Abstract
Various types of zinc (Zn) complexes have been developed as promising antidiabetic agents in recent years. However, the pharmacological action of Zn complex is not elucidated because the biodistribution of the complex in a living organism has not been studied. Nuclear medicine imaging is superior technology for the noninvasive analysis of the temporal distribution of drug candidates in living organisms. Gamma-ray emission imaging (GREI), which was developed by our laboratory as a novel molecular imaging modality, was adopted to visualize various γ-ray–emitting radionuclides that are not detected by conventional imaging techniques such as positron emission tomography and single-photon emission computed tomography. Therefore, we applied GREI to a biodistribution assay of Zn complexes. In the present study, 65Zn was produced in the natCu(p,n) reaction in an azimuthal varying field cyclotron for the GREI experiment. The distribution was then noninvasively visualized using GREI after the intravenous administration of a 65Zn-labeled di(1-oxy-2-pyridinethiolato)zinc [Zn(opt)2], ZnCl2, and di(l-histidinato)zinc. The GREI images were validated using conventional invasive assays. This novel study showed that GREI is a powerful tool for the biodistribution analysis of antidiabetic Zn complexes in a living organism. In addition, accumulation of 65Zn in the cardiac blood pool was observed for [Zn(opt)2], which exhibits potent antidiabetic activity. These results suggest that the slow elimination of Zn from the blood is correlated to the antidiabetic activity of [Zn(opt)2].
Abbreviations: DM, Diabetes mellitus; GREI, Gamma-ray emission imaging; PET, Positron emission tomography; SPECT, Single-photon emission computed tomography; [Zn(opt)2], Di(1-oxy-2-pyridinethiolato)zinc; [Zn(His)2], Di(l-histidinato)zinc; DMSO, Dimethyl sulfoxide
Keywords: GREI, 65Zn-labeled Zn complex, Biodistribution
Highlights
-
•
GREI was applied to the biodistribution analysis of Zn complexes.
-
•
The characteristic accumulation of 65Zn for [Zn(opt)2] was successfully visualized.
-
•
Long retention in the blood may be related in the antidiabetic effect of [Zn(opt)2].
1. Introduction
Medicinal inorganic chemistry is a discipline in which metal complexes are used in therapeutic and diagnostic medicine [1], [2], [3]. The zinc (Zn) complexes are promising metallodrugs for the treatment of type 2 diabetes mellitus [4], [5]. In recent years, several Zn complexes have been discovered that have potent antidiabetic activity in experimental diabetic animals [5], [6], [7]. For example, a novel Zn complex showed potent hypoglycemic activity at a dose lower than the clinical dose of zinc acetate used to treat Wilson's disease [7]. It is presumed that the Zn atom is the component of the complex responsible for its hypoglycemic activity since ligands such as low-molecular-weight organic compounds are generally inactive. The complexation of Zn with a ligand improves its gastrointestinal absorption, enabling its efficient delivery to target tissues [7], [8], [9]. The coordination mode of the Zn complex also influences its pattern of tissue distribution [9], [10]. However, the pharmacological action of Zn complexes is not fully elucidated because their biodistribution in vivo has not been extensively studied. Therefore, the development of noninvasive methods for analyzing the distribution of Zn complexes in vivo will accelerate the discovery of safe and efficacious Zn complexes for treatment of type 2 diabetes.
The nuclear medicine imaging technique is useful in early drug development for analyzing the biokinetics of drug candidates in experimental animals and humans (i.e., clinical microdose studies) [11], [12]. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are widely used to noninvasively visualize the movements of radionuclide-labeled molecules [13], [14]. However, PET is limited to determine 511 keV γ-rays that originate from positron-emitting radionuclide while PET is more sensitive and accurate than other molecular imaging techniques. In SPECT imaging, γ-rays with low energy (<300 keV) emitted from the radionuclide are detected. Therefore, these conventional apparatuses are not suitable for the measurement and visualization of the in vivo behavior of the Zn complex because 65Zn chosen as a radionuclide for the labeling emits γ-ray with high energy of 1116 keV.
On the other hand, the gamma-ray emission imaging (GREI) apparatus developed in our laboratory enables the spectroscopic imaging of wide energy-range γ-rays (200–2000 keV) by using a semiconductor Compton camera system [15]. The in vivo imaging of different γ-ray–emitting radionuclides, such as 65ZnCl2, 85SrCl2, iodinated (131I) methylnorcholesterol and others, was achieved using GREI [15], [16], [17]. Successful demonstrations of the multiple molecular imaging inspired us to apply GREI to the biodistribution analysis of the Zn complex in a living organism. This study was the first to noninvasively investigate the kinetic behaviors of the di(1-oxy-2-pyridinethiolato)zinc complex ([Zn(opt)2]), which has potent antidiabetic activity, using GREI after an intravenous administration of 65Zn-labeled [Zn(opt)2]. The distribution was compared to those of ZnCl2 and the di(l-histidinato)zinc complex ([Zn(His)2]), which does not exhibit antidiabetic activity.
2. Experimental
2.1. Animals
Ten-week-old male C57BL/6J mice were purchased from CLEA Japan, Inc. All animals were housed under a 12-h light/dark cycle in a temperature-controlled animal room and were allowed free access to food and tap water. All animal experiments were approved by the Ethics Committee on Animal Care and Use of RIKEN and were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2. Probe preparation
The 65Zn nuclide was produced in the natCu(p,n) reaction (nat: natural isotopic abundance) in a RIKEN azimuthal varying field cyclotron. A metallic copper foil (chemical purity: 99.99%) 220 mg/cm2 thick was irradiated by a 14-MeV proton beam with an intensity of 15 µA. After the irradiation, 65Zn was chemically separated from the Cu target by an anion-exchange method. Radionuclide purity>99% was determined by the γ-ray spectrometry using a calibrated Ge detector. The specific radioactivity>0.2 GBq/g was estimated by inductively coupled plasma mass spectrometry for a control sample that was treated with the same chemical process as the irradiated Cu target.
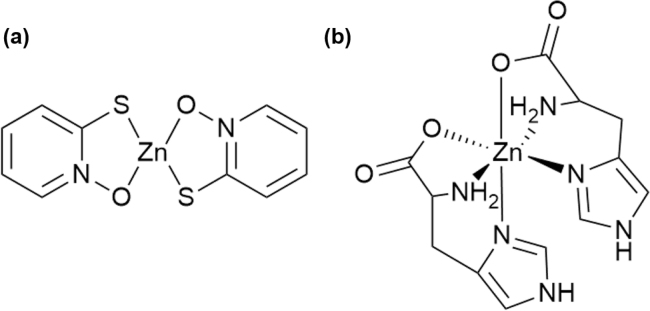
[Zn(opt)2] and [Zn(His)2] were prepared as previously described [8], [18], and the complexes were determined by elemental analyses and infrared spectra. Their predicted coordination structures are shown in Fig. 1 [19], [20].
Fig. 1.
Proposed coordination structures of the di(1-oxy-2-pyridinethiolato)Zn and di(l-histidinato)Zn complexes. Charges are omitted for simplicity.
Purified 65Zn dissolved in 30 μL of saline (Otsuka Pharmaceutical Co. Ltd.) and [Zn(opt)2] dissolved in 70 μL of dimethyl sulfoxide (DMSO; Wako Pure Chemical Industries Ltd.) or [Zn(His)2] dissolved in 70 μL of saline were stirred at room temperature over night to exchange cold Zn and 65Zn [7], [9], [10]. The 65ZnCl2 solution, a mixture of 65Zn dissolved in 30 μL of saline and ZnCl2 dissolved in 70 μL of DMSO, was prepared immediately prior to administration. The solutions were prepared to contain 10 mg Zn/mL as well as 10 MBq/mL or 2.5 MBq/mL for the GREI experiment or biodistribution analysis, respectively.
2.3. GREI experiment
Eleven-week-old male C57BL/6J mice were intravenously administered a single dose of 65Zn-labeled [Zn(opt)2] ([65Zn(opt)2]), 65Zn-labeled ZnCl2 (65ZnCl2), or 65Zn-labeled [Zn(His)2] ([65Zn(His)2]) via the tail vein at 1.0 mg Zn/kg of body weight. Each mouse was fixed on a board and placed just under the imaging head. Fifteen minutes after the injection, the GREI experiments were carried out under isoflurane anesthesia for 8 h. The acquired data were recorded in list mode with real-, and live-time information. The distribution images were reconstructed from the acquired data by the adoption of the image-reconstruction methods as previously described [15].
2.4. Biodistribution analysis
Eleven-week-old male C57BL/6J mice were divided into [65Zn(opt)2]-, 65ZnCl2-, and [65Zn(His)2]-treated groups, and the 65Zn-labeled compounds were intravenously administered at a dose of 1.0 mg Zn/kg of body weight. Four hours after the single intravenous administration, the mice were sacrificed under isoflurane anesthesia. Blood was collected, and the organs of interest (heart, pancreas, liver, kidney, stomach) were removed. Their radioactivities due to 65Zn were measured using a calibrated Ge detector.
3. Results
3.1. Visualization of 65Zn-labeled Zn complexes in the whole body by GREI
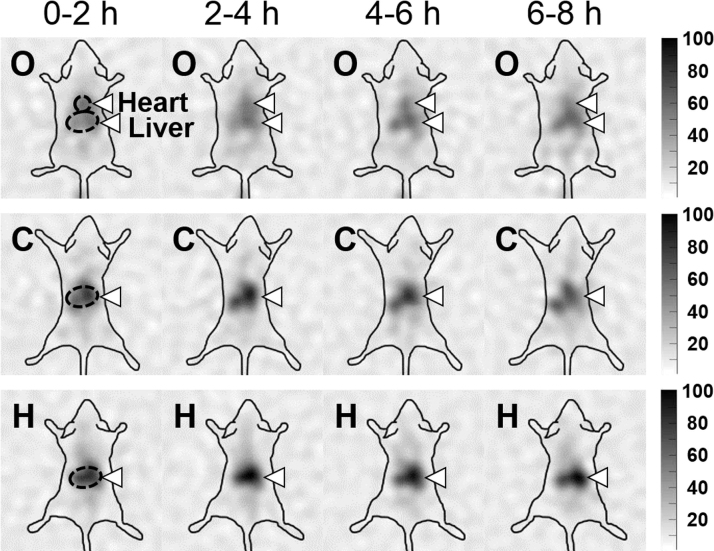
The distribution images of 65Zn were reconstructed every 2 h after the administration of [65Zn(opt)2], 65ZnCl2, or [65Zn(His)2] (Fig. 2). The arrows indicate the characteristic accumulation of 65Zn. High accumulation of all compounds was observed in the liver. Moreover, a different distribution of 65Zn among the studied Zn compounds was obtained in the distribution images: in the heart, high accumulation of [65Zn(opt)2] was noted, whereas little accumulation was observed for 65ZnCl2 and [65Zn(His)2].
Fig. 2.
Distribution images of 65Zn in C57BL/6J mice after the administration of the 65Zn-labeled Zn compounds ([65Zn(opt)2] (O), 65ZnCl2 (C), or [65Zn(His)2] (H)) reconstructed every 2 h. The arrows indicate the characteristic accumulation of 65Zn.
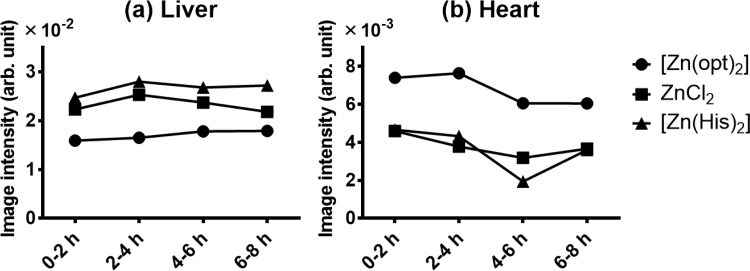
The regions of interest were drawn around the heart and liver and the numerical values of the distributions was calculated by integrating the pixel values inside them to compare the values in the heart and liver of each mouse (Fig. 3). Image intensity gradients in such organs for each compound were maintained for 8 h and indicated that 65Zn accumulation for [65Zn(opt)2] was much higher in the heart and lower in the liver than those for other studied compounds.
Fig. 3.
Image intensities of the 65Zn accumulated in the liver (a) and heart (b) extracted from each time flame of the images for [65Zn(opt)2], 65ZnCl2, and [65Zn(His)2].
3.2. Biodistribution of 65Zn
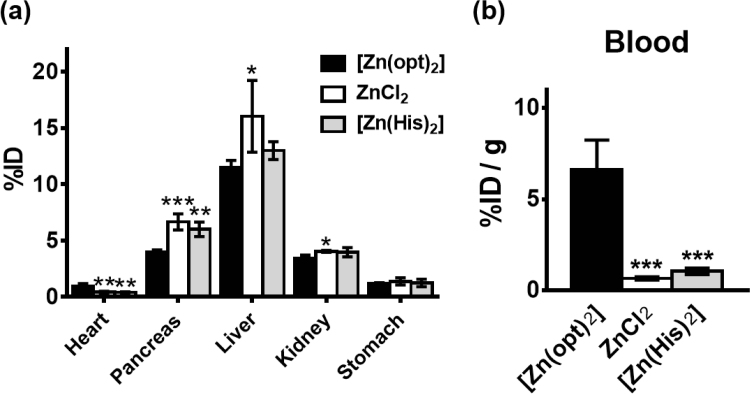
The radioactivities due to 65Zn in the resected organs and blood were determined 4 h after the administration of [65Zn(opt)2], 65ZnCl2, or [65Zn(His)2] (Fig. 4). As shown in Fig. 4a, the uptake of 65Zn in the liver was the highest, whereas the uptake rate (expressed as percent injected dose [%ID]) in the liver was at least twice as high as those in other organs for all compounds. The uptake rate of 65Zn in the liver, pancreas, and kidney of [65Zn(opt)2]-treated mice were reduced compared with the uptake rate in each organ of 65ZnCl2- and [65Zn(His)2]-treated mice. On the other hand, the uptake rate in the heart tissue of [65Zn(opt)2]-treated mice was significantly higher than those of 65ZnCl2- and [65Zn(His)2]-treated mice. In addition, the concentration of 65Zn (expressed as %ID/g tissue) in blood samples as [65Zn(opt)2] was much higher than that as 65ZnCl2 or [65Zn(His)2] (Fig. 4b).
Fig. 4.
The uptake rate of 65Zn in each tissue (a) and in the blood (b) at 4 h after an intravenous administration of di(1-oxy-2-pyridinethiolato)Zn ([65Zn(opt)2]), 65ZnCl2, or di(l-histidinato)Zn ([65Zn(His)2]). Data are expressed as mean values±SD for four mice. For the statistical evaluation, one-way analysis of variance with Bonferroni's test was used. *P<0.05, **P<0.01, ***P<0.001 versus [65Zn(opt)2]. %ID, percent injected dose.
4. Discussion
Biodistribution analyses of drug candidates enable the evaluation and prediction of their biological effects. For the assessment of the Zn complexes, tissue distribution patterns of exogenous Zn have been invasively analyzed by the determination of 65Zn radioactivity in each tissue removed from the experimental animals after the administration of the 65Zn-labaled Zn complex [7], [9], [10]. In general, the conventional method requires much time, high cost, and significant labor to assess drug candidate distribution. On the other hand, the molecular imaging technique enables a noninvasive and temporary study in the same living animals, decreases its statistical variance, and reduces the number of subjects. Therefore, we developed a noninvasive method for analyzing the distribution of 65Zn-labeled Zn complexes in live mice using GREI. The conventional invasive assays were also performed to validate the GREI images.
In GREI experiments, we were the first to successfully visualize the difference in distribution among the 65Zn-labeled compounds after their intravenous injection (Fig. 2). The distribution images of 65Zn for all compounds in the liver and heart were in agreement with the results of the invasive assay (Fig. 3, Fig. 4). The accumulation of 65Zn increased in the liver of 65ZnCl2- and [65Zn(His)2]-treated mice, while the image intensities in the [65Zn(opt)2]-treated mouse was low. This tendency was also observed in the uptake rate of 65Zn in the liver by the invasive assay. In addition, the accumulation of 65Zn in the heart of [65Zn(opt)2]-treated mouse observed in the GREI image corresponds to the results that both 65Zn blood concentration and 65Zn uptake into the heart tissue in [65Zn(opt)2]-treated mice were higher than those in 65ZnCl2-, [65Zn(His)2]-treated mice. It is well known that the values of the thoracic pool (blood volume in the heart and lungs) amount to about 30% of the total circulatory blood volume [21]. The accumulation in the heart would be mainly derived from the enormously high concentration of 65Zn in the blood of [65Zn(opt)2]-treated mice because the uptake rates of 65Zn in the heart tissue were low. These results suggest that the distribution images of 65Zn reflected the biodistributions determined by the invasive method.
Interestingly, the characteristic accumulation of 65Zn was observed in the heart of [65Zn(opt)2]-treated mouse. The invasive assay exhibited that the accumulation was mainly derived from the radioactivity of 65Zn in the cardiac blood pool. It was reported that a relatively long retention time of 65Zn in the blood leads to a sustained release of Zn, which may be an important factor in the exertion of its antidiabetic activity [7], [10]. The presented results suggested that the slow elimination of Zn from blood is related to the high antidiabetic activity of [Zn(opt)2].
In conclusion, here we successfully demonstrated that GREI enables the noninvasive analysis of 65Zn distribution for [65Zn(opt)2], 65ZnCl2, and [65Zn(His)2] in intact live mice as well as the visualization of the differences in 65Zn distribution among these compounds. The accumulation of 65Zn in the cardiac blood pool was observed for [Zn(opt)2] as an antidiabetic drug candidate and suggested that the slow elimination of Zn from the blood may be an important factor related to its antidiabetic activity. We are currently investigating the correlation between the distribution pattern of the Zn complex and its antidiabetic activity, in addition to developing the GREI system to realize faster and more quantitative imaging analyses.
Acknowledgment
This work was supported by a Grant-in-Aid for the Japan Society for the Promotion of Science Fellows to M. Munekane (No. 26·8219).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.12.004.
Appendix A. Supplementary material
Supplementary material
References
- 1.Farrell N. Metal complexes as drugs and chemotherapeutic agents. Chem. Soc. Rev. Compr. Coord. Chem. II. 2003;9:809–840. [Google Scholar]
- 2.Sakurai H., Yoshikawa Y., Yasui H. Current state for the development of metallopharmaceutics and anti-diabetic metal complexes. Chem. Soc. Rev. 2008;37:2383–2392. doi: 10.1039/b710347f. [DOI] [PubMed] [Google Scholar]
- 3.Bergamo A., Gaiddon C., Schellens J.H.M., Beijnen J.H., Sava G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012;106:90–99. doi: 10.1016/j.jinorgbio.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai H., Katoh A., Kiss T., Jakusch T., Hattori M. Metallo-allixinate complexes with anti-diabetic and anti-metabolic syndrome activities. Metallomics. 2010;2:670–682. doi: 10.1039/c0mt00025f. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa Y., Yasui H. Zinc complexes developed as metallopharmaceutics for treating diabetes mellitus based on the bio-medicinal inorganic chemistry. Curr. Top. Med. Chem. 2012;12:210–218. doi: 10.2174/156802612799078874. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki S., Munekane M., Kitamura Y., Hiromura M., Kamino S., Yoshikawa Y., Saji H., Enomoto S. Development of new zinc dithiosemicarbazone complex for use as oral antidiabetic agent. Biol. Trace Elem. Res. 2013;154:111–119. doi: 10.1007/s12011-013-9704-x. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto S., Yasui H., Yoshikawa Y. Development of a novel antidiabetic zinc complex with an organoselenium ligand at the lowest dosage in KK-Ay mice. J. Inorg. Biochem. 2013;121:10–15. doi: 10.1016/j.jinorgbio.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa Y., Murayama A., Adachi Y., Sakurai H., Yasui H. Challenge of studies on the development of new Zn complexes (Zn(opt)2) to treat diabetes mellitus. Metallomics. 2011;3:686–692. doi: 10.1039/c1mt00014d. [DOI] [PubMed] [Google Scholar]
- 9.Adachi Y., Yoshida J., Kodera Y., Kiss T., Jakusch T., Enyedy E.A., Yoshikawa Y., Sakurai H. Oral administration of a zinc complex improves type 2 diabetes and metabolic syndromes. Biochem. Biophys. Res. Commun. 2006;351:165–170. doi: 10.1016/j.bbrc.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Murakami H., Yasui H., Yoshikawa Y. Pharmacological and pharmacokinetic studies of anti-diabetic tropolonato-Zn(II) complexes with Zn(S2O2) coordination mode. Chem. Pharm. Bull. 2012;60(9):1096–1104. doi: 10.1248/cpb.c12-00078. [DOI] [PubMed] [Google Scholar]
- 11.Matthews P.M., Rabiner E.A., Passchier J., Gunn R.N. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 2011;73:175–186. doi: 10.1111/j.1365-2125.2011.04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner C.C., Langer O. Approaches using molecular imaging technology – use of PET in clinical microdose studies. Adv. Drug Deliv. Rev. 2011;63:539–546. doi: 10.1016/j.addr.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willimann J.K., van Bruggen N., Dinkelborg L.M., Gambhir S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 14.Ray P. Multimodality molecular imaging of disease progression in living subjects. J. Biosci. 2011;36:499–504. doi: 10.1007/s12038-011-9079-0. [DOI] [PubMed] [Google Scholar]
- 15.Motomura S., Enomoto S., Haba H., Igarashi K., Gono Y., Yano Y. Gamma-ray Compton imaging of multitracer in biological samples using strip germanium telescope. IEEE Trans. Nucl. Sci. 2007;54:710–717. [Google Scholar]
- 16.Motomura S., Kanayama Y., Haba H., Watanabe Y., Enomoto S. Multiple molecular simultaneous imaging in a live mouse using semiconductor Compton camera. J. Anal. At. Spectrom. 2008;23:1089–1092. [Google Scholar]
- 17.Motomura S., Kanayama Y., Hiromura M., Fukuchi T., Ida T., Haba H., Watanabe Y., Enomoto S. Improved imaging performance of a semiconductor Compton camera GREI makes for a new methodology to integrate bio-metal analysis and molecular imaging technology in living organisms. J. Anal. At. Spectrom. 2013;28:934–939. [Google Scholar]
- 18.Yoshikawa Y., Ueda E., Suzuki Y., Yanagihara N., Sakurai H., Kojima Y. New insulinomimetic zinc(II) complexes of α-amino acids and their derivatives with Zn(N2O2) coordination mode. Chem. Pharm. Bull. 2001;49:652–654. doi: 10.1248/cpb.49.652. [DOI] [PubMed] [Google Scholar]
- 19.Barnett B.L., Kretschmar H.C., Hartman F.A. Structural characterization of bis(N-oxopyridine-2-thionato)zinc(II) Inorg. Chem. 1977;16:1834–1838. [Google Scholar]
- 20.Kretsinger R.H., Cotton F.A. The crystal and molecular structure of di-(l-histidino)-zinc(II) dihydrate. Acta Cryst. 1963;16:651–657. [Google Scholar]
- 21.Nylin G., Celander H. Determination of blood volume in the heart and lungs and the cardiac output through the injection of radiophosphorus. Circulation. 1950;1:76–83. doi: 10.1161/01.cir.1.1.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material