potent inducer of SV graft spasm in patients with DM. Furthermore, protein level of AT1R in the membrane fraction of human SV smooth muscle cells from DM patients was significantly increased as compared with that in non-DM patients, but not the protein level of 5-HT2A receptors. These results suggest that the mechanism for hyperreactivity to AngII in the human SV from DM patients is due to, at least in part, the increase in the amount of AT1R on the membrane of SV smooth muscle cells.
The incidence of DM is increasing globally, and cardiovascular complications are the most common causes of mortality and morbidity among DM patients [13]. Alternations in vascular reactivity play a key role in diabetic cardiovascular complications [14]. Vascular reactivity under DM conditions has been the subject of many studies. Endothelial dysfunction among DM patients has been well-documented and recognized as an important factor leading to decreased vasodilatation and increased vasoconstriction [15]. By contrast, the dysfunction of vascular smooth muscle in DM patients is less clear and remains to be fully explored [14], [16], [17]. DM is also a well-established risk factor for early and late adverse outcomes after CABG. The SV remains the most widely used conduit in CABG, although it has shorter patency than arterial conduits [18]. One of the most serious problems in CABG is bypass graft spasm, which can lead to premature occlusion and increased severe adverse complications [4], [5]. As shown in Fig. 1A, the low concentration of 5-HT-induced vasoconstriction (less than 100 nM) in the endothelium-denuded human SV was significantly augmented in the DM group than in the non-DM group, in a similar manner to that observed in our previous report [7]. In addition, the vasoconstrictive hyperreactivity to 5-HT persisted even with stimulation by a high concentration of 5-HT (more than 100 nM).
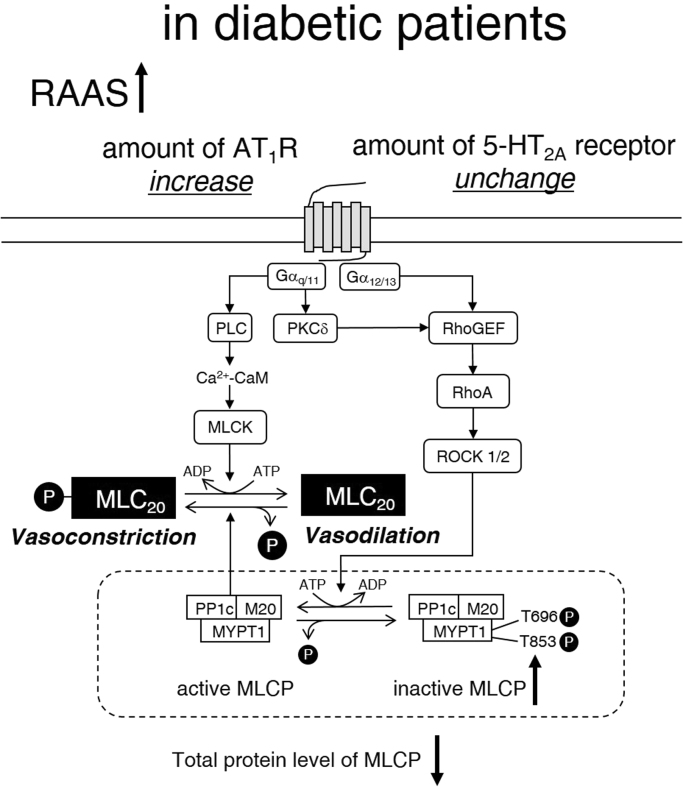
5-HT2A receptors are expressed on the cell membrane of the human SV and contribute to 5-HT-induced vasoconstriction [19], [20]. At the grafted area, platelets are susceptible to activation because endothelial cells entirely break off from the vascular wall of the grafted vessels during the CABG process [22]. Activated platelets release several chemical mediators including 5-HT. The platelet-released 5-HT may induce vasospasm of the graft mediated by the 5-HT2A receptor [21]. In our present study, vasoconstriction induced by >1 μM 5-HT was greater than that induced by 60 mM KCl in both the DM and non-DM groups. These results strongly suggest that 5-HT could be a potent inducer of vasospasm after CABG. As shown in Fig. 3, vasoconstriction is controlled by both activities of myosin light chain kinase and myosin light chain phosphatase (MLCP). 5-HT2A receptor and AT1R are coupled to Gq, phospholipase C, RhoA/Rho kinase, and the downstream intracellular signal transduction cascade. When RhoA/ROCK pathway is activated by Gq coupled receptors, such as 5-HT2A receptor or AT1R, the myosin phosphatase target subunit 1 (MYPT1), a subunit of MLCP, is phosphorylated, and the phosphorylation inhibits MLCP activity. We previously reported that in the resting state of the human endothelium-denuded SV, total tissue protein levels of 5-HT2A receptor, RhoA, ROCK1 and ROCK2 did not differ between the DM and non-DM groups. However, the total protein level of MYPT1 was significantly lower in the DM group than in the non-DM group. Furthermore, the ratio of P(Thr696)-MYPT1 to total MYPT1 was significantly higher in the DM group than in the non-DM group. These results suggest that the hyperreactivity to 5-HT or AngII in the SV smooth muscle of patients with DM is not only due to the enhanced phosphorylation of MLCP but also due to the defective protein level of MLCP [7]. Similar hyperreactivity of vasoconstriction in response to noradrenaline in the renal artery was observed with the upregulation of Rho kinase in high glucose- and streptozotocin-induced DM model rats [22]. This hyperreactivity was elicited not only by the dysfunction of vascular endothelial cells but also the degeneration of the vascular smooth muscle itself because the augmentation of vasoconstriction was detected in endothelium-denuded, as well as intact arteries.
Fig. 3.
Additional hypothesis on the mechanisms of diabetic SV smooth muscle hyperreactivity as described previously (Biochem Biophys Res Commun, 412, 323–327, 2011). PLC, phospholipase C; PKCδ, protein kinase Cδ; CaM, calmodulin; MLCK, Ca2+-CaM-dependent myosin light chain kinase; MLC20, 20-kDa regulatory myosin light chain; PP1c, a catalytic subunit of type 1 protein phosphatase; M20, 20-kDa non-catalytic subunit of MLCP; MYPT1, myosin phosphatase target subunit 1 of MLCP; RhoGEF, GDP-GTP exchange factor of RhoA. Under DM conditions the protein level of AT1R in the membrane fraction is increased. Thus, the hyperreactivity to AngII is due to not only the qualitative and quantitative deterioration of MLCP but also the protein level of membrane AT1R increase.
It has been reported that insulin resistance, the main defects in type 2 DM, upregulates the RAAS [8], [9]. In the present study, AngII caused the vasoconstriction of endothelium-denuded SV in a concentration-dependent manner (Fig. 1B). The AngII-induced maximal tension of the SV in the DM group was approximately 80% of that induced by 60 mM KCl. On the other hand, the AngII-induced maximal tension of the SV in the non-DM group was approximately 20% of that induced by 60 mM KCl. These results suggest that AngII is also a potent inducer of graft spasm after CABG in DM patients but not in non-DM patients. The increasing rates of AngII-induced vasoconstriction in the DM group to the non-DM group were significantly greater than those of 5-HT-induced vasoconstriction (Table). This augmentation of vasoconstrictive hyperreactivity to AngII can be partially explained by the enhanced RhoA/ROCK pathway and the defective protein level of MLCP, because the AT1R is coupled with Gq/11 and G12/13, similar to the 5-HT2A receptor [7]. However, it still not clear why there is a difference between the hyperreactivity to 5-HT and AngII induced by DM in the SV vasoconstrictive responses. Hence, we examined the protein level of 5-HT2A receptor and AT1R in the membrane fraction of SV smooth muscle harvested from DM or non-DM patients. The membrane AT1R level in the DM group was significantly higher than that in the non-DM group (Fig. 2). On the other hand, the membrane level of 5-HT2A receptor in the DM group was comparable with that in the non-DM group. These results suggest that the hyperreactivity to AngII of the human SV from DM patients is due to the increase in the membrane AT1R localization, in addition to the quantitative and qualitative degradation of MLCP (Fig. 3).
The use of angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) is particularly common among patients with hypertension. In a Japanese hospital, approximately half of patients undergoing CABG were administered these angiotensin system inhibitors. However, the effect of ACEI/ARB therapy on the clinical outcomes of patients undergoing CABG is still controversial. A prospective cohort study indicated that ACEI treatment after CABG was associated with improved in-hospital outcomes [23]. By contrast, another clinical trial showed that routine early initiation of quinapril, an ACEI, did not appear to improve the clinical outcome 3 years after CABG among patients at low risk of cardiovascular events [24]. In the report that showed the improvement effect of ACEI on in-hospital outcomes [23], the rate of DM patients in ACEI-administered and non-administered groups were 37.6% and 25.0%, respectively (P<0.05). However, in the report which did not show an improvement in clinical outcomes [24], the rate of DM patients in ACEI-administered and non-administered group were 9.5% and 10.4%, respectively (P=NS). Based on our present study, the controversial effect of ACEI, as reported previously, may depend on the ratio of patients with DM in the research subjects.
In conclusion, DM is associated with a high risk of SV spasm early after CABG due to hyperreactivity to vasocontractile ligands. In particular, AngII plays important roles in the pathogenesis of SV spasm in DM patients, but not in non-DM patients, early after CABG, which is often accompanied by increasing RAAS activity. Thus, the use of ACEI or ARB in patients undergoing CABG, particularly among DM patients, undoubtedly contributes to the prevention of SV graft spasm, aside from their established benefits such as antihypertensive and cardio-protective effects.
Acknowledgements
This study was supported in part by MEXT KAKENHI Grant Number 25462154. We thank Ms. Ayana Abe for her assistance in the technical aspects of our study.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.03.008.
Appendix A. Supplementary materials
Supplementary Material
References
- 1.Mohr F.W., Morice M.C., Kappetein A.P., Feldman T.E., Ståhle E., Colombo A., Mack M.J., Holmes D.R., Jr., Morel M.A., Van Dyck N., Houle V.M., Dawkins K.D., Serruys P.W. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomized, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 2.Farkouh M.E., Domanski M., Sleeper L.A., Siami F.S., Dangas G., Mack M., Yang M., Cohen D.J., Rosenberg Y., Solomon S.D., Desai A.S., Gersh B.J., Magnuson E.A., Lansky A., Boineau R., Weinberger J., Ramanathan K., Sousa J.E., Rankin J., Bhargava B., Buse J., Hueb W., Smith C.R., Muratov V., Bansilal S., King S., 3rd, Bertrand M., Fuster V. FREEDOM Trial investigators, strategies for multivessel revascularization in patients with diabetes. N. Engl. J. Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 3.Thourani V.H., Weintraub W.S., Stein B., Gebhart S.S., Craver J.M., Jones E.L., Guyton R.A. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Am. Thorac. Surg. 1999;67:1045–1052. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 4.Chanda J., Canver C.C. Reversal of preexisting vasospasm in coronary-artery conduits. Am. Thorac. Surg. 2001;72:476–480. doi: 10.1016/s0003-4975(01)02799-0. [DOI] [PubMed] [Google Scholar]
- 5.Fabricius A.M., Gerber W., Hanke M., Garbade J., Autschbach R., Mohr F.W. Early angiographic control of perioperative ischemia after coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2001;19:853–858. doi: 10.1016/s1010-7940(01)00692-3. [DOI] [PubMed] [Google Scholar]
- 6.Lopes R.D., Hafley G.E., Allen K.B., Ferguson T.B., Peterson E.D., Harrington R.A., Mehta R.H., Gibson C.M., Mack M.J., Kouchoukos N.T., Califf R.M., Alexander J.H. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N. Engl. J. Med. 2009;361:235–244. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo Y., Kuwabara M., Tanaka-Totoribe N., Kanai T., Nakamura E., Gamoh S., Suzuki A., Asada Y., Hisa H., Yamamoto R. The defective protein levels of myosin light chain phosphatase (MLCP) in the isolated saphenous veins, as a vascular conduit in coronary artery bypass grafting (CABG), harvested from patients with diabetes mellitus (DM) Biochem. Biophys. Res. Commun. 2011;412:323–327. doi: 10.1016/j.bbrc.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 8.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z. The renin-angiotensin system and insulin resistance. Curr. Diab. Rep. 2007;7:34–42. doi: 10.1007/s11892-007-0007-5. [DOI] [PubMed] [Google Scholar]
- 10.Osgood M.J., Harrison D.G., Sexton K.W., Hocking K.M., Voskresensky I.V., Komalavilas P., Cheung-Flynn J., Guzman R.J., Brophy C.M. Role of renin-angiotensin system in the pathogenesis ofintimal hyperplasia: therapeutic potential for prevention of vein graft failure? Ann. Vasc. Surg. 2012;26:1130–1144. doi: 10.1016/j.avsg.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai T., Kuwabara M., Tanaka-Totoribe N., Nakamura E., Matsuo Y., Gamoh S., Suzuki A., Asada Y., Hisa H., Yamamoto R. Insulin induces internalization of the plasma membrane 5-hydroxytryptamin2A (5-HT2A) Receptor in the isolated human endothelium-denuded saphenous vein via the phosphatidylinositol 3-kinase pathway. J. Phamacol. Sci. 2012;118:178–185. doi: 10.1254/jphs.11172fp. [DOI] [PubMed] [Google Scholar]
- 12.Gamoh S., Kanai T., Tanaka-Totoribe N., Ohkura M., Kuwabara M., Nakamura E., Yokota A., Yamasaki T., Watanabe A., Hayashi M., Fujimoto S., Yamamoto R. Water-soluble jack-knife prawn extract inhibits 5-hydroxytryptamine-induced vasoconstriction and platelet aggregation in humans. Food Funct. 2015;6:444–449. doi: 10.1039/c4fo00716f. [DOI] [PubMed] [Google Scholar]
- 13.Lam T., Bums K., Dennis M., Cheung N.W., Gunton J.E. Assessment of cardiovascular risk in diabetes: Risk scores and provocative testing. World J. Diabetes. 2015;6:634–641. doi: 10.4239/wjd.v6.i4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Z., Su W., Allen S., Pang H., Daugherty A., Smart E., Gong M.C. COX-2 up-regulation and vascular smooth muscle contractile hyperactivity in spontaneous diabetic db/db mice. Cardiovasc. Res. 2005;67:723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Hadi H.A., Suwaidi J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007;3:853–876. [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischhacker E., Esenabhalu V.E., Spitaler M., Holzmann S., Skrabal F., Koidl B., Kostner G.M., Graier W.F. Human diabetes is associated with hyperreactivity of vascular smooth muscle cells due to altered subcellular Ca2+distribution. Diabetes. 1999;48:1323–1330. doi: 10.2337/diabetes.48.6.1323. [DOI] [PubMed] [Google Scholar]
- 17.Okon E.B., Chung A.W., Rauniyar P., Padilla E., Tejerina T., McManus B.M., Luo H., van Breemen C. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 18.Hortmann H.C., Oliveira H.G., Rabello R.R., Rocha E.A., Oliveira S.C. Comparison of patency between radial artery and saphenous vein in a coronary artery bypass grafting post operative with return of the symptoms. Rev. Bras. Cir. Cardiovasc. 2010;25:218–223. doi: 10.1590/s0102-76382010000200014. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura E., Tanaka N., Kuwabara M., Yamashita A., Matsuo Y., Kanai T., Onitsuka T., Asada Y., Hisa H., Yamamoto R. Relative contributions of 5-hydroxytryptamine (5-HT) receptor subtypes in 5-HT-induced vasoconstriction of the distended human saphenous vein as a coronary artery bypass graft. Biol. Pharm. Bull. 2011;34:82–86. doi: 10.1248/bpb.34.82. [DOI] [PubMed] [Google Scholar]
- 20.Gamoh S., Hisa H., Yamamoto R. 5-hydroxytryptamine receptors as targets for drug therapies of vascular-related diseases. Biol. Pharm. Bull. 2013;36:1410–1415. doi: 10.1248/bpb.b13-00317. [DOI] [PubMed] [Google Scholar]
- 21.Miyata K., Shimokawa H., Higo T., Yamawaki T., Katsumata N., Kandabashi T., Tanaka E., Takamura Y., Yogo K., Egashira K., Takeshita A. Sarpogrelate, a selective 5-HT2A serotonergic receptor antagonist, inhibits serotonin-induced coronary artery spasm in a porcine model. J. Cardiovasc. Pharmacol. 2000;35:294–301. doi: 10.1097/00005344-200002000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Li T., Yang G.M., Zhu Y., Wu Y., Chen X.Y., Lan D., Tian K.L., Liu L.M. Diabetes and hyperlipidemia induce dysfunction of VSMCs: contribution of the metabolic inflammation/miRNA pathway. Am. J. Physiol. Endocrinol. Metab. 2015;308:E257–E269. doi: 10.1152/ajpendo.00348.2014. [DOI] [PubMed] [Google Scholar]
- 23.Drenger B., Fontes M.L., Miao Y., Mathew J.P., Gozal Y., Aronson S., Dietzel C., Mangano D.T. Patterns of use of perioperative angiotensin- converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in-hospital morbidity and mortality. Circulation. 2012;126:261–269. doi: 10.1161/CIRCULATIONAHA.111.059527. [DOI] [PubMed] [Google Scholar]
- 24.Rouleau J.L., Warnica W.J., Baillot R., Block P.J., Chocron S., Johnstone D., Myers M.G., Calciu C.D., Dalle-Ave S., Martineau P., Mormont C., van Gilst W.H. IMAGINE (Ischemia Management with Accupril post-bypass Graft via Inhibition of the coNverting Enzyme) investigators, effects of angiotensin-converting enzyme inhibition in low-risk patients early after coronary artery bypass surgery. Circulation. 2008;177:24–31. doi: 10.1161/CIRCULATIONAHA.106.685073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material