Abstract
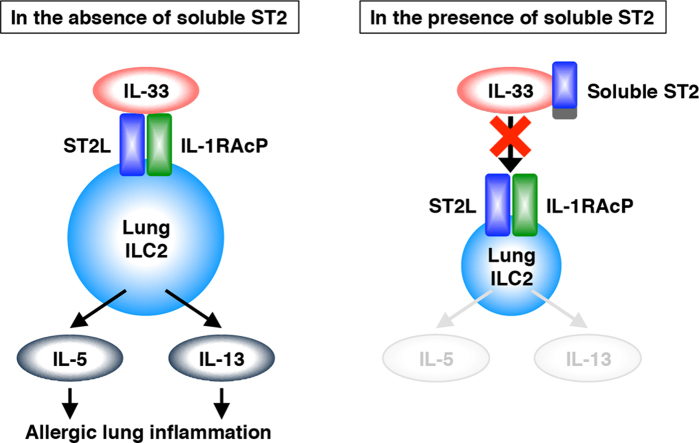
Type 2 innate lymphoid cells (ILC2) in lungs produce interleukin (IL)-5 and IL-13 in response to IL-33 and may contribute to the development of allergic diseases such as asthma. However, little is known about negative regulators and effective inhibitors controlling ILC2 function. Here, we show that soluble ST2, a member of the IL-1 receptor family, suppresses the effect of IL-33 on lung ILC2 in vitro. Stimulation with IL-33 to naïve ILC2 induced morphological change and promoted cell proliferation. In addition, IL-33 upregulated expression of cell surface molecules including IL-33 receptor and induced production of IL-5 and IL-13, but not IL-4. Pretreatment with soluble ST2 suppressed IL-33-mediated responses of ILC2. The results suggest that soluble ST2 acts as a decoy receptor for IL-33 and protects ILC2 from IL-33 stimulation.
Abbreviations: ILC2, type 2 innate lymphoid cells; IL, interleukin; Th2, type 2 helper T; NF-κB, nuclear factor-kappa B; MAPK, mitogen-activated protein kinases; JNK, c-Jun amino-terminal kinases; ERK, extracellular signal-regulated kinases; FSC, forward scatter; SSC, side scatter; CFSE, carboxyfluorescein diacetate succinimidyl ester
Keywords: Soluble ST2, Interleukin-33, Type 2 innate lymphoid cells, Decoy receptor
Graphical abstract
Highlights
-
•
Expression of IL-33 receptor in lung ILC2 is upregulated by IL-33 stimulation.
-
•
Soluble ST2 functions as a decoy receptor for IL-33.
-
•
Soluble ST2 protects naïve lung ILC2 from stimulation by IL-33.
1. Introduction
Expression of the ST2 gene is induced in serum-stimulated fibroblasts and differentiated immune cells including type 2 helper T (Th2) cells and mast cells [1], [2], [3], [4]. Two isoforms of the ST2 protein are produced; a soluble form (soluble ST2) and a transmembrane form (ST2L), and both belong to the interleukin (IL)-1 receptor family. Soluble ST2 lacks the transmembrane and intracellular portions and is secreted from the cells. Previous studies have reported that the serum level of soluble ST2 is increased in autoimmune diseases and pulmonary diseases, including eosinophilic pneumonia, asthma, and idiopathic pulmonary fibrosis [5], [6], [7], [8]. On the other hand, ST2L forms a receptor with the aid of the IL-1 receptor accessory protein (IL-1RAcP) for IL-33, which is a member of the IL-1 cytokine family [9], [10]. IL-33 binds to its receptor to trigger the activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPK) including c-Jun amino-terminal kinases (JNK), p38 MAPK (p38), and extracellular signal-regulated kinases (ERK). The activation results in production of Th2-associated cytokines including IL-5 and IL-13, which induce eosinophil maturation and mucus production, respectively. In a mouse model, airway inflammation was induced by administration of IL-33 [9], [11]. In addition, transgenic mice overexpressing IL-33 have been shown to exhibit airway inflammation [12]. These results suggest that the IL-33/ST2L axis is associated with the development of airway inflammation.
Type 2 innate lymphoid cells (ILC2), originally referred to as natural helper (NH) cells, were discovered as a novel target of IL-33 [13]. Recent studies reported that lung ILC2 participated in the induction of airway inflammation in influenza virus-infected mice and papain-administrated mice [14], [15]. These studies indicate that lung ILC2 are a possible therapeutic target for airway inflammation. However, negative regulators and effective inhibitors controlling ILC2 function have not yet been identified. We previously reported that soluble ST2 bound to IL-33 directly and inhibited its binding activity for the IL-33 receptor by using cells of the murine thymoma cell line EL-4 that were stably transfected with ST2L (ST2L/EL-4 cells) [16]. However, it has not been determined whether soluble ST2 affects natural IL-33-target cells such as ILC2. Here, we isolated lung ILC2 from naïve BALB/c mice and examined the responses of ILC2 to stimulation with IL-33. We also demonstrated that soluble ST2 suppressed the IL-33-mediated responses of lung ILC2.
2. Materials and methods
2.1. Mice
BALB/c mice were purchased from Japan SLC Inc. (Shizuoka, Japan) and housed in the animal research facility of Jichi Medical University. All experiments were approved by the Animal Research Ethics Board of Jichi Medical University.
2.2. Antibodies and reagents
Monoclonal antibodies against CD16/CD32, Alexa Fluor 700-conjugated CD45.2, fluorescein isothiocyanate-conjugated lineage cocktail (CD3ε, Gr-1, CD11b, CD45R, and Ter119), CD19, and CD49b, phycoerythrin (PE)-conjugated CD127 and IL-5, peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated CD25, allophycocyanin-conjugated c-Kit and IL-4, PE-Cy7-conjugated Sca-1 were purchased from BioLegend. Streptavidin-conjugated brilliant violet (BV) 421, carboxyfluorescein diacetate succinimidyl ester (CFSE), zombie NIR dye, and brefeldin-A were also purchased from BioLegend. Monoclonal antibodies against biotinylated T1/ST2 and PerCP-eFluor710-conjugated IL-13 were purchased from MD Bioproducts and eBioscience, respectively. Propidium iodide (PI) was purchased from BD Biosciences. Murine IL-2 and IL-33 were purchased from PeproTech.
2.3. Cell culture
Human embryonic kidney 293T (HEK293T) cells were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich) containing 10% fetal bovine serum (FBS). ST2L/EL-4 cells were cultured in RPMI 1640 medium (Sigma-Aldrich) containing 5% FBS, 50 μM 2-mercaptoethanol (2-ME), and 6 μg/ml blasticidin [16]. Lung ILC2 were cultured in RPMI1640 medium containing 10% FBS, 50 μM 2-ME, and IL-2 (20 ng/ml).
2.4. Purification of recombinant soluble ST2
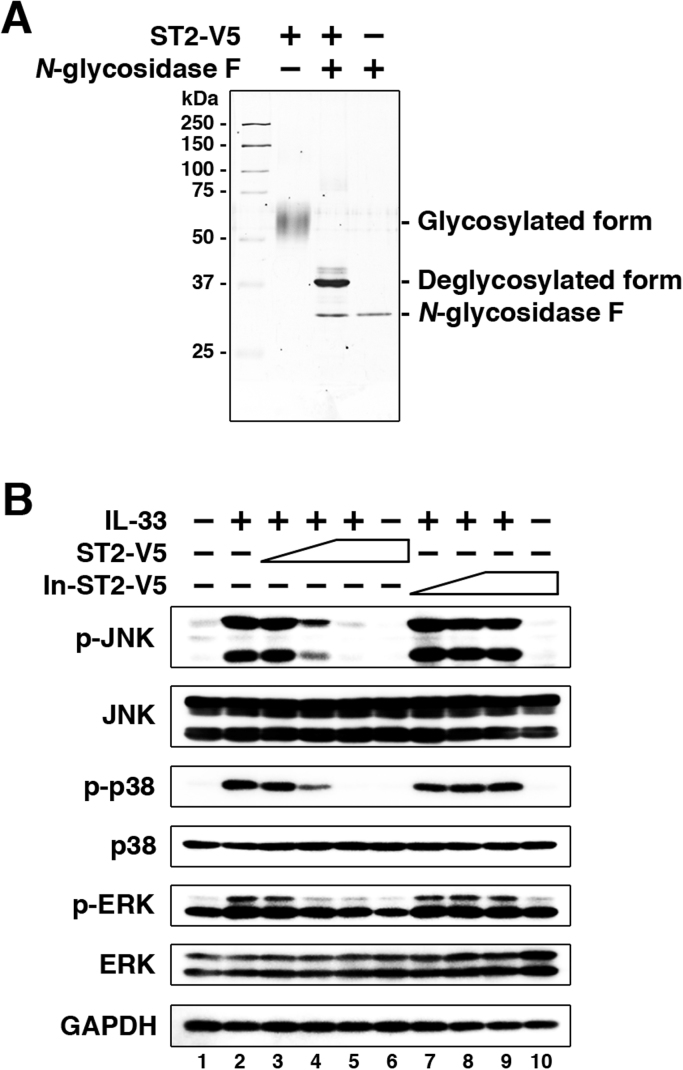
Recombinant murine soluble ST2 tagged with V5 and His (ST2-V5) was transiently expressed in HEK293T cells and purified from serum-free culture supernatants as described previously [17]. Deglycosylation of ST2-V5 with N-glycosidase F (Roche) and silver staining were performed as described previously [18]. For preparation of inactivated ST2-V5 (In-ST2-V5), ST2-V5 was treated at 100 °C for 30 min.
2.5. Isolation of lung ILC2
Lungs were perfused with phosphate-buffered saline (PBS), and then treated with collagenase IV (Gibco) and DNase I (Sigma-Aldrich). Lung lymphocytes were separated by centrifugation with Percoll (GE Healthcare). The lymphocytes were incubated with anti-CD16/CD32 antibody for blocking Fc receptor and then stained with fluorescent-labeled antibodies against CD45.2, CD3ε, Gr-1, CD11b, CD45R, Ter119, CD19, CD49b, CD127, CD25, c-Kit, and Sca-1. Dead cells were stained with PI. ILC2s were sorted using a Special Order Research Products (SORP) FACSAria II (BD Biosciences) with a purity of more than 95%. Purified ILC2 were rested for 36 h before stimulation.
2.6. Stimulation of ST2L/EL-4 cells and lung ILC2
ST2L/EL-4 cells (1×106 cells) were treated with ST2-V5 or In-ST2-V5 for 1 h and then stimulated with IL-33 (10 ng/ml) for 15 min. The cell lysates were prepared for western blotting. Lung ILC2 (4×103 cells) were treated with ST2-V5 or In-ST2-V5 for 1 h and then stimulated with IL-33 (10 ng/ml) for 24 or 96 h. In the proliferation assay, lung ILC2 were labeled with CFSE and then cultured for 96 h. The culture supernatants and the cells were harvested for analyses of cytokine production, proliferation, and cell surface molecules. The cells were also stained with Diff-Quik (Siemens Healthcare Diagnostics) and observed with a Biozero BZ-9000 (Keyence).
2.7. Western blotting
ST2L/EL-4 cells were lysed in RIPA buffer (50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 1% (v/v) Nonidet P-40, 0.5% (w/v) deoxycholate, 0.1% (w/v) sodium dodecyl sulfate (SDS)) plus 20 mM β-glycerophosphate, 10 mM sodium fluoride, 1 mM sodium orthovanadate, and protease inhibitor cocktail (Roche). The protein samples were separated by electrophoresis on SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were probed with antibodies against JNK, phosphorylated (p-) JNK, p38, p-p38, ERK, p-ERK (Cell Signaling Technology), and GAPDH (Santa Cruz Biotechnology), followed by horseradish peroxidase-conjugated anti-rabbit IgG (Cell Signaling Technology) and anti-mouse IgG antibodies (Bio-Rad Laboratories) in Can Get Signal reagents (Toyobo). The signals were detected with Immobilon western detection reagents (Millipore) and ImageQuant LAS4000 (GE Healthcare).
2.8. Flow cytometry
Lung ILC2 were incubated with anti-CD16/CD32 antibody prior to staining with fluorescent-labeled antibodies. For the proliferation assay, CFSE-labeled ILC2 were stained with biotinylated anti-T1/ST2 antibody, then staining with anti-Sca-1 antibody, streptavidin-conjugated BV421, and zombie NIR dye. For intracellular cytokine staining, ILC2 were treated with brefeldin-A for 5 h prior to harvest, followed by fixation with 4% (w/v) paraformaldehyde and permeabilization with PBS containing 0.5% (w/v) saponin and 0.5% (w/v) bovine serum albumin. The cells were stained with antibodies against IL-4, IL-5, and IL-13. Cell surface molecules of ILC2 were detected with biotinylated anti-T1/ST2 antibody and streptavidin-conjugated BV421 in addition to antibodies for ILC2 sorting. Dead cells were stained with PI. The cells were analyzed on a SORP FACSAria II and LSRFortessa (BD Biosciences). Data analysis was performed using FlowJo software (Tree Star).
2.9. Enzyme-linked immunosorbent assays
IL-5 and IL-13 in culture supernatants were measured using ELISA kits (Biosource International Inc. and eBioscience) according to the manufacturer's instructions.
2.10. Statistical analysis
Data were analyzed by the Tukey–Kramer test and represented as the means±SEM. A value of p<0.01 was considered to be significant.
3. Results
3.1. Soluble ST2 blocked the activation of intracellular signaling by IL-33 stimulation
We first determined the best molar ratio of soluble ST2 and IL-33 for the effective suppression of IL-33 stimulation. In purified recombinant soluble ST2 tagged with V5 and His (ST2-V5), the glycosylated and the unglycosylated forms were detected as a broad band of 55–65 kDa and a sharp band of 37 kDa, respectively (Fig. 1A). The molecular weights of ST2-V5 and commercially available IL-33 were calculated as 37,000 and 18,000, respectively. For these experiments, a cell line stably expressing ST2L, ST2L/EL-4, was used to ensure a sufficient number of cells, because the total number of lung ILC2 is only 1.5×104 cells per naïve mouse [15]. In addition, heat-inactivated ST2-V5 (In-ST2-V5) was prepared as a control of ST2-V5. ST2L/EL-4 cells were pretreated with various amounts of ST2-V5 or In-ST2-V5, and stimulated with a constant amount of IL-33, followed by analysis of intracellular signaling (Fig. 1B). The molar ratios of ST2-V5:IL-33 and In-ST2-V5:IL-33 were 1:5, 1:1, and 5:1 in culture media. Stimulation with IL-33 induced phosphorylation of JNK, p38, and ERK in the absence of ST2-V5 and In-ST2-V5. The phosphorylations were reduced by pretreatment with ST2-V5 in a dose-dependent manner and abrogated by exposure to ST2-V5:IL-33 at a molar ratio of 5:1. In contrast, pretreatment with In-ST2-V5 had no effect on the phosphorylation of kinases at any molar ratio. These results demonstrate that ST2-V5 blocks the activation of intracellular signaling in ST2L/EL-4 cells by IL-33 stimulation. Based on these results, we fixed the molar ratio of ST2-V5:IL-33 and In-ST2-V5:IL-33 at 5:1 in the following experiments.
Fig. 1.
The effect of soluble ST2 on the IL-33 signaling pathway in EL-4 cells that stably express ST2L. (A) Purified ST2-V5 (100 ng) was left untreated or treated with N-glycosidase F and developed on SDS polyacrylamide gel, followed by silver staining. (B) ST2L/EL-4 cells were pretreated with ST2-V5 or In-ST2-V5 (4.1, 20.7, and 103.6 ng/ml, corresponding to 0.11, 0.56, and 2.80 nM, respectively) for 1 h and then stimulated with IL-33 (10 ng/ml, corresponding to 0.56 nM) for 15 min. PBS was added as a control for ST2-V5, In-ST2-V5, and IL-33. Phosphorylation and expression levels of the indicated proteins were detected by western blotting. Results are representative of three independent experiments. p-, phosphorylated.
3.2. Soluble ST2 suppressed IL-33-mediated morphological change and proliferation of lung ILC2
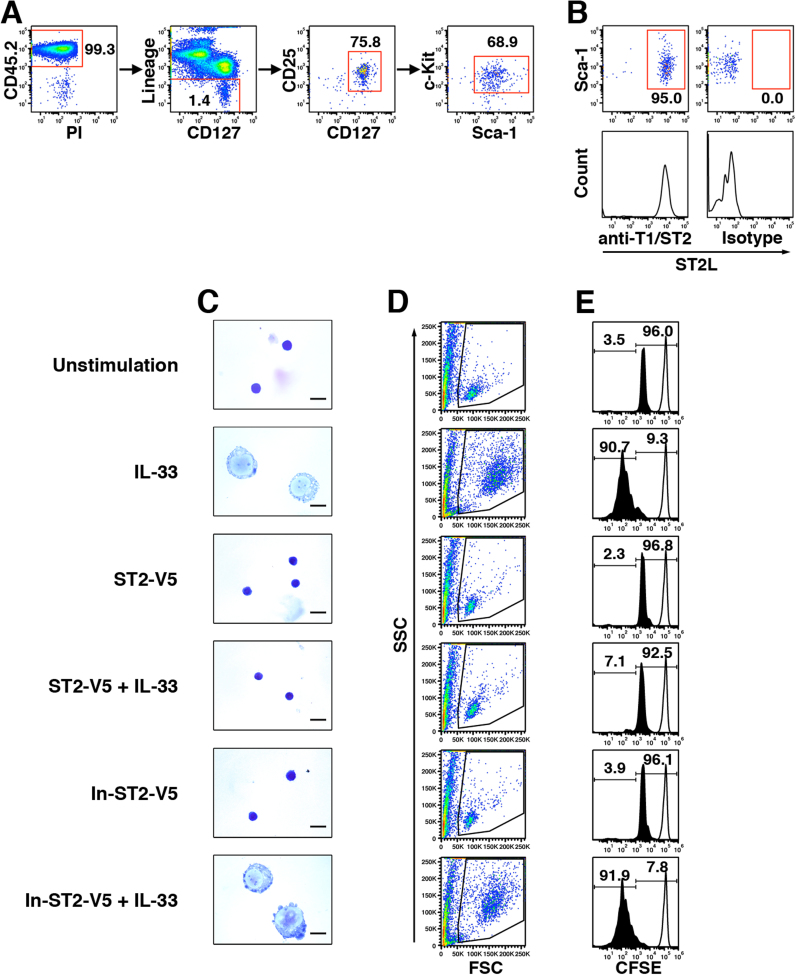
Previous studies characterized murine lung ILC2 as a CD45.2-positive cell population expressing CD127, CD25, c-Kit, and Sca-1, but not lineage markers (Lineage) including CD3ε, CD19, CD45R, CD49b, CD11b, Gr-1, and Ter119 [15], [19]. To use ILC2 for evaluation of ST2-V5, we isolated lung ILC2 as a CD45.2+Lineage-CD127+CD25+c-Kit+Sca-1+ population from naïve BALB/c mice by cell sorting (Fig. 2A). Flow cytometry analysis showed that more than 95% of isolated lung ILC2 expressed ST2L on the cell surface (Fig. 2B). Cell viability of lung ILC2 in the absence of IL-2 decreased during resting duration after cell sorting (data not shown). Therefore, we added IL-2 in culture medium for lung ILC2 at all times.
Fig. 2.
Soluble ST2 suppressed IL-33-mediated morphological change and proliferation of lung ILC2. (A) Sorting strategy of naïve lung ILC2. After exclusion of doublets in live lung lymphocytes, ILC2 were isolated as a CD45.2+Lineage-CD127+CD25+c-Kit+Sca-1+ population by cell sorting. (B) Expression of ST2L on isolated ILC2 was analyzed with antibodies against T1/ST2 (anti-T1/ST2) and isotype control (Isotype) by flow cytometry. The numbers indicate the percentage of gated cells in the respective gating step. (C) Naïve lung ILC2 were pretreated with ST2-V5 or In-ST2-V5 (103.6 ng/ml, corresponding to 2.80 nM) for 1 h and then stimulated with IL-33 (10 ng/ml, corresponding to 0.56 nM) in medium containing IL-2 (20 ng/ml) for 96 h. PBS was added as a control for ST2-V5, In-ST2-V5, and IL-33. Cells were mounted on glass slides and stained with Diff-Quik. Scale bars, 10 μm. (D and E) CFSE-labeled lung ILC2 were pretreated and stimulated as in Panel C. CFSE dilution was analyzed by flow cytometry. (D) Profile of FSC/SSC in CFSE-labeled lung ILC2. (E) Cell division of CFSE-labeled lung ILC2. The solid-lined and black-filled histograms represent cells cultured for 0 and 96 h, respectively. Right- and left-side numbers indicate the percentage of single- and more-divided population, respectively. Results are representative of three independent experiments.
To investigate the effect of IL-33 on naïve lung ILC2, we first observed the cell morphology of ILC2 (Fig. 2C). Lung ILC2 were small under an unstimulated condition (addition of IL-2 alone), being similar in size to fat-associated NH cells [13]. The nucleus occupied most of the cell volume, whereas the cytoplasm was scarce. Stimulation with IL-33 induced morphological change of ILC2. IL-33-stimulated ILC2 exhibited an increase in overall cell size due to an expansion of both the nucleus and cytoplasm. In addition, a few nucleoli appeared in the nucleus and vacuoles existed in the cytoplasm. IL-33-mediated morphological change was prevented by pretreatment with ST2-V5, but not by pretreatment with In-ST2-V5.
Next, we analyzed the cell proliferation of ILC2. Naïve ILC2 were labeled with CFSE and then stimulated with IL-33. Cell proliferation was analyzed as CFSE dilution by flow cytometry. The results of the flow cytometric analysis showed that IL-33-stimulated ILC2 increased in size and granularity in the forward scatter (FSC) and side scatter (SSC) profiles compared with the unstimulated cells (Fig. 2D). The results for the FSC/SSC profile coincided with those of the microscopic observation. CFSE dilution monitoring revealed the left-shifting of a single population from a parent population at 0 h (before proliferation) in unstimulated cells (Fig. 2E). A similar pattern was also observed in ILC2 treated with ST2-V5 or In-ST2-V5 alone. On the other hand, more than 90% of IL-33-stimulated ILC2 exhibited several divided populations, indicating that proliferation was promoted. The IL-33-dependent proliferation was suppressed by pretreatment with ST2-V5, but not by pretreatment with In-ST2-V5. Neither ST2-V5 alone nor In-ST2-V5 alone induced aberrant morphological change and proliferation. These results indicate that pretreatment with ST2-V5 is effective for suppressing the IL-33-induced morphological change and proliferation of ILC2.
3.3. Soluble ST2 suppressed IL-33-mediated expression of cytokine receptors and cytokine production in lung ILC2
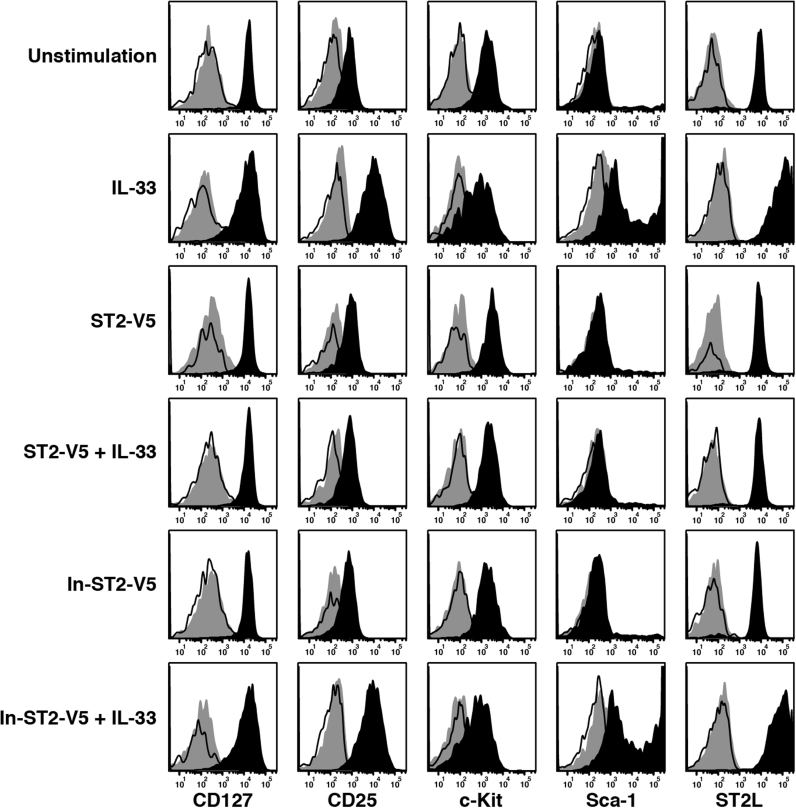
We further investigated the expression of cell surface molecules and cytokines in lung ILC2. Expression of CD127, CD25, c-Kit, Sca-1, and ST2L was analyzed by flow cytometry after 96 h of IL-33 stimulation (Fig. 3). The expression of cell surface molecules in the unstimulated cells was not different from that in the cells just after cell sorting. Stimulation with IL-33 induced upregulation of CD25, Sca-1, and ST2L expression and slight downregulation of CD127 and c-Kit expression. Pretreatment with ST2-V5 abrogated the IL-33-induced modulation in the expression of cell surface molecules.
Fig. 3.
IL-33 regulated expression of cell surface molecules on lung ILC2. Naïve lung ILC2 were pretreated and stimulated as in Fig. 2 (C). The cells were stained with fluorescent-labeled antibodies and then analyzed for expression of CD127, CD25, c-Kit, Sca-1, and ST2L on the CD45.2+Lineage- population by flow cytometry. The solid-lined, gray-filled, and black-filled histograms represent unstained, isotype antibody-stained, and specific antibody-stained cells, respectively. Results are representative of three independent experiments.
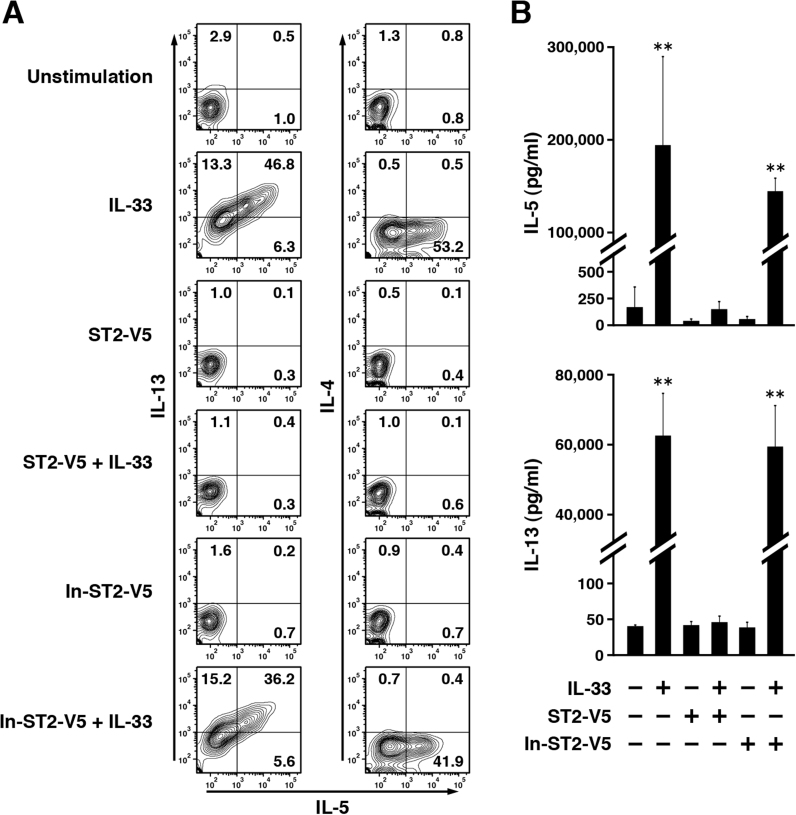
When stimulated with IL-33, ILC2 are known to produce IL-5 and IL-13. To assess the cytokine production of lung ILC2, we detected intracellular and secreted cytokines by flow cytometry and ELISA, respectively. Intracellular cytokines were detected after 24 h of IL-33 stimulation to reduce the influence of factors secreted from ILC2 (Fig. 4A). Stimulation with IL-33 increased a population producing IL-5 and IL-13 simultaneously. However, IL-4 production was not induced under any condition. Cytokine levels in the culture supernatants were measured after 96 h of IL-33 stimulation. Consistent with the results of the intracellular cytokine staining, IL-5 and IL-13 were secreted into the culture media in large amounts (Fig. 4B). On the other hand, IL-4 was present only at very low or undetectable levels (data not shown). IL-33-dependent IL-5 and IL-13 production were decreased significantly by pretreatment with ST2-V5, whereas pretreatment with In-ST2-V5 did not suppress cytokine production (Fig. 4A and B). These results indicate that ST2-V5 suppresses the IL-33-induced expression of proteins, including the expression of cytokines and cytokine receptors in lung ILC2.
Fig. 4.
Soluble ST2 suppressed IL-33-mediated production of IL-5 and IL-13 in lung ILC2. (A) Naïve lung ILC2 were pretreated as in Fig. 2 (C) and then stimulated with IL-33 for 24 h. Intracellular IL-4, IL-5, and IL-13 were detected by flow cytometry. The numbers in each quadrant indicate the percentage of cells. Results are representative of three independent experiments. (B) Naïve lung ILC2 were pretreated as in Fig. 2 (C) and then stimulated with IL-33 for 96 h. Culture supernatants were collected, and then applied to ELISA for measurement of IL-5 and IL-13. The data are shown as the means±SEM (**, p<0.01, IL-33 or In-ST2-V5+IL-33 versus other groups).
4. Discussion
Here we showed that soluble ST2 suppressed the effect of IL-33 on lung ILC2 derived from naïve BALB/c mice. IL-33 induced morphological change of naïve lung ILC2 dramatically. IL-33-stimulated lung ILC2 exhibited augmented proliferation and upregulated expression of cytokine receptors and Th2-associated cytokines. Pretreatment with soluble ST2 significantly suppressed IL-33-induced responses.
We demonstrated that a five-molar excess of soluble ST2 competed away the IL-33-mediated responses in ST2L/EL-4 cells and lung ILC2. Structural analysis using nuclear magnetic resonance (NMR) showed that the ternary complex of IL-33/ST2L/IL-1RAcP formed a stoichiometry of 1:1:1 [20]. The NMR-based study also proposed a complex formation model, in which IL-33 bound to ST2L first and then the IL-33/ST2L complex recruited IL-1RAcP. Soluble ST2 corresponds to the extracellular portion of ST2L, including the IL-33-binding site [1], [2]. Based on the structural analysis, it was predicted that equal amount of soluble ST2 and IL-33 formed into complexes. However, a sufficient suppressive effect was achieved when the ST2-V5/IL-33 molar ratio was 5:1 in our system (Fig. 1). We also found that the suppression of IL-33 function lasted for at least 4 days even though ST2-V5 was added only once before IL-33 stimulation (Fig. 2, Fig. 3, Fig. 4). The results suggested that soluble ST2 bound to IL-33 stably and continuously.
IL-33 is involved in the onset of allergic reaction. IL-33 is released from epithelial cells and vascular endothelial cells of the lungs in response to allergens, chemical stimuli, and mechanical damage [21], [22]. Resident ILC2 in lungs express ST2L constitutively and can respond rapidly to IL-33 stimulation. In isolated naïve lung ILC2, IL-33 induced an expansion of cell size with the proliferation and a large amount of IL-5 and IL-13 production (Figs. 2 and 4). IL-33 also induced further expression of CD25 and ST2L, which correspond to IL-2 receptor and IL-33 receptor, respectively (Fig. 3). Previous study in fat-associated NH cells showed that IL-2 signal participates in the cell proliferation without changing surface phenotype [13]. Therefore, amplification of these receptors may cause further activation of lung ILC2 by IL-2 and IL-33 stimulation, leading to airway inflammation with eosinophil infiltration and mucus accumulation. In patients with asthma, serum soluble ST2 has been shown to increase temporarily in correlation with the severity of exacerbation and to decrease during remission [7]. The level of IL-33 has also been correlated with the severity of exacerbation [23]. These studies suggest that fluctuation in soluble ST2 and IL-33 production may influence the disease course in asthma. In addition, a human genome-wide association study showed that the IL1RL1 gene (the ST2 gene) and the IL33 gene were associated with susceptibility to asthma [24], [25]. Thus, regulation of the expression and function of IL-33, ST2L, and soluble ST2 will be important for amelioration of the symptoms of asthma. Our findings indicate that soluble ST2 plays a role in protecting ILC2 from IL-33 stimulation and thereby maintaining them in a naïve state (Fig. 2, Fig. 3, Fig. 4). Therefore, soluble ST2 may be a candidate for a therapeutic agent of asthma. Recent studies have reported that ILC2 contributed to the development of atopic dermatitis and obesity [26], [27], [28], [29]. The suppressive effect of soluble ST2 may also be effective for these diseases, although further studies will be needed to confirm that soluble ST2 can suppress disease in vivo.
In summary, we demonstrated that soluble ST2 functions as a decoy receptor of IL-33 using naïve lung ILC2 in vitro. Pretreatment with soluble ST2 suppressed various responses of lung ILC2 to IL-33 stimulation. Our findings may be helpful for regulating the progression in allergic diseases such as asthma.
Acknowledgments
We are grateful to Dr. Toshimi Yoshida and Dr. Jin Mo Park (Harvard Medical School) for their advice in cell sorting and helpful discussion. We thank all members of the Department of Biochemistry (Jichi Medical University) for their discussion. We also thank the Core Center of Research Apparatus (Jichi Medical University) for the management of SORP FACSAria II and LSRFortessa. ImageQuant LAS4000 and LSRFortessa were subsidized by JKA through its promotion funds from KEIRIN RACE. This work was supported by a Research Grant for Medical 2011 from the Takeda Science Foundation (Grant number M2011, to Hayakawa M.) and a Grant-in-Aid for Challenging Exploratory Research from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant number 26670161, to Hayakawa H.).
Footnotes
Transparency Document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.02.002.
Appendix A. Transparency Document
Transparency Document
Transparency Document
Supplementary material
References
- 1.Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa K., Takagi T., Tsukamoto T. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- 3.Yanagisawa K., Naito Y., Kuroiwa K. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J. Biochem. 1997;121:95–103. doi: 10.1093/oxfordjournals.jbchem.a021577. [DOI] [PubMed] [Google Scholar]
- 4.Gächter T., Werenskiold A.K., Klemenz R. Transcription of the interleukin-1 receptor-related T1 gene is initiated at different promoters in mast cells and fibroblasts. J. Biol. Chem. 1996;271:124–129. doi: 10.1074/jbc.271.1.124. [DOI] [PubMed] [Google Scholar]
- 5.Kuroiwa K., Arai T., Okazaki H. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem. Biophys. Res. Commun. 2001;284:1104–1108. doi: 10.1006/bbrc.2001.5090. [DOI] [PubMed] [Google Scholar]
- 6.Oshikawa K., Kuroiwa K., Tokunaga T. Acute eosinophilic pneumonia with increased soluble ST2 in serum and bronchoalveolar lavage fluid. Respir. Med. 2001;95:532–533. doi: 10.1053/rmed.2001.1080. [DOI] [PubMed] [Google Scholar]
- 7.Oshikawa K., Kuroiwa K., Tago K. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am. J. Respir. Crit. Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 8.Tajima S., Oshikawa K., Tominaga S. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124:1206–1214. doi: 10.1378/chest.124.4.1206. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz J., Owyang A., Oldham E. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Chackerian A.A., Oldham E.R., Murphy E.E. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 11.Kondo Y., Yoshimoto T., Yasuda K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 12.Zhiguang X., Wei C., Steven R. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol. Lett. 2010;131:159–165. doi: 10.1016/j.imlet.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Moro K., Yamada T., Tanabe M. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 14.Monticelli L.A., Sonnenberg G.F., Abt M.C. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halim T.Y., Krauss R.H., Sun A.C. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa H., Hayakawa M., Kume A. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J. Biol. Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 17.Takezako N., Hayakawa M., Hayakawa H. ST2 suppresses IL-6 production via the inhibition of IkappaB degradation induced by the LPS signal in THP-1 cells. Biochem. Biophys. Res. Commun. 2006;341:425–432. doi: 10.1016/j.bbrc.2005.12.206. [DOI] [PubMed] [Google Scholar]
- 18.Takagi T., Yanagisawa K., Tsukamoto T. Identification of the product of the murine ST2 gene. Biochim. Biophys. Acta. 1993;1178:194–200. doi: 10.1016/0167-4889(93)90009-e. [DOI] [PubMed] [Google Scholar]
- 19.Halim T.Y., MacLaren A., Romanish M.T. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Lingel A., Weiss T.M., Niebuhr M. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors—insight into heterotrimeric IL-1 signaling complexes. Structure. 2009;17:1398–1410. doi: 10.1016/j.str.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefrançais E., Cayrol C. Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. Eur. Cytokine Netw. 2012;23:120–127. doi: 10.1684/ecn.2012.0320. [DOI] [PubMed] [Google Scholar]
- 22.Cayrol C., Girard J.P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Préfontaine D., Lajoie-Kadoch S., Foley S. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J. Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 24.Torgerson D.G., Ampleford E.J., Chiu G.Y. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat. Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirota T., Takahashi A., Kubo M. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat. Genet. 2011;43:893–896. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai Y., Yasuda K., Sakaguchi Y. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl. Acad. Sci. USA. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salimi M., Barlow J.L., Saunders S.P. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brestoff J.R., Kim B.S., Saenz S.A. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molofsky A.B., Van Gool F., Liang H.E. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency Document
Transparency Document
Supplementary material