Abstract
Background
INTERMACS patient Profiles and Modifiers are descriptors of patient illness severity prior to durable VAD implant. It is unknown how individual U.S. institutions and practitioners assign Profiles and if Modifiers improve on risk discrimination.
Methods
Web-based survey respondents (n=212) answered questions about the INTERMACS Profile assignment process within their institution. For five hypothetical clinical scenarios, respondents assigned the best Profile. The INTERMACS registry (2009–2014) was then queried and hazard ratios (HR, 95% confidence interval) for mortality between Profiles and based on the presence of temporary circulatory support (TCS), Frequent Flyer (FF), or Arrhythmia Modifiers were calculated.
Results
Respondents included 131 (62%) cardiologists, 30 (14%) surgeons, and 51 (24%) physician extenders/coordinators. Institutional Profile assignment was variable (63% assigned by cardiologists/surgeons; 10% by research coordinators; 27% by physician extenders). Profile assignments within hypothetical patient scenarios were heterogeneous, especially for contiguous Profiles.
One year survivals for Profiles 1, 2, and 3 were 77±1.2%, 80±0.7%, and 84±0.7%, respectively (p<0.001). While Profile 1 patients had worse adjusted survival than Profiles 3 (p=0.001), survival for Profiles 1 vs. 2 was similar (adjusted HR 1.01 [0.88–1.12]). The TCS (adjusted HR 1.1 [0.94–1.2]) and Arrhythmia (adjusted HR 1.1 [0.97–1.2]) modifiers were not predictive of mortality but the FF modifier was (HR 1.3 [1.02–1.63]).
Conclusions
Substantial heterogeneity exists in the process and assignment of INTERMACS Profiles. This heterogeneity could impact mortality estimates used for risk stratification. Only the FF modifier appears to improve risk discrimination beyond that of known risk factors. Adding objective descriptors may reduce Profile heterogeneity.
Introduction
Risk stratification of candidates for left ventricular assist device (LVAD) implant is critical for appropriate LVAD candidate selection to help foster good patient outcomes and ensure appropriate resource utilization. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) patient Profiles were devised to categorize patients for the purposes of risk assessment prior to LVAD implant (1). The Profiles, ranging from 1–7, describe a patient’s clinical status in terms of hemodynamic stability (critical cardiogenic shock to stable), inotrope use (inotrope dependent vs. not), and functional capacity (inpatient, housebound, walking wounded). In 2009, Modifiers were added to enhance Profile description and include the temporary circulatory support (TCS), Arrhythmia and Frequent Flyer modifiers (1). The TCS modifier is meant to be assigned to patients in Profiles 1–3 supported in the preVAD period on an intraaortic balloon pump (IABP, used for shock and not elective support), extracorporeal membrane oxygenation (ECMO), or temporary ventricular (right or left) support (1). The Arrhythmia modifier can be applied to any profile and includes patients with recurrent ventricular tachyarrhythmias that evoke clinical compromise (1). Finally, the Frequent Flyer (FF) modifier applies to patients in Profile 3 at home on inotropes and patients in Profiles 4–7 (1). The FF modifier denotes a patient with frequent emergency room (ER) visits or hospitalizations (≥2 ER/hospital visits in 3 months or 3 visits in 6 months) for heart failure management, including use of IV diuretics, inotropes, and/or ultrafiltration (1).
INTERMACS Profiles have been shown by many to be predictive of outcome following LVAD implant (2–3). However, the accuracy of risk stratification relies on uniform application of patient Profiles to patients of similar preoperative status. Like NYHA class, patient Profiles are based largely on subjective descriptors and the reliability of Profile assignment and mechanism of institutional profile assignment in U.S. VAD centers is not known. Further, the implication of Modifier ascription has not been examined and it is unknown if they portend poorer outcome.
The aims of this study are to examine 1) how U.S. VAD institutions assign INTERMACS patient Profiles; 2) the reliability of INTERMACS patient Profile assignment; and 3) whether INTERMACS Modifiers provide prognostic information that is additive to INTERMACS patient Profile and other known correlates of risk.
Methods
Query of INTERMACS Profile Assignment within the U.S
A web-based survey (via www.surveymonkey.com) was sent out via the International Society for Heart and Lung Transplant (ISHLT) Connect (http://community.ishlt.org/home) message board and by email to ISHLT cardiologists, surgeons, nurse practitioners/physician assistants (referred herein as physician extenders), VAD coordinators and research coordinators. Responses from practitioners who lived outside the U.S. (n=18) and by a perfusionist (n=1) were excluded from the analysis, leaving 212 total U.S. respondents. Responses from the same program were permitted (n=10 instances).
Survey participants were asked to assign their job category (as above), intuitional yearly VAD volume, and VAD center type (destination therapy (DT) alone or DT and bridge to transplant (BTT)). The following questions were asked about their institutional handling of patient INTERMACS Profiles: 1) Who assigns the INTERMACS Profile to patients within your institution? (Options: a) cardiologist, b) surgeon, c) cardiologist or surgeon, d) physician extender, e) research coordinator, f) Other). 2) Prior to assigning a patient INTERMACS profile, do you routinely discuss INTERMACS categorization with the transplant/VAD committee to generate a consensus? (Options: a) Yes, b) No, c) Sometimes but not always).
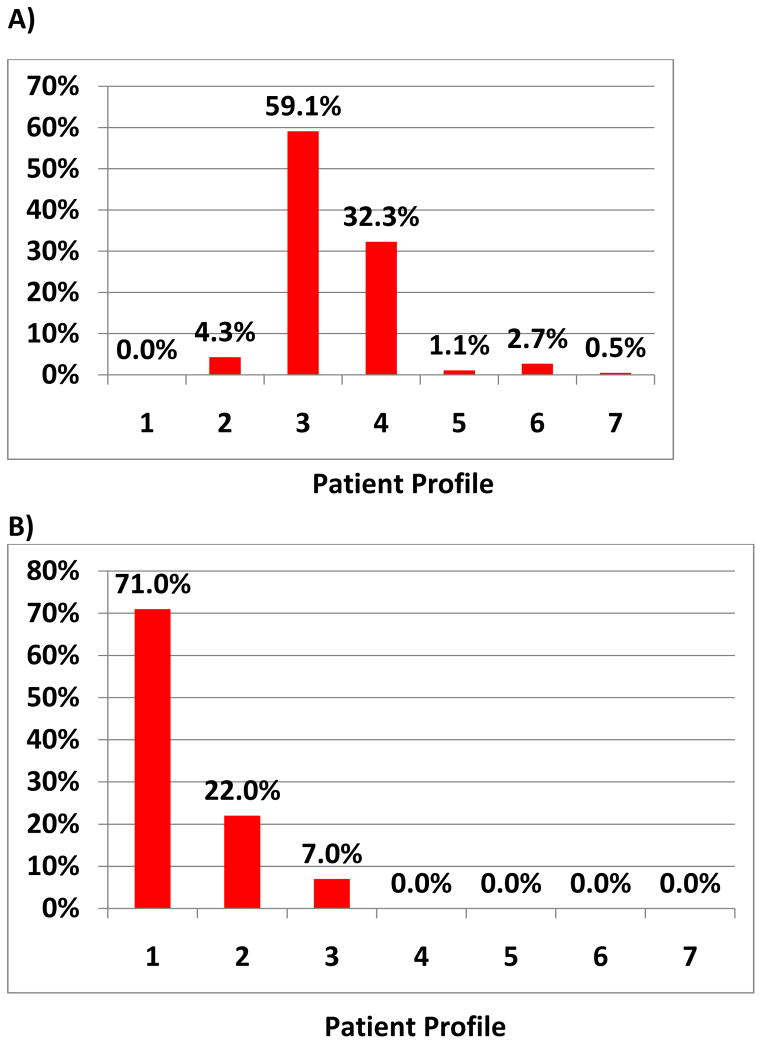
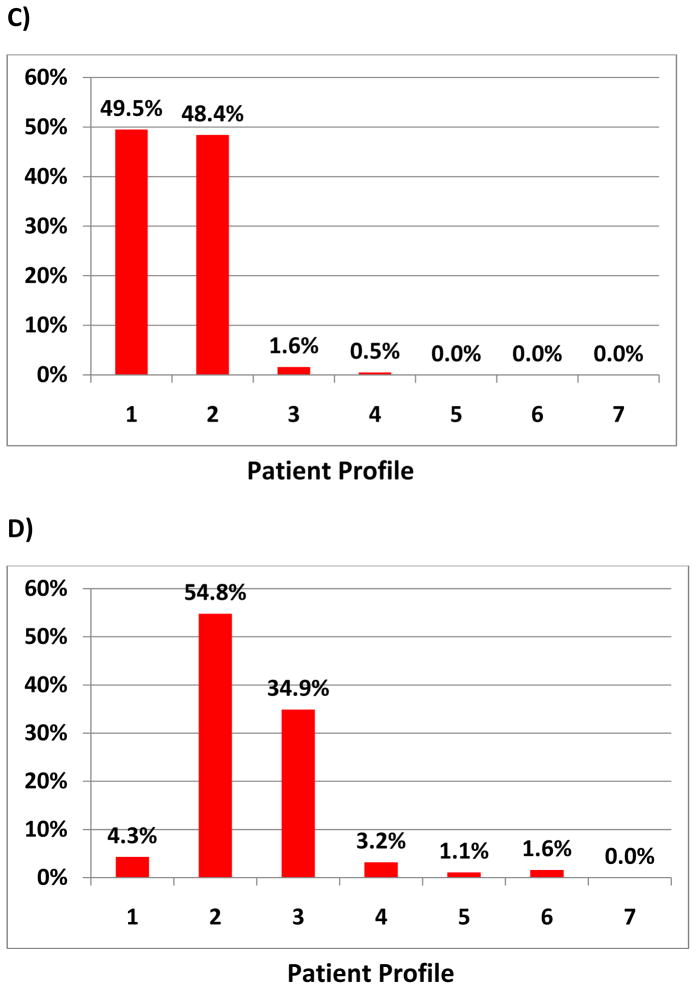
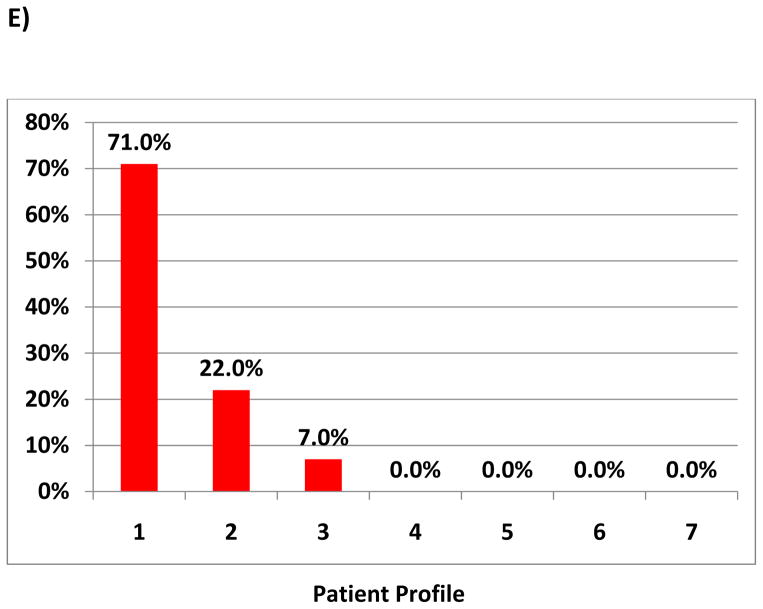
Survey participants were then provided with 5 patient scenarios (table 1) and asked to assign the best INTERMACS Profile based on the information provided. Frequency of each Profile for each scenario was tallied.
Table 1.
Survey Clinical Scenarios.
| Case Description | |
|---|---|
| Scenario A | 56 year old male with chronic nonischemic systolic heart failure admitted from clinic with NYHA class IIIb–IV symptoms and 20 pound weight gain on 100 mg torsemide. Not on home inotropes. 2nd CHF admission in 6 months. Baseline Cr 1.0 mg/dL. Admission Cr 1.5, ALT 20, INR 1.0. Currently on the telemetry (nonICU) floor on 0.125 mcg/kg/min milrinone and Lasix 10 mg/hr gtt. On day of operation, Cr 1.1 mg/dL, ALT 28 IU/L, INR 1.0. Swan on 0.125 mcg/kg/min milrinone following medical Rx: RA 9, PA 65/21, WP 20, CI 2.3. |
| Scenario B | 56 year old male with acute nonischemic biventricular heart failure in shock on admission who was placed on ECMO then bilateral Centrimags (Thoratec Corporation, Pleasanton, CA). Currently, patient is in the ICU on ventilator support and stable on vasopressin 0.02 units/min, milrinone 0.5 mcg/kg/min, and amiodarone. His mean arterial pressure is 77 mmHg and his heart rate is 110 bpm. He is making urine with a Cr of 1.2 mg/dL (baseline 1.5), ALT 33, INR 1.1. |
| Scenario C | 56 year old male with chronic nonischemic heart failure (EF <10%) admitted with 20 pound weight gain, nausea and vomiting with SBP 83/68 and HR 120 bpm. He is on 100 mg daily torsemide at home. First admit in a year. Not on home inotropes. Currently, patient is in the ICU on an intraaortic balloon pump on vasopressin 0.04 units/min, milrinone 0.5 mcg/kg/min, dobutamine 5 mcg/kg/min and levophed 12 mcg/min. His blood pressure is 85/65 mmHg and his heart rate is 125 bpm. Swan numbers:, RA 14, PA 53/30, WP 27, CI 1.5. He is making urine with a lot of IV diuretics. Labs day of operation: Cr 1.7 mg/dL (1.0 is his baseline), His INR is 1.5 (no warfarin), ALT 100. He is NOT vented. |
| Scenario D | 56 year old male with chronic nonischemic systolic heart failure admitted from clinic with NYHA class IIIb–IV symptoms and 20 pound weight gain on 100 mg torsemide. Baseline Cr 1.0 increasing to 1.5 mg/dL on admission. Currently in the ICU on milrinone 0.5 mcg/kg/min and Lasix 15 mg/hr gtt. No pressors, IABP or ventilator. Labs on day of operation: ALT 90, INR 1.3, Cr 2.1. Swan on 0.5 mcg/kg/min milrinone: RA 20, PA 45/36, WP 32, CI 1.6 |
| Scenario E | 56 year old male with chronic nonischemic systolic heart failure admitted from home for scheduled VAD implant. Discharged 2 weeks ago after presenting with 20 pound weight gain on 100 mg torsemide with baseline Cr 1.4 peaking at 1.7 mg/dL on prior admission. NOT on home inotropes. 2nd admission in 6 months. Currently on the tele (nonICU) floor. Labs day of operation: ALT 20, INR 0.8, Cr 1.5. Admission swan numbers OFF milrinone: RA 11, PA 65/28, WP 27, CI 1.6. |
Abbreviations: ALT= alanine aminotransferase (IU/L), Cr=creatinine (mg/dL), ECMO=extracorporeal membrane oxygenation, IABP=intraaortic balloon pump, ICU= intensive care unit, NYHA= New York Heart Association, RA=right atrial pressure (mmHg), PA= pulmonary artery pressure (mmHg), WP= wedge pressure (mmHg), CI=cardiac index, (L/min/m2), SBP=systolic blood pressure (mmHg), HR=heart rate (beats per minute).
Characteristics of Patient Profiles within INTERMACS and those with Modifiers
Data from 10,802 patients entered into INTERMACS who underwent continuous flow (CF) LVAD support (2009–2014) were analyzed. Preoperative clinical characteristics and demographics and frequencies of preoperative vasoactive medication use, hemodialysis, ventilator support, and ascription of temporary circulatory support (TCS, for Profiles 1–3), Arrhythmia (for all Profiles) and Frequent Flyer (for Profiles 3–7) modifiers were compared across Profiles. TCS is defined by INTERMACS as IABP, temporary LVAD or RVAD, and ECMO (4). IABP support in INTERMACS can be coded for TCS and nonTCS indications such that all patients on IABP support are not necessarily classified as requiring TCS. For example, some centers may use IABP support for prophylactic use and/or to provide hemodynamic support during induction of anesthesia, which would be coded as IABP “yes” and TCS “no”.
For TCS modifier analyses, patients with unknown/blank TCS modifier statuses were excluded (n=338), leaving n=8432 Profile 1–3 patients for TCS modifier-related analyses. There were 23 patients listed as “no TCS use” in INTERMACS who were incongruously documented to have some form of TCS (n=17 ECMO and n=6 temporary LVAD) within 48 hours of LVAD. These patients were therefore reassigned to the TCS “yes” group. There were 23 patients assigned in Profiles 4–7 to a TCS “yes” status; INTERMACS only allows for Profiles 1–3 to receive this modifier. Thus, the 23 patients were reassigned to the Profile 3 “TCS yes” group. Finally, there were 326 patients listed as having been on TCS without a documented TCS type. These patients were included generically as TCS patients, but not further analyzed by TCS subcategory.
The main clinical outcomes of interest were differences in survival between 1) contiguous INTERMACS patient Profiles and 2) based on use of INTERMACS Modifiers.
Statistical Analysis
Categorical variables were tallied as frequencies and were compared with Fishers exact testing or Pearson’s X2 p values for >2×2 comparisons. Continuous variables were assessed for normality using histograms and are reported as mean ± standard error or median [25th, 75th], as appropriate, unless otherwise specified. Possible differences between groups were assessed by Student’s t or Mann-Whitney testing, as appropriate. For all comparisons, a p ≤0.05 was considered statistically significant.
Odds ratios for operative mortality (within 90 days of implant) were generated with logistic regression. Kaplan-Meier survival estimates were calculated, censoring patients at the time of transplant or explant for recovery. Survival was compared with Log Rank testing. Cox regression was used to compare adjusted mortality in patients based on INTERMACS Profiles, focusing on contiguous Profiles using pairwise comparisons. Mortality comparisons were adjusted for known clinical risks, including year of implant, age category, sex, prior cardiac surgery, body mass index, destination therapy, use of inotropes, vasopressors, preoperative (within 48 hours) dialysis, ventilator support and preoperative creatinine, INR, and albumin (2–6). Cox regression was also used to assess the associated mortality risk of having the TCS, Arrhythmia, or Frequent Flyer modifier preoperatively, controlling for INTERMACS Profile and the above known risk correlates. Hazard ratios (HR) [95% confidence interval] are provided.
This analysis was approved by the INTERMACS Data Access, Analysis, and Publication (DAAP) committee. For the INTERMACS analysis, patients provided consent and institutional IRB approval was granted.
Results
Query of INTERMACS Profile Assignment within the U.S
Of the 212 U.S. survey respondents, 62% (n=131) were cardiologist, 14% (n=30) were surgeons, 16% (n=33) were VAD coordinators or extended providers and 8% (n=18) were research coordinators. Few respondents were from DT only VAD centers (n=14, 2%). The remaining respondents were at centers that provided VAD implants for both BTT and DT indications. Of these (n=198), 22% implanted >50 VADs a year, 38% implanted 31–50 VADs a year, 19% implanted 15–30 VADs a year, and 14% implanted <15 VADs a year.
The process for INTERMACS Profile assignment within the U.S. is variable across centers. The treating surgeon/cardiologist assigns INTERMACS Profile in 63% of centers. In 27% of centers, either the VAD coordinator or extended provider caring for the patient assigns the patient’s Profile, while 10% of centers leave Profile assignment to the institution research coordinator. When asked “Do you routinely discuss INTERMACS Profile with the VAD committee to generate a group Profile consensus for INTERMACS entry,” only 24% answered “yes,” 39% said “sometimes” and 37% said “no.”
Clinical Scenarios and INTERMACS Profile Assignment
There was marked heterogeneity noted in assignment of INTERMACS Profile (table 1 and Figure 1A–E). The greatest difference in responses tended to be amongst patients on TCS or in shock prior to VAD implant. In scenario B, 71% of respondents classified a patient initially on ECMO then stabilized with bilateral CentriMag (Thoratec Corporation, Pleasanton, CA) pumps as an INTERMACS Profile 1. However, 22% and 7% classified the patient as Profiles 2 and 3, respectively. There was also heterogeneity in practitioner response. An INTERMACS 1 status was assigned by 65% of cardiologist, 73% of surgeons, 69% of research coordinators and 49% of extended providers. An INTERMACS 2 status was assigned by 19% of cardiologists, 13% of surgeons, 15% of coordinators and 30% of extended providers (p<0.001).
Figure 1. Frequency of Profile Assignment for Given Patient Scenario.
The scenarios are provided in Table 1 and correspond to A–E.
The patient in scenario C on two inotropes and vasopressors, with poor cardiopulmonary hemodynamics and signs of renal and hepatic dysfunction was assigned INTERMACS Profiles 1 and 2 by 49.5% respondents and 48.4% of respondents, respectively. An INTERMACS 1 Profile was assigned by 53% of cardiologists, 33% of surgeons, 15% of research coordinators and 33% of extended providers. A Profile 2 was assigned by 35% of cardiologists, 60% of surgeons, 77% of research coordinators and 49% of extended providers (p<0.001). Statistically significant differences in assignment of INTERMACS Profile by provider existed for each scenario (p<0.05, data not shown).
INTERMACS Profile heterogeneity also appears to be greatest in contiguous Profiles. In scenario A, the non-intensive care unit (ICU) patient with stable vitals and end organs and a cardiac index of 2.3 on 0.125 mcg/kg/min of milrinone was characterized as Profile 3 in 59% and Profile 4 in 32%. In scenario D, the ICU patient with high filling pressures, a low cardiac index on 0.5 mcg/kg/min milrinone with acute renal failure, and mild transaminitis and coagulopathy was classified by 54.8% as INTERMACS Profile 2 and 34.9% as Profile 3.
Characteristics of Patient Profiles within INTERMACS
In INTERMACS, there were 10,802 patients implanted with continuous flow devices from 2009–2014. Table 2 shows patient clinical characteristics and demographics by INTERMACS Profile. As expected, INTERMACS Profile 1 patients had greater requirements for preoperative ventilator support, vasopressor use, and were more likely to have received dialysis prior to VAD implant. Further, they were more likely to have been supported with TCS. Profile 2 patients required less mechanical and pharmacologic support than Profile 1 patients, but had a higher frequency of preoperative vasopressor, ventilator and IABP use than Profiles 3–7. Characteristics of patients in Profiles 3–7 were overall similar with small clinical differences in each of the above factors.
Table 2.
Patient Clinical Characteristics and Demographics by INTERMACS Profile
| Profile 1 (n=1584) | Profile 2 (n=4089) | Profile 3 (n=3097) | Profile 4–7 (n=2032) | p value | |
|---|---|---|---|---|---|
|
| |||||
| Age Group, yrs | <0.001 | ||||
| <50 | 536 (34%) | 1084 (27%) | 747 (24%) | 360 (18%) | |
| 50–69 | 930 (59%) | 2498 (61%) | 1834 (59%) | 1279 (63%) | |
| ≥70 | 118 (7.4%) | 507 (12%) | 516 (17%) | 393 (19%) | |
|
| |||||
| Male | 1202 (76%) | 3200 (78%) | 2447 (79%) | 1655 (81%) | 0.001 |
|
| |||||
| ISCM | 806 (51%) | 1753 (43%) | 1393 (45%) | 1133 (56%) | <0.001 |
|
| |||||
| BTT | 375 (24%) | 1239 (30%) | 748 (24%) | 439 (22%) | <0.001 |
|
| |||||
| Creatinine, mg/dL | 1.5 ± 0.02 | 1.5 ± 0.01 | 1.4 ± 0.01 | 1.4 ± 0.02 | <0.001 |
|
| |||||
| Albumin, g/dL | 3.0 ± 0.02 | 3.4 ± 0.01 | 3.5 ± 0.01 | 3.7 ± 0.02 | <0.001 |
|
| |||||
| Cardiac Arrest* | 291 (18%) | 130 (3.2%) | 40 (1.3%) | 20 (1.0%) | <0.001 |
|
| |||||
| Ventilator Support | 422 (27%) | 123 (3.0%) | 36 (1.2%) | 30 (1.5%) | <0.001 |
|
| |||||
| Dialysis | 80 (5.1%) | 58 (1.4%) | 17 (0.5%) | 9 (0.4%) | <0.001 |
|
| |||||
| Inotrope | 1384 (87%) | 3795 (93%) | 2872 (93%) | 1625 (80%) | <0.001 |
|
| |||||
| Vasopressor | 360 (23%) | 207 (5.0%) | 69 (2.2%) | 35 (1.7%) | <0.001 |
|
| |||||
| IABP | 827 (52%) | 1144 (28%) | 433 (14%) | 356 (18%) | <0.001 |
|
| |||||
| Temp-LVAD | 37 (2.3%) | 39 (1.0%) | 31 (1.0%) | 22 (1.1%) | <0.001 |
|
| |||||
| Temp-RVAD | 11 (0.7%) | 3 (0.1%) | 1 (0.1%) | 0 (0%) | <0.001 |
|
| |||||
| ECMO | 215 (14%) | 27 (0.7%) | 5 (0.2%) | 0 (0%) | <0.001 |
N(%) or mean ± standard error shown.
denotes cardiac arrest during index admission prior to VAD
Abbreviations: ALT= alanine aminotransferase, BTT=Bridge to transplant, ECMO=extracorporeal membrane oxygenation within 48 hours of operation, IABP= intraaortic balloon pump within 48 hours of operation, ISCM=ischemic cardiomyopathy.
One year post-LVAD survival for the INTERMACS cohort was 82±2.2%. One year survivals for patients in Profiles 1, 2, 3, and 4–7 were 77±1.2%, 80±0.7%, 84±0.7%, and 83±0.9%, respectively (p<0.001). On pairwise comparison of survival, there was a significant difference in survival between INTERMACS Profile 1 vs. Profile 3 (p<0.001) and Profile 1 vs. the combined Profiles 4–7 (p<0.001). There was also a significant difference between INTERMACS Profile 2 vs 3 (p<0.001) and Profile 2 vs the combined Profiles 4–7 (p=0.031). However, there was no significant difference between Profiles 1 vs. Profile 2 (p=0.066).
After controlling for known preoperative factors for VAD mortality (2–6), patients in INTERMACS Profile 1 (adjusted HR 1.23 [1.02–1.48] p=0.027) and Profile 2 (adjusted HR 1.24 [1.07–1.44], p=0.008) had ~20% higher adjusted mortality than patients in Profiles 4–7 (p<0.001, table 3). Patients in Profile 3 had lower mortality than Profile 1 (adjusted HR 0.77 [0.66–0.91], p=0.001) and Profile 2 (adjusted HR 0.77 [0.69–0.86, p<0.001]. However, there was no difference in adjusted survival when comparing INTERMACS Profiles 1 vs. 2 (adjusted HR 1.01 [0.88–1.12], p=0.88).
Table 3.
Multivariable Predictors of Mortality after LVAD
| Adjusted Mortality Hazard Ratio [95% CI] | p value | |
|---|---|---|
|
| ||
| Implant 2009–10 | 1.24 [1.12–1.37] | <0.001 |
|
| ||
| Age ≥70 | 1.40 [1.25–1.58] | <0.001 |
|
| ||
| Female | 1.16 [1.05–1.30] | 0.005 |
|
| ||
| DT | 1.32 [1.20–1.45] | <0.001 |
|
| ||
| BMI, kg/m2 | 1.01 [1.00–1.01] | 0.058 |
|
| ||
| Prior sternotomy | 1.37 [1.25–1.50] | <0.001 |
|
| ||
| Vasopressor use | 1.19 [1.00–1.41] | 0.056 |
|
| ||
| Inotrope use | 0.95 [0.83–1.09] | 0.47 |
|
| ||
| Dialysis | 1.85 [1.42–2.41] | <0.001 |
|
| ||
| Ventilator support | 1.17 [0.97–1.42] | 0.11 |
|
| ||
| INTERMACS Profile | <0.001 | |
| 1 | 1.23 [1.02–1.48] | 0.027 |
| 2 | 1.24 [1.07–1.44] | 0.004 |
| 3 | 0.95 [0.81–1.11] | 0.53 |
| 4–7 (reference) | ---- | ---- |
|
| ||
| Creatinine, per mg/dL | 1.14 [1.09–1.19] | <0.001 |
|
| ||
| Albumin, per g/dL | 0.92 [0.87–0.98] | 0.008 |
|
| ||
| INR | 1.05 [0.96–1.16] | 0.31 |
BMI= body mass index, CI= confidence interval, DT= destination therapy, INR= international normalized ratio
Patient Characteristics and post LVAD Survival based on Modifier
The TCS Modifier
The TCS modifier can be applied to patients in Profiles 1–3. Of the 8,432 patients with a documented TCS modifier status (yes/no) in INTERMACS, 2207 (26%) patients were documented to be on TCS support prior to VAD. TCS devices included temporary RVAD support (1%, n=15), temporary LVAD support (6%, n=129), ECMO (11%, n=248), and IABP support (63%, n=1687). While most patients (44%) on TCS were categorized as INTERMACS Profile 1, 41% and 15% were assigned Profile 2 and 3 statuses, respectively.
Patient characteristics for those with and without preoperative TCS support and by INTERMACS Profile are shown in Tables 4 (appendix). While TCS patients were younger than nonTCS patients, patients on TCS had higher frequencies of other high risk characteristics including preoperative cardiac arrest, preoperative vasopressor, dialysis and ventilator use, and lower serum albumin.
Table 4, appendix.
Characteristics of patients who have TCS Modifier in INTERMACS by INTERMACS Profile
| Entire Cohort | Profile 1 | Profile 2 | Profile 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TCS Yes (n=2207) | TCS No (n=6225) | TCS Yes (n=982) | TCS No (n=555) | TCS Yes (n=895) | TCS No (n=2991) | TCS Yes (n=330) | TCS No (n=2679) | p-value | |
|
| |||||||||
| Age | |||||||||
| Group, yrs† | <0.001 | ||||||||
| <50 | 665 (30%) | 1615 (26%) | 324 (33%) | 194 (35%) | 257 (28%) | 781 (26%) | 84 (25%) | 640 (24%) | |
| 50–69 | 1349 (61%) | 3703 (59%) | 590 (48%) | 314 (56%) | 556 (62%) | 1810 (60%) | 203 (62%) | 1579 (59%) | |
| ≥70 | 193 (8.8%) | 907 (15%) | 68 (7%) | 47 (9%) | 82 (10%) | 400 (14%) | 43 (13%) | 460 (17%) | |
|
| |||||||||
| Male | 1725 (78%) | 4870 (78%) | 751 (77%) | 413 (74%) | 706 (79%) | 2340 (78%) | 268 (81%) | 2117 (79%) | 0.84 |
|
| |||||||||
| BMI, kg/m2 | 28.3 ± 0.1 | 28.5 ± 0.1 | 28.7 ± 0.2 | 27.8 ± 0.3 | 27.9 ± 0.2 | 28.5 ± 0.1 | 28.5 ± 0.4 | 28.7 ± 0.1 | 0.26 |
|
| |||||||||
| ISCM | 1076 (49%) | 2740 (44%) | 543 (55%) | 244 (44%) | 379 (42%) | 1291 (43%) | 154 (47%) | 1206 (45%) | <0.001 |
|
| |||||||||
| BTT | 596 (27%) | 1676 (27%) | 219 (22%) | 145 (26%) | 276 (31%) | 897 (30%) | 91 (28%) | 634 (24%) | <0.001 |
|
| |||||||||
| Prior Sternotomy | 1479 (67%) | 4188 (67%) | 120 (12%) | 48 (8.6%) | 74 (8.3%) | 246 (8.2%) | 37 (11%) | 243 (9.1%) | 0.006 |
|
| |||||||||
| Creatinine, mg/dL | 1.4 ± 0.04 | 1.4 ± 0.1 | 1.5 ± 0.03 | 1.6 ± 0.04 | 1.4 ± 0.03 | 1.5 ± 0.01 | 1.4 ± 0.04 | 1.4 ± 0.01 | 0.14 |
|
| |||||||||
| Albumin, g/dL† | 3.1 ± 0.02 | 3.5 ± 0.01 | 2.9 ± 0.03 | 3.2 ± 0.03 | 3.2 ± 0.02 | 3.4 ± 0.01 | 3.3 ± 0.05 | 3.5 ± 0.02 | <0.001 |
|
| |||||||||
| INR† | 1.4 ± 0.01 | 1.3 ± 0.01 | 1.4 ± 0.02 | 1.4 ± 0.02 | 1.4 ± 0.01 | 1.3 ± 0.01 | 1.3 ± 0.02 | 1.3 ± 0.01 | <0.001 |
|
| |||||||||
| Cardiac Arrest*,† | 289 (13%) | 157 (2.5%) | 214 (22%) | 72 (13%) | 57 (6.4%) | 64 (2.1%) | 18 (5.5%) | 21 (0.8%) | <0.001 |
|
| |||||||||
| Ventilator† Support | 353 (16%) | 203 (3.3%) | 298 (30%) | 115 (21%) | 43 (4.8%) | 70 (2.3%) | 12 (3.6%) | 18 (0.7%) | <0.001 |
|
| |||||||||
| Dialysis† | 83 (3.8%) | 66 (1.1%) | 53 (5.4%) | 25 (4.5%) | 28 (3.1%) | 28 (0.9%) | 2 (0.6%) | 13 (0.5%) | <0.001 |
|
| |||||||||
| Inotrope† | 1950 (88%) | 5788 (93%) | 855 (87%) | 486 (88%) | 828 (93%) | 2789 (93%) | 267 (81%) | 2513 (94%) | <0.001 |
|
| |||||||||
| Vasopressor † | 371 (17%) | 250 (4.0%) | 259 (26%) | 94 (17%) | 96 (11%) | 104 (4%) | 16 (5%) | 52 (2%) | <0.001 |
|
| |||||||||
| IABP† | 1390 (63%) | 919 (15%) | 567 (58%) | 236 (43%) | 628 (70%) | 457 (15%) | 195 (59%) | 226 (8.4%) | <0.001 |
|
| |||||||||
| Temp-LVAD | 129 (5.8%) | --- | 37 (3.8%) | -- | 39 (4.4%) | -- | 53 (19%) | -- | -- |
|
| |||||||||
| Temp-RVAD | 15 (0.7%) | --- | 11 (1.1%) | -- | 3 (0.3%) | -- | 1 (0.3%) | -- | -- |
|
| |||||||||
| ECMO | 248 (11%) | --- | 215 (22%) | -- | 27 (3%) | -- | 6 (2%) | -- | -- |
n(%) or mean ± standard error mean shown n(%) shown.
denotes cardiac arrest during index admission prior to VAD.
p <0.05 for comparison between TCS yes and no in whole cohort.
Abbreviations: INR = International normalized ratio, BMI= body mass index, BTT=bridge to transplant, ECMO=extracorporeal membrane oxygenation within 48 hours of operation, IABP= intra-aortic balloon pump within 48 hours of operation, ISCM=ischemic cardiomyopathy,
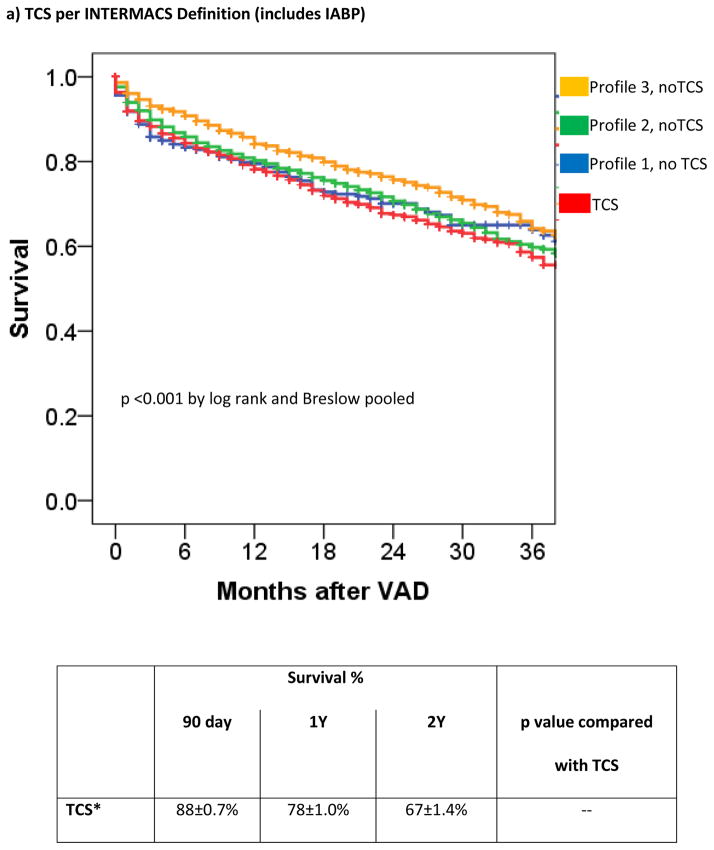
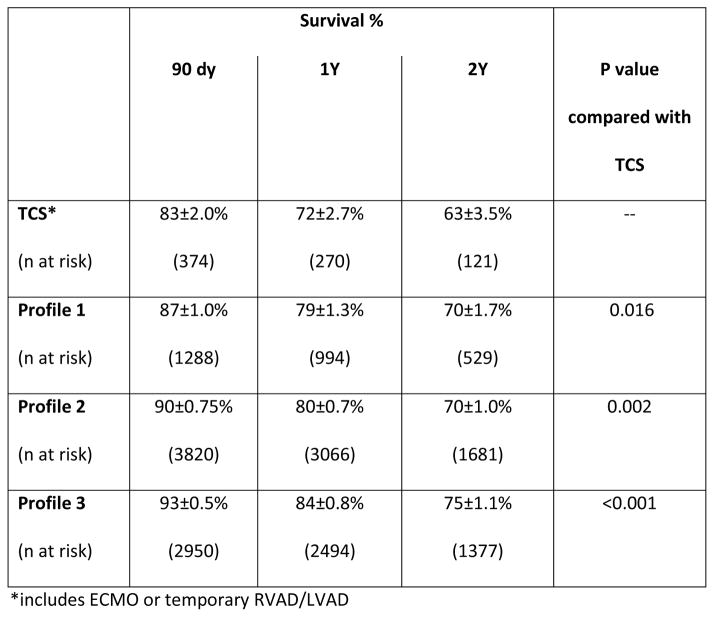
Within the group of patients eligible for TCS (Profiles 1–3, n=8432), post-LVAD survival at 1 year for those who were on TCS at the time of durable VAD implant was 78±1.0% compared with 82±0.6% in those without TCS (HR 1.21 [1.09–1.34] p <0.001). The odds of operative death (death within 90 days) for those on TCS was 1.3 [1.13–1.55] higher than those without preoperative TCS. For the patients (n=7636) who survived the operative period, there was no difference in overall survival between those on TCS and those without TCS support (HR 1.12 [0.98–1.29], p=0.10).
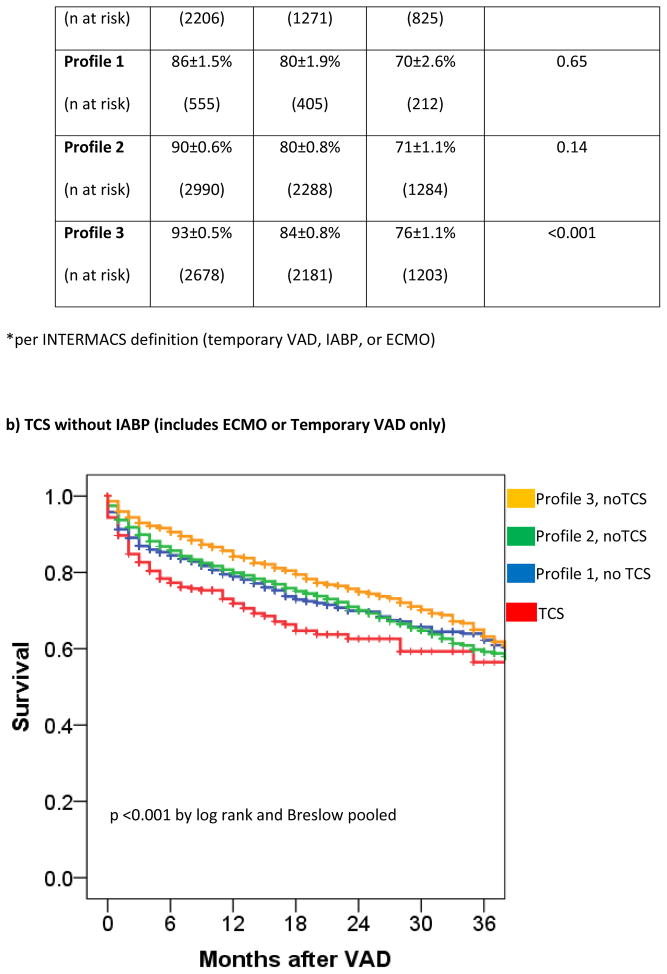
The patients without TCS were then grouped according to INTERMACS Profile and patients on TCS were analyzed in aggregate. Survival in patients on TCS approximated that of Profile 1 and 2 patients not receiving TCS (figure 2a) with hazard ratios of 1.05 [0.71–1.28] (p=0.56) and 1.09 [0.98–1.2] (p=0.13) for TCS vs Profile 1 (without TCS) and TCS vs Profile 2 (without TCS), respectively. Within Profile 1, 6-month survival with TCS was 82±1.3% compared with 83±1.6% in Profile 1 patients without TCS (p=0.27 for overall survival). Within Profile 2, 6-month survival with TCS was 85±1.3% compared with 87±0.6% in Profile 2 patients without TCS (p=0.49 for overall survival). Patients in Profile 3 without TCS had a 29% lower risk of death (HR 0.71 [0.63 – 0.80], p < 0.0001) than the combined TCS cohort.
Figure 2. Survival of INTERMACS Registry Patients Stratifying INTERMACS Profile or by TCS.
TCS is compared to survival in nonTCS patients according to INTERMACS Profile. TCS survival is shown according to the INTERMACS TCS definition (IABP (for shock), ECMO, or percutaneous VAD) (a) or according to the revised definition of ECMO or Temporary VAD (b).
After controlling for INTERMACS Profile and other previously outlined risk factors for mortality (2–6), the requirement for preoperative TCS was not predictive of postVAD outcome (adjusted HR 1.09[0.96–1.23], p=0.17).
Limiting TCS to ECMO and/or temporary RVAD/LVAD support (n=374), survival at 1 year for those with a history of ECMO/temporary VAD support was 72% compared with 81% in those without TCS (HR 1.50 [1.2–1.8], p <0.001). The odds of operative death in TCS patients was 1.98 [1.49–2.63]. Of those who survived the operative period, preoperative TCS use did not confer worse overall survival (HR 1.14 [0.84–1.55] for TCS vs. no TCS).
Survival on TCS was inferior to that of nonTCS patients in INTERMACS Profiles 1 and 2 without TCS (Figure 2b). The adjusted hazard ratio of a preoperative ECMO/temporary VAD requirement was 1.38 [1.10–1.73, p=0.006].
Arrhythmia Modifier
Of 10,802 patients, n=2963 (27%) were ascribed with the Arrhythmia “yes” modifier status, 7326 (68%) the Arrhythmia “no” modifier status, and 512 (4.7%) had a blank Arrhythmia status. Patient characteristics for those with and without the Arrhythmia modifier for Profiles 1–7 are shown in Table 5 (appendix). Patients with arrhythmias tended to be older, have a prior CAD diagnosis, and required more vasopressors and TCS with a greater frequency of preoperative cardiac arrest then non-arrhythmia patients. Arrhythmia patients were less likely to be on inotropes.
Appendix Table 5.
Characteristics of Patients by INTERMACS Modifier Status
| Arrhythmia | Frequent Flyer | |||
|---|---|---|---|---|
| Yes (n=2963) | No (n=7326) | Yes (n=1032) | No (n=1354) | |
|
| ||||
| Age Group, yrs | † | |||
| <50 | 619 (21%) | 1986 (27%) | 202 (20%) | 253 (19%) |
| 50–69 | 1867 (63%) | 4360 (60%) | 612 (59%) | 849 (63%) |
| ≥70 | 477 (16%) | 980 (13%) | 218 (21%) | 252 (18%) |
|
| ||||
| Male | 5652 (77%)† | 2448 (83%) | 815 (79%) | 1110 (82%) |
|
| ||||
| ISCM | 1438 (49%)† | 3414 (47%) | 521 (50%)† | 767 (57%) |
|
| ||||
| BTT | 760 (26%) | 1904 (26%) | 190 (18%)† | 225 (22%) |
|
| ||||
| Creatinine, mg/dL | 1.4±0.01 | 1.4±0.01 | 1.4±0.02 | 1.4±0.02 |
|
| ||||
| Albumin, g/dL | 3.4±0.01 | 3.4±0.01 | 3.6±0.02 | 3.7±0.02 |
|
| ||||
| Cardiac Arrest* | 257 (8.7%)† | 199 (2.7%) | 9 (0.9%) | 13 (1.0%) |
|
| ||||
| Ventilator Support | 245 (8.3%)† | 344 (4.6%) | 5 (0.5%)† | 27 (2%) |
|
| ||||
| Dialysis | 55 (1.9%) | 104 (1.4%) | 9 (0.9%) | 5 (0.4%) |
|
| ||||
| Inotrope | 2258 (76%)† | 6033 (82%) | 705 (68%)† | 408 (30%) |
|
| ||||
| Vasopressor | 220 (7.6%)† | 422 (5.8%) | 16 (1.6%) | 23 (1.8%) |
|
| ||||
| IABP | 797 (27%) | 1851 (25%) | 120 (12%)† | 266 (20%) |
|
| ||||
| Temp-LVAD | 43 (1.5%)† | 73 (1.0%) | 5 (0.5%) | 17 (1.3%) |
|
| ||||
| Temp-RVAD | 8 (0.3%)† | 6 (0.1%) | 0 | 0 |
|
| ||||
| ECMO | 92 (3.1%)† | 117 (1.6%) | 1 (0.1%) | 1 (0.1%) |
n(%) shown or mean±standard error.
denotes cardiac arrest during index admission prior to VAD.
p <0.05 for yes vs. no within a Modifier category
Abbreviations: ALT= alanine aminotransferase, BTT=Bridge to transplant, ECMO=extracorporeal membrane oxygenation within 48 hours of operation, IABP= intraaortic balloon pump within 48 hours of operation, ISCM=ischemic cardiomyopathy, Temp= temporary
Survival at 12 months in those with arrhythmias was 79±0.8% compared with 82±0.5% in those without arrhythmias prior to VAD implant (HR 1.16 [1.06–1.27], p=0.001). However, after controlling for other known correlates of risk (1–3), an affirmative Arrhythmia modifier status was not associated with worse outcome (adjusted HR 1.07 [0.97–1.17], p=0.20) after VAD implant.
Frequent Flyer
In INTERMACS, there were 5129 (48%) patients in Profiles 3–7. A Frequent Flyer (FF) status (yes or no) was assigned to 2,386 (47%). Of those with a FF status recorded, 1032 (43%) were defined as FFs. Patient characteristics for those with and without the FF Modifier (Profiles 3–7) are shown in Table 5 (appendix). FF patients were more likely to be DT, on inotropes and less likely to have an IABP in place at the time of VAD.
Survival at 12 months in FFs in Profiles 3–7 was 82±1.3% compared with 83±1.1% in those in Profiles 3–7 without the modifier (HR 1.13 [0.95–1.33], p=0.17). Adjusting for INTERMACS Profile and other correlates of risk (2–6), the FF modifier was predictive of overall INTERMACS mortality (adjusted HR 1.27 [1.00–1.60, p=0.047) and mortality in those restricted to Profiles 3–7 (adjusted HR 1.29 [1.02–1.63], p=0.034).
Discussion
INTERMACS patient Profiles are important for characterizing patient risk prior to VAD implant. However, we found marked differences in how U.S. VAD centers assign INTERMACS Profile prior to VAD implant and heterogeneity in Profile assignment frequency for a given patient scenario. While patients with the FF modifier had increased mortality beyond that of INTERMACS Profile and other known risk factors, the TCS and Arrhythmia modifiers as presently defined did not further discriminate risk. This suggests enhancements can be made to improve VAD candidate risk discrimination using the INTERMACS patient Profile classification system.
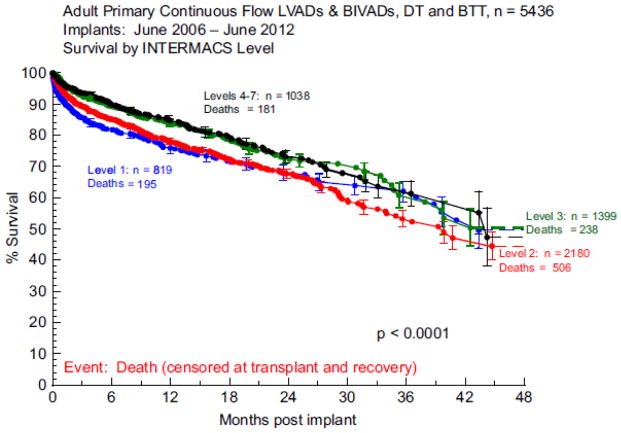
The INTERMACS Profiles were devised during the development of the INTERMACS database to describe a patient status preoperatively to “facilitate communication with colleagues, adjustment for preoperative risk, and clarification of target populations for devices (1).” The Profiles were re-evaluated in 2009 with a few clarifications, and the Frequent Flyer and TCS modifiers were added to the pre-existing Arrhythmia modifier (1). The INTERMACS Profiles largely succeeded in their aims and have been shown to be predictive of VAD candidate outcome (2–3) but important limitations warrant consideration. Boyle and colleagues looked at BTT and DT patients at 3 centers and found better survival in patients in Profiles 4–7 vs Profile 1 (3). However, Profile 1 patients did not have a significantly different survival than patients in Profiles 2–3 (p=0.18) but study power was limited. In INTERMACS annual reports, patient Profile overall has repeatedly been shown to be predictive of outcome (2,5). However, a difference between contiguous Profiles has never been shown. In fact, in the Fifth annual report, the survival curves (Figure 3) for patients in Profiles 1 vs 2 overlap (5) in a sample with ample power (n=3000). In the present analysis, after controlling for other correlates of risk, there was no significant difference between patients in Profile 1 vs. Profile 2, with significant differences noted only when Profiles 1 and 2 were compared with clearly less sick patients in Profiles 3–7. Thus, while Profiles are predictive of outcome, precise mortality estimates for similar patient Profiles may not be achievable.
Figure 3. Actuarial Survival Stratified by INTERMACS Profile in the 5th Annual Report.
Reprinted with Permission. JHLT 2013;32:141–56.
Heterogeneity in patient Profile assignment displayed in this analysis may partially account for the lack of significant differences in patient survival between contiguous Profiles. We found that assignment of patient Profiles is highly variable in the U.S., ranging from physicians caring for the patient to research coordinators who may have less exposure to a patient’s day to day status. Few centers discuss patient Profiles prior to INTERMACS registry data entry to achieve a consensus. Using patient scenarios, we also found very clear variability in how patients are classified within U.S. centers. This variability occurred not only between nonphysician providers and doctors, but also between physicians. To enhance the quality of risk stratification by INTERMACS Profiles, we feel a consensus between the treating surgeon and cardiologist should be achieved and subsequently reported to institutional INTERMACS research coordinators prior to INTERMACS data entry.
Variability was also notable in patients on TCS and between contiguous patient Profiles. The variability in the TCS classification may be partially explained because INTERMACS allows application of a less ill Profile (e.g. Profile 2–3) for previously Profile 1 patients who are deemed stable upon operating room entry following application TCS. Yet, no study has shown that preoperative TCS actually improves survival, let alone achieves outcomes equal to that of a Profile 2–3 patient (7). The variability in contiguous patient Profiles may be driven by the subjectivity of the Profile descriptions. Differentiating “stable but inotrope dependent” (Profile 3) from “resting symptoms” (Profile 4) when an inotrope wean is not attempted while inpatient is not feasible. Likewise, clinicians may have different thresholds for what is deemed “sliding on inotropes” (Profile 2) and “crash and burning” (Profile 1). Improvement in patient Profile classification variability could be gained by placing more objective measures within a Profile description.
Modifiers
While Modifiers were added to improve patient characterization, only the FF modifier portended worse outcome beyond that of INTERMACS Profile and other known risk factors. However, the validity of the FF risk ascription is challenged by INTERMACS FF modifier data quality such that only 47% of Profiles 3–7 had the FF yes/no designation recorded. Presuming a mortality association exists, the FF modifier may assist in risk stratification of the “less ill” VAD candidate- a group of patients for whom data is presently lacking. Typical risk factors for VAD mortality (e.g. vasopressors, renal failure) are less prevalent in Profiles 3–7. For similar reasons, the HeartMate II Risk Score may be less discriminative of risk in patients categorized within Profiles 3–7 (8). In addition to further studies on the FF modifier, the Medical Arm of Mechanically Assisted Circulatory Support (MEDAMACS) and the Registry Evaluation of Vital Information for VADs in Ambulatory Life (REVIVAL) studies may help better elucidate outcomes in this less ill population (9, 10).
While predictive of outcome in univariable analyses, the TCS and Arrhythmia modifiers were not predictive of survival when controlling for other known risks. The TCS modifier may suffer in part from absent or inappropriately assigned data. Some patients listed as on TCS had no TCS type listed and some patients on percutaneous VADs had a blank TCS modifier status. We suspect there is also an underreporting of percutaneous LVAD use within the INTERMACS registry. Of the 10,082 patients included in our registry only 129 (1.2%) were reported to have had temporary LVAD support. Given reports of increased utilization of percutaneous LVADs (11) this underreporting may be due to inadequate education of data entry personnel at each clinical site, as well as poor distinction of what a temporary “LVAD” is in the 48-hour pre-operative period. Finally, many patients with the TCS modifier had IABPs as their only form of support. Once IABP was removed from the TCS definition, the TCS modifier conferred ~40% higher mortality. We feel the TCS modifier definition should be amended to include only ECMO and temporary VAD support. Given the marked survival disadvantage of TCS, we feel that patients who present in shock regardless of presumed stability following TCS should be assigned a Profile 1-TCS status.
The Arrhythmia modifier was suggestive of increased risk in uncontrolled analysis. The clinical profile of patients with the “yes” Arrhythmia modifier was that of sicker patients, requiring more vasopressors, ventilator and dialysis support than those without high burden arrhythmias. Ventricular arrhythmias often limit the impact of interventions such as IABP and inotrope support in patients in the preoperative setting so the Arrhythmia modifier status may uncover under-resuscitated patients. However, the Arrhythmia offered no further risk discrimination when other correlates of risk, including INTERMACS Profile, were taken in to account.
Limitations
This analysis combined survey data and INTERMACS data. While most consider a 25% survey response rate high, data may be subjected to response bias. Since INTERMACS is a database limited to U.S. centers, we did not include survey data from non-U.S. centers. Thus, these results may not apply outside the U.S. Finally, INTERMACS data flaws exist, as highlighted above. However, this is typical of large registries and small areas of data disintegrity are unlikely to have marked implications on data conclusions. However, for the Frequent Flyer modifier analyses where larger data deficiencies exist, results should be interpreted with caution.
Conclusion
While INTERAMCS Profiles are predictive of risk, important limitations of the Profile designations exist and it is not presently possible to accurately differentiate risk between contiguous Profiles. Variability in Profile assignment and the subjectivity of Profile descriptors may account for some of the reduced risk discrimination of Profiles. To reduce some of this heterogeneity in assignment, we feel that a consensus from the treating physicians should be achieved prior to data entry in to INTERMACS. Refinement of Profiles with less subjective (and more objective measures) descriptors may improve risk prediction. The Arrhythmia modifier as presently defined does not improve risk discrimination in INTERMACS and should be omitted or revised. Finally, the TCS modifier offers the greatest heterogeneity in INTERMACS profile assignment and failed to predict outcome in its current definition. We feel that TCS should only include ECMO and temporary VAD devices and patients on TCS should be assigned an INTERMACS 1 Profile.
Footnotes
Disclosures: All authors receive institutional research funding from the Thoratec Corporation (Pleasanton, CA) and HeartWare (Framingham, MA). Dr. Cowger received travel support (<$1000) from Thoratec in 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS Profiles of advanced heart failure: The current picture. J Heart Lung Transplant. 2009;28:535–41. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. J Heart Lung Transplant. 2014;33:555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–7. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 4.INTERMACS Manual of Operations and Procedures. Retrieved from https://www.uab.edu/medicine/intermacs/images/protocol_4.0/Data_Collection_Forms_2-4-15/3_Pre_Implant.pdf.
- 5.Kirklin JK, Naftel DC, Kormos EL, et al. Fifth INTERMACS annual report: Risk factor analysis form more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Cowger J, Sundareswaran K, Rogers J, et al. The HeartMate II Risk Score: Predicting survival in candidates for left ventricular assist device support. J Am Coll Cardiol. 2013;61:313–21. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 7.Shah P, Stulak J, Pagani F, et al. Clinical Outcomes of Heart Failure Patients with Cardiogenic Shock Treated with Temporary Circulatory Support Prior to Durable LVAD Implant. ASAIO. 2015 doi: 10.1097/MAT.0000000000000309. in press. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SS, Nahumi N, Han J, et al. Preoperative mortality risk assessment in patients with continuous flow left ventricular assist devices: application of the HeartMate II risk score. J Heart Lung Transplant. 2014;33:675–81. doi: 10.1016/j.healun.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Steward GC, Stevenson LW. INTERMACS and MedaMACS: How with they guide future therapy. Current Cardiology Reports. 2013;15:394. doi: 10.1007/s11886-013-0394-y. [DOI] [PubMed] [Google Scholar]
- 10.University of Michigan. clinicaltrials.gov [internet] Bethesda (MD): National Library of Medicine (US); 2000. Registry Evaluation of Vital Information for VADs in Ambulatory Life (REVIVAL) [cited 2015 October 23]. Available from https://clinicaltrials.gov/ct2/show/NCT01369407 NLM Identifier: NCT01369407. [Google Scholar]
- 11.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support. Incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–15. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]