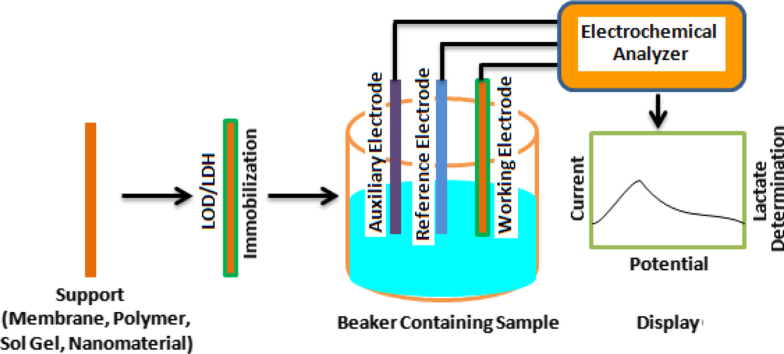
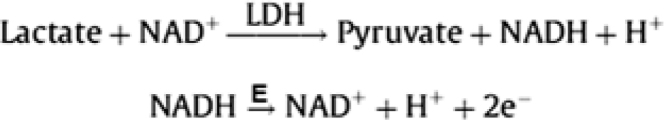Abstract
Lactate detection plays a significant role in healthcare, food industries and is specially necessitated in conditions like hemorrhage, respiratory failure, hepatic disease, sepsis and tissue hypoxia. Conventional methods for lactate determination are not accurate and fast so this accelerated the need of sensitive biosensors for high-throughput screening of lactate in different samples. This review focuses on applications and developments of various electrochemical biosensors based on lactate detection as lactate being essential metabolite in anaerobic metabolic pathway. A comparative study to summarize the L-lactate biosensors on the basis of different analytical properties in terms of fabrication, sensitivity, detection limit, linearity, response time and storage stability has been done. It also addresses the merits and demerits of current enzyme based lactate biosensors. Lactate biosensors are of two main types – lactate oxidase (LOD) and lactate dehydrogenase (LDH) based. Different supports tried for manufacturing lactate biosensors include membranes, polymeric matrices-conducting or non-conducting, transparent gel matrix, hydrogel supports, screen printed electrodes and nanoparticles. All the examples in these support categories have been aptly discussed. Finally this review encompasses the conclusion and future emerging prospects of lactate sensors.
Keywords: Lactate, Biosensor, Lactate oxidase, Lactate dehydrogenase, Nanomaterials
Graphical abstract
Highlights
-
•
Different enzymes used in lactate bio sensing have been studied.
-
•
Support used for fabrication biosensors have been discussed.
-
•
The linearity range, response time, detection limit, etc. have been studied.
-
•
Merits and demerits of different supports are also discussed.
1. Introduction
Lactate concentration has been widely used as a key parameter in the clinical diagnostics for assessing patient health conditions and study of diseases and for continuous surveillance in surgery [1], [2], sports medicine [3], shock/trauma [4] and food industry [5], [6]. The baseline lactate level in blood ranges from 0.5 to 1.5 mmol/l at rest [1] but can rise up to 25 mmol/L during the intense exertion [7]. Lactate is a key metabolite of the anaerobic metabolic pathway. When the energy demand by tissues cannot be met by aerobic respiration, an increase in lactate concentration will occur from the anaerobic metabolism. Without adequate clearance by liver and kidney, the accumulated concentration of lactic acid results in lactic acidosis [8]. So the production and utilization of lactate are tightly controlled by lactate homeostasis in a healthy person. The lactate balance is significant to acid–base homeostasis, as formation of lactate is associated with enhancement in proton concentration inside the cells and the utilization of lactate and regeneration of bicarbonate are required to counter balance the production of proton and the loss of bicarbonate [9]. Such finely tuned interplay of parameters that influences the delicate lactate balancing system is necessary because our body is viable only within an extremely narrow range of pH (between pH 7.2 and 7.4) [10]. Clinically, causes of lactic acidosis can be classified by two ways: type A disorders, in which there is decreased tissue oxygenation such as with shock, left ventricular failure, sepsis and poisoning with carbon monoxide and cyanide; or type B disorders, caused by certain drugs/toxins, along with systemic disease, including failure of renal and hepatic system, diabetes and malignancy or inborn error metabolism [11]. According to Medicare data and published literature in the year 2000, an estimated 4.4 million patients were admitted to ICU in US annually, in which about 25% of the above patients suffered from sepsis (this is due to procoagulant and systemic inflammatory response) [12]. When this condition is combined with one or more vital organ dysfunctions, the patient will develop severe sepsis. The mortality rate of sepsis is estimated around 30–50%, which corresponds to at least 225,000 deaths annually [13], [14]. Recent studies have shown that early lactate clearance could improve the treatment outcome in severe sepsis and septic shock, which could potentially lead to the decrease in sepsis related mortalities [15]. Therefore, a patient's blood lactate level may act as alarm signal for the severity of illness and may also be used to improve the diagnosis and treatments of a broad range of diseases. Lactate also has a significant importance in sports medicine, especially for determining physical fitness in athletics. Level of lactate in blood during exercise is used as an indicator for the athletic training status and fitness since elevated levels of blood lactate results in decrease level of pH in blood finally resulting in fatigue.
The importance of lactate estimation also can be found in many food and fermentative industry. Fermentative products such as fermented milk products, wine, cured meat and fish and also pickled vegetables produce lactate. Due to this fact, lactate is commonly used as a specific indicator of the presence of bacterial fermentation, thus as an indicator for the freshness and quality of the food [16].
As mentioned above, it is clear that lactate is a metabolite that has evoked a great interest in many industries for importance of detecting and measuring its existence in various media. Analytical methods, which are most commonly employed for lactate determination, are high performance liquid chromatography (HPLC) [17] with other methods being fluorometry [18], colorimetric test [19], [20], chemiluminescence [21] and magnetic resonance spectroscopy [22], [23]. Although these methods provide results, these suffer from drawbacks like time consuming requirement of sample pre-treatment, costly due to requirement of expensive machinery and trained manpower. However, biosensors can overcome these limitations. When compared with various methods available for L-lactate detection, biosensing methods possess the advantages of being simple, direct, and real-time with no need of sample preparation (except perhaps for dilution of the sample), combining rapid response with high specificity, economical and are user-friendly [24].
In this present review, we briefly introduced the development and applications of L-lactate biosensors. We also summarized the L-lactate based biosensors by comparing their different analytical properties in terms of fabrication, sensitivity, detection limit, linearity, response time and storage stability. Along with this the merits and demerits of current enzyme based lactate biosensors have been highlighted. Finally this review encompasses the conclusion and future emerging prospects of lactate sensors.
2. Enzymes involved in lactate biosensors
The concept of an enzyme coupled biosensing electrode involves placement of an enzyme in close proximity to an electrode surface. The enzyme involved must catalyse the reaction which involves consuming of electroactive reactant or generation of electroactive species. The depletion or production process is then monitored and gives a direct measurement of the analyte concentration.
Different enzymes have attracted the attention in lactate detection as the test case for several biological applications. In the fabrication of L-lactate biosensors, the most commonly used biological recognition element are L-lactate dehydrogenase (LDH) and L-lactate oxidase (LOD) due to prevalence of simple enzymatic reaction involved and considerably simple sensor design fabrication.
2.1. Lactate oxidase (LOD)
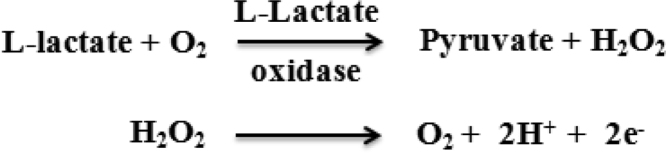
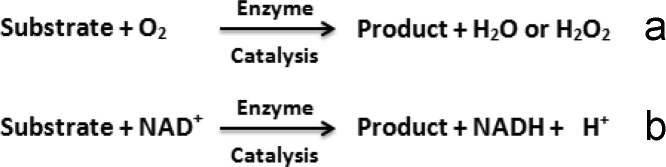
L-LOD (EC 1.13.12.4), a globular flavoprotein can be obtained from a variety of bacterial sources, such as Pediococcus, Aerococcus viridans, and Mycobacterium smegmatis. It catalyzes the oxidation of L-lactate to pyruvate in the presence of dissolved oxygen and forms hydrogen peroxide, which is electrochemically active and can be either reduced or oxidized to give a current proportional to the L-lactate concentration [25], [26].
The principle of an electrochemical biosensor based on LOD reaction has been described below.
However, the hydrogen peroxide produced by enzymatic reaction requires a high oxidation potential which in turn leads to interference caused by electro-oxidizable species. Furthermore, the fluctuation of oxygen concentration in the solution brings the system complexity, affects the detection limit and leads to extra costs, and makes miniaturization a difficult task for practical applications. One way to overcome the issues related to oxygen concentration is to use L-lactate dehydrogenase.
2.2. Lactate dehydrogenase (LDH or LD)
LDH, a quaternary protein is found in animals, plants, and prokaryotes. It has important medical significance as it present throughout the tissues, such as blood cells and heart muscle. It is released during tissue damage, so it basically acts as a marker of common injuries and disease.
L-LDH (EC 1.1.1.27 and EC 1.1.1.28) has a high catalytic activity for conversion of L-lactate to pyruvate and NADH. LDH belongs to class of oxidoreductases that catalyze the oxidation of α-hydroxyacids. Being a dehydrogenase, it transfers a hydride from one molecule to another.
L-LDH is also widely used as an important candidate in biosensor fabrication [27], [28], [29]. There has been continuous improvement of LDH based biosensors with the regular improvement in use of different transducer and the availability of highly purified enzyme. LDH based biosensors work in the presence of a coenzyme (NAD or NADP) which acts as mediator to shuttle the electrons between the enzyme and electrode. LDH converts L-lactate into pyruvate and NAD to NADH. On the electrode surface, NADH is oxidized under the influence of the applied potential and the oxidation current is directly proportional to the L-lactate concentration in the solution. The principle of an electrochemical biosensor based on LDH reaction and oxidation of NADH to generate electrons (e−) is shown in Fig. 1
Fig. 1.
Electrochemical reactions involved in LOD biosensors.
3. Biosensors
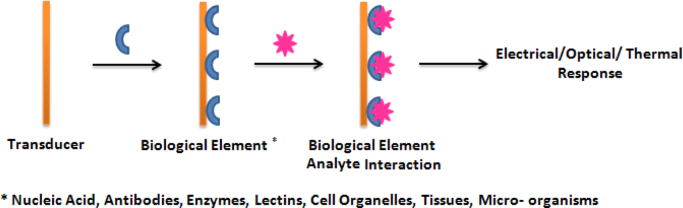
Biosensor is a sensing device that comprised of a biological entity as an important part of assembly during the analyte detection. A biosensor is comprised of two elements: (i) biological element and (ii) transducer element. The biological component (bioreceptor) comprises the specific receptors which are modeled after biological systems to interact with the analytes of interest. This interaction is measured by a sensor which outputs a measurable signal proportional to the presence of the target analyte in the sample. So a variety of biological elements including nucleic acids, antibodies, enzyme, lectins, cell organelles, tissues and microorganism have been immobilized for biosensing. Biosensors exploit specificity and sensitivity of the above said molecules as an analyte.
The signal, generated as a result of interaction between the immobilized biological element and the analyte may be electrical, optical, thermal, etc. which are transformed by the means of suitable transducer element into a measurable electrical parameter such as current or voltage. The biosensor selectivity against the target analyte is determined by bio-recognition layer, while sensitivity is dependent on transducer ( Fig. 2, Fig. 3).
Fig. 2.
Electrochemical reactions involved in LDH biosensors.
Fig. 3.
Basic principle of electrochemical biosensor.
By far, compared to various methods available for the detection of lactate such as colorimetric method, chemiluminescence, fluorimetric method, high performance liquid chromatography (HPLC) and magnetic resonance spectroscopy, amperometric lactate biosensors have gained the most significant developments provided by the advantage like simplicity, portability, rapid response, high specificity, low cost, minimal or no sample preparation and user-friendly operation.
3.1. Basic principle of electrochemical biosensors
Amperometric biosensor exploits the current produced when an oxidation or reduction reaction occurs at an electrode; current response generated during the reaction is directly proportional to concentration of that species present in the particular sample. So it functions by the measurement of the current produced on application of potential between working and reference electrodes which results with the electrocatalytic oxidation or reduction of the involved electroactive species. Magnitude of this current is directly correlated to the concentration of a redox-active reagent or product in an enzymatic reaction. Generally biosensor for detection of L-lactate in tissue or blood focuses on enzymatic amperometric sensors owing to their simple design and performance. The enzyme involved here must have the ability to catalyze a reaction involving the analyte with the consumption of an electroactive reactant and/or the production of an electroactive product. The depletion or production process is then monitored amperometrically and gives a direct measurement of the analyte concentration.
Generally amperometric biosensors are based on enzymes that, either consume oxygen e.g. all the oxidases, or generation of hydrogen peroxide (oxidases producing water are excluded), or indirect production of reduced form of β-nicotinamide adenine dinucleotide such as dehydrogenases, with the catalytic breakdown of substrate [30]. The general equations of the above mentioned types of amperometric biosensors are given below in Fig. 4:
Fig. 4.
Schematic representation showing generation of different products.
3.2. Electrode material
Amperometric measurements are usually carried out with an assembly containing electrochemical cell with the three electrode system, comprising of a working having immobilized enzyme, reference electrode and a counter current electrode. The working electrode is the main electrode which is fabricated using different electroactive materials. The electron transfer occurs at the surface of the working electrode. The selection of working electrode primarily depends on the redox behavior of the target analyte and the background current over the potential region required for the measurement. A constant potential is maintained at a working electrode or an array of working electrodes (metal or carbon based material) [31]. The reference electrode (Ag/AgCl or saturated calomel electrodes) provides a stable and reproducible potential, to which the working electrode potential is compared. In the three electrodes system the potential applied is controlled by using a reference electrode, the current flow through the solution between the working and counter electrodes is measured. The counter electrode maintains stability. In the construction of L-lactate biosensors, gold and platinum are the most common electrode materials. For example, it was found that with dehydrogenases, platinum working electrodes are less prone to fouling than glassy carbon electrodes [32]. On the other hand, carbon based working electrodes are used in the form of carbon paste, carbon powder, carbon fiber, glassy carbon electrodes, screen printed electrodes with graphite based printing ink or graphite electrodes. As carbon electrodes result in slow transfer of electrons, they are of less exploited in the L-lactate based fabrication of biosensors [33].
The electrode surface area can also be modified in order to increase the sensitivity of enzymatic biosensors. This involves increase in the electrode surface area which leads to loading of higher amounts of the enzyme in the recognition layer. Nanomaterials play important role in increasing the electrode surface area and include nanoparticles like carbon nanotubes (CNTs) [34], [35], gold nanoparticles (AuNPs) [36], [37], platinum nanoparticles (PtNPs) [38], [39], metal oxides based nanoparticles i.e., niobium oxide [40] or molybdenum oxide [41] and semiconducting material based nanoparticles such as zinc oxide [42].
4. Enzyme(s) immobilization
Immobilization of enzyme to act as bioreceptor is one of the most important steps in the development of enzyme based biosensor. The main target is to obtain the optimum bio-stability and reaction efficiencies of the enzyme. There are some important factors that must be taken into account while immobilizing the enzyme. The key factor is the performance of an enzyme electrode in terms of sensitivity, selectivity, lifetime, linear range, response time and stability. The susceptibility to interfering agents must also be considered as it depends strongly on the immobilization methods of enzymes. The enzyme should be immobilized in the active form and the activity must be preserved with less reduction in the specificity for the analyte. Its stability also has to be maintained or preferably increased to ensure the stability of the biosensor in terms of reusability and long term storage. The types of matrix (support) where the enzyme will be immobilized also need to be taken into account. The choice and condition of matrix must be suitable for the enzyme to retain its activity. A variety of enzyme immobilization methods are present including physical adsorption, membrane confinement, covalent binding, cross link formation, electrical polymerization and finally monolayer formation by self-assembly. Physical adsorption method relies on non-specific physical interaction between the enzyme protein and the surface of the matrix (such as glass, plastic and cellulose) brought about by mixing of a concentrated solution of enzyme with the solid. The fundamental interacting forces are the weak bonds such as van der waal, hydrogen bonding and salt linkages. But this immobilization technique is not reproducible and reliable method because of the problems associated with leaching during long-term storage [43]. Physical entrapment involves entrapping the enzyme in polymer matrices or sol–gel matrices. In this, the enzyme is either mixed with sol–gel precursors or with monomers which are cross-linked either via electropolymerization, photo crosslinking, cross-linking using gamma irradiation, isocyanate and other agents, or the enzymes are entrapped using electrostatic interactions. In sol–gel method, a silica gel network is formed around the enzyme of interest and chemical condensation [44], [45]. Such coverage with the coating serves to extend the linear range by reduction of the local substrate concentration and to eliminate potential interferences (e.g. coexisting electroactive species). On the other hand, chemical coupling of enzyme provides stable biosensing surfaces that are resistant to wide ranges of pH, temperature and ions to ensure a greater long-term enzymatic stability. However, a covalent bond of the enzyme to the matrix will decrease its activity. Thus, the binding process must occur under conditions that do not denature the enzyme [46].
The crosslinking process uses a bifunctional agent to form a bridge between different biocatalytic proteins. Glutaraldehyde is one example of crosslinking reagents that give greater stability to the immobilized enzyme although, inevitably, some inactivation does occur as the chemicals used in crosslinking may block the active site present on the enzyme. Care should be taken to ensure that the crosslinks retain the molecule and the leaching does not occur [29]. The electro polymerization method involves polymerization in the presence of an electric current. The desired polymer film is subjected to suitable oxidation potential of the monomer or consecutive cyclic voltammetry is used in a suitable positive scan potential region. This immobilization technique is known to exclude interfering species from the electrode surface. It also prevents electrode fouling which in turns helps to protect the enzyme structure [47], [28]. The noble metal surfaces can also be functionalized by self-assembled monolayer (SAM) as this procedure exploits the organic molecules (aliphatic and aromatic) which contain amines, disulfides, alkanesilanes (R–S–S–R), sulfides (R–S–R) as free anchorage group for enzyme immobilization. The process consists of the deposition of controlled and stable packed monolayers directly onto the transducer surface [48]. In order to maintain high stability of the layer, generally long-chain (C12 or higher) n-alkylthiols or silanes are used because the use of a shorter chain length results in decrease in stability and less structural organization [49]. In above immobilization method, the spatial arrangement and the orientation of the enzymes can be controlled and also provide absence of diffusion barriers, but major limitation that exists is to produce reproducible layering of enzymes [50].
5. Electron-transfer system
Amperometric biosensor functioning is based on the measurement of current which is related to the transfer of electrons from the active site of the immobilized enzyme to the working electrode surface [131]. Generally this electron transfer reaction at the time of catalysis of biological molecules is very slow at ordinary electrodes surface. So the electrode material used for immobilization of bio recognition molecules in a biosensor should be such that it provides a good electron transport capacity. Different strategies can be used regarding the construction of amperometric enzyme electrodes to facilitate the direct electron transfer process in L-lactate detection. These include the use of mediator-modified enzymes, electrodes modified by membrane for electron transfer, by conducting or non-conducting-polymer matrices, by sol–gel based supports, by hydrogel supports, by screen printed support or use of nanoparticles as electrode materials. Some of these strategies are reviewed and their applications to L-lactate biosensors are described.
Biocompatible membrane with different porosities serves as a common immobilization matrix due to their applications being as a barrier for interference, works as an antifouling electrode and protecting the enzyme structure and may lead to increased linear response. The thickness of the membrane also affects the rate of electron transfer which in turn can be adjusted during the deposition of the membrane layer by the amount of charge applied. Romero et al. fabricated a lactate oxidase based amperometric sensor immobilized on a mucin/albumin hydrogel matrix with nafion membrane which served as a protecting layer on the electrode surface [70]. They obtained remarkable higher operational stability and sensitivity (0.537±0.007) mA M−1. Their study showed that nafion membrane acted as a facilitator of electron transfer and hence accelerated the electron transfer. Moreover it also served as a barrier to the ascorbic acid and thus minimizing its interference effect.
Conducting polymers have gradually drawn attention as a material for immobilization of biological molecules to enhance sensor performance. The electrically conducting polymers have attractive properties which results in improved electron transport capacity, stability and sensitivity of biosensor. Rahman et al. developed a highly stable and sensitive lactate sensor based on poly-5,2′-5′,2′′-terthiophene-3′-carboxylic acid (pTTCA)/MWCNT composite film modified Au electrode [28]. The carboxylic acid group functionalized conductive polymer allowed enormous active sites onto the biosensing layer which resulted in immobilization of LDH through covalent bond formation and acted as the electron transferring medium during electrocatalytic oxidation of NADH. In this the LDH immobilized on the pTTCA/MWCNT composite film reduces NAD+ to NADH during the biochemical reaction with L-lactate. This resulted in oxidation of NADH at the working electrode releasing two electrons at 0.3 V. The pTTCA/MWCNT composite film acted as an electron acceptor and hence gets reduced. This composite film modified electrode merged the properties of MWCNT and conductive polymer and showed good electrocatalytic oxidation response at an applied potential of 0.3 V versus Ag/AgCl with sensitivity of 0.0106 µA/µM and detection limit of 1 μM, based on a signal/noise ratio of 3. The performance of the sensor was excellent in terms of sensitivity, selectivity, response time, and reproducibility. Furthermore, the resulting sensor allowed its application for measurement of lactate concentration in human serum and commercial milk.
Sol gel based matrices have also been used for biosensing. These are biocompatible porous structures which provide space for integrating nanostructured particles which in turn play an important role in accelerating electron transfer. Parra-Alfambra et al. presented an electrochemical lactate biosensor by integrating gold nanoparticles (AuNPs) and enzyme lactate oxidase (LOx) into a sol–gel polymeric matrix obtained from (3-mercaptopropyl)-trimethoxysilane (MPTS) [101]. This resulted in a favorable microenvironment for LOx by preserving catalytic activity and incorporation of AuNPs which loaded large amount LOx and provided direct electron transfer at the working electrode. The sensor exhibited a linear response to L-lactic acid in the range of 50 μM–0.25 mM, with a high sensitivity of 3.4 µA mM−1 and a detection limit of 4.0 μM.
Hydrogel matrices serve as a water swellable polymer networks in which the biorecognition elements are embedded. Zanini et al. reported lactate biosensor based on immobilization of L-lactate oxidase in a laponite/chitosan hydrogel on a glassy carbon electrode with ferrocene–methanol (FcMe) used as artificial mediator [86]. The aim was to develop best hydrogel composition. The biosensor showed excellent analytical response with a low response time (~5 s). The sensitivity of (0.326±0.003) A cm−2 M−1 and detection limit of (3.8±0.2)×10−6 M was obtained. The proposed sensor was suitable for quantification of lactate concentration in dairy items and alcoholic beverages.
Screen printed electrodes (SPE) are miniaturized disposable device that are manufactured by depositing multiple successive layers of different ink on solid substrate. Screen printed carbon electrode surface can be easily modified with the mediators, conducting polymers or nanostructures for their use in biosensors as a transducer to improve electron transfer rate. Pianoa et al. prepared a sensor based on screen printed electrode for lactate measurement in serum [77]. The screen-printed carbon electrode was modified with meldola blue-reinecke salt. Meldola's Blue-Reinecke Salt acted as an artificial electron mediator which aided the faster electron transfer rate between the SPCE electrode surface and the LDH enzyme. The biosensor showed a linear response over the range 0.55–10 mM and obtained a good reproducibility at an applied potential of +0.05V. The sensor showed a response time of 10 s.
With the advancement in nanotechnology, nanostructured based electrodes have gained considerable recognition in the regulation of analytical characteristics of bio-sensing devices. Nanostructured based electrodes show unique characteristics such as large surface-to-volume ratio, high surface reaction energy, high sensitivity, high catalytic efficiency, fast response time and strong adsorption ability for stable immobilization of biomolecules. They acts as a direct electrical connectors between the bulk electrode material and the redox center of enzyme and there by facilitating the electron transfer. Nanomaterials, such as zinc oxide nanostructures (ZNO), carbon nanotubes (CNT) and gold nanoparticles (AuNPs) are most intensively studied in fabricating lactate biosensors. Nesakumar et al. fabricated lactic acid biosensor by immobilizing lactate dehydrogenase enzyme with chitosan onto the ZnO nanorods [132]. This Au/NanoZnO/LDH electrode was shown to have detection limit of 4.73 nmol L−1 and sensitivity of 1.832 µA µmol−1 L with a response time of less than 1 s. The high iso electric point (~9.5) of ZnO nanorods resulted in easy enzyme immobilization and direct electron transfer between the active sites of the LDH and the electrode surface and showed a linear response in the range of 0.2–0.8 µmol L−1. The stability of this LDH biosensor was found to be 28 days. Thus it can be concluded that the analytical characteristic of a lactate biosensor can be optimized through the control of the electron transfer process.
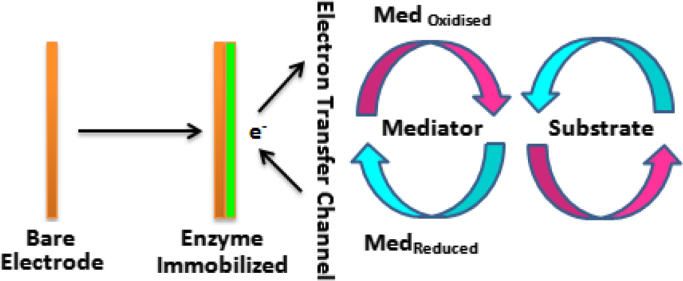
6. Mediators
On immobilizing the enzyme to the transducer, trapping of the cofactor occurs at the active site of the enzyme which is situated at some distance within enzyme, as a result of which it is incapable to respond contact with the surface of the working electrode. To cope up with the above problem a mediator is used. Mediator is basically an artificial electroactive species that works as an electron transferring agent by acting as a shuttle or a bridge between the cofactor and the electrode. The mediator is reduced to Mred by the cofactor, and then oxidized back into Mox when it comes into contact with the anode, which is polarized at the appropriate potential. There are various advantages of using mediators such as prevention of electrode fouling. Along with this, the very slow rates of electron transfer to electrode surfaces, as is the case for many redox enzymes, can be enhanced by the use of mediators [51]. The use of mediator helps in lowering down of the reduction potential during the sensing strategy but also suffers from the drawback that some of the species, which are oxidizable when the potential is applied, can interfere in the detection process due to the over potential which is necessary for the direct oxidation of NADH or H2O2 generated by the enzymatic reaction [52] (Fig. 5).
Fig. 5.
Schematic representation showing biosensor operating with mediator.
In order to choose the suitable mediator, it is important to consider the basic potential of the compounds involved in the course of reaction. The formal potential of the mediator should be close to that of the bio component being studied [53]. For ensuring the healthy electron transfer, mediator must be in oxidized as well as reduced forms. This means that mediator must remain immobilized near the electrode. However, the main problem with most mediators is their solubility in an aqueous solution and their toxicity, especially when target is an in vivo application.
Mediators used in lactate biosensors can be categorised into 3 types as conducting polymers, transition metal compounds and organic dyes based mediators. The conducting polymers based mediators include polymers such as poly(aniline)–poly(acrylate) [54], poly(aniline)–poly(vinyl sulfonate) [55], poly(pyrrole) [56], poly(pyrrole)–poly(vinyl sulfonate) and poly(vinylpyrrolidone) [57]. Transition metal compounds based mediators include complexes with Prussian blue [58], [59], cobalt phthalocyanine [60], [61], hydroxymethyl ferrocene [62], [63], osmium complexes with a variety of redox polymers [64]. The third category include organic dyes based mediators such as methylene green [65], Meldola blue [40], [66], tetrathiafulvalene [67] or quinine groups [68]. For example, polymerized flavin adenine dinucleotide (FAD) was also exploited as mediator in lactate biosensors as it is enzyme friendly. The biosensors were prepared using enzymes such as lactate dehydrogenase (LDH), lactate oxidase (LOD), or baker's yeast (BY) immobilized at the surface of the electrode. The sensors showed good linearity, stability and sensitivity [69].
7. Performance factors
Set up information of different Lactate based biosensors are compared in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and the respective performance characteristics are discussed. The classification in the table is done on the basis of different supports used for immobilizing the enzymes LDH or LOD in lactate biosensors. The basic parameters that control the performance of Lactate sensor in terms of sensitivity, operational stability, response time, linearity and detection limit are compiled in the α along with their commercial applications.
Table 1.
Various properties of membrane based L-lactate biosensors.
| Enzyme; origin; amount used | Immobilization matrix; Working electrode material | Type of transducer; immobilization method | Sensitivity | Detection limit | Linearity | Applications/inhibitors | Response time (s) | Storage stability (days) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| L-LDH (no EC given); rabbit muscle; 5/10/20U | CA membrane/Meldola blue, NADP; carbon, screen printing ink electrode | Amperometric; NR | 24.38 nA mmol−1 L | 0.5–8 mmol L−1 | 1–20 mmol L−1 | NR; ascorbate, pyruvate | NR | 3 | [73] |
| LOD (EC 1.1.3.2); Pediococcus sp.; 0.15U | Polypyrrole (PPYox) membrane /Pt electrode | Amperometric; entrapment | 7.2±0.1 nA Mmol−1 L | NR | NR | Tomato juice; acetaminophen, ascorbate, urate | NR | 60 | [75] |
| LOD (no EC given); Aerococcus viridans; 22UmL | Semi-permeable membrane using a SIRE technology; Pt electrode | Amperometric; NR | NR | 0.05 mmol L−1 | 0–0.1 mmol L−1 | Tomato paste, baby food; NR | 70 | NR | [76] |
| L-LDH (EC 1.1.1.27); rabbit muscle; 3.34UmL−1; pyruvate oxidase (EC 1.2.3.3); Aerococcus viridans; 1.40 U mL−1; salicylate hydroxylase (EC 1.14.13.1); Pseudomonas sp; 0.56 U mL−1 | Poly(carbamoyl) sulfonate (PCS) hydrogel on a Teflon membrane/Pt | Amperometric; NR | 3.05–276.35 mA mmol−1 L | 4.3 mmol L−1 | 0–400 μM | Healthy supplements, soda, sport drinks, yoghurt milk; electroactive substances | 2 | 11 | [78] |
| LOD(no EC given);Pedicoccus species ;20–40 units/mg | PolyDVB/EVB-coated composit membranes; Au coated polyester support | Amperometric; cross-linking with glutaraldehyde | NR | NR | 0–2 mM | Blood samples; NR | 1 | 21 | [72] |
| LDH; (EC 1.1.1.27); Porcine heart;257 U/mg | MBRS-SPCE/cellulose acetate membrane/screenprinted carbon electrode | FIA/amperometric; NR | NR | NR | 0.55–10 mM | Serum samples; NR | NR | 17 | [77] |
| LOD (no EC given); NR; NR | Amucin/albumin hydrogel matrix /Nafion membrane;Pt electrode | Amperometric;NR | 0.537±0.007 µA M−1 | 0.8 µM | 2 –1000 μM | Blood sample; NR | NR | 150 | [70] |
| LOD (no EC given); NR; NR | Mesoporous silica/screen-printed Prussian Blue (PB)-/hydrophilic porous membrane; NR | Electrochemical; NR | 150–1.1 mM | NR | NR | NR | NR | NR | [71] |
| LOD (no EC given);(Pediococcussp.); NR HRP(no EC given); (Type II from horseredish); NR | CNT/polysulfon membrane; screen printed electrode | Amperometric; NR | NR | 0.05 mg L−1 | 0.1–5 mg L−1 | Wine and beer samples; NR | NR | NR | [74] |
CA: Cellulose acetate.
Pt: Platinum.
SIRE: Sensors based on injection of the recognition element.
DVB: Divinylbenzene.
EVB: Ethylvinylbenzene.
MBRS-SPCE: Meldola's Blue-Reinecke salt- screen-printed carbon electrode.
CNT: Carbon nano tube.
NR: Not reported.
Table 2.
Comparison of analytical properties of non-conducting polymer matrices based L-lactate biosensors.
| Enzyme; origin; amount used | Immobilization matrix; working electrode material | Type of transducer; immobilization method | Sensitivity | Detection limit | Linearity | Applications/inhibitors | Response time (s) | Storage stability (days) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| LOD (no EC given); Pediococcus sp.; 5.5 U | Poly-vinyl alcohol (PVA) matrix on platinized graphite electrode | Amerometric; crosslinking | 1.71 mµA mmol−1 | 10 mµM | 2×10−5–4×10−3 M | Dairy products; NR | 10–45 | 90 | [82] |
| LOD (no EC given); Pediococcus sp.; 2.04 U | Sol–gel film derived from MTEOS/Os-polymer /GCE | Amperometric; NR | 1.02 mA mmol−1 L | 0.05 mmol L−1 | 0.1–9 mmol L−1 | NR; NR | 10 | 7 | [84] |
| LOD (EC 1.1.3.2); Pediococcus sp.; 17 U | Hydrogel based osmium complexes in redox polymer/GCE | Amperometric; NR | NR | NR | 0–1 mmol L−1 | NR; NR | NR | NR | [85] |
| LOD (no EC given); NR; 5 U | Poly(carbamoyl)sulfonate (PCS) hydrogel/screen-printed graphite/nafion/Pt electrode | Amerometric; NR | 0.682–0.321 nA mmol−1 L | 0.5 mmol L−1 | 0–0.5/0–1 mmol L−1 | Dairy products and human whole blood and serum; NR | 50 | 42 | [83] |
| LOD (no EC given); Pediococcus sp.; NR | Chitosan/poly-vinyl imidazole Os (PVI-Os)/CNT/gold electrode | Amerometric; NR | NR | 0.6 μM | 0–600 μM | Food analysis; NR | NR | NR | [81] |
| LOD (no EC given); NR; NR | Amucin/albumin hydrogel matrix/Nafion Polymer; Pt electrode | Amperometric; NR | 0.537±0.007 µA M−1 | 0.8 μM | 2–1000 μM | Blood sample; NR | NR | 150 | [70] |
| LOD(no EC given); NR; 0.5 U mL−1 | Laponite-chitosan hydrogel/GCE | Amperometric, NR | (0.326±0.003) A cm −2 M−1 | (3.8±0.2)×10−6 M | NR | Alcoholic beverages and dairy products; NR | 5 | NR | [86] |
| LOD (no EC given); Pediococcus sp.; NR HRP (no EC given); horseredish (Type II); NR | Chitosan/MWCNT/ferrocyanide/gold electrode | Amperomertic; NR | NR | 1.66 μM | 5–340 μM | Real food samples and beverages; NR | 15 | NR | [80] |
| LOD (no EC given); NR; NR | Nafion/cobalt phthalocyanine/poly-vinyl alcohol/screen-printed carbon electrode | Amperometric; NR | NR | NR | 18.3 μM–1.5 mM | NR; NR | 90 | 270 | [60] |
MTEOS: Methyltriethoxysilane.
GCE: Glassy Carbon Electrode.
PVI-Os: Poly Vinyl Imidazole-Osmium.
MWCNT: Multiwalled Carbon Nanotube.
Table 3.
Shows properties of different conducting polymer based L-lactate biosensors.
| Enzyme; origin; amount used | Immobilization matrix; working electrode material | Type of transducer; immobilization method | Sensitivity | Detection limit | Linearity | Applications/inhibitors | Response time (s) | Storage stability (days) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| D-LDH (EC 1.1.1.28); Leuconostoc mesenteroides ssp. cremoris; 23–187 U mg−1 | PEI/NAD+3/carbon paste electrode | Amperometric; NR | NR | 30 mmol L−1 | 0.05–5 mmol L−1 | NR; pyruvate, DL-α- hydroxybutyric acid | NR | 31 | [90] |
| L-LDH (EC 1.1.1.27); rabbit muscle; 16–80 U mg−1 Glutamic pyruvic transaminase (GPT) (EC 2.6.1.2); pig heart; 0–5 U mg−1 | PPD film/carbon paste electrode | Amperometric, NR | 0.56–1.1 mA Mmol−1 L | 0.03–0.6 mmol L−1 | 0.5–77 µmmol L−1/0.5–8.5 µmmol L−1 | Cider; ascorbate, urate, L-cysteine, glutathione, | 40–80 | 1 | [93] |
| LOD (no EC given); Pediococcus sp.; NR | Polyanion doped PPY film/Pt electrode | Amperometric, NR | 5 µA mmol−1 L cm−2 | 5 mmol L−1 | 0–2/0/16/0–30 mmol L−1 | NR; NR | 20–30 | 50 | [56] |
| L-LDH (EC 1.1.1.27); rabbit muscle; 1–4.5 U LOD (EC 1.1.3.2); Pediococcus sp.; 0.1–0.8 U | PANI/ITO coated glass plate | Amperometric, adsorption | 5.5–38.5 µA mmol−1 L | 5×10−5 M | 0.1-1to 1–4 mmol L−1 | NR; NR | NR | 21 | [43] |
| L-LDH (EC 1.1.1.27); rabbit muscle; 0.1 mg | PPY–PVS/NR | Amperometric, crosslinking with glutaraldehyde | NR | NR | 0.5–6 mmol L−1 | NR; ascorbate, citrate, glucose, glutamate | 40 | 14 | [91] |
| L-LDH (EC 1.1.1.27); rabbit muscle; 4.5 U LOD (EC 1.1.3.2); Pediococcus sp.; 0.1 U | PANI film/ ITO coated glass plate | Amperometric, adsorption | NR | 0.05–1 mmol L−1 | 0.1–1 to 1–4 mmol L−1 | NR | NR | 21 | [92] |
| L-LDH (EC 1.1.1.27); Bacillus Stearothermophilus.; NR | PANI–PAA/GCE | Amperometric; NR | NR | NR | 0.4–0.55 mol L−1 | NR; NR | NR | NR | [55] |
| LDH (no EC given); NR; NR | MWCNT/ P3 MT polymer/GCE | Amperometric, NR | NR | 5.6×10−7 M | 1.0×10−6–5.0×10−4 M | NR; NR | NR | NR | [35] |
| L-LDH (no EC given); rabbit muscle;811 U/mg | pTTCA/MWCNT composite film/Au electrode | Amperometric, covalent | 0.0106 | 1 | 5–90 | Human serum samples, milk; NR | NR | 30 | [28] |
NAD: nicotinamide adenine dinucleotide.
PPD: Poly-(o-phenylenediamine).
PANI/ITO: Polyaniline.
PPY–PVS: Polypyrrole–Polyvinylsulphonate.
P3 MT: Poly (3-methylthiophene).
PAA: Polyacrylate.
pTTCA: Poly-5,2′-5′,2″-terthiophene-3′-carboxylic acid.
Table 4.
Shows properties of different sol–gel based L-lactate biosensors.
| Enzyme; origin; amount used | Immobilization matrix; working electrode material | Type of transducer; immobilization method | Sensitivity | Detection limit | Linearity | Applications/inhibitors | Response time (s) | Storage stability (days) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| LOD (no EC given); Pediococcus sp.; 2.04 U | Sol–gel film derived from MTEOS/Os-polymer/GCE | Amperometric; NR | 1.02 mA mmol−1 L | 0.05 mmol L−1 | 0.1–9 mmol L−1 | NR; NR | 10 | 7 | [84] |
| LOD (no EC given); NR; 26–40 mg mL−1 | Sol–gel based Graphite/platinized carbon | Amperometric; NR | 870–2800 µA mol−1 L | 4 nmol L−1 | 0–0.3 mmol L−1 | Wine; NR | NR | 14 | [103] |
| LDH (no EC given); NR; NR | Carbodiimide coupling/sol–gel based | Electrochemical; covalent | 1.47 µA/mM | 1.5 μM | NR | NR; NR | NR | 7 | [102] |
| LDH (no EC given); NR; NR | (APTMS)/porous sol–gel/Pt electrode | Amperometric; NR | NR | NR | 5×10−5–5×10−3 M | NR; NR | NR | 30 | [99] |
| L-LDH (EC 1.4.1.3); rabbit muscle or chicken heart; 200 U mL−1 | Graphite epoxy/Graphite screen printing ink (sol–gel) | Amperometric; NR | 80 mA mol−1 L | 0.87 mmol L−1 | 1–1.2 mmol L−1 | NR; NR | 30s | 7 | [98] |
| LOD (no EC given); NR; NR | Silica sol–gel film/MWCNT/GCE | Amperometric; NR | 6.031 μA mM−1 | 0.3×10−3 mM | 0.2–2.0 mM | Blood samples; NR | NR | NR | [96] |
| LDH (no EC given); NR; NR | Sol–gel 3-D silicate network derived from 3-(mercaptopropyl)trimethoxysilane (MPTS)/AuNPs | Amperometric; NR | 0.446 nA/nM | 100 nM | 0–0.8 mM | NR; NR | NR | NR | [37] |
| LOD (no EC given); NR; NR | Sol–gel film/MWCNTs/Pt nano/GCE | Amperometric; adsorption | 6.36 μA mM−1 | NR | 0.2–2.0 mM | Blood samples; NR | NR | 28 | [97] |
| LOD; NR; NR | Sol–gel based MWCNTs/Pt-nano electrode | Amperometric; covalent | 6.36 μA/mmol/L | NR | 0.2–2.0 mmol/L | NR; NR | 5 | 28 | [39] |
| LOD; (no EC given); NR; NR | Sol–gel based Prussian Blue modified electrode | Amperometric; NR | 0.18 A M−1 cm−2 | NR | NR | Noninvasive diagnostics and food quality control; NR | NR | NR | [100] |
| LOD (no EC given); NR; NR | Sol–gel derived from MPTS/AuNPs | Amperometric; NR | 3.4 µA mM−1 | 4.0 μM | 50 μM–0.25 mM | NR; NR | NR | NR | [101] |
| LOD (no EC given); NR; NR | Silica sol gel coated with Niobium oxide/carbon paste electrode | Amperometric; adsorption | 6.5×10−7 mol L−1. | NR | 0.1–5.0 mmol L−1 | Blood samples; NR | NR | 45 | [40] |
(APTMS): 3-(aminopropyl)trimethoxysilane.
AuNPs: Gold nanoparticles.
MPTS: 3-(mercaptopropyl)-trimethoxysilane.
Table 5.
Shows properties of different hydro-gel based L-lactate biosensors.
| Enzyme; origin; amount used | Immobilization matrix; Working electrode material | Type of transducer; immobilization method | Sensitivity | Detection limit | Linearity | Applications/inhibitors | Response time (s) | Storage stability (days) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| LOD (no EC given); Pediococcus sp.; NR | Hydrogel derived with PVP/Au electrode | Amperometric; NR | NR | NR | 0.25–1.5 mmol L−1 | NR; ascorbate | NR | NR | [57] |
| LOD (EC 1.1.3.2); Pediococcus sp.; 17 U | Hydrogel based osmium complexes in redox polymer/GCE | Amperometric; NR | NR | NR | 0–1 mmol L−1 | NR; NR | NR | NR | [85] |
| L-LDH (EC 1.1.1.27); rabbit muscle; 3.34 U mL−1 pyruvate oxidase (EC 1.2.3.3) Aerococcus viridans; 1.40 U mL−1 salicylate hydroxylase (EC 1.14.13.1); Pseudomonas sp.; 0.56 U mL−1; | Poly(carbamoyl) sulfonate (PCS) hydrogel on a Teflon membrane/Pt | Amperometric; NR | 3.05–276.35 mA mmol−1 L | 4.3 mmol L−1 | 0 and 400 μM | Healthy supplements, soda, sport drinks, yoghurt milk; electroactive substances | 2 | 11 | [78] |
| LOD (no EC given); NR; 0.55 U mL−1 | Laponite hydro gel/GCE | Amperometric; NR | 0.33±0.01 A cm−2 M−1 | NR | NR | Wine and dairy products; NR | 10 | NR | [104] |
| LOD (no EC given); NR; 0.5 U mL−1 | Laponite-chitosan hydrogel/GCE | Amperometric; NR | (0.326±0.003) A cm−2 M−1 | 3.8±0.2×10−6 M | NR | Alcoholic beverages and dairy products; NR | 5 | NR | [86] |
PVP: polyvinyl pyridine.
Table 6.
Shows properties of different Screen printed electrode based L-lactate biosensors.
| Enzyme; origin; amount used | Immobilization matrix; working electrode material | Type of transducer; immobilization method | Sensitivity | Detection limit | Linearity | Applications/ inhibitors | Response time (s) | Storage stability (days) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| L-LDH (no EC given); rabbit muscle; 5/10/20 U | CA membrane/ Meldola blue, NAD; carbón screen printing ink electrode | Amperometric; NR | 24.38 nA mmol−1 L | 0.5–8 mmol L−1; | 1–20 mmol L−1 | NR; ascorbate, pyruvate | NR | 3 | [73] |
| LOD (no EC given); NR; 5 U | Poly(carbamoyl)sulfonate (PCS) hydrogel / screen-printed graphite /nafion/Pt electrode | Amerometric; NR | 0.682–0.321 nA mmol−1 L | 0.5 mmol L−1 | 0–0.5/0–1 mmol L−1 | Dairy prod-ucts and human whole blood and serum; NR | 50 | 42 | [83] |
| LOD (EC 1.1.3.4); NR; 2.4–3.5 U mg−1 peroxidase (EC 1.11.1.7) horseradish.; 5.4 U mg−1 | Carbón screen printing ink electrode | Amerometric; NR | 0.27–1.30 nA mol−1 L | 10 mmol L−1 | 5–40 mmol L−1 to 20–250 mmol L−1 | Milk, white cheese and yoghurt; NR | NR | 14 | [107] |
| LOD (no EC given); NR; 5 U | Carbon screen printing ink electrode | Amerometric; NR | NR | 1 mmol L−1 | 1–50 mmol L−1 | Lactic fermenting beverage; NR | 50 | 300 | [108] |
| L-LDH (EC 1.4.1.3); rabbit muscle or chicken heart; 200 U mL−1 | Graphite epoxy/Graphite screen printing ink (sol–gel) | Amperometric; NR | 80 mA mol−1 L | 0.87 mmol L−1 | 1–1.2 mmol L−1 | NR; NR | 30 | 7 | [98] |
| L-LDH (EC.1.1.1.27); NR; NR | SWCNTSs/Variamine blue/screen-printed electrode | Amperometric; entrapment | NR | 1 µM | 5–450 μM | Probiotic curd | NR | NR | [106] |
| LDH; (EC 1.1.1.27); Porcine heart; 257 U/mg | MBRS-SPCE/cellulose acetate membrane/screen printed carbon electrode | Amperometric; NR | NR | NR | 0.55–10 mM | Serum samples; NR | NR | 17 | [77] |
| LOD (no EC given); NR; NR | Mesoporous silica/screen-printed Prussian Blue (PB)-/hydrophilic porous membrane; NR | Electrochemical; NR | 150–1.1 mM | NR | NR | NR | NR | NR | [71] |
| LOD (no EC given); NR; NR | Nafion/cobalt phthalocyanine/screen-printed carbon electrode | Amperometric, NR | NR | NR | 18.3 μM–1.5 mM | NR; NR | 90 | 270 | [60] |
| LOD (no EC given); Pediococcus sp.; NR HRP (no EC given); horseradish. (Type II); NR | CNT/polysulfon membrane; screen printed electrode | Amperometric; NR | NR | 0.05 mg L−1 | 0.1–5 mg L−1 | Wine and beer samples; NR | NR | NR | [74] |
Table 7.
Shows properties of different NPs based L-lactate biosensors.
| Enzyme; origin; amount used | Immobilization matrix; Working electrode material | Type of transducer; Immobili-zation method | Sensiti-vity | Detection limit | Linearity | Applications/ Inhibitors | Respo-nse time (sec) | Storage stability (days) | Refere-nce |
|---|---|---|---|---|---|---|---|---|---|
| LDH (EC 1.1.1.27);NR; 876 U mg−1 | Meldola blue/MWCNT electrode | Amperometric; crosslinking with glutaraldehyde | 3.46 µA cm−2 mmol L−1 | 7.5×10−6 mol L−1 | 0.10–10 mmol L−1. | Blood samples; NR | NR | NR | [66] |
| LDH (no EC given); NR; NR | AuNPs/MPTS | Amperometric; NR | 0.446 nA/nM | 100 nM | 0–0.8 mM | NR; NR | NR | NR | [37] |
| LOD (no EC given); NR; NR | MWCNT/GCE | Amperometric; NR | 6.031 μA mM−1 | 0.3×10−3 mM | 0.2–2.0 mM | Blood samples; NR | NR | NR | [96] |
| LDH(no EC given); NR; NR | PAA/Si4N3 (ND) surface | Potentiometric; covalent | 49.7 mV | 2×10−7 M | 10−5 M. | Blood samples; NR | 60 | NR | [113] |
| LDH (EC 1.1.1.27); rabbit muscle; 811 U mg−1 | MWCNT/CHIT composite/GCE | Amperometric; entrapment | 0.0083 A M−1 cm−2 | 0.76 μM | 5–120 μM | NR; NR | 3 | 7 | [110] |
| LOD(no EC given); Pediococcus sp.; NR | Chitosan/poly-vinyl imidazole Os (PVI-Os)/ CNT/ gold electrode | Amerometric; NR | NR | 0.6 μM | 0–600 μM | Food analysis; NR | NR | NR | [81] |
| LOD (no EC given); NR; NR | MWCNTs/Ptnano/GCE | Amperometric, adsorption | 6.36 μA mM−1 | NR | 0.2–2.0 mM | Blood samples; NR | NR | 28 | [97] |
| LOD (no EC given); NR; NR | H2Ti3O7 nanotube network | Electrochemical | 0.24 µA cm−2 mM−1 | NR | 0.5–14 mM | NR; NR | 5 | NR | [114] |
| LDH (no EC given); NR; NR | MWCNT/ P3 MT polymer/GCE | Amperometric; NR | NR | 5.6×10−7 M | 1.0×10−6–5.0×10−4 M | NR; NR | NR | NR | [35] |
| LOD(E.C.1.1.3.2);Aerococcus species; 42 U mg−1 | Pt-black Nanoparticles/ gold electrode | Amperometric;NR | 1.43 µA mM−1 | NR | 1–20 mM | Serum samples; NR | 50 | 60 | [112] |
| L-LDH (no EC given); rabbit muscle; 811 U/mg | pTTCA/MWNT composite film/Au electrode | Amperometric, Covalent | 0.0106 | 1 | 5–90 | Human serum samples, milk; NR | NR | 30 | [28] |
| L-LDH (EC.1.1.1.27); NR; NR | SWCNTSs/Variamine blue | Amperometric, entrapment | NR | 1 µM | 5–450 μM | Probiotic curd; NR | NR | NR | [106] |
| LOD (no EC given); NR; NR | ZNO/mwCNT | NR; NR | NR | NR | NR | NR; NR | NR | NR | [42] |
| LOD (no EC given); NR; NR | Sol–gel based MWCNTs/Pt-nano electrode | Amperometric; covalent | 6.36 μA/mmol/L | NR | 0.2–2.0 mmol/L | NR; NR | 5 | 28 | [39] |
| LOD (no EC given); NR; NR | nitrogen-doped CNT/GCE | Amperometric; NR | 0.040±0.002 A M−1 cm–2 | 4.1±1.6 μM | 14–325 μM | NR; NR | NR | 90 | [34] |
| LOD (no EC given); NR; NR | NanoZnO-MWCNTs/GCE | Electrochemiluminescence; NR | NR | 4 nmol L−1 | 0.01–10 μmol L−1 and 10–200 μmol L−1 | Blood samples; NR | NR | NR | [118] |
| LOD (no EC given); NR; NR | Niobium oxide/carbon paste electrode | Amperometric, adsorption | 6.5×10−7 mol L−1. | NR | 0.1–5.0 mmol L−1 | Blood samples; NR | NR | 45 | [40] |
| LOD (no EC given); Pediococcus sp.; NR HRP (no EC given); horseradish (Type II); NR | CNT/polysulfon screen printed electrode | Amperometric; NR | NR | 0.05 mg L−1 | 0.1–5 mg L−1 | Wine and beer samples; NR | NR | NR | [74] |
| L-LOD (E.C. 1.1.3.2); Pediococcus sp.; 40 U mg−1 | ZnO nanorods/Au coated glass substrates | Potentiometric; cross-linking with glutraldehyde | 41.33±1.58 mV/decade | 1×10−4–1×100 mM | 0.01–0.4 and 1.0–5.0 | Drugs, food and other biological samples; NR | 10 | 21 | [117] |
| LOD (EC 1.1.3.2); Pediococus sp.; 38 units mg−1 | MoO3/Au coated SiO2 electrode | Electrochemical; NR | 0.87 µA/mM | 0.15 mM | 0.5–8mM | NR; NR | 10 | NR | [41] |
| LOD (no EC given); Pediococus sp.; NR HRP (Type II from horseredish) | Chitosan/MWCNT/ferrocyanide/ gold electrode | Amperomertic; NR | NR | 1.66 μM | 5–340 μM | Real food samples and beverages; NR | 15 | NR | [80] |
| LOD (no EC given);NR | ZnO nanotetrapods | Electrochemical; electrostatic adsorption | 28.0 μA cm−2 mM−1 | 1.2 Μm | 3.6 μM–0.6 mM | NR; NR | NR | NR | [120] |
| LDH; (EC 1.1.1.27) rabbit’s muscle; 140 U mg−1 | Fe3O4/MWCNT/GCE | Electrochemical; covalent | 7.67 µA mM−1 | 5 µM | 50-500 µM | Human serum sample; NR | NR | 14 | [109] |
| LDH (no EC given); rabbit's muscle; NR | Nano-CeO2/GCE electrode | Amperometric; NR | NR | 50 μM | 200–2000 µM | Blood samples; NR | 4 | NR | [116] |
| LOD(no EC given); NR; NR | TiO2-NPs/PRG/GCE | NR; NR | 6.0 μA mM−1 | 0.6 Μm | 2.0 μM–0.40 mM | NR; NR | NR | NR | [115] |
| LOD (no EC given); Pediococcus sp.; NR | ZNO nanowires | Amperometric; NR | 15.6 µA cm−2 mM−1 | 12 μM | 12–1200 μM | NR; NR | NR | NR | [119] |
| L-LDH (no EC given); rabbit muscle; 130 U/mg | Au/Nano ZnO electrode | Electrochemical; covalent | 1.832 µA µmol−1 L | 4.73 nmol L−1 | 0.2–0.8 µmol L−1 | Food products; urea | 1 | 23 | [132] |
CHIT: Chitosan.
PAA: Polyacrylic acid.
Si4N3: Silicon nitride.
ND: Nanostructured.
ZnO: Zinc oxide.
MoO3: Molybdenum trioxide.
The detection limit or limit of detection (LOD) is an important performance characteristic in optimum setup design of lactate biosensors and is defined as the lowest concentration of the substrate being measured, which gives a minimum detectable difference signal (reduction in activity) that can either be determined by statistical approach which is equal to 2 or 3 standard deviations (S.D.) of the mean response of the blank samples (zero concentration of the inhibitor) or based on signal-to-noise approach [133]. Lower detection limit is an important characteristic in validation of biosensor performance. By miniaturiazing the size of biosensor and improving the binding affinity of biological molecules this characteristic can be gradually improved. Ibupoto et al. reported a lactic acid biosensor based on lactate oxidase enzyme immobilized by cross linking with gluteraldehyde onto the ZnO nanorods [117]. This Au/NanoZnO/LOD bio-electrode was shown to have a detection limit (LOD) of 1×10−4 mmol L−1 and sensitivity of 41.33±1.58 mV decade−1 with linearity over 0.1–1 mmol L−1. This biosensor was found to be stable for more than three weeks with a response time of 10 s. This Au/Nano ZnO electrode showed low detection limit and higher sensitivity towards lactate due to the ZnO nanostrucutred based bioelectrode.
Sensitivity of the electrode is another important characteristic in validation of lactate sensor performance. The sensitivity corresponds to the slope of the calibration curve and working electrode area. Various factors govern the role of increasing the sensitivity of biosensor. As sensitivity of the biosensor is function of the physical electrode design and the biological molecule immobilized. The electrode surface area in general can be modified in order to increase the sensitivity of enzymatic biosensors. This involves increase in the electrode surface area which leads to loading of higher amounts of the enzyme in the recognition layer. Nanomaterials play important role in increasing the electrode surface area and include nanoparticles like carbon nanotubes (CNTs) [34], [35], gold nanoparticles (AuNPs) [36], [37], platinum nanoparticles (PtNPs) [38], [39], metal oxides based nanoparticles i.e., niobium oxide [40] or molybdenum oxide [41] and semiconducting material based nanoparticles such as zinc oxide [42]. Nesakumar et al. synthesized ZnO nanorods for lactate detection in food products [132]. The ZnO nanorods provided an increased surface area for immobilizing the lactate dehydrogenase enzyme. This Au/NanoZnO/LDH electrode was shown to have detection limit of 4.73 nmol L−1and sensitivity of 1.832 µA µmol−1 L with a response time of less than 1 s. The high iso electric point (~9.5) of ZnO nanorods resulted in easy enzyme immobilization and direct electron transfer between the active sites of the LDH and the electrode surface and showed a linear response in the range of 0.2–0.8 µmol L−1. The stability of this LDH biosensor was found to be 28 days. Another way to increase the sensitivity of enzymatic biosensors is by the use of mediators. Mediators play important role which aid in electron transfer from the biological molecule to the working electrode surface to improve biosensor sensitivity. Pereira et al. fabricated a multi-wall carbon-nanotube based lactate biosensor by incorporating meldola blue as a mediator to shuttle electrons between NADH and working electrode under the catalysis of LDH [66]. With use of the mediator, the biosensor operating potential was reduced to 0.0 V versus SCE. The sensor showed excellent sensitivity of 3.46 µA cm−2 mmol L−1 with wide linear response range of 0.10–10 mmol L−1. This demonstrated that this biosensor could be used for direct measurements of lactate in blood samples. Another approach to amplify the sensitivity of enzymatic biosensors is by the use of bienzymatic system which involves the coimmobilization of lactate oxidase and lactate dehydrogenase enzymes [92]. Chaubey et al. coimmoblized lactate oxidase (LOD) and lactate dehydrogenase (LDH) onto electrochemically prepared polyaniline (PANI) films. This PANI/LOD/LDH bienzyme electrode system showed improved signal amplification by substrate recycling, making it possible to detect L-lactate at lower concentrations (0.1–1 mM). The stability of PANI/LOD/LDH bienzyme electrode was found to be 21 days. However, in most cases all of these approaches do have their certain limitations.
Enhancement of operational stability of the biosensor without the loss of sensitivity is one of the problems being faced for lactate detection. Operational stability is generally dependant on the type of biosensor used. Disposable sensors show stability for seconds to several minutes whereas the reusable sensors may show stability for several days to several months. The biological element used for the lactate sensor generally affects the long term stability. The biological activity of enzymes is affected by its environmental conditions [134], methods used for its immobilization on the transducer. The types of matrix (support) where the enzyme is immobilized must be suitable for the enzyme to retain its activity for proper operational stability of sensor. A variety of enzyme immobilization methods may be used such as physical adsorption [43], entrapment which involves entrapping the enzyme in polymer matrices [85], [43] or sol–gel matrices [44], [45], membrane confinement [70], covalent binding [46], cross link formation [29], monolayer formation by self-assembly [48]. Recently nanostructured based electrodes show unique characteristics of strong adsorption ability for stable immobilization of biomolecules which in turn aids in improved storage stability. Teymourianc et al. obtained very good storage stability by loading LDH on the Fe3O4/MWCNTs nanocomposite film by coprecipitation procedure [109]. Fe3O4 nanoparticles showed redox properties similar to those of mediators used for fast electron transfer between NADH and electrode at a low potential of 0.0 mV vs. Ag/AgCl in neutral pH media. The sensor showed a linear response up to 300 μM with a detection limit of 0.3 μM. The initial activity of proposed electrode was found to be reserved (96%) for more than 2 weeks when kept in air at room temperature. The high intrinsic stability of this modified electrode was due to the chemical and mechanical stability of Fe3O4/MWCNTs.
To obtain wide linear range is an important characteristic that determines sensor performance. It is defined as the range of analyte concentration in which the sensor can operate and the sensor response changes linearly with this concentration. Jena et al. developed a novel amperometric biosensor for L-lactate measurement [37]. Entrapment of LDH and Gold nanoparticles with the sol–gel 3-D silicate network derived from 3-(mercaptopropyl)trimethoxysilane (MPTS) resulted in wide linear range of 0–0.8 mM. The sensor showed excellent sensitivity of 0.446 nA/nM with a detection limit of 100 nM. This biosensor linearly responded to L-lactate in the range of 0–0.8 mM.
Quantification limit (LOQ), response time, repeatability, reproducibility and accuracy are other factors responsible for characterization of biosensor performance. The quantification limit (LOQ) is a reliable parameter of quantitative assay to quantitatively determine the lowest amount of substrate in a sample with suitable precision and accuracy [133]. Nesakumar et al. reported a lactic acid biosensor by immobilizing lactate dehydrogenase enzyme with chitosan onto the ZnO nanorods [132]. This Au/NanoZnO/LDH electrode was shown to have sensitivity of 1.832 µA µmol−1 L, linear response in the range of 0.2–0.8 µmol L−1 with a response time of less than 1 s. The detection limit (LOD) was found to be 4.73 nmol L−1and quantification limit (LOQ=3.33×LOD) of 15.75 nmol L−1. The stability of this LDH biosensor was found to be 28 days.
Short response time and fast repeatability are generally the requirement for an optimal working biosensor. Response time is basically the time taken by transducer to detect the measurable changes in the biological response to a detectable input signal. Response time of the biosensor depends on various factors such as the rate of substrate diffusion through the solution to the membrane surface for catalysis at the active site of the enzyme, the rate of product diffusion to the working electrode surface and mediators used which results in fast electron transfer [135]. Pianoa et al. fabricated a sensor based on screen printed electrode for lactate measurement in serum [77]. The screen-printed carbon electrode was modified with meldola blue-reinecke salt. Meldola's Blue-Reinecke Salt acted as an artificial electron mediator which aided the faster electron transfer rate between the SPCE electrode surface and the LDH enzyme. The biosensor showed a linear response over the range 0.55–10 mM and obtained a good reproducible signal at an applied potential of +0.05 V. The sensor showed a response time of 10 s. Rahaman et al. also obtained good reproducibility for pTTCA/MWNT/LDH/NAD+ sensor with a relative standard deviation (RSD) of 4.3% [28]. The sensor-to-sensor and run-to-run reproducibility were found to be 1.7% and 1.1%, respectively by conducting a series of five successive measurements of lactate for about 5 µM in 0.1 mM PB.
Repeatability refers to number of times the sensor can be operated and is ready for the next reading. On the other hand reproducibility of a sensor refers to the predictable results that can be obtained by the repeated usages. Kriz et al. developed amperometric lactate sensor based on entrapment of lactate oxidase with a semi-permeable membrane using a SIRE technology on Pt electrode. The concentration of dissolved L-lactate was used to measure freshness in the food products [76]. They studied repeatability for tomato paste and baby food and the measurements were found to be 2.5% (RSD, n=15) and 4.0% (RSD, n=15) and for reproducibility it was 13.0% (RSD, n=45) and 3.0% (RSD, n=45), respectively.
In order to determine the accuracy of a biosensor the measured values obtained by the developed sensor are compared with another reference method to determine the correlation between the two. Pereira et al. fabricated lactate biosensor based on LDH/MWCNT-MB electrode working at a potential of 0.0 V [66]. The sensor showed excellent sensitivity of 3.46 µA cm−2 mmol L−1 with wide linear response range of 0.10–10 mmol L−1. The results obtained were in good correlation with the reference spectrophotometric studies. It was found that the averages obtained by the two methods were statistically equal in the confidence level of 95% by applying t-Student test to verify the averages obtained by two methods and hence the proposed biosensor was found to be accurate.
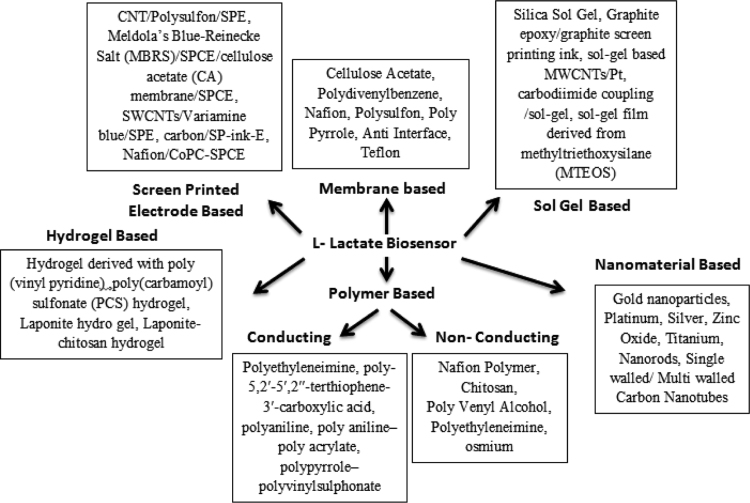
8. Classification of L-lactate biosensors
The classification of L-lactate biosensors on the basis of different supports used for immobilizing the enzymes LDH or LOD.
L-Lactate biosensors having immobilized LDH works along with coenzyme (NAD or NADP). The enzyme will convert L-lactate into pyruvate and NAD to NADH. On the electrode surface, NADH will be oxidized under the influence of the applied potential and the oxidation current will be directly proportional to the L-lactate concentration in the solution.
L-Lactate biosensors on the other hand having immobilized LOD catalyzes the reaction involved in oxidizing substrate L-lactate to the pyruvate. This reaction occurs in the presence of dissolved oxygen. Hydrogen peroxide is generated as a result of above reaction; which is electrochemically active and can be reduced or oxidized. The current generated as a result of oxidation or reduction is proportional to the concentration of L-lactate present in the sample (Fig. 6).
Fig. 6.
Different supports available for fabrication of working electrode.
8.1. Membrane used in fabrication of L- lactate biosensors
Membranes with different porosities can be used to attach the enzyme in a close proximity of the electrode surface in lactate biosensors. This basically involves the immobilization of an enzyme on a biocompatible natural or artificial membrane and attaching the membrane to the transducer for improving the sensitivity and selectivity of the fabricated biosensor. The membrane based biosensors provides an inexpensive, portable and rapid disposable way to determine lactate concentration. It also helps in maintaining the enzymatic stability and provides an increased shelf life of the biosensor. In addition to this it also solves various limitations such as the direct enzymatic immobilization to the transducer, prevents loss of enzyme, allows mass production and hence reduction in time of the enzymatic response which in turn helps to improve the reproducibility of the biosensor signals.
8.1.1. Supports used for immobilization
Amucin/albumin hydrogel matrix/Nafion membrane [70], mesoporous silica/screen-printed Prussian Blue (PB)/hydrophilic porous membrane [71], polydivinylbenzene/ethylvinylbenzene (DVB/EVB) coated membranes [72], cellulose acetate (CA) membrane [73], CNT/polysulfon membrane [74], polypyrrole (PPYox) anti-interference membrane [75], Semi-Permeable Membrane Using a SIRE Technology/Pt electrode [76], Meldola's Blue-Reinecke Salt (MBRS-SPCE)/cellulose acetate membrane/screen printed carbon electrode [77], poly(carbamoyl) sulfonate (PCS) hydrogel on a Teflon membrane/Pt [78].
8.1.2. Merits
Membrane shows high degree of flexibility and mechanical durability over a wider pH range, highly selective preventing the passage of the interfering substances, and finally help in the response amplification.
8.1.3. Demerits
Suffer from the problem of membrane fouling. The pores of semipermeable membrane could be blocked which may lead to hindrance in the passage of solute. Table 1 shows various properties of membrane based L-lactate biosensors.
8.2. Polymeric matrices based LDH biosensors
Polymers provides an application in the field of electronic measuring devices, especially in biosensors, due to their ability to form perm selective coating on the surface of the working electrode which reduces or prevents the interfering compounds to penetrate the sensing layer on the surface of the electrode. Theses polymeric matrices are flexible, biocompatible and are less expensive [79].
8.2.1. Non-conducting polymer matrices based L-lactate biosensors
Non-conducting polymers are also widely used as supporting films on the electrode surface for LDH/LOD immobilization. These non-conducting polymer materials basically possess different functional groups, such as amines, carboxylic acids, thiols and others before or after modification of the surface with the help of different oxidizing agents. They can be easily being prepared in laboratory.
8.2.1.1. Different supports which are used for immobilization
Amucin/albumin hydrogel matrix/Nafion Polymer Pt electrode [70], Chitosan/MWCNT/ferrocyanide/gold electrode [80], Chitosan/poly-vinyl imidazole Os (PVI-Os)/CNT/gold electrode [81], poly-vinyl alcohol (PVA) matrix on platinized graphite electrodes [82], polyethyleneimine (PEI) and poly(carbamoyl)sulfonate (PCS) hydrogel/screen-printed graphite/nafion/Pt electrode [83], sol–gel film derived from MTEOS/Os-polymer /GCE [84], hydrogel based osmium complexe [Os(byp)2ClPyCH2NH Poly(allylamine)] redox polymer/GCE [85], Laponite-chitosan hydrogel/GCE [86], Nafion/cobalt phthalocyanine poly-vinyl alcohol/screen-printed carbon electrode [60].
8.2.1.2. Merits
Can be prepared easily; provide a favorable microenvironment for the enzyme molecules to enhance storage stability, variety of supports can be synthesized due to the possibility chemical modifications.
8.2.1.3. Demerits
Support may acts as barrier between electron and transducer surface which then influence the sensitivity of biosensor. Table 2 provides a comparison of analytical properties of all non-conducting polymer based L-lactate biosensors.
8.2.2. Conducting polymer matrices based L-lactate biosensors
Conducting polymers are the organically conjugated polymers are highly conductivity as a result of presence of extended π-orbital system [87]. They are mostly synthesized by reactions involving dehydrogenation which involves oxidative coupling of monocyclic precursors. There various properties such as electrical conductivity, mechanical properties can be modified by chemical modeling. These can be electrochemically synthesized on very small size of electrode having substrate of any shape and size with the help of the polymer film and also used for electro polymerization for enzyme entrapment [88], [89].
8.2.2.1. Different supports which are used for immobilization
Polyethyleneimine (PEI)/Carbon paste electrode [90], poly (3-methylthiophene) (P3 MT)/MWCNT/glassy carbon electrode (GCE) [35], poly-5,2′-5′,2′′-terthiophene-3′-carboxylic acid (pTTCA)/MWCNT composite film/Au electrode [28], polyaniline (PANI) films/indium–tin-oxide (ITO) coated glass plates as working electrode [43], poly-aniline–poly acrylate (PANI–PAA) film/GCE [55], polypyrrole–polyvinylsulphonate (PPY–PVS) [91], polyanion doped poly(pyrrole) film/Pt electrode [56], polyaniline (PANI)/ITO [92], poly(o-phenylenediamine) (PPD)/carbon electrode [93].
8.2.2.2. Merits
Biocompatible in neutral aqueous environment, enhances sensitivity and selectivity as a result of good electrical conductivity and their electron transfer channel.
8.2.2.3. Demerits
High cost, difficulties regarding fabrication, doping results in lack of mechanical stability and shorter life span, etc. Table 3 shows properties of different conducting polymer based L-lactate biosensors.
8.3. Sol–gel based lactate biosensor
Sol–gel method comprises of the entrapment and immobilization of enzyme in a porous, optically transparent gel matrix. It is one of the important supports being used which are known to attract the attention for the fabrication of modified electrodes. It provides a versatile way for immobilization due to the presence of organic or inorganic orthosilicates formed by wet chemical technique. It provides M–O or M–OH–M bridges which helps in enzyme immobilization [94]. In the above strategy, the sol (or solution) converts to a gel-like network around the enzyme and consists of both solid phase and liquid phase [95].
8.3.1. Sol gel supports used for immobilization of enzyme
Sol–gel 3-D silicate network derived from 3-(mercaptopropyl)trimethoxysilane (MPTS)/GNPs [37], silica sol gel film/MWCNT/ GCE [96], sol–gel film/MWCNTs/Ptnano/GCE [97], silica sol gel coated with Niobium oxide/carbon paste electrode [40], Graphite epoxy/graphite screen printing ink(sol–gel) [98], 3-(aminopropyl)trimethoxysilane, 2-(3,4-epoxycyclohexyl)ethyl-trimethoxysilane (APTMS)/porous sol–gel/platinum-based electrode [99], sol–gel based MWCNTs/Pt-nano electrode [39], sol–gel based Prussian Blue modified electrode [100], sol–gel 3D polymeric network derived from (3-mercaptopropyl)-trimethoxysilane (MPTS)/gold nanoparticles (AuNPs) [101], carbodiimide coupling/sol–gel based [102], sol–gel based Graphite/Platinised carbon [103], sol–gel film derived from methyltriethoxysilane (MTEOS)/osmium redox polymer (Os-polymer) /GCE [84].
8.3.2. Merits
Ease of preparation, chemically inert, tunable porosity, show photo-chemical and thermal stability; prevent leakage of the enzyme in the medium.
8.3.3. Demerits
Non-conductive, involvement of diffusional limitation steps, less sensitive, the interaction between the catalyst–matrix and kinetics involved is not clear. Table 4 shows properties of different sol–gel based L-lactate biosensors.
8.4. Hydrogel based lactate biosensor
Hydrogel basically can be described as three dimensional polymeric network in which all polymer chains are interconnected and are present in water as a dispersion medium. The polymeric chains are hydrophilic and highly adsorbent in nature. Hydrogels can be categorized as natural hydrogel and synthetic hydrogel. The synthetic one are more in use due to the applications like higher water absorption capacity and long working life.
8.4.1. Hydro gel supports used for immobilization of enzyme
Hydrogel derived with poly(vinyl pyridine) complexed with [Os(4,4′- dimethoxy-2,2′-bipyridine)2Cl]+/2+/Au electrode [57], hydrogel based osmium complexes [Os(byp)2ClPyCH2NH Poly(allylamine)] redox polymer/GCE [85], poly(carbamoyl) sulfonate (PCS) hydrogel on a Teflon membrane/Pt [78], Laponite hydro gel/GCE [104], Laponite-chitosan hydrogel/GCE [86].
8.4.2. Merits
Provides biocompatible microenvironment for enzymes, show high sensitivity, provides sufficient permeability for both solvent and substrate molecules.
8.4.3. Demerits
Difficulty in handling due to low mechanical strength. Table 5 shows properties of different hydro-gel based L-lactate biosensors.
8.5. Screen printed electrode (SPE) based lactate biosensors
Screen printing methodology provides an attractive and widely used technique for fabrication of biosensors. It comprises of a chemically inert substrate on which three electrode system, including working electrode, reference electrode and counter electrode, are printed using screen printing strategy [105]. The biological molecules or the biological receptor in their active form is immobilized on this chemically inert substrate.
8.5.1. Supports used for immobilization of enzyme
CA membrane/Meldola blue, NADP; carbon Screen printing ink electrode [73], CNT/polysulfon/SPE [74], Meldola's Blue-Reinecke Salt (MBRS)/SPCE/cellulose acetate (CA) membrane/SPCE [77], mesoporous silica/screen-printed Prussian Blue (PB) /hydrophilic porous membrane [71], poly(carbamoyl)sulfonate (PCS) hydrogel/screen-printed graphite/nafion/Pt electrode [83], SWCNTs/Variamine blue/SPE [106], carbon/SP-ink-E [107], [108], Graphite epoxy/graphite SP-ink-(sol–gel) Electrode [98], Nafion/CoPC-SPCE [60].
8.5.2. Merits
Provides an economical, portable and disposable electrode system, suitable for working with micro-volumes and are easier to prepare and modify, have excellent specificity and selectivity.
Table 6 shows properties of different Screen printed electrode based L-Lactate biosensors.
8.6. Nanoparticles (NPs) based L-lactate biosensors
Nanoparticles can also be used for effective electrical wiring of LDH or LOD enzyme for L-lactate detection. Various nanomaterials, such as zinc oxide nanostructures (ZNO), carbon nanotubes (CNT) and gold (Au) nanoparticles have been used as electrical connectors between the electrode and the redox center of enzyme. Wide variety of nanoparticles has been used in lactate biosensors due to their advantage both in terms of stability and in improving the sensitivity. As a result of valuable properties of large surface-to-volume ratio, excellent surface reaction activity, high electro catalytic efficiency and highly adsorbing property, nanomaterials are an important candidate for enzyme immobilization in fabrication of lactate biosensors.
8.6.1. Nanoparticles supports used for immobilization of enzyme
Nanoparticles, such as multi walled carbon nanotubes (MWCNT)/Meldola blue [66], MWCNT/poly (3-methylthiophene) (P3 MT) polymer modified glassy carbon electrode (GCE) [35], nitrogen-doped CNT [34], ferrocene CNT/polysulfon screen printed electrode [74], pTTCA/MWNT composite film/Au electrode [94], MWCNT/GCE [96], Fe3O4/MWCNT/GCE [109], MWCNT/CHIT composite/GCE [110], SWCNTSs/Variamine blue [106], Chitosan/MWCNT/ferrocyanide/gold electrode [80], Chitosan/poly-vinyl imidazole Os (PVI-Os)/CNT [81], MWCNTs/Pt-nano electrode [39], gold nanoparticles (GNPs)/sol–gel 3-D silicate network derived from 3-(mercaptopropyl)trimethoxysilane (MPTS) [37], gold nanorods [111], platinum nanoparticles (Pt-black Nanoparticles) with gold electrode [112], sol–gel film/MWCNT/Pt nanoparticle [97], Polyacrylic acid (PAA)/Si3N4 nanostructured [113], Hydrogen titanate (H2Ti3O7) nanotube network [114], nanoparticles of metal oxides such as niobium oxide/carbon paste electrode [40], Titanium dioxide nanoparticles (TiO2-NPs)/photocatalytically reduced graphene (PRG)/GCE [115], Cerium oxide Nano-CeO2/GCE electrode [116], molybdenum oxide nanowires, MoO3/Au coated SiO2 electrode [41] and nanoparticles of semiconducting materials such as zinc oxide(ZNO)/MWCNT [42], ZNO nanorod/glass coated substrate [117], nano-ZnO-MWCNTs/GCE [118], Au/Nano ZnO electrode [132], ZNO nanowires [119], ZnO nanotetrapods [120].
8.6.2. Merits
Nanoparticles play an extensive role in fabrication of novel nanostructured biosensors their sensing applications such as excellent electron transfer ability; they possess small size and large surface area, they are highly electroactive and chemically stable in aqueous as well as non-aqueous environment. Among these ZnO, CNTs, AuNPs based are widely used to immobilize enzymes, resulting from their good biocompatibility and preservability of enzymatic activities. Table 7 shows properties of different NPs based L-lactate biosensors.
9. Practical applications of L-lactate biosensors
There is an urgent requirement of L-lactate biosensors in medical sector for the accurate and easy determination of lactate level. It is very necessary to monitor L-lactate concentration in the serum which plays an important role in clinical diagnosis of patients, in intensive care unit and during surgery. Lactic acid production can increase in the body due to conditions such as strenuous exercise or other conditions-such as heart failure [121], hemorrhage [10], respiratory failure [122], hepatic disease [123], a severe infection (sepsis), or shock [124], tissue hypoxia [125]. The level of lactic acid can also increase when liver is acutely damaged or diseased, as normally liver is responsible for breakdown of lactic acid in the body [126]. So determination of lactate level with the aid of biosensor can help to predict the outcome in patients admitted to intensive care unit [127]. In sports medicine sector also, L-lactate biosensors plays important role as blood lactate level acts as an indicator for training status and fitness during exercise for athletes. So the lactate concentration is determined not only in whole blood but also in sweat, saliva, and interstitial fluid. Monitoring of lactate level by the help of biosensor is not only limited to clinical diagnostics but is also crucial in the food industry especially in the fermentation of dairy products. The presence of amount of Lactate levels may have a great impact on stability, flavor, freshness, storage lifetime and quality of milk and other dairy products, as well as fruits, vegetables [76], [128], meat [129] and wine [130]. So L-lactate concentration plays an important role in clinical diagnostics, medicine validation, and food analysis. Lactate based biosensors acts as rapid and reliable method for the on-site detection of the concentration of lactate. Moreover when compared with traditional HPLC and other methods, biosensors provide advantages of being very selective, sensitive, disposable, do not require extensive instrumentation, inexpensive and work with complete automation and provide rapid results.
10. Conclusion and future prospects
The numerous development and research efforts to devise an ultrasensitive biosensors based on lactate monitoring provides an insight of its important role in clinical diagnosis and food analysis. Among the various lactate detection methods, enzyme based electrochemical lactate sensors are of the predominant type due to their sensitivity, low detection limits, comparatively simple fabrication, user friendly, portability, reliability and reasonable cost. However, more research is needed regarding implantable lactate sensor, which is usually implanted in blood vessel (intravascular) or under the skin (subcutaneous) and may fulfill the demand of continuous monitoring of lactate. This would basically prevent the requirement of sample dilution and would be fruitful for the patients in hospitals under critical monitoring. Although these type of sensors faces challenge regarding the biocompatibility, slow dynamic response and the technical problems. Nevertheless with an advent of nanotechnological advancement, nanoparticles promises to be suitable supports as working electrodes for the lactate detection in terms of miniaturization and development of semi-invasive or finally non-invasive sensing devices for commercialization. Nanomaterials (metal-oxide based, SWCNTs/MWCNTs, gold (Au) nanoparticles) due to their advantages such as in terms of large surface-to-volume ratio, high IEP values, high surface reaction activity, high catalytic efficiency and strong adsorption ability provides a better surface for lactate immobilization. Indeed, the booming research interest of lactate sensors will continue with increasing prevalence in clinical diagnosis, food analysis and the experimental research acquired through investigation of nanostructured lactate sensors will soon revolutionize the biosensor industry in future.
Acknowledgment
One of the authors thank the financial support from the Department of Science & Technology, New Delhi, for providing INSPIRE fellowship as SRF-P (IF110655).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.11.010.
Appendix A. Supplementary material
Supplementary material
References
- 1.Kost G.J. New whole blood analyzers and their impact on cardiac and critical care. Crit. Rev. Clin. Lab. Sci. 1993;30:153–202. doi: 10.3109/10408369309084667. [DOI] [PubMed] [Google Scholar]
- 2.Bakker J., Gris P., Coffernils M., Kahn R.J., Vincent J.L. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am. J. Surg. 1996;171:221–226. doi: 10.1016/S0002-9610(97)89552-9. [DOI] [PubMed] [Google Scholar]
- 3.Parra A., Casero E., Vazquez L., Pariente F., Lorenzo E. Design and characterization of a lactate biosensor based on immobilized lactate oxidase onto gold surfaces. Anal. Chim. Acta. 2006;555:308–315. [Google Scholar]
- 4.Wang J. Amperometric biosensors for clinical and therapeutic drug monitoring: a review. J. Pharm. Biomed. Anal. 1999;19:47–53. doi: 10.1016/s0731-7085(98)00056-9. [DOI] [PubMed] [Google Scholar]
- 5.Pilloton R., Nwosu T.N., Mascini M. Amperometric determination of lactic acid. Applications on milk samples. Anal. Lett. 1988;21:727–740. [Google Scholar]
- 6.Mazzei F., Azzoni A., Cavalieri B., Botre F., Botre C. A multi-enzyme bioelectrode for the rapid determination of total lactate concentration in tomatoes, tomato juice and tomato paste. Food Chem. 1996;55:413–418. [Google Scholar]
- 7.Goodwin M.L., Harris J.E., Hernandez A., Gladden L.B. Blood lactate measurements and analysis during exercise: a guide for clinicians. J. Diabetes Sci. Technol. 2007;1:558–569. doi: 10.1177/193229680700100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uribarri J., Oh M.S., Carroll H.J. D-lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine. 1998;77:73–82. doi: 10.1097/00005792-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kemp G. Lactate accumulation, proton buffering, and pH change in ischemically exercising muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R895–R901. doi: 10.1152/ajpregu.00641.2004. [DOI] [PubMed] [Google Scholar]
- 10.Valenza F., Aletti G., Fossali T., Chevallard G., Sacconi F., Irace M., Gattinoni L. Lactate as a marker of energy failure in critically ill patients: hypothesis. Crit. Care. 2005;9:588–593. doi: 10.1186/cc3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreisberg R.A. Pathogenesis and management of lactic acidosis. Annu. Rev. Med. 1984;35:181–193. doi: 10.1146/annurev.me.35.020184.001145. [DOI] [PubMed] [Google Scholar]
- 12.Pronovost P.J., Rinke M.L., Emery K., Dennison C., Blackledge C., Berenholtz S.M. Interventions to reduce mortality among patients treated in intensive care units. J. Crit. Care. 2004;19:158–164. doi: 10.1016/j.jcrc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Angus D.C., Zwirble W.T.L., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the united states: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Fowler R.A., Hill-Popper M., Stasinos J., Petrou C., Sanders G.D., Garber A.M. Cost-effectiveness of recombinant human activated protein C and the influence of severity of illness in the treatment of patients with severe sepsis. J. Crit. Care. 2003;18:181–191. doi: 10.1016/j.jcrc.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen H.B., Rivers E.P., Knoblich B.P., Jacobsen G., Muzzin A., Ressler J.A., Tomlanovich M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004;32:1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro F., Silanikove N. Rapid and accurate determination of d- and l-lactate, lactose and galactose by enzymatic reactions coupled to formation of a fluorochromophore: application in food quality control. Food Chem. 2010;119:829–833. doi: 10.1016/j.foodchem.2011.04.074. [DOI] [PubMed] [Google Scholar]
- 17.Milagres M.P., Brandao S.C., Magalhaes M.A., Minim V.P., Minim L.A. Development and validation of the high performance liquid chromatography–ion exclusion method for detection of lactic acid in milk. Food Chem. 2012;135:1078–1082. doi: 10.1016/j.foodchem.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 18.Galban J., de Marcos S., Castillo J.R. Fluorometric-enzymatic lactate determination based on enzyme cytochrome b2 fluorescence. Anal. Chem. 1993;65:3076–3080. doi: 10.1021/ac00069a022. [DOI] [PubMed] [Google Scholar]
- 19.Barker S.B., Summerson W.H. The colorimetric determination of lactic acid in biological material. J. Biol. Chem. 1941;138:535–554. [Google Scholar]
- 20.Heinemann B. A rapid colorimetric method for the determination of lactic acid in milk. J. Dairy Sci. 1940;23:969–972. [Google Scholar]
- 21.Wu F., Huang Y., Huang C. Chemiluminescence biosensor system for lactic acid using natural animal tissue as recognition element. Biosens. Bioelectron. 2005;21:518–522. doi: 10.1016/j.bios.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Soares D.P., Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin. Radiol. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Ren J., Dean Sherry A., Malloy C.R. Noninvasive monitoring of lactate dynamics in human forearm muscle after exhaustive exercise by (1)H-magnetic resonance spectroscopy at 7 tesla. Magn. Reson. Med. 2013;70:610–619. doi: 10.1002/mrm.24526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolaus N., Strehlitz B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim. Acta. 2008;160:15–55. [Google Scholar]
- 25.Meyerhoff C., Bischof F., Mennel F.J., Sternberg F., Bican J., Pfeiffer E.F. On line continuous monitoring of blood lactate in men by a wearable device based upon an enzymatic amperometric lactate sensor. Biosens. Bioelectron. 1993;8:409–414. doi: 10.1016/0956-5663(93)80025-k. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer D., Moller B., Klimes N., Szeponik J. Amperometric lactate oxidase catheter for real-time lactate monitoring based on thin film technology. Biosens. Bioelectron. 1997;12:539–550. doi: 10.1016/s0956-5663(97)00014-6. [DOI] [PubMed] [Google Scholar]
- 27.Gue A.M., Tap H., Gros P., Maury F. A miniaturised silicon based enzymatic biosensor: towards a generic structure and technology for multi-analytes assays. Sens. Actuators B. 2002;82:227–232. [Google Scholar]
- 28.Rahman M.M., Shiddiky M.J., Rahman M.A., Shim Y.B. A lactate biosensor based on lactate dehydrogenase/nictotinamide adenine dinucleotide (oxidized form) immobilized on a conducting polymer/multiwall carbon nanotube composite film. Anal. Biochem. 2009;384:159–165. doi: 10.1016/j.ab.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 29.Arnaldo C., Marina R., Alexandre K., Denise V., Lauro T. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sens. Actuators B. 2006;124:269–276. [Google Scholar]
- 30.Mamas P., Miltiades I.K. Enzyme based amperometric biosensors for food analysis. Electroanalysis. 2002;14:241–261. [Google Scholar]
- 31.Thevenot D.R., Toth K., Durst R.A., Wilson G.S. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001;16:121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 32.Blaedel W.J., Engstrom R.C. Reagentless enzyme electrodes for ethanol, lactate, and malate. Anal. Chem. 1980;52:1691–1697. [Google Scholar]
- 33.Salimi A., Noorbakhsh A., Mamkhezri H., Ghavami R. Electrocatalytic reduction of H2O2 and oxygen on the surface of thionin incorporated onto MWCNTs modified glassy carbon electrode: application to glucose detection. Electroanalysis. 2007;19:1100–1108. [Google Scholar]
- 34.Goran J.M., Lyon J.L., Stevenson K.J. Amperometric detection of l-lactate using nitrogen-doped carbon nanotubes modified with lactate oxidase. Anal. Chem. 2011;83:8123–8129. doi: 10.1021/ac2016272. [DOI] [PubMed] [Google Scholar]
- 35.Agui L., Eguilaz M., Pena-Farfal C., Yanez-Sedeno P., Pingarron J.M. Lactate dehydrogenase biosensor based on an hybrid carbon nanotube-conducting polymer modified electrode. Electroanalysis. 2009;21:386–391. [Google Scholar]
- 36.Gamero M., Pariente F., Lorenzo E., Alonso C. Nanostructured rough gold electrodes for the development of lactate oxidase-based biosensors. Biosens. Bioelectron. 2010;25:2038–2044. doi: 10.1016/j.bios.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Jena B.K., Raj C.R. Amperometric l-lactate biosensor based on gold nanoparticles. Electroanalysis. 2007;19:816–822. [Google Scholar]
- 38.Yu Y.Y., Yang Y., Gu H., Zhou T.S., Shi G.Y. Size-tunable Pt nanoparticles assembled on functionalized ordered mesoporous carbon for the simultaneous and on-line detection of glucose and l lactate in brain microdialysate. Biosens. Bioelectron. 2013;41:511–518. doi: 10.1016/j.bios.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 39.He X.R., Yu J.H., Ge S.G., Zhang X.M., Lin Q., Zhu H., Feng S., Yuan L., Huang J.D. Amperometric l-lactate biosensor based on sol–gel film and multi-walled carbon nanotubes/platinum nanoparticles enhancement. Chin. J. Anal. Chem. 2010;38:57–61. [Google Scholar]
- 40.Pereira A.C., Kisner A., Tarley C.R.T., Kubota L.T. Development of a carbon paste electrode for lactate detection based on Meldola's blue adsorbed on silica gel modified with niobium oxide and lactate oxidase. Electroanalysis. 2011;23:1470–1477. [Google Scholar]
- 41.Shakir I., Shahid M., Yang H.W., Cherevko S., Chung C.H., Kang D.J. MoO3 nanowire-based amperometric biosensor for l-lactate detection. J. Solid State Electrochem. 2012;16:2197–2201. [Google Scholar]
- 42.Wang Y.T., Bao Y.J., Lou L., Li J.J., Du W.J., Zhu Z.Q., Peng H., Zhu J.Z. A novel l-lactate sensor based on enzyme electrode modified with ZnO nanoparticles and multiwall carbon nanotubes. J. Electroanal. Chem. 2011;661:8–12. [Google Scholar]
- 43.Chaubey A., Pande K.K., Singh V.S., Malhotra B.D. Co-immobilization of lactate oxidase and lactate dehydrogenase on conducting polyaniline films. Anal. Chim. Acta. 2000;407:97–103. [Google Scholar]
- 44.Pierre A.C. The sol–gel encapsulation of enzymes. Biocatal. Biotransform. 2004;22:145–170. [Google Scholar]
- 45.Li C.I., Lin Y.H., Shih C.L., Tsaur J.P., Chau L.K. Sol–gel encapsulation of lactate dehydrogenase for optical sensing of l-lactate. Biosens. Bioelectron. 2002;17:323–330. doi: 10.1016/s0956-5663(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 46.Palmisano F., DeBenedetto G.E., Zambonin C.G. Lactate amperometric biosensor based on an electrosynthesized bilayer film with covalently immobilized enzyme. Analyst. 1997;122:365–369. [Google Scholar]
- 47.Emr S.A., Yacynych A.M. Use of polymer film in amperometric biosensors. Electroanalysis. 1995;7:913–923. [Google Scholar]
- 48.Wink T., Van Zuilen S.J., Bult A., Van Bennekom W.P. Self-assembled monolayers for biosensors. Analyst. 1997;122:43R–50R. doi: 10.1039/a606964i. [DOI] [PubMed] [Google Scholar]
- 49.Gooding J.J., Hibbert D.B. The application of alkanethiols selfassembled monolayers to enzyme electrodes. Trends Anal. Chem. 1999;18:525–532. [Google Scholar]
- 50.Rahman M.M. Fabrication of l-lactate biosensor based on redox species mediated lactate oxidase using micro-device. J. Biol. Med. Res. 2010;1:09–14. [Google Scholar]
- 51.Antiochia R., Cass A.E.G., Palleschi G. Purification and sensor applications of an oxygen insensitive, thermophilic diaphorase. Anal. Chim. Acta. 1997;345:17–28. [Google Scholar]
- 52.Yoon H.C., Kim H.S. Electrochemical characteristics of a carbon-based thick-film l-lactate biosensor using l-lactate dehydrogenase. Anal. Chim. Acta. 1996;336:57. [Google Scholar]
- 53.Fultz M.L., Durst R.A. Mediator compounds for the electrochemical study of biological redox systems: a compilation. Anal. Chim. Acta. 1982;140:1–18. [Google Scholar]
- 54.Simon E., Halliwell C.M., Toh C.S., Cass A.E.G., Bartlett P.N. Oxidation of NADH produced by a lactate dehydrogenase immobilised on poly(aniline)–poly(anion) composite films. J. Electroanal. Chem. 2002;538–539:253–259. [Google Scholar]
- 55.Halliwell C.M., Simon E., Toh C.S., Bartlett P.N., Cass A.E.G. Immobilisation of lactate dehydrogenase on poly(aniline)–poly(acrylate) and poly(aniline)–poly(vinyl sulphonate) films for use in a lactate biosensor. Anal. Chim. Acta. 2002;453:191–200. [Google Scholar]
- 56.Khan G.F., Wernet W. Design of enzyme electrodes for extended use and storage life. Anal. Chem. 1997;69:2682–2687. [Google Scholar]
- 57.Kenausis G., Taylor C., Katakis I., Heller A. Wiring' of glucose oxidase and lactate oxidase within a hydrogel made with poly(vinyl pyridine) complexed with [Os(4,4′- dimethoxy-2,2′-bipyridine)2Cl]+/2+ J. Chem. Soc. 1996;92:4131–4136. [Google Scholar]
- 58.Salazar P., Martin M., O'Neill R.D., Roche R., Gonzalez-Mora J.L. Biosensors based on Prussian blue modified carbon fibers electrodes for monitoring lactate in the extracellular space of brain tissue. Int. J. Electrochem. Sci. 2012;7:5910–5926. [Google Scholar]
- 59.Salazar P., Martin M., O'Neill R.D., Roche R., Gonzalez-Mora J.L. Surfactant-promoted Prussian Blue-modified carbon electrodes: enhancement of electro-deposition step, stabilization, electrochemical properties and application to lactate microbiosensors for the neurosciences. Colloids Surf. B Biointerfaces. 2012;92:180–189. doi: 10.1016/j.colsurfb.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 60.Shimomura T., Sumiya T., Ono M., Ito T., Hanaoka T. Amperometric l-lactate biosensor based on screen-printed carbon electrode containing cobalt phthalocyanine, coated with lactate oxidase-mesoporous silica conjugate layer. Anal. Chim. Acta. 2012;714:114–120. doi: 10.1016/j.aca.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 61.Schmitt R.E., Molitor H.R., Wu T.S. Voltammetric method for the determination of lactic acid using a carbon paste electrode modified with cobalt phthalocyanine. Int. J. Electrochem. Sci. 2012;7:10835–10841. [Google Scholar]
- 62.Parra A., Casero E., Vazquez L., Jin J., Pariente F., Lorenzo E. Microscopic and voltammetric characterization of bioanalytical platforms based on lactate oxidase. Langmuir. 2006;22:5443–5450. doi: 10.1021/la060184g. [DOI] [PubMed] [Google Scholar]
- 63.Serban S., ElMurr N. Redox-flexible NADH oxidase biosensor: a platform for various dehydrogenase bioassays and biosensors. Electrochim. Acta. 2006;51:5143–5149. [Google Scholar]
- 64.Mao L.Q., Yamamoto K. Amperometric on-line sensor for continuous measurement of hypoxanthine based on osmiumpolyvinylpyridine gel polymer and xanthine oxidase bienzyme modified glassy carbon electrode. Anal. Chim. Acta. 2000;415:143–150. [Google Scholar]
- 65.Kulys J., Wang L.Z., Maksimoviene A. l-Lactate oxidase electrode based on methylene green and carbon paste. Anal. Chim. Acta. 1993;274:53–58. [Google Scholar]
- 66.Pereira A.C., Aguiar M.R., Kisner A., Macedo D.V., Kubota L.T. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola blue coimmobilized on multi-wall carbon-nanotube. Sens. Actuators B Chem. 2007;124:269–276. [Google Scholar]
- 67.Marzouk S.A.M., Cosofret V.V., Buck R.P., Yang H., Cascio W.E., Hassan S.S.M. A conducting salt-based amperometric biosensor for measurement of extracellular lactate accumulation in ischemic myocardium. Anal. Chem. 1997;69:2646–2652. doi: 10.1021/ac970020s. [DOI] [PubMed] [Google Scholar]
- 68.Haccoun J., Piro B., Tran L.D., Dang L.A., Pham M.C. Reagentless amperometric detection of l-lactate on an enzyme-modified conducting copolymer poly(5-hydroxy-1,4-naphthoquinone-co-5-hydroxy- 3-thioacetic acid-1,4-naphthoquinone) Biosens. Bioelectron. 2004;19:1325–1329. doi: 10.1016/j.bios.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Leonida M.D., Starczynowski D.T., Waldman R., Aurian- Blajeni B. Polymeric FAD used as enzyme-friendly mediator in lactate detection. Anal. Bioanal. Chem. 2003;376:832. doi: 10.1007/s00216-003-1991-1. [DOI] [PubMed] [Google Scholar]
- 70.Romero M.R., Ahumada F., Garay F., Baruzzi A.M. Amperometric biosensor for direct blood lactate detection. Anal. Chem. 2010;82:5568–5572. doi: 10.1021/ac1004426. [DOI] [PubMed] [Google Scholar]
- 71.Shimomura T., Sumiya T., Ono M., Itoh T., Hanaoka T. An electrochemical biosensor for the determination of lactic acid in expiration. Procedia Chem. 2012;6:46–51. [Google Scholar]
- 72.Bridge K., Davis F., Collyer S.D., Higson S.P.J. Flexible ultrathin polyDVB/EVB composite membranes for the optimization of a Lactate Sensor. Electroanalysis. 2007;19:567–574. [Google Scholar]
- 73.Sprules S.D., Hart J.P., Wring S.A., Pittson R. A reagentless, disposable biosensor for lactic acid based on a screen-printed carbon electrode containing Meldola's Blue and coated with lactate dehydrogenase, NAD+ and cellulose acetate. Anal. Chim. Acta. 1995;304:17–24. [Google Scholar]
- 74.Perez S., Fabregas E. Amperometric bienzymatic biosensor for l-lactate analysis in wine and beer samples. Analyst. 2012;137:3854–3861. doi: 10.1039/c2an35227c. [DOI] [PubMed] [Google Scholar]
- 75.Palmisano F., Rizzi R., Centonze D., Zambonin P.G. Simultaneous monitoring of glucose and lactate by an interference and cross-talk free dual electrode amperometric biosensor based on electropolymerized thin films. Biosens. Bioelectron. 2000;15:531–539. doi: 10.1016/s0956-5663(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 76.Kriz K., Kraft L., Krook M., Kriz D. Amperometric determination of l-lactate based on entrapment of lactate oxidase on a transducer surface with a semi-permeable membrane using a SIRE technology based biosensor. Application: tomato paste and baby food. J. Agric. Food Chem. 2002;50:3419. doi: 10.1021/jf0114942. [DOI] [PubMed] [Google Scholar]
- 77.Pianoa M., Serbanb S., Pittsonb R., Dragoa G.A., Hartc J.P. Amperometric lactate biosensor for flow injection analysis based on a screen-printed carbon electrode containing Meldola's Blue-Reinecke salt, coated with lactate dehydrogenase and NAD+ Talanta. 2010;82:34–37. doi: 10.1016/j.talanta.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 78.Kwan R.C., Hon P.Y., Mak K.K., Renneberg R. Amperometric determination of lactate with novel trienzyme/poly(carbamoyl) sulfonate hydrogel-based sensor. Biosens. Bioelectron. 2004;19:1745–1752. doi: 10.1016/j.bios.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Cosnier S. Biosensors based on electropolymerized films: new trends. Anal. Bioanal. Chem. 2003;377:507–520. doi: 10.1007/s00216-003-2131-7. [DOI] [PubMed] [Google Scholar]
- 80.Monosik R., Stredansky M., Greif G., Sturdik M. A rapid method for determination of L-lactic acid in real samples by amperometric biosensor utilizing nanocomposite. Food Control. 2012;23:238–244. [Google Scholar]
- 81.Cui X., Li C.M., Zang J., Yu S. Highly sensitive lactate biosensor by engineering chitosan/PVI-Os/CNT/LOD network nanocomposite. Biosens. Bioelectron. 2007;22:3288–3292. doi: 10.1016/j.bios.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Hajizadeh K., Halsall H.B., Heineman W.R. Immobilization of lactate oxidase in a poly (vinyl alcohol) matrix on platinized graphite electrodes by chemical cross-linking with isocyanate. Talanta. 1991;38:37–47. doi: 10.1016/0039-9140(91)80007-m. [DOI] [PubMed] [Google Scholar]
- 83.Patel N.G., Erlenkotter A., Cammann K., Chemnitius G.C. Fabrication and characterization of disposable type lactate oxidase sensors for dairy products and clinical analysis. Sensors Actuators B: Chem. 2000;67:134–141. [Google Scholar]
- 84.Park T.M., Iwuoha E.I., Smyth M.R., Freaney R., McShane A.J. Sol–gel based amperometric biosensor incorporating an osmium redox polymer as mediator for detection of l-lactate. Talanta. 1997;44:973–978. doi: 10.1016/s0039-9140(96)02164-9. [DOI] [PubMed] [Google Scholar]
- 85.Danilowicz C., Corton E., Battaglini F., Calvo E.J. An Os(byp)2ClPyCH2NH Poly(allylamine) hydrogel mediator for enzyme wiring at electrodes. Electrochim. Acta. 1998;43:3525–3531. [Google Scholar]
- 86.Zanini V.P., de Mishima B.L., Solis V. An amperometric biosensor based on lactate oxidase immobilized in Laponite-chitosan hydrogel on a glassy carbon electrode. Application to the analysis of l-lactate in food samples. Sens. Actuators B Chem. 2012;155:75–80. [Google Scholar]
- 87.F. Scholz, A New Era in Electrochemistry, Gyorgy: Inzelt, Monographs in Electrochemistry, Springer, 2008, pp. 1–6.
- 88.Rahman M.A., Kumar P., Park D.S., Shim Y.B. Electrochemical sensors based on organic conjugated polymers. Sensors. 2008;8:118–141. doi: 10.3390/s8010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guiseppi-Elie A., Wallace G.G., Matsue T. 2nd ed. Marcel Dekker; New York: 1998. Handbook of Conducting Polymers; pp. 963–991. [Google Scholar]
- 90.Shu H.C., Mattiasson B., Persson B., Nagy G., Gorton L., Sahni S., Geng L., Boguslavsky L., Skotheim T. A reagentless amperometric electrode based on carbon paste, chemically modified with d-lactate dehydrogenase, NAD(+), and mediator containing polymer for d-lactic acid analysis: I. construction, composition, and characterization. Biotechnol. Bioeng. 1995;46:270–279. doi: 10.1002/bit.260460310. [DOI] [PubMed] [Google Scholar]
- 91.Chaubey A., Gerard M., Singhal R., Singh V.S., Malhotra B.D. Immobilization of lactate dehydrogenase on electrochemically prepared polypyrrole–polyvinylsulphonate composite films for application to lactate biosensors. Electrochim. Acta. 2000;46:723–729. [Google Scholar]
- 92.Chaubey A., Pande K.K., Pandey M.K., Singh V.S. Signal amplification by substrate recycling on polyaniline/lactate oxidase/lactate dehydrogenase bienzyme electrodes. Appl. Biochem. Biotechnol. 2001;96:239–248. doi: 10.1385/abab:96:1-3:239. [DOI] [PubMed] [Google Scholar]
- 93.Lobo-Castanon M.J., Miranda-Ordieres A.J., Tunon-Blanco P. A bienzyme-poly-(o-phenylenediamine)-modified carbon paste electrode for the amperometric detection of L-lactate. Anal. Chim. Acta. 1997;346:165–174. [Google Scholar]
- 94.Sarma A.K., Vatsyayan P., Goswani P., Minteer S.D. Recent advances in material science for developing enzyme electrodes. Biosens. Bioelectron. 2009;24:2313–2322. doi: 10.1016/j.bios.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 95.Blackman C.S., Parkin I.P. Polymers for biosensors construction. Chem. Mater. 1997;9:2354–2375. [Google Scholar]
- 96.Huang J., Song Z., Li J., Yang Y., Shi H., Baoyan Wu, Anzai J., Osa T., Chen Q. A highly-sensitive l-lactate biosensor based on sol-gel film combined with multi-walled carbon nanotubes (MWCNTs) modified electrode. Mater. Sci. Eng.: C. 2007;27:29–34. [Google Scholar]
- 97.Huang J., Li J., Yang Y., Wang X., Wu B., Anzai J., Osa Tetsuo, Chen Q. Development of an amperometric l-lactate biosensor based on l-lactate oxidase immobilized through silica sol–gel film on multi-walled carbon nanotubes/platinum nanoparticle modified glassy carbon electrode. Mater. Sci. Eng. C. 2008;28:1070–1075. [Google Scholar]
- 98.Prieto-Simon B., Fabregas E., Hart A. Evaluation of different strategies for the development of amperometric biosensors for l-lactate. Biosens. Bioelectron. 2007;22:2663–2668. doi: 10.1016/j.bios.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 99.Gomes S.P., Odlozilikova M., Almeida M.G., Araujo A.N., CMCM Couto, Montenegro M., CBSM Application of lactate amperometric sol–gel biosensor to sequential injection determination of l-lactate. J. Pharm. Biomed. 2007;43:1376–1381. doi: 10.1016/j.jpba.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 100.Yashina E.I., Borisova A.V., Karyakina E.E., Shchegolikhina O.I., Vagin M.Y., Sakharov D.A., Tonevitsky A.G., Karyakin A.A. Sol–gel immobilization of lactate oxidase from organic solvent: toward the advanced lactate biosensor. Anal. Chem. 2010;82:1601–1604. doi: 10.1021/ac9027615. [DOI] [PubMed] [Google Scholar]
- 101.Parra-Alfambra A.M., Casero E., Petit-Dominguez M.D., Barbadillo M., Pariente F., Vazquez L., Lorenzo E. New nanostructured electrochemical biosensors based on three-dimensional (3-mercaptopropyl)-trimethoxysilane network. Analyst. 2011;136:340–347. doi: 10.1039/c0an00475h. [DOI] [PubMed] [Google Scholar]
- 102.Lin C.L., Shih C.L., Chau L.K. Amperometric l-lactate sensor based on sol–gel processing of an enzyme-linked silicon alkoxide. Anal. Chem. 2007;79:3757–3763. doi: 10.1021/ac061972d. [DOI] [PubMed] [Google Scholar]
- 103.Albareda-Sirvent M., Hart A.L. Preliminary estimates of lactic and malic acid in wine using electrodes printed from inks containing sol–gel precursors. Sens. Actuators B. 2002;87:73–81. [Google Scholar]
- 104.Zanini V.P., de Mishima B.L., Labbe P., Solis V. An l-lactate amperometric enzyme electrode based on l-lactate oxidase immobilized in a Laponite gel on a glassy carbon electrode. Application to dairy products and red wine. Electroanalysis. 2010;22:946–954. [Google Scholar]
- 105.Li M., Li Y.T., Li D.W., Long Y.T. Recent developments and applications of screen-printed electrodes in environmental assays – a review. Anal. Chim. Acta. 2012;734:31–44. doi: 10.1016/j.aca.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 106.Radoi A., Moscone D., Palleschi G. Sensing the lactic acid in probiotic yogurts using an l-lactate biosensor coupled with a microdialysis fiber inserted in a flow analysis system. Anal. Lett. 2010;43:1301–1309. [Google Scholar]
- 107.Ghamouss F., Ledru S., Ruille N., Lantier F., Boujtita M. Bulk-modified modified screen-printing carbon electrodes with both lactate oxidase (LOD) and horseradish peroxide (HRP) for the determination of l-lactate in flow injection analysis mode. Anal. Chim. Acta. 2006;570:158. doi: 10.1016/j.aca.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 108.Sato N., Okuma H. Amperometric simultaneous sensing system for d-glucose and l-lactate based on enzyme-modified bilayer electrodes. Anal. Chim. Acta. 2006;565:250–254. [Google Scholar]
- 109.Teymourianc H., Salimia A., Rahman H. Low potential detection of NADH based on Fe3O4 nanoparticles/multiwalled carbon nanotubes composite: fabrication of integrated dehydrogenase-based lactate biosensor. Biosens. Bioelectron. 2012;33:60–68. doi: 10.1016/j.bios.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 110.Tsai Y.C., Chen S.Y., Liaw H.W. Immobilization of lactate dehydrogenase within multiwalled carbon nanotube-chitosan nanocomposite for application to lactate biosensors. Sens. Actuators B. 2007;125:474–481. [Google Scholar]
- 111.Yang H.W., Kim D.C., Yoo S.H., Park S., Kang D.J. Constructing LBL-assembled functional bio-architecture using gold nanorods for lactate detection. Mater. Res. Bull. 2012;47:3056–3060. [Google Scholar]
- 112.Liu C.X., Liu H.M., Yang Q.D., Tian Q., Cai X.X. Development of amperometric lactate biosensor modified with Pt-black nanoparticles for rapid assay. Chin. J. Anal. Chem. 2009;37:624–628. [Google Scholar]
- 113.Lupu A., Valsesia A., Bretagnol F., Colpo P., Rossi F. Development of a potentiometric biosensor based on nanostructured surface for lactate determination. Sens. Actuators B Chem. 2007;127:606–612. [Google Scholar]
- 114.Yang M.L., Wang J., Li H.Q., Zheng J.G., Wu N.Q. A lactate electrochemical biosensor with a titanate nanotube as direct electron transfer promoter. Nanotechnology. 2008;19:075502. doi: 10.1088/0957-4484/19/7/075502. [DOI] [PubMed] [Google Scholar]
- 115.Elena C., Concepcion A., Petit-Domingueza Maria Dolores, Vazquezc L., Parra-Alfambraa A.M., Merinod P., alvarez-Garciac S., Andresc A., Suareza E., Parientea F., Lorenzoa E. Lactate biosensor based on a bionanocomposite composed of titanium oxide nanoparticles, photocatalytically reduced graphene, and lactate oxidase. Microchim. Acta. 2014;181:79–87. [Google Scholar]
- 116.Nesakumar N., Sethuraman S., Krishnan U.M., Rayappan J.B. Fabrication of lactate biosensor based on lactate dehydrogenase immobilized on cerium oxide nanoparticles. J. Colloid Interface Sci. 2013;410:158–164. doi: 10.1016/j.jcis.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 117.Ibupoto Z.H., Shah S.M.U.A., Khun K., Willander M. Electrochemical l-lactic acid sensor based on immobilized ZnO nanorods with lactate oxidase. Sensors. 2012;12:2456–2466. doi: 10.3390/s120302456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haghighi B., Bozorgzadeh S. Fabrication of a highly sensitive electro chemiluminescence lactate biosensor using ZnO nanoparticles decorated multiwalled carbon nanotubes. Talanta. 2011;85:2189–2193. doi: 10.1016/j.talanta.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 119.Zhao Y., Yan X., Kang Z., Fang X., Zheng X., Zhao L., Du H., Zhang Y. Zinc oxide nanowires-based electrochemical biosensor for l-lactic acid amperometric detection. J. Nanopart. Res. 2014;16:2398. [Google Scholar]
- 120.Lei Y., Luo N., Yan X., Zhao Y., Zhang G., Zhang Y. A highly sensitive electrochemical biosensor based on zinc oxide nanotetrapods for l-lactic acid detection. Nanoscale. 2012;4:3438–3443. doi: 10.1039/c2nr30334e. [DOI] [PubMed] [Google Scholar]
- 121.Karlsson J., Willerson J.T., Leshin S.J., Mullins C.B., Mitchell J.H. Skeletal-muscle metabolites in patients with cardiogenic-shock or severe congestive heart failure. Scand. J. Clin. Lab. Investig. 1975;35:73–79. [PubMed] [Google Scholar]
- 122.De Backer D., Creteur J., Zhang H.B., Norrenberg M., Vincent J.L. Lactate production by the lungs in acute lung injury. Am. J. Respir. Crit. Care Med. 1997;156:1099–1104. doi: 10.1164/ajrccm.156.4.9701048. [DOI] [PubMed] [Google Scholar]
- 123.Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit. Care. 2002;6:322–326. doi: 10.1186/cc1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sayeed M.M., Murthy P.N.A. Adenine nucleotide and lactate metabolism in the lung in endotoxin shock. Circ. Shock. 1981;8:657–666. [PubMed] [Google Scholar]
- 125.Rimachi R., de Carvahlo F.B., Orellano-Jimenez C., Cotton F., Vncent J.L., De Backer D. Lactate/pyruvate ratio as a marker of tissue hypoxia in circulatory and septic shock. Anaesth. Intensive Care. 2012;40:427–432. doi: 10.1177/0310057X1204000307. [DOI] [PubMed] [Google Scholar]
- 126.Kruse J.A., Zaidi S.A., Carlson R.W. Significance of blood lactate levels in critically ill patientswith liver disease. Am. J. Med. 1987;83:77–82. doi: 10.1016/0002-9343(87)90500-6. [DOI] [PubMed] [Google Scholar]
- 127.Smith I., Kumar P., Molloy S., Rhodes A., Newman P.J., Grounds R.M., Bennett E.D. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001;27:74–83. doi: 10.1007/s001340051352. [DOI] [PubMed] [Google Scholar]
- 128.Thakur M.S., Ragavan K.V. Biosensors in food processing. J. Food Sci. Technol. 2013;50:625–641. doi: 10.1007/s13197-012-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stekelenburg F.K., Kant-Muermans M.L.T. Effects of sodium lactate and other additives in a cooked ham product on sensory quality and development of a strain of Lactobacillus curvatus and Listeria monocytogenes. Int. J. Food Microbiol. 2001;66:197–203. doi: 10.1016/s0168-1605(00)00521-3. [DOI] [PubMed] [Google Scholar]
- 130.Shkotova L.V., Goriushkina T.B., Tran-Minh C., Chovelon J., Soldatkin A.P. Amperometric biosensor for lactate analysis in wine and must during fermentation. Mater. Sci. Eng. C. 2008;28:943–948. [Google Scholar]
- 131.Habermuller K., Mosbach M., Schuhmann W. Electron-transfer mechanisms in amperometric biosensors. Fresenius J. Anal. Chem. 2000;366:560–568. doi: 10.1007/s002160051551. [DOI] [PubMed] [Google Scholar]
- 132.Nesakumar N., Thandavan K., Sethuraman S., Krishnan U.M., Rayappan J.B.B. An electrochemical biosensor with nanointerface for lactate detection based on lactate dehydrogenase immobilized on zinc oxide nanorods. Colloid Interface Sci. 2014;414:90–96. doi: 10.1016/j.jcis.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 133.Armbruster D.A., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008;1:S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 134.Abel P.U., Von woedtke T., Schulz B., Bergann T., Schwock A. Stability of immobilized enzymes as biosensors for continuous application in vitro and in vivo. J. Mol. Catal. B – Enzym. 1999;7:93. [Google Scholar]
- 135.C.H. Suelter, L. Kricka, Methods of Biochemical Analysis: Bioanalytical Applications of Enzymes, vol. 36, 2006.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material