Summary
Many anticoagulant drugs inhibiting proteins of the coagulation cascade have been developed. The main targets of anticoagulant drugs are thrombin and factor Xa; inhibiting these factors delays thrombus growth, thus preventing thrombosis while increasing bleeding risk. A balance between thrombosis and bleeding is ensured in the ‘therapeutic window’ of the anticoagulant drug concentration range. Novel anticoagulant drugs and combinations thereof are being developed. We rank coagulation factors as potential anticoagulant drug targets in combination with thrombin inhibitors, aptamer HD1 and bivalirudin, providing a background for several promising dual target treatment strategies.
The thrombin generation test was used to assess the whole coagulation cascade in normal and factor-deficient human blood plasma. Potential therapeutic windows were estimated for coagulation factors, ranking them as targets for anticoagulant drugs. Thrombin and factor Xa have been revealed as the most promising targets, which fully agrees with the current drug development strategy. Inhibitors of factors Va and VIIa are expected to have narrow therapeutic windows. Inhibitors of factors VIIIa and IXa are expected to have a moderate anticoagulant effect. Factors XI and XII are poor targets for anticoagulant drugs. Compared with plasma that is deficient in factor II, the thrombin inhibitors bivalirudin and aptamer HD1 had increased activity. Both inhibitors were tested in deficient plasma providing a model of potential drug combination. The most promising combinations were anti-thrombin with anti-V/Va and also anti-thrombin with anti-IX/IXa. Each combination had an incremental dose-effect dependence that is promising from the standpoint of the therapeutic window.
Keywords: Anticoagulant, Bivalirudin, Coagulation cascade, DNA aptamer, Thrombin
Highlights
-
•
Coagulation factors are ranked as anticoagulant targets.
-
•
Several promising combinations of anticoagulant and thrombin inhibitor are proposed.
-
•
The most promising combinations are anti-thrombin with anti-V/Va or anti-IX/IXa.
1. Introduction
The coagulation cascade together with platelets provide thrombus formation in several seconds after a vessel injury. The coagulation cascade yields fibrin fibers that tighten the plug formed from aggregating platelets. Both processes are tightly regulated by several feedback mechanisms (accelerating as well as inhibitory) to ensure a controlled, prompt response. Genetic alterations, inflammation, obesity, cancer, immobilization, and other causes lead to conditions of excessive thrombus formation [1], [2], [3], [4]. Oral anticoagulant drugs and platelet inhibitors have been developed to prevent thrombotic events in high-risk patients. Parenteral anticoagulant drugs have been developed for surgical procedures [3], [5], [6]. The causes of thrombosis are manifold and treatment thus requires adapted approaches employing distinct medications and combinations thereof. Therapeutic combinations of several platelet inhibitors and also combinations of platelet inhibitors with an anticoagulant drug have currently passed clinical trials [3], [7], [8], [9]. Other combinations still have to be explored providing safer and more efficient thrombosis treatment.
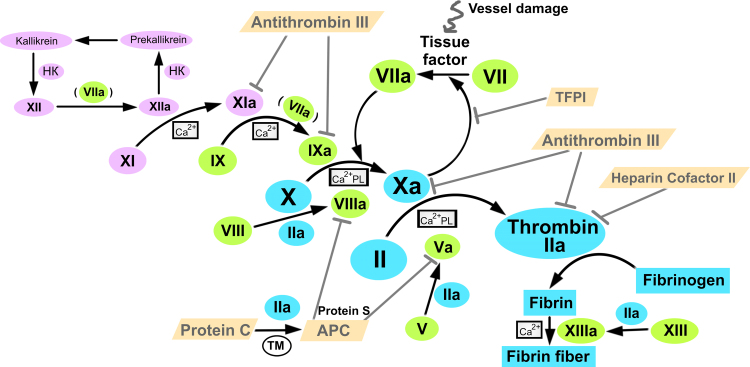
The coagulation cascade is triggered with the tissue factor that is exposed upon vessel damage. The tissue factor triggers factor VII activation to factor VIIa, which in turn catalyzes the activation of factor X to factor Xa, which catalyzes the conversion of prothrombin into thrombin. Thrombin and also factors VIIa and Xa catalyze several positive regulatory loops that significantly accelerate thrombin generation (see Fig. 1). The main function of thrombin in vivo is to catalyze the conversion of fibrinogen into fibrin, which spontaneously associates into a net of fibrin fibers. Excessive thrombin is mainly neutralized by antithrombin III, a serpin-family protein acting like a suicide substrate. A set of inhibitors is responsible for disabling the cascade after a proper fibrin fiber net is formed; among them are heparin cofactor II inhibiting thrombin, activated protein C with protein S inhibiting factors Va and VIIIa, as well as TFPI inhibiting the factor Xa-dependent activation of factor VII [11], [12], [13].
Fig. 1.
The coagulation cascade is a sophisticated regulatory network controlling the formation of fibrin fiber. Tissue factor triggers coagulation under vessel damage, whereas factor XII induces supplementary contact activation intrinsic pathway. HK – high molecular weight kininogen, PL – phospholipid surface, TM – thrombomodulin, APC – activated protein C, TFPI – tissue factor pathway inhibitor. The scheme is derived from a diagram from Enzyme Research Laboratories [10].
The thrombin generation test (TGT) is a sophisticated technique to study the coagulation cascade in detail. The TGT allows real-time tracing of thrombin produced in a blood plasma sample. The technique traces thrombin generation and its subsequent inactivation using a fluorogenic substrate. The thrombin generation profile is sensitive to anticoagulant drugs and hemophilia-related disorders and is a sharp tool for diagnostics and for research issues [14], [15], [16], [17], [18], [19]. For example, the TGT has recently been used to test therapeutic combinations of edoxaban, a factor Xa inhibitor, with the platelet inhibitors clopidogrel and ticagrelor [18].
A wide variety of thrombin inhibitors have been developed, representing all possible classes of inhibitors [20]. Bivalirudin and aptamer HD1 are short-acting anticoagulant drugs for the intravenous administration during surgical procedures. Bivalirudin is a peptide of 20 amino acids, which binds thrombin through the interaction with both the active site and the fibrinogen-binding site [21], [22], whereas the aptamer HD1 is a short DNA of 15 nucleotides which is structured in a guanine quadruplex, and binds thrombin through fibrinogen-binding site only [23], [24]. Both anticoagulant drugs are highly specific and have linear dose-effect dependencies at micromolar concentrations in blood plasma [24], [25].
The development of dual targeting therapy based on direct inhibitors of thrombin and of some other coagulation factors could be beneficial, providing a decrease of therapeutic doses (synergism) and a decrease of bleeding rates (non-linear dose-effect dependence). As a first estimation, we have used the TGT to assess anticoagulant effect of thrombin inhibitors, bivalirudin and aptamer HD1, on blood plasma with coagulation factor deficiency. As a result, we have estimated the inhibitory capacity of potential anticoagulant drug combinations providing a background for the development of novel dual target treatment strategies.
2. Materials and methods
Inorganic salts and Tris were purchased from MP Biomedicals (France). Bivalirudin trifluoroacetate was purchased from Selleck Chemicals, USA; DNA aptamer HD1, 5′-ggttggtgtggttgg-3′, was synthesized by Evrogene (Russian Federation). Standard platelet-poor human plasma (normal plasma) and all deficient human plasma were purchased from Siemens (Germany). The Technothrombin® TGA Kit, the whole set of reagents for the TGT, was purchased from Technoclone (Austria).
2.1. Thrombin generation test
An Infinite® 200 Pro microplate reader equipped with fluorescent and thermostatic modules (Tecan GmbH, Switzerland) was used to perform the TGT. The calibration procedure and measurements were performed according to the manufacturer's protocol. For the control experiments, 40 μl of normal plasma was placed in a well of a 96-well microliter plate (Greiner Bio-One, USA), 50 μl of fluorogenic substrate solution with calcium cations was then added, and 10 μl of trigger was finally added to start the coagulation cascade. The microplate reader was thermostatically controlled at 37 °C. The time intervals between measurements were 1 min, and the respective excitation and emission wavelengths were 360 nm and 460 nm. The experiment lasted until the fluorescence plateau was reached. The RCL reagent was used as a trigger containing tissue factor embedded in phospholipid microvesicles. Each measurement was performed at least three times.
Immunodepleted deficient human plasma was used to assess the effect of the particular factor deficiency. The manufacturer declared a particular factor-deficient human plasma to have ≤1% of the activity of the particular factor. Mixtures of normal plasma (approx. 100% content of the factor) and factor-deficient plasma (approx. 0% content of the factor) were created to simulate the particular factor contents of 0%, 2%, 10%, 25%, 50%, and 100%. Each mixture was tested as described above. Plasma deficient in one of the factors, II, V, VII, VIII, IX, X, XI, and XII, was tested.
The thrombin inhibitors bivalirudin and DNA aptamer HD1 were tested in normal, deficient plasma, and their mixtures described above. The inhibitor was added to the plasma sample in a volume of ≤4 μl to minimize alterations of the reaction mixture volume. To assemble the spatial structure of DNA aptamer HD1, its solution in 10 mM KCl was heated for 5 min at 95 °C and cooled to room temperature before each experiment. To study the inhibitory effects on normal plasma, the final inhibitor concentration in plasma was set in a range of 0.5–10 µM. To study the inhibitory effects on deficient plasma, the final concentration was 2 µM for bivalirudin and 4 µM for the DNA aptamer HD1. Each plasma sample was tested with the inhibitor as described above.
2.2. Data treatment
Thrombin generation curves were derived from the fluorescence curves and parameterized using the Technothrombin® TGA Evaluation Software (Technoclone, Austria). All subsequent data manipulations including statistical analysis to calculate means and standard deviations from three individual measurements were performed using Origin 8.1 (OriginLab, USA). The same software was used to construct all graphs.
3. Results and discussion
3.1. Thrombin generation in deficient plasma
Data treatment of the TGT yields a dependence of thrombin concentration on reaction time (Fig. 2A). The curve represents two main processes: thrombin generation de novo and its subsequent complete inactivation by cascade inhibitors. The curve can be described by a set of characteristic parameters:
-
1.
Lag time is the time of generating the first thrombin amounts (about 2–10 nM) after cascade triggering.
-
2.
Peak thrombin concentration is the maximum enzyme concentration resulting from a balance between the thrombin generation and inactivation processes.
-
3.
Time to peak corresponds to the time of maximum thrombin concentration.
-
4.
Velocity index represents the effective rate of thrombin generation between lag time and time to peak.
-
5.
Endogenous thrombin potential (ETP) is the area under the curve; ETP reflects the total enzymatic work of thrombin, which depends on the thrombin amount and its lifetime in plasma.
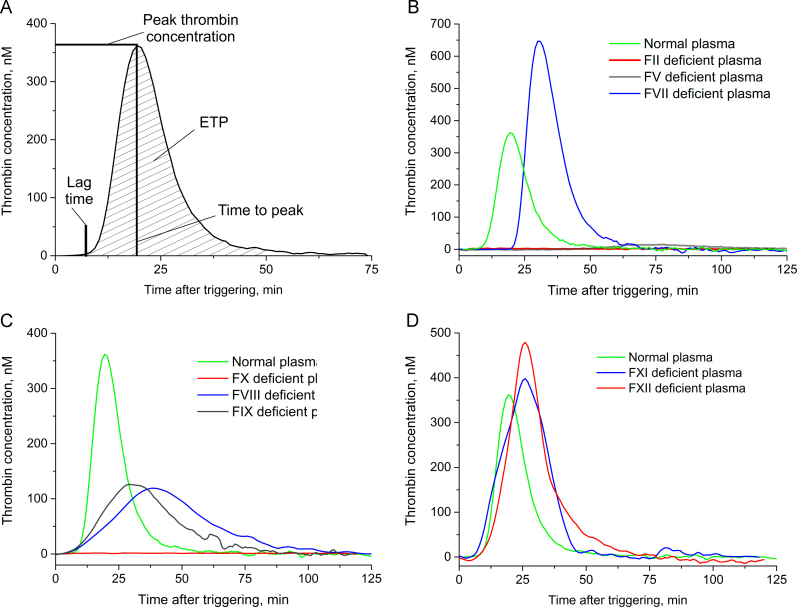
Fig. 2.
Thrombin generation in normal and deficient plasma. A – TGT parameters; B – thrombin generation in normal, factor II-, factor V-, and factor VII-deficient plasma; C – thrombin generation in normal, factor VIII-, factor IX-, and factor X-deficient plasma; D – thrombin generation in normal, factor XI-, and factor XII-deficient plasma.
Poor coagulation is characterized by decreased peak thrombin concentration, velocity index, and ETP, while lag time and time to peak increase.
In this study, we characterized normal and eight deficient plasma with the TGT assay by Technothrombin® (Fig. 2). Previously, Duchemin et al. [26] published results for the thrombin generation in deficient plasma using calibrated automated thrombography. The main difference between these two assays is a trigger. Duchemin et al. used 1 or 5 pM of tissue factor and 4 µM of phospholipid vesicles [26]. Technothrombin® reagent differs in ten-fold higher tissue factor content [27]. Both assays provide similar TGT results in normal plasma [28]. Here, we provide data for the comparison of two assays in deficient plasma.
Deficient plasma were produced using immunodepletion that could affect the contents of some non-target coagulation factors, providing some additional effect on thrombin generation. This gives one more reason for the careful comparison of new data with results published previously.
Prothrombin (factor II) and factor X are the most critical proteins for thrombin generation because the key reaction comprises prothrombin cleavage by factor Xa. The absence of either factor II or factor X leads to the absence of thrombin (Fig. 2B and C). Another protein with a great impact on thrombin generation is factor V, a cofactor of prothrombin cleavage with factor Xa; its absence dramatically decreases thrombin generation (Fig. 2B).
The absence of either factor VIII or factor IX has a moderate effect on thrombin generation, cutting peak thrombin concentration by more than half and slightly increasing the time to peak (Fig. 2C). Factors VIII and IX participate in the additional activation pathway of factor X.
Factors XI and XII have a low impact on thrombin generation as they are unnecessary for tissue-factor-triggered coagulation (Fig. 2D). All the data agree with the first experiments using the TGT on deficient plasmas [26].
Thrombin generation in factor VII deficient plasma (Fig. 2B) differs from the data of Duchemin et al. [26]. Factor VII is crucial for tissue-factor-triggered thrombin generation because it is the protein that interacts with the tissue factor, and the tissue factor–factor VIIa complex activates factor X. A high level of thrombin generation in factor VII deficient plasma can be explained if the residual content of factor VII in the immunodepleted plasma suffices to trigger the cascade under a high tissue factor concentration. Retardation of cascade triggering is shown with a doubled lag time, while the lag times of factor VIII- and factor IX-deficient plasma are the same as in normal plasma (Fig. 2B and C).
In contrast, Duchemin et al. [26] used a low tissue factor concentration to trigger the deficient plasma that yielded a significantly weaker thrombin generation. Hence, high trigger amounts could compensate for a low factor VII concentration and yield normal thrombin generation. These data are in agreement with van't Veer et al. [29], who showed that 100 pM of factor VIIa is sufficient for thrombin generation under a tissue factor concentration of 100 pM, but not 10 pM. These results highlight the ability of the cascade to function properly, even under the considerable deficiency of some proteins.
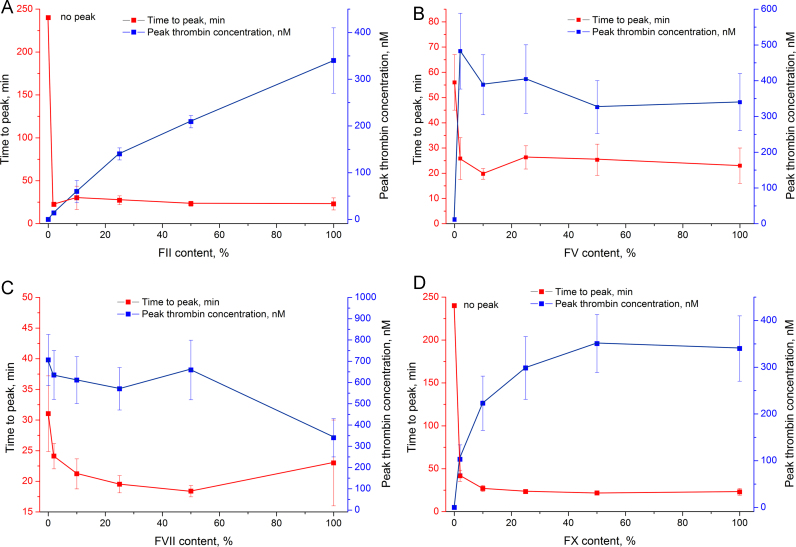
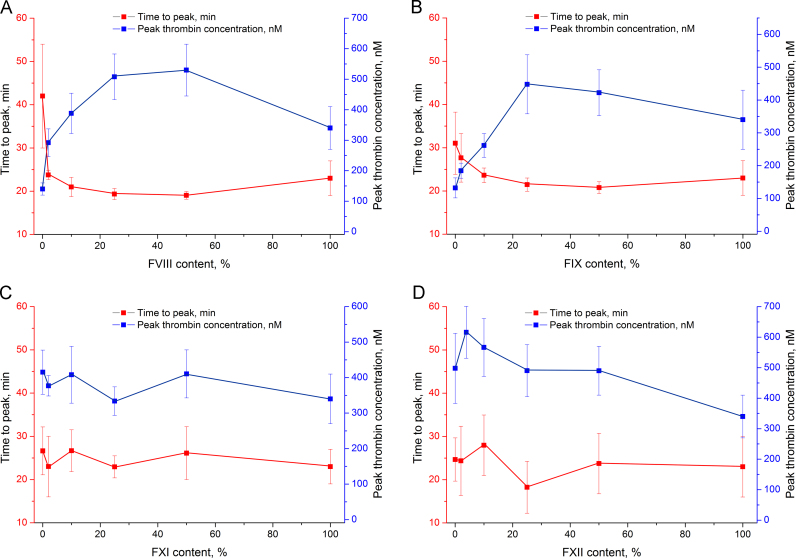
More detailed experiments were conducted to assess the dependence of thrombin generation on the particular factor content. Normal plasma (approx. 100% of the particular factor) and factor-deficient plasma (approx. 0% of the particular factor) were mixed, yielding a set of samples with nominal 0%, 2%, 10%, 25%, 50%, and 100% content of the particular factor. Peak thrombin concentration and time to peak were chosen as the most indicative parameters; their alteration upon changing the particular factor content is shown in Fig. 3, Fig. 4. The whole set of data is listed in Table 1.
Fig. 3.
Time to peak and peak thrombin concentration in plasma with different factor content: A – factor II, B – factor V, C – factor VII, and D – factor X deficient plasma (no peak – time to peak exceeds 240 min).
Fig. 4.
Time to peak and peak thrombin concentration in plasma with different factor content: A – factor VIII-, B – factor IX-, C – factor XI-, and D – factor XII-deficient plasma.
Table 1.
TGT parameters in plasma with factor content varied from 100% (normal plasma) to 0% (factor deficient plasma). The values are expressed in percentages from the values for normal plasma. n.m. – not measurable.
| Deficient factor | TGT parameters | Amounts of normal plasma |
|||||
|---|---|---|---|---|---|---|---|
| 100% | 50% | 25% | 10% | 2% | 0% | ||
| Factor II | Lag time | 100 | 90 | 100 | 110 | 150 | 380 |
| Peak thrombin concentration | 100 | 62 | 41 | 18 | 4 | n.m. | |
| Time to peak | 100 | 100 | 120 | 130 | 100 | n.m. | |
| ETP | 100 | 79 | 58 | 24 | 3 | n.m. | |
| Factor V | Lag time | 100 | 120 | 120 | 140 | 170 | 400 |
| Peak thrombin concentration | 100 | 100 | 120 | 110 | 140 | 4 | |
| Time to peak | 100 | 110 | 110 | 90 | 110 | 240 | |
| ETP | 100 | 110 | 130 | 110 | 120 | 3 | |
| Factor VII | Lag time | 100 | 70 | 75 | 95 | 130 | 190 |
| Peak thrombin concentration | 100 | 190 | 170 | 180 | 190 | 210 | |
| Time to peak | 100 | 80 | 85 | 95 | 100 | 130 | |
| ETP | 100 | 150 | 150 | 160 | 160 | 170 | |
| Factor VIII | Lag time | 100 | 60 | 60 | 60 | 68 | 75 |
| Peak thrombin concentration | 100 | 150 | 150 | 110 | 86 | 41 | |
| Time to peak | 100 | 83 | 84 | 91 | 103 | 180 | |
| ETP | 100 | 140 | 150 | 135 | 116 | 70 | |
| Factor IX | Lag time | 100 | 70 | 70 | 66 | 73 | 85 |
| Peak thrombin concentration | 100 | 120 | 130 | 77 | 54 | 39 | |
| Time to peak | 100 | 90 | 93 | 102 | 120 | 130 | |
| ETP | 100 | 120 | 130 | 90 | 82 | 67 | |
| Factor X | Lag time | 100 | 92 | 106 | 120 | 270 | 490 |
| Peak thrombin concentration | 100 | 100 | 90 | 66 | 30 | n.m. | |
| Time to peak | 100 | 95 | 100 | 120 | 180 | n.m. | |
| ETP | 100 | 108 | 105 | 90 | 41 | n.m. | |
| Factor XI | Lag time | 100 | 68 | 59 | 65 | 76 | 66 |
| Peak thrombin concentration | 100 | 120 | 100 | 120 | 110 | 120 | |
| Time to peak | 100 | 110 | 100 | 110 | 100 | 120 | |
| ETP | 100 | 120 | 110 | 135 | 120 | 140 | |
| Factor XII | Lag time | 100 | 58 | 55 | 56 | 53 | 65 |
| Peak thrombin concentration | 100 | 140 | 140 | 170 | 210 | 150 | |
| Time to peak | 100 | 100 | 85 | 120 | 100 | 110 | |
| ETP | 100 | 120 | 120 | 150 | 150 | 130 | |
The decrease in prothrombin content linearly reduced peak thrombin concentration, while time to peak drastically increased in the sample with 0% prothrombin (Fig. 3A). Factor X deficiency had a similar effect, but the curve of peak thrombin concentration was nonlinear, and its major curvature was observed in the 0–25% range of factor X content (Fig. 3D). The factor V curve was also nonlinear with major changes in the 0–2% range of factor V content (Fig. 3B). Extremely low peak thrombin concentration corresponds to only 2.5-fold increase of time to peak. Factor VII deficiency slightly increased time to peak and peak thrombin concentration (Fig. 3C).
Factors VIII and IX promote factor X activation. Deficiency of these factors decreased peak thrombin concentration 2–3-fold in the 0–25% range of factor content. Time to peak was doubled in the case of factor VIII deficiency; factor IX deficiency had a weaker effect on the parameter (Fig. 4A and B). Both factor XI and XII had negligible effects on peak thrombin concentration and time to peak in all concentration ranges (Fig. 4C and D).
A low content of the key coagulation factors except prothrombin decreased peak thrombin concentration nonlinearly with different curvatures; prothrombin deficient plasma showed a linear dependence. Time to peak increased abruptly at extremely low factor content.
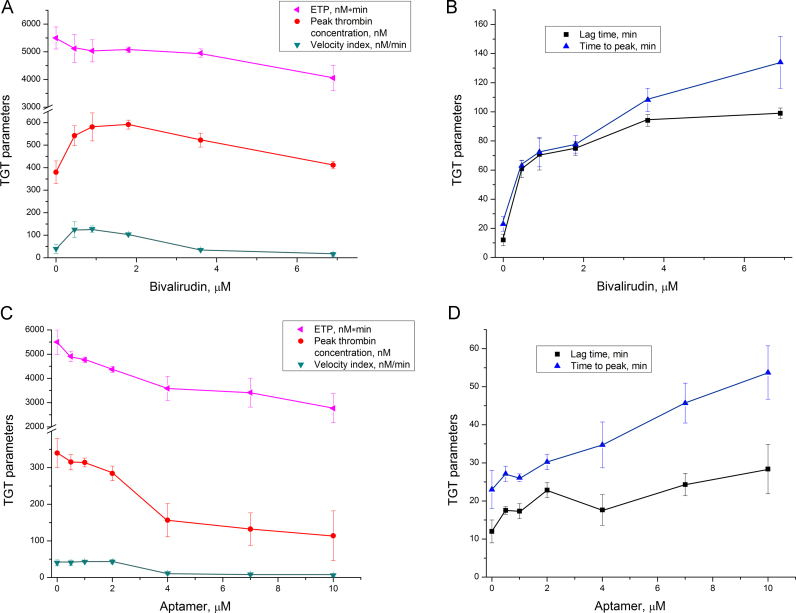
3.2. Thrombin generation in plasma with thrombin inhibitors
Bivalirudin and the DNA aptamer HD1 represent promising biodegradable classes of thrombin inhibitors with high specificity and low toxicity. These inhibitors affected thrombin generation differently. Both inhibitors increased lag time and time to peak in normal plasma; the effect of bivalirudin was approximately five times greater than that of the aptamer (Fig. 5). Bivalirudin substantially delayed the beginning of thrombin generation; just after the first thrombin amounts appeared, bulk thrombin amounts formed. Peak thrombin concentration was increased in the samples with bivalirudin, while ETP decreased slightly. These parameters indicate a steepening of the thrombin generation curve; the same amount of thrombin is produced in a shorter time period. Similar results were previously published by Tanaka et al. [17]. The aptamer inhibited the overall process, resulting in a decrease of ETP, peak thrombin concentration, and velocity index, as well as the increase of lag time and time to peak (Fig. 5C and D). In contrast, partial factor II deficiency decreased ETP, peak thrombin concentration, and velocity index, while lag time and time to peak were affected only in the total absence of prothrombin (Fig. 3A).
Fig. 5.
Thrombin generation in normal plasma in the presence of thrombin inhibitors: A, B – bivalirudin; C, D – aptamer HD1.
These inhibitors affect thrombin generation differently, and both differ from factor II deficient plasma in the TGT. These data indicate that thrombin-inhibitor complexes actively participate in protein–protein interactions forming nonproductive interactomes and thus provide an additional inhibitory action. Some confirmation could be found in Danforth et al. [30]; a 60% content of either factor V or IX or X alone did not increase time to peak, while 60% content of all three factors increased time to peak by 1.5-fold. Moderate inhibition of several coagulation factors could thus yield a pronounced effect. Studying the effect of thrombin inhibitors on deficient plasmas could elucidate thrombin feedbacks that are inhibited with aptamer and/or bivalirudin.
3.3. Thrombin generation in deficient plasmas with thrombin inhibitors
Bivalirudin and DNA aptamer HD1 were tested in factor deficient plasma with a nominal content of the particular factor of 0%, 2%, 10%, 25%, 50%, and 100%. The two inhibitors affected the TGT parameters differently. Time to peak was chosen as the main parameter for the comparison because it was increased by both inhibitors, while it was unchanged for most of the plasma samples and increased drastically in some samples with 0% nominal content of the particular factor.
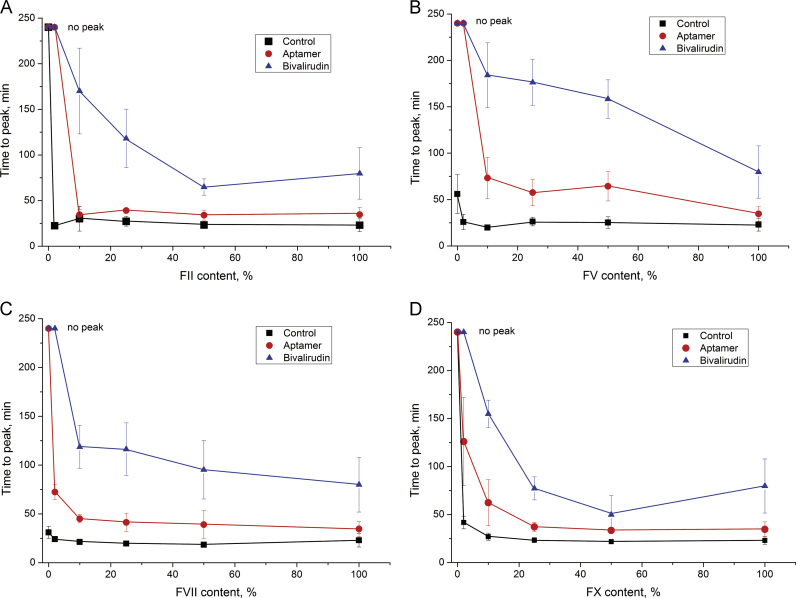
Thrombin inhibitors highlighted the ranking of the coagulation factors: bivalirudin and aptamer greatly decreased thrombin generation in the samples with a low content of factors II, V, VII, and X (Fig. 6). All of these factors are essential for tissue-factor-triggered coagulation. The most interesting results were obtained for plasma deficient in factors V or VII. Both plasma with a particular factor content of about <1% had delayed thrombin generation (control experiments), and bivalirudin and aptamer completely inhibited thrombin generation in these plasma. The effects of the thrombin inhibitors on factor V-deficient plasma were the most pronounced among all other samples. A possible reason for this phenomenon could be competition between the inhibitor and factor V for the thrombin exosite I. This exosite is necessary for factor V binding and activation with thrombin [31]. The aptamer binds the same thrombin exosite I; moreover, it binds the pro-exosite I of prothrombin. The latter binding mode leads to competition with factor Va in the prothrombin activation complex and thus inhibits thrombin generation [23]. These multilevel inhibitory actions of the aptamer could be a reason for the obvious difference between the time-to-peak alteration in factor II deficient plasma and in normal plasma with the aptamer. Bivalirudin also targets thrombin exosite I [21] and potentially could inhibit factor V activation with thrombin, being a more efficient anticoagulant drug than the aptamer.
Fig. 6.
Thrombin generation inhibited with bivalirudin and aptamer HD1 with different factor contents: A – factor II-, B – factor V-, C – factor VII-, and D – factor X-deficient plasma (no peak – time to peak exceeds 240 min).
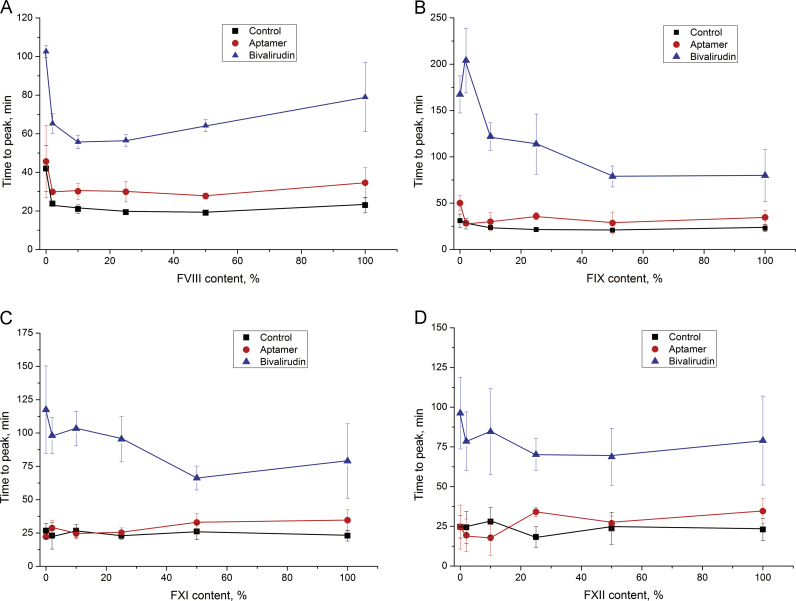
Factor VIII is also activated by thrombin, and this process could be inhibited by inhibitors of the thrombin exosite I. However, a deficiency of factor VIII per se, as well as its combination with thrombin inhibitors, affected thrombin generation only moderately (Fig. 7A). Similar effects were observed for factor IX-deficient plasma; bivalirudin inhibited thrombin generation more efficiently than the aptamer (Fig. 7B).
Fig. 7.
Thrombin generation inhibited with bivalirudin and aptamer HD1 with different factor content: A – factor VIII-, B – factor IX-, C – factor XI-, and D – factor XII-deficient plasma.
There were no statistically significant differences in the samples with different contents of factors XI and XII (Fig. 7C and D). Both factors had minor effects on tissue-factor-triggered coagulation, and the thrombin inhibitors did not yield any substantial changes in thrombin generation in the presence of a deficiency of these factors.
3.4. Potential anticoagulant drugs for dual targeting treatment
Plasma deficient in a particular factor is a model system for the anticoagulant action if we suppose that the inhibitor turns off all factor activities being an equivalent to the absence of the factor. But thrombin inhibitors provide an example of a multilevel inhibitory action. Bivalirudin and DNA aptamer HD1 bind thrombin exosite I and inhibit protein–protein interactions that are mediated by this site. Nevertheless, the thrombin-inhibitor complex still uses other thrombin sites for protein–protein interactions forming nonproductive interactomes. Free thrombin in fact competes with the thrombin-inhibitor complex for binding factors V and VIII. Another possibility for multilevel inhibition is the binding of prothrombin, which interferes with thrombin generation.
Considering a simple model of factor inhibition, we could rank coagulation proteins using the TGT with tissue factor as the trigger: (1) factors that are required for thrombin generation (II, V, VII, and X), (2) factors that are significant for thrombin generation (VIII and IX), and (3) factors that are irrelevant for tissue-factor-triggered thrombin generation (XI and XII). The first and second groups are potential anticoagulant drug targets. Inhibitors of proteins in the first group could totally prevent thrombin generation. Inhibitors of factors II/IIa and X/Xa could provide any extent of thrombin generation decrease, potentially yielding a wide therapeutic window. While inhibitors of factors V/Va and VII/VIIa could provide an “all or none” effect, they have limited therapeutic value because their therapeutic window is potentially narrow. Inhibitors of proteins in the second group could provide only a 2–3-fold decrease of thrombin generation; inhibition of factors IX/IXa is expected to impact thrombin generation more than inhibition of factors VIII/VIIIa. The potential therapeutic window is questionable because the efficacy of the inhibitors is initially restricted. These conclusions fully agree with the current drug market: all direct anticoagulant drugs target either factor Xa or thrombin (IIa); the RNA aptamer to factor IXa reached Phase III of the clinical trials [32]. Inhibitors of other factors stand aside, as they are currently in research and development.
Bivalirudin and aptamer HD1 demonstrated that inhibitors can exhibit a multilevel inhibitory mode. This ability is a result of two circumstances: (1) thrombin is involved in multiple feedbacks, and (2) the ligand could bind prothrombin, inhibiting its conversion to the thrombin. The first circumstance is unique to thrombin, but the second could be used to design new inhibitors of other factors.
Although anticoagulant drugs function in various ways, some anticoagulant drug combinations could be evaluated. We have assessed possible combinations based only on thrombin inhibitors. The most potent and promising combination could be created by combining thrombin and factor V/Va inhibitors; pronounced synergetic effects are expected in the case of partial inhibition of factor V with a wide therapeutic window. Combining thrombin inhibitors with inhibitors of factors VII/VIIa and X/Xa seems less promising because there is no effect plateau; the TGT parameters change rapidly as the factor content decreases. Factor IX is especially interesting because its restricted effect on thrombin generation could be accentuated with thrombin inhibitors. This combination could substantially decrease thrombin generation without the risk of preventing it completely. This feature could be extremely relevant for therapeutic applications. A thorough study of real anticoagulant drug combinations will elucidate specific features of the coagulation cascade functioning and help to find new, potent, and safe anticoagulant drug formulations.
Conflict of interest
The authors declare that they have no conflict of interest. This work was supported by the Russian Foundation for Basic Research, Grant nos. 14-04-32006 and 14-04-01757. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
E. Zavyalova contributed to the study design, performed experiments, analyzed data, and wrote the manuscript; A. Kopylov contributed to the study design and discussions of the results.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.11.011.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Mackman N. New insights into the mechanisms of venous thrombosis. J. Clin. Investig. 2012;122:2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furie B., Furie B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo D.J., Ferreiro J.L. Antiplatelet and anticoagulant therapy for atherothrombotic disease: the role of current and emerging agents. Am. J. Cardiovasc. Drugs. 2013;13:233–250. doi: 10.1007/s40256-013-0022-7. [DOI] [PubMed] [Google Scholar]
- 4.Page C., Pitchford S. Platelets and allergic inflammation. Clin. Exp. Allergy. 2014;44:901–913. doi: 10.1111/cea.12322. [DOI] [PubMed] [Google Scholar]
- 5.Weitz J.I. Expanding use of new oral anticoagulants. F1000 Prime Rep. 2014;6:93. doi: 10.12703/P6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mousa S.A. Novel anticoagulant therapy: principle and practice. Methods Mol. Biol. 2010;663:157–179. doi: 10.1007/978-1-60761-803-4_5. [DOI] [PubMed] [Google Scholar]
- 7.Iannopollo G., Camporotondo R., De Ferrari G.M., Leonardi S. Efficacy versus safety: the dilemma of using novel platelet inhibitors for the treatment of patients with ischemic stroke and coronary artery disease. Ther. Clin. Risk Manag. 2014;10:321–329. doi: 10.2147/TCRM.S39216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber K., Bates E.R., Valgimigli M. Antiplatelet and anticoagulation agents in acute coronary syndromes: what is the current status and what does the future hold? Am. Heart J. 2014;168:611–621. doi: 10.1016/j.ahj.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Lane D.A., Raichand S., Moore D. Combined anticoagulation and antiplatelet therapy for high-risk patients with atrial fibrillation: a systematic review. Health Technol. Assess. 2013;17:1–188. doi: 10.3310/hta17300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coagulation cascade by Enzyme Research Laboratories (cited 15.04.15). Available from: 〈http://www.enzymeresearch.co.uk/coag.htm〉.
- 11.Steffel J., Lüscher T.F., Tanner F.C. Tissue factor in cardiovascular diseases. Molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 12.Jesty J., Beltrami E. Positive feedbacks of coagulation their role in threshold regulation. Arterioscler. Thromb. Vasc. Biol. 2005;25:2463–2469. doi: 10.1161/01.ATV.0000187463.91403.b2. [DOI] [PubMed] [Google Scholar]
- 13.Geddings J.E., Mackman N. Recently identified factors that regulate hemostasis and thrombosis. Thromb. Haemost. 2014;111:570–574. doi: 10.1160/TH13-10-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Dieri R., de Laat B., Hemker H.C. Thrombin generation: what have we learned? Blood Rev. 2012;26:197–203. doi: 10.1016/j.blre.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Young G., Sorensen B., Dargaud Y. Thrombin generation and whole blood viscoelastic assays in the management of hemophilia: current state of art and future perspectives. Blood. 2013;121:1944–1950. doi: 10.1182/blood-2012-08-378935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castoldi E., Rosing J. Thrombin generation tests. Thromb. Res. 2011;127:S21–S25. doi: 10.1016/S0049-3848(11)70007-X. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K.A., Szlam F., Sun H.Y. Thrombin generation assay and viscoelastic coagulation monitors demonstrate differences in the mode of thrombin inhibition between unfractionated heparin and bivalirudin. Anesth. Analg. 2007;105:933–939. doi: 10.1213/01.ane.0000278868.23814.3b. [DOI] [PubMed] [Google Scholar]
- 18.Honda Y., Morishima Y. Thrombin generation induced by tissue factor plus ADP in human platelet rich plasma: a potential new measurement to assess the effect of the concomitant use of an oral factor Xa inhibitor edoxaban and P2Y12 receptor antagonists. Thromb. Res. 2015;135(5):958–962. doi: 10.1016/j.thromres.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Campo G., Pavasini R., Pollina A. Thrombin generation assay: a new tool to predict and optimize clinical outcome in cardiovascular patients? Blood Coagul. Fibrinolysis. 2012;23:680–687. doi: 10.1097/MBC.0b013e328355111f. [DOI] [PubMed] [Google Scholar]
- 20.Zavyalova E., Kopylov A. Multiple inhibitory kinetics reveal an allosteric interplay among thrombin functional sites. Thromb. Res. 2015;135(1):212–216. doi: 10.1016/j.thromres.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Priestle J.P., Rahuel J., Rink H. Changes in interactions in complexes of hirudin derivatives and human a-thrombin due to different crystal forms. Protein Sci. 1993;2:1630–1642. doi: 10.1002/pro.5560021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warkentin T.E., Koster A. Bivalirudin: a review. Expert Opin. Pharmacother. 2005;6(8):1349–1371. doi: 10.1517/14656566.6.8.1349. [DOI] [PubMed] [Google Scholar]
- 23.Kretz C.A., Stafford A.R., Fredenburgh J.C., Weitz J.I. HD1, a thrombin-directed aptamer, binds exosite 1 on prothrombin with high affinity and inhibits its activation by prothrombinase. J. Biol. Chem. 2006;281:37477–37485. doi: 10.1074/jbc.M607359200. [DOI] [PubMed] [Google Scholar]
- 24.Zavyalova E., Golovin A., Timoshenko T. DNA aptamers for human thrombin with high anticoagulant activity demonstrate target- and species-specificity. Curr. Med. Chem. 2012;19:5232–5237. doi: 10.2174/092986712803530575. [DOI] [PubMed] [Google Scholar]
- 25.Kimmelstiel C., Zhang P., Kapur N.K. Bivalirudin is a dual inhibitor of thrombin and collagen-dependent platelet activation in patients undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2011;4:171–179. doi: 10.1161/CIRCINTERVENTIONS.110.959098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duchemin J., Pan-Petesch B., Arnaud B. Influence of coagulation factors and tissue factor concentration on the thrombin generation test in plasma. Thromb. Haemost. 2008;99:767–773. doi: 10.1160/TH07-09-0581. [DOI] [PubMed] [Google Scholar]
- 27.Hron G., Kollars M., Binder B.R. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296(4):397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 28.Chandler W.L., Roshal M. Optimization of plasma fluorogenic thrombin-generation assays. Am. J. Clin. Pathol. 2009;132(2):169–179. doi: 10.1309/AJCP6AY4HTRAAJFQ. [DOI] [PubMed] [Google Scholar]
- 29.van ‘t Veer C., Golden N.J., Mann K.G. Inhibition of thrombin generation by the zymogen factor VII: implications for the treatment of hemophilia A by factor VIIa. Blood. 2000;95(4):1330–1335. [PubMed] [Google Scholar]
- 30.Danforth C.M., Orfeo T., Everse S.J. Defining the boundaries of normal thrombin generation: investigations into hemostasis. PLoS One. 2012;7:e30385. doi: 10.1371/journal.pone.0030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bukys M.A., Orban T., Kim P.Y. The structural integrity of anion binding exosite I of thrombin is required and sufficient for timely cleavage and activation of factor V and factor VIII. J. Biol. Chem. 2006;281:18569–18580. doi: 10.1074/jbc.M600752200. [DOI] [PubMed] [Google Scholar]
- 32.Denas G., Pengo V. Investigational anticoagulants for hematological conditions: a new generation of therapies. Expert Opin. Investig. Drugs. 2013;22:1281–1294. doi: 10.1517/13543784.2013.821463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material