Abstract
The purpose of this study was to investigate how CD44 impaired Akt phosphorylation, EGR-1 expression and cell proliferation. E6.1 Jurkat cells, which lack endogenous CD44 expression, were engineered to express CD44. Previously we showed that Akt is hypophosphorylated, EGR-1 expression is reduced and proliferation is impaired in CD44 expressing E6.1 Jurkat cells. The cell cycle was studied using flow cytometry and the role of calcium (Ca2+) in Akt phosphorylation and EGR-1 expression was investigated using Western blotting. Phosphatase activity was assessed using a commercially available kit. CD44 expressing cells showed disruption at the G1 to S transition. Chelation of Ca2+ from the culture media impaired Akt phosphorylation and EGR-1 expression in both CD44 expressing cells and the open vector control. Moreover, Ni2+ disrupted cell proliferation in both cell types suggesting Ca2+ import through calcium release activated calcium channels (CRAC). Staining of cells with fura-2 AM showed significantly higher Ca2+ in CD44 expressing cells as compared with the vehicle control. Finally, non-calcium mediated phosphatase activity was significantly greater in CD44 expressing cells. We propose that the enhanced phosphatase activity in the CD44 cells increased the dephosphorylation rate of Akt; at the same time, the increased intracellular concentration of Ca2+ in the CD44 cells ensured that the phosphorylation of Akt remains intact albeit at lower concentrations as compared with the vector control. Reduced Akt phosphorylation resulted in lowered expression of EGR-1 and hence, reduced the cell proliferation rate.
Keywords: CD44, Acute Lymphoblastic Leukemia, Calcium, Jurkat, Proliferation
Highlights
-
•
CD44 expression reduced Akt phosphorylation and EGR-1 expression.
-
•
CD44 expressing E6.1 Jurkat cells showed increased intracellular Ca2+ concentrations as compared with vector controls.
-
•
Akt phosphorylation and EGR-1 expression were Ca2+ dependent.
-
•
Enhanced phosphatase activity in the CD44 expressing E6.1 Jurkat cells increased the dephosphorylation of Akt; at the same time the increased intracellular Ca2+ concentration ensured that the phosphorylation of Akt remains intact albeit at lower concentrations as compared with the vector control.
-
•
Reduced Akt phosphorylation resulted in lowered expression of EGR-1 and reduced the cell proliferation rate.
1. Introduction
CD44 is a type I transmembrane glycoprotein. Although classically considered to mediate trafficking via its adhesive interactions with hyaluronan [1], [2], [3], CD44 has also been shown to regulate cell signaling and cell proliferation. CD44 lacks a signaling domain, however it can facilitate signal transduction by serving as a platform for molecular recruitment and assembly [4].
Several reports in a variety of cell types have shown that CD44 can augment cell proliferation responses. For example, CD44 can trigger mobilization of Ca2+ in aortic endothelial cells which in turn leads to their proliferation [5]. On the other hand, negative regulation of cell proliferation by CD44 has been reported only rarely. Gadhoum et al. showed that CD44 inhibited the proliferation of NB4 cells [6]. More recently, we have evaluated the proliferation of E6.1 Jurkat cells engineered to express the standard isoform of CD44 [7]. Importantly, E6.1 Jurkat cells do not endogenously express CD44. We found that CD44 significantly reduced cell proliferation and down-regulated early growth response-1 (EGR-1) protein. EGR-1 is a zinc finger transcription factor that can activate the promoters of many genes whose products can influence cell proliferation [8], [9]. Transfection of control E6.1 Jurkat cells with EGR-1 siRNA also inhibited cell proliferation, confirming its role. Disruption of the PI3K/Akt pathway with pharmacological inhibitors reduced EGR-1 expression and cell proliferation, mirroring the properties of CD44 expressing E6.1 Jurkat cells. Finally, Akt was hypophosphorylated in cells expressing CD44 showing its potential role in negatively regulating Akt activation. Results from these studies suggested a novel pathway in which CD44 can negatively regulate cell proliferation by Akt inactivation and down-regulated EGR-1 expression [7]. On the other hand, the mechanism of Akt inactivation was not determined.
Protein phosphatase 2A (PP2A) is known to be an important regulator of the cell cycle [10]. Moreover, Akt is one of its molecular targets [11]. Because PP2A has been shown to associate with CD44 in T cell leukemia [12] we hypothesized that Akt was being inactivated in CD44 expressing E6.1 Jurkat cells via phosphatase activity. Indeed, we found that calcium independent phosphatase activity was significantly enhanced in the CD44 expressing cells, but not the vector control cells. At the same time, Ca2+ from the culture environment ensured that the phosphorylation of Akt remains intact albeit at lower concentrations as compared with the vector control. Interestingly, Yasuoka et al. has previously reported that Ca2+ may also increase the expression of PP2A [13]. This may further suggest a role for Ca2+ in both inductive and suppressive proliferative functions in our system. In conclusion, our results suggest that reduced Akt phosphorylation due to phosphatase activity resulted in lowered expression of EGR-1 and hence, reduced the cell proliferation rate in CD44 expressing E6.1 Jurkat cells.
2. Materials and methods
2.1. Cell lines and tissue culture
E6.1 Jurkat cells were engineered to express CD44 or an open vector control as described previously [7]. Expression of CD44 was confirmed by flow cytometry (Supplementary Fig. 1). Cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin, 0.25 µg/mL amphotericin B, and 800 µg/mL Hygromycin B. Calcium was removed from media via ethylene glycol tetraacetic acid (EGTA) chelation at a concentration of 2.5 mM. LY294002 (Sigma-Aldrich #L9908) was used at a concentration of 10 µM for 24 h. Wortmannin (Sigma-Aldrich #W1628) was used at 5 µM for 24 h. Cantharidic acid (Enzo Life Sciences #BML-PR106-0010) was used at either 100 nM, 250 nM, or 500 nM as indicated for 24 h in Western blotting and 48 h in proliferation experiments.
2.2. Cell cycle analysis
Cells were synchronized by serum starvation for 24 h before each experiment. One million cells per mL were suspended in 70% ethanol and incubated at 20 °C for 2 h. Cells were centrifuged and washed in PBS twice before being incubated in 60 µg/mL propidium iodide (Sigma-Aldrich #P4170) and 10 µg/mL RNase A (Sigma-Aldrich #R6513) for 1 h at 37 °C. Cells were then analyzed via flow cytometry utilizing a Beckman Coulter Cytomics FC500.
2.3. BrdUrd incorporation
Cells were synchronized in the G1 phase by serum starvation for 24 h prior to experiments. 5-Bromo-2′-deoxyuridine (BrdUrd) was added to media at 10 µM and cells were cultured under normal conditions for 30 min. Samples were stained for BrdUrd according to kit manufacturer instructions (BD Biosciences #557891) before analysis via flow cytometry.
2.4. Proliferation assays
One hundred thousand cells per group were plated into 96 well plates in 200 µL of media. Cells were allowed to proliferate for 24 h or 48 h. Proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay (Promega #G5421). To rule out the possibility that an observed decrease in proliferation was due to a difference in viability, trypan blue staining was performed before and after incubation to assure that viability was maintained throughout the incubation period. Inducible calcium release activated channel (CRAC) inhibition was performed by adding 50 µM NiSO4 (Sigma-Aldrich #227676) to culture media.
2.5. Western blotting
Ten million cells were harvested and lysed with CelLytic M (Sigma-Aldrich #C2978) per the manufacturer's instructions. Protein concentration was assessed via the Bradford assay and equal amounts of protein were loaded onto a 4–20% acrylamide gel for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Protein was transferred from SDS-PAGE gels onto nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween 20 for 2 h before overnight incubation in primary antibody at 4 °C. EGR-1 was detected at 1:1000 dilution with anti-hEGR1 antibody (R&D Systems #AF2818). Phospho-Akt was detected at 1:1000 dilution with anti-pAkt S473 antibody (Cell Signaling #9271). Total Akt was detected at 1:1000 with anti-Akt antibody (Cell Signaling #9272). Beta-actin was used as a loading control and detected at 1:1000 dilution with anti-beta-actin antibody (Cell Signaling #4967). Blotting with secondary goat anti-rabbit antibodies conjugated to alkaline phosphatase (Invitrogen) was performed before visualization with a UVP GelDoc-It imaging system.
2.6. Intracellular calcium measurements
One million cells per mL were suspended in PBS without calcium. Fura-2-AM was loaded into the cells at 6 µM and then incubated for 15 min at 37 °C. The suspension was diluted a further one fourth with PBS and incubated for 15 min at 37 °C. After washing, cells were suspended in a buffer containing 140 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 20 mM HEPES, 1 mM NaH2PO4, and 5.5 mM glucose at pH 7.4. Cells were placed on coverslips coated in poly-l-lysine and incubated for 10 min at 37 °C before imaging. Intracellular calcium was measured at 37 °C by the ratiometric technique using fura-2-AM (excitation at 340 nm and 380 nm, emission at 510 nm) (Invitrogen, Carlsbad, CA) as described by utilizing a Nikon Eclipse TE2000-5 microscope and NIS-Elements AR3.2 software (Nikon Instruments, Melville, NY) [14]. The Grynkiewicz equation was used to convert the 340 nm/380 nm ratio to internal levels of calcium in nanomolar concentrations [15].
2.7. Phosphatase assay
Phosphatase activity in the cells was measured using the kit from Enzo Life Sciences (#BML-AK804-0001). Briefly, cell protein extracts were chilled on ice in protease inhibitor lysis buffer. Extracts were then desalted using a resin column and the amount of recovered protein was estimated by the Bradford method. Equal concentrations of proteins were added to wells containing RII phosphopeptide substrate. Wells containing EGTA were used to determine calcineurin activity. Results were expressed as nmoles of phosphate per well after development with BIOMOL Green and absorption at 655 nm.
2.8. JC-1 mitochondrial staining
One million cells per sample were stained with 5μM JC-1 dye from Invitrogen (#T3168) by incubating at 37 °C for 15 min. Cells were spun down and washed once in warm PBS. An ISS PC1 spectrofluorometer was used to measure the fluorescence intensity of each cell sample at an ex/em of 485 nm/530 nm for the monomeric form of JC-1 and an ex/em of 535 nm /590 nm for the aggregate form of JC-1.
2.9. Statistical analysis
Two groups were compared by the 2-tailed Student's t-test and more than two groups by the ANOVA with Tukey's multiple comparisons test. A p-value of less than 0.05 was considered significant. All results are shown as the mean±SD. Each experiment was repeated thrice with similar results in the experiments.
3. Results
3.1. CD44 expression causes cell cycle arrest
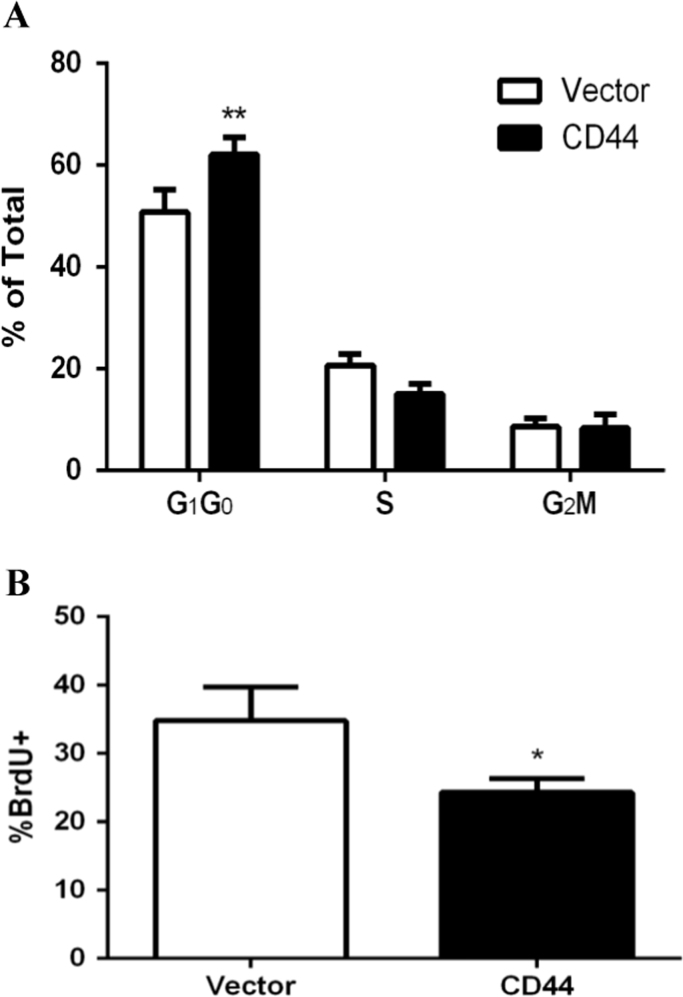
Our laboratory has previously shown that EGR-1 regulates the proliferation of E6.1 Jurkat cells and that CD44 expression decreases the expression of EGR-1 [7]. The doubling time for CD44 expressing E6.1 Jurkat cells is 34.67±0.58 h as compared with a doubling time of 23.00±1.00 h for E6.1 Jurkat cells lacking CD44 expression (values are presented as the means±standard deviations). This significant increase in doubling time (P<0.01) is evidence that CD44 significantly perturbs E6.1 Jurkat cell proliferation. Due to EGR-1's importance in regulating the cell cycle [16], [17], [18], [19] we wanted to examine potential dysregulations of the cell cycle due to CD44 expression. E6.1 Jurkat cells expressing CD44 were found to have a higher accumulation of cells in the G1/G0 phase of the cell cycle as compared with the vector control (Fig. 1A). To determine if there was a deficiency in CD44 expressing cells when progressing through the G1 to S phase checkpoint we directly measured the rate at which the cells transitioned between phases. Vector control and CD44 expressing E6.1 Jurkat cells were synchronized in the G1 phase of the cell cycle and then allowed to proliferate for 30 min in the presence of BrdUrd. CD44 expressing cells showed a slower transition from the G1 phase into the S phase when compared with the vector control cells (Fig. 1B). These data show that CD44 expression triggers a disruption in cell cycle at the G1 to S phase transition, which is consistent with a decrease in EGR-1 expression which is a transcription factor responsible for the expression of early genes within the cell cycle.
Fig. 1.
CD44 expressing Jurkat T cells are arrested in phase G1. (A) Cell cycle analysis was performed via propidium iodide staining and flow cytometry. Results show representative data from four repeated experiments. (B) BrdUrd incorporation was measured in cells synchronized in the G1 phase of the cell cycle after being allowed to proliferate for 30 min. Results show representative data from four repeated experiments. Asterisks indicated statistical significance as assessed using the 2-tailed Student's t-test (*p<0.05, **p<0.01).
3.2. CD44 expression induces calcium influx
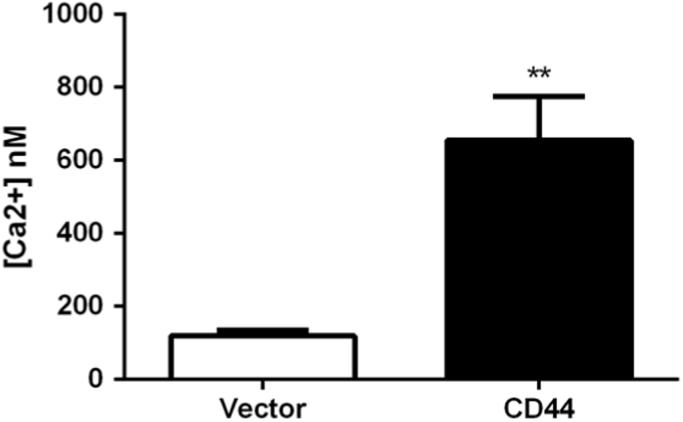
Cells were loaded with Fura-2 on poly-l-lysine coated slides in order to measure their intracellular calcium concentrations. E6.1 Jurkat cells expressing CD44 were found to have significantly more intracellular calcium as compared with the vector control cells (Fig. 2, Supplementary Fig. 2). These results showed that CD44 expression disrupts calcium homeostasis.
Fig. 2.
CD44 expression induces calcium influx. Resting intracellular calcium concentration was assessed by ratiometric imaging of fura-2-AM loaded cells. Multiple images were taken for ratiometric assessment. The asterisk indicates the statistical significance using the 2-tailed Student's t-test (n=40 cells; **p<0.01).
3.3. CD44 expression induced Ca2+ influx uses CRAC channels to regulate proliferation and Ca2+ is required for proper cell cycling
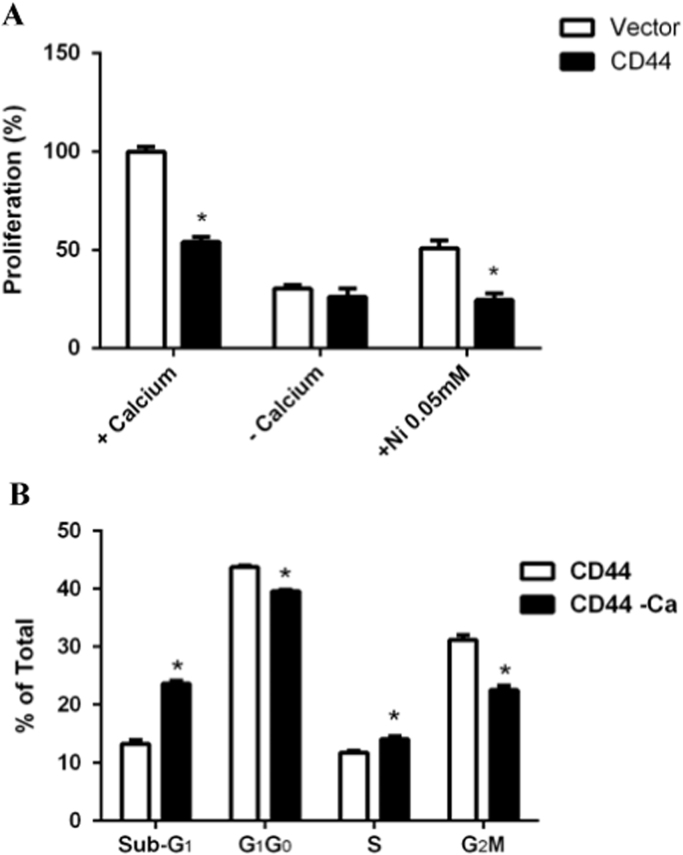
To determine if E6.1 Jurkat cells were utilizing Ca2+ release activated calcium channels (CRAC) for their calcium influx we cultured cells with the inhibitor, Ni2+ [20], [21], [22]. While both CD44 expressing and vector control E6.1 Jurkat cells were both inhibited by Ni2+ the vector control cells proliferated twice as much as the CD44 expressing cells. These results show that E6.1 Jurkat cells require Ca2+ for their proliferation (Fig. 3A). To determine if excess calcium influx was responsible for the delay in the cell cycle seen in Fig. 1 we synchronized CD44 expressing E6.1 Jurkat cells and cultured them with and without extracellular calcium. CD44 expressing E6.1 Jurkat cells required Ca2+ for their proliferation and showed significantly greater death rates without Ca2+ (i.e., the increased sub-G1 phase of the cell cycle; Fig. 3B).
Fig. 3.
CD44 expression induced Ca2+ influx uses CRAC channels to regulate proliferation and Ca2+ is required for proper cell cycling. (A) Cell proliferation with extracellular calcium, without extracellular calcium, and with Ni2+, an inhibitor of CRAC channels under normal extracellular calcium levels. Results are normalized to percent of the vector control cells with calcium. (B) Cell cycle analysis was performed on CD44 expressing cells via propidium iodide staining and flow cytometry after incubation in calcium containing (open bars) or calcium free (closed bars) media for 24 h after synchronization. Asterisks indicate the statistical significance using the 2-tailed Student's t-test (*p<0.05).
3.4. EGR-1 expression is regulated by Ca2+
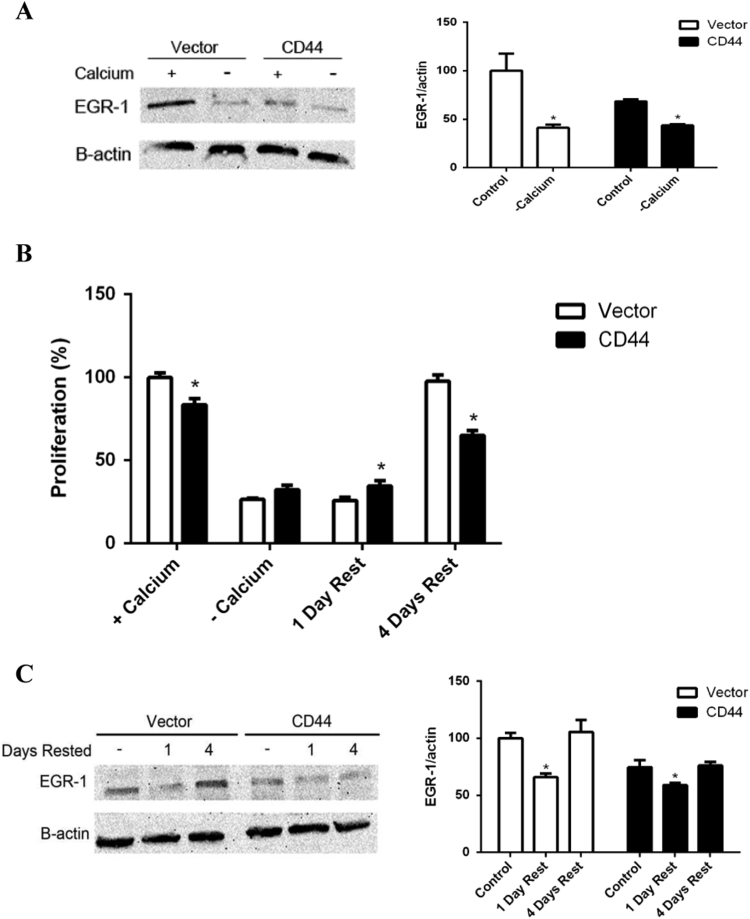
EGR-1 expression is regulated in part by Ca2+ signaling [23], [24]. In order to determine if EGR-1 is regulated by calcium in E6.1 Jurkat cells, we cultured them and the vector control cells with and without Ca2+. EGR-1 expression was reduced in both vector control cells and CD44 expressing cells (Fig. 4A), indicating that Ca2+ is required for EGR-1 expression. Upon culture in media without Ca2+ for 24 h, both CD44 expressing and vector control cells proliferated less than those cultured in the presence of Ca2+ (Fig. 4B). In order to determine if this decrease in proliferation due to Ca2+ deprivation was a permanent or transient effect, we restored Ca2+ to cells which had been deprived of extracellular Ca2+. After one day of restored Ca2+ in the media, both the CD44 expressing and vector control E6.1 Jurkat cells showed proliferation equivalent to cells without available Ca2+ (Fig. 4C). These results indicate that Ca2+ is required for both the vector control and CD44 expressing E6.1 Jurkat cells. After four days of restored Ca2+, EGR-1 expression proliferation returned to its baseline in both CD44 expressing and vector control cells (Fig. 4D). These data show that Ca2+ is required for EGR-1 expression and proliferation.
Fig. 4.
EGR-1 expression is regulated by Ca2+. (A) Western blot for EGR-1 expression with and without calcium. A representative blot is shown as the left panel and cumulative densitometry values are shown as the right panel. (B) Cell proliferation before, during, and after a 24 h period without extracellular calcium. Results are normalized to percent of control cell line with calcium. There was no difference between Vector and CD44 cell lines without calcium (p>0.05). (C) Western blotting for EGR-1 expression before removal of extracellular calcium, after one day of rest following 24 h without extracellular calcium, and after four days of rest following 24 h without extracellular calcium. A representative blot is shown as the left panel and cumulative densitometry values are shown as the right panel. Densitometry values from three different Western blots were used to calculate the means ± SD. Asterisks indicated statistical significance using the 2-tailed Student's t-test (A and B) or the ANOVA with Turkey's multiple comparisons test (C) (*p<0.05;**p<0.01).
3.5. CD44 regulates EGR-1 through Ca2+ signaling and the Akt pathway
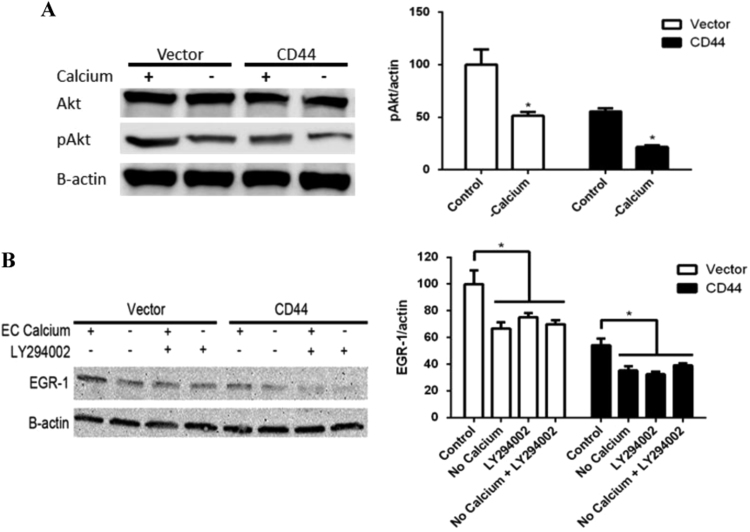
To investigate the possibility that CD44 expression regulates Akt phosphorylation through Ca2+ signaling, we cultured cells with and without Ca2+ and measured the phosphorylation of Akt. As we have previously shown, Akt was hypophosphorylated in cells expressing CD44 under normal conditions [7]. When Ca2+ signaling is removed through chelation with EGTA, both the vector control cells and CD44 expressing Jurkat T cells have hypophosphorylated Akt (Fig. 5A). The observed decrease due to the removal of calcium was proportional in each cell line, with both control and CD44 expressing cells having approximately half as much phosphorylated Akt.
Fig. 5.
CD44 regulates EGR-1 through Ca2+ signaling and the Akt pathway. (A) Western blot for total Akt and phosphorylated Akt with and without extracellular calcium. A representative blot is shown as the left panel and cumulative densitometry values are shown as the right panel. (B) Western blot for EGR-1 expression with extracellular calcium (EC calcium), without EC calcium, with an inhibitor of Akt activation, and without EC calcium and an Akt inhibitor. A representative blot is shown as the left panel and cumulative densitometry values are shown as the right panels. Densitometry values from three different Western blots were used to calculate the means±SD. Asterisks indicated statistical significance using the 2-tailed Student's t-test (A) or the ANOVA with Turkey's multiple comparisons test (B) (*p<0.05).
To determine if Akt regulation of EGR-1 expression occurs upstream or downstream of Ca2+ based EGR-1 expression, we cultured cells with Akt inhibitor LY294002 in addition to the removal of extracellular calcium. Importantly, LY294002 was non-toxic to cells. Moreover, Wortmannin provided similar results as previously reported [7]. EGR-1 expression was reduced in control and CD44 expressing Jurkat cells in response to lack of extracellular Ca2+, Akt inhibition, and both the lack of extracellular Ca2+ and Akt inhibition (Fig. 5B). We interpret these data to suggest that CD44 expression results in decreased Ca2+ mediated Akt phosphorylation through another mechanism, potentially increased phosphatase activity.
3.6. CD44 increases phosphatase activity in E6.1 Jurkat cells
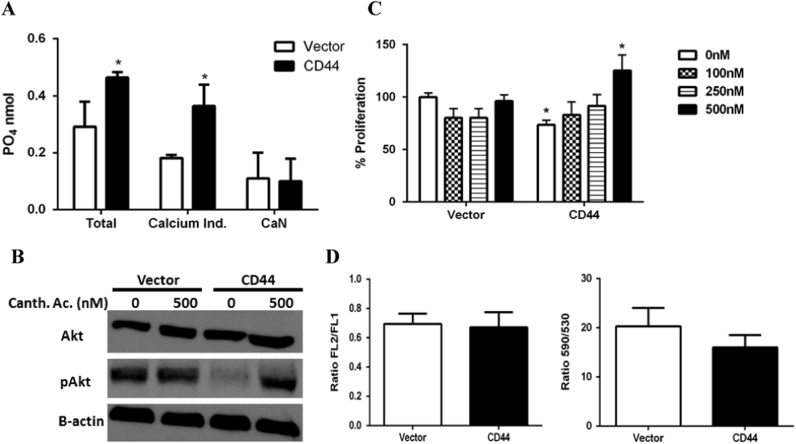
In the above experiments, Ca2+ was shown to play a role in both EGR-1 expression and Akt phosphorylation. Because CD44 expressing cells showed significantly higher concentrations of Ca2+, how can we explain Akt hypophosphorylation and reduced EGR-1 expression? Rajasagi et al. has shown that CD44 is associated with the expression of phosphatase enzymes [12]. We therefore hypothesized that CD44 expressing E6.1 Jurkat cells could potentially express higher activities of phosphatases. As shown in Fig. 6A, total phosphatase activity was significantly higher in CD44 expressing cells as compared with the vector control. Calcineurin, which is a Ca2+ dependent phosphatase did not have significantly different activities between the CD44 expressing and non-expressing cells. Yasuoka et al. showed that elevated Ca2+ levels in myocardiac H9c2 cells decreases the activation of Akt by inducing PP2A expression and activity [13]. Similarly, as shown in Fig. 6B, we found that use of the PP2A inhibitor cantharidic acid resulted in increased phosphorylation of Akt after 24 h in CD44 expressing cells, but not the vector control cells. Inhibition of PP2A with cantharidic acid also rescued the proliferation of CD44 expressing E6.1 Jurkat cells, and the increase in proliferation over the control baseline is not observed in similarly treated vector control cells (Fig. 6C). Finally, the manuscript by Rajasagi et al. also shows that CD44 association with PP2A in EL4 T lymphoma cells results in apoptosis via mitochondrial depolarization [12]. By contrast, we found that CD44 expression in Jurkat E6.1 cells does not induce apoptosis [7] nor does it impact mitochondria polarization [12] (Fig. 6D). Thus, CD44 appears to have different effects based on the genetic background of the cell type under investigation.
Fig. 6.
CD44 increases phosphatase activity in E6.1 Jurkat cells. (A) A phosphatase activity assay specific for PP1, PP2A, calcineurin, and PP2C. Total represents all measured phosphatase activity. Calcium Independent (Calcium Ind.) is the activity of PP1, PP2A, and PP2C. CaN is the activity of just calcineurin. (B) Western blot for total Akt and phosphorylated Akt with cantharidic acid. The Western blot was repeated 3 times with similar results. (C) Proliferation of cells with different concentrations of cantharidic acid over 48 h. Results are normalized to untreated vector control cells. (D) JC-1 staining for mitochondrial depolarization as measured by flow cytometry (left panel) and spectrofluorometry (right panel). Results are expressed as the ratio of red mitochondrial associated dye to green cytosol associated dye. Asterisks indicated statistical significance using the 2-tailed Student's t-test (A) or the ANOVA with Turkey's multiple comparisons test (C) (*p<0.05).
4. Discussion
We previously have shown that expression of CD44 in Jurkat E6.1 cells significantly impaired cell proliferation by reducing Akt activation and EGR-1 expression. The purpose of these current studies was to determine the mechanism whereby CD44 inactivates Akt, down-regulates EGR-1 and impairs cell proliferation. Here, we advance our previous studies by showing that CD44 expression enhances the uptake of Ca2+ and increases the activity of phosphatase enzymes.
Chelation of Ca2+ significantly reduced Akt phosphorylation and EGR-1 expression in both CD44 expressing and control vector cells. Although it is also possible that EGR-2 and EGR-3, which can serve as negative regulators for EGR-1 expression play a role in its expression, our finding that LY294002 impairs EGR-1 expression strongly suggests that the PI3K/Akt pathway is the major pathway for EGR-1 expression in E6.1 Jurkat cells. CD44 expressing E6.1 Jurkat cells showed an increase in the S phase of the cell cycle when the extracellular calcium was removed and increased cell death. This result provides further support to Fig. 3A, reinforcing the notion that extracellular Ca2+ is required for proliferation in CD44 expressing E6.1 Jurkat cells. Finally, cell proliferation was significantly impaired by Ni2+ which is an inhibitor of CRAC channels. Therefore, we reasoned that Ca2+ is responsible for the Akt mediated expression of EGR-1 in both CD44 expressing and vector control Jurkat E6.1 cells and for their proliferation.
Analyses showed that CD44 dysregulated Ca2+ homeostatsis as compared with the vector control cells. The concentration of intracellular Ca2+ in CD44 expressing cells was nearly three times higher than the intracellular concentration of Ca2+ in vector control cells. Because Ca2+ was shown to be requisite for Akt phosphorylation and EGR-1 expression it would seem reasonable to postulate that CD44 expressing cells should have higher concentrations of activated Akt and EGR-1 and proliferate at a greater rate than vector control cells. On the contrary, we have observed that Akt was hypophosphorylated and EGR-1 expression was reduced in CD44 expressing cells. At the same time, we also found a significant increase in the activities of phosphatases in the CD44 expressing cells. The phosphatase PP2A has been immunoprecipitated with CD44 in EL4 T lymphoma cells and cross-linkage of CD44 has been shown to activate PP2A in EL4 T lymphoma cells [12]. Increased intracellular calcium has been shown in H9c2 myocardiac cells to increase the expression and activity of PP2A, resulting in decreased Akt phosphorylation [13]. We found that inhibition of PP2A with cantharidic acid resulted in increased phosphorylation of Akt similar to the vector control cells. Cantharidic acid had no overall effect on the phosphorylation of Akt in the control cells, suggesting a CD44 specific effect on PP2A activity. We propose that the increased phosphatase activity in the CD44 cells increases the dephosphorylation rate of Akt. Previous investigations have shown that phosphatases can directly dephosphorylate Akt [25], [26]. At the same time, the increased intracellular concentration of Ca2+ in the CD44 cells ensures that the phosphorylation of Akt remains intact albeit at lower concentrations as compared with the vector control cells. We propose that the lower concentration of active Akt in the CD44 expressing cells results in lower concentrations of EGR-1, hence reduced proliferation rates.
E6.1 Jurkat cells are a T cell lymphoblastic leukemia line. Previous investigations have shown that there is a link between CD44 expression and T cell lymphoblastic leukemia. Cavalcanti et al. found that CD44 expression was detected in 77% of patients with disease and was correlated with mediastinal mass, adrenomegaly, infiltration of the central nervous system and other organs. This group proposed that CD44 could be regarded as a marker for tissue infiltration in T cell leukemia [27]. Similarly, Viskova et al. found that high levels of CD44 expression were linked to a highly aggressive subtype of acute lymphoblastic leukemia [28]. Khan et al. found that CD44 surface expression predicted relapse in children with acute lymphoblastic leukemia [26]. More recently Kamazani et al. found that CD44 expression in acute lymphoblastic leukemia was correlated with higher white blood cell counts, a higher percentage of blast cells in the bone marrow, and that CD44 expression could serve as predictor of responsiveness to therapy [27]. Although we currently do not know the impact of CD44 expression on the in vivo lethality of E6.1 Jurkat cells, current experiments in mice are planned in our laboratory to determine its effect.
In conclusion, we have reported a unique functional role for CD44 in regulating cell proliferation. Results and experimental systems described in this study provide the technical and conceptual foundation for further investigating the role of CD44 in cell proliferation. Future experiments showing the effect of CD44 expression in vivo may elucidate the mechanistic relationship between CD44 expression in acute lymphoblastic leukemia and the negative outcomes associated with CD44 in clinical studies.
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.03.016.
Appendix A. Supplementary material
Supplementary materials
.
Supplementary figures 1 and 2.
.
References
- 1.DeGrendele H., Estess P., Picker L.J., Siegelman M.H. CD44 and its ligand hayluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J. Exp. Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeGrendele H.C., Estess P., Siegelman M.H. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 3.Mohamadzadeh M., DeGrendele H., Arizpe H., Estess P., Siegelman M. Proinfammatory stimuli regulate endothelial hyaluronan expression and CD44-HA-dependent primary adhesion. J. Clin. Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponta H., Sherman L., Herrlich P.A. CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 5.Singleton P.A., Bourguignon L.Y. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp. Cell Res. 2004;295:102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Gadhoum Z., Leibovitch M.P., Qi J., Dumenil D., Durand L., Leibovitch S., Smadja-Joffe F. CD44: a new means to inhibit acute myeloid leukemia cell proliferation via p27Kip1. Blood. 2004;103:1059–1068. doi: 10.1182/blood-2003-04-1218. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L.S., Ma H.W., Greyner H.J., Zuo W., Mummert M.E. Inhibition of cell proliferation by CD44: Akt is inactivated and EGR-1 is down-regulated. Cell Prolif. 2010;43:385–395. doi: 10.1111/j.1365-2184.2010.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao X., Koski R.A., Gashler A., McKiernan M., Morris C.F., Gaffney R., Hay R.V., Sukhatme V.P. Identification and characterization of the egr-1 gene product, a DNA-binding zince finger protein induced by differentiation and growth signals. Mol. Cell. Biol. 1990;10:1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X., Mahendran R., Guy G.R., Tan Y.H. Detection and characterization of cellular EGR-1 binding to its recognition site. J. Biol. Chem. 1993;268:16949–16957. [PubMed] [Google Scholar]
- 10.Perrotti D., Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013;14:e229–e238. doi: 10.1016/S1470-2045(12)70558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Z. Surmeli, P. Gursoy, A.P. Erdogan, E. Bozkurt, H. Atmaca, S. Uzunoglu, C. Sezgin, U.A. Sanli, R. Uslu, B. Karaca, Combination of zoledronic acid and serine/threonine phosphatase inhibitors induces synergistic cytotoxicity and apoptosis in human breast cancer cells via inhibition of PI3K/Akt pathway, Tumour Biology, 2015. [DOI] [PubMed]
- 12.Rajasagi M., von Au A., Singh R., Hartmann N., Zoller M., Marhaba R. Anti-CD44 induces apoptosis in T lymphoma via mitochondrial depolarization. J. Cell. Mol. Med. 2010;14:1453–1467. doi: 10.1111/j.1582-4934.2009.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasuoka C., Ihara Y., Ikeda S., Miyahara Y., Kondo T., Kohno S. Antiapoptotic activity of akt is down-regulated by Ca2+ in myocardiac H9c2 cells. Evidence of Ca(2+)-dependent regulation of protein phosphatase 2Ac. J. Biol. Chem. 2004;279:51182–51192. doi: 10.1074/jbc.M407225200. [DOI] [PubMed] [Google Scholar]
- 14.Dibas A.I., Rezazadeh S.M., Vassan R., Mia A.J., Yorio T. Mechanism of vasopressin-induced increase in intracellular Ca2+ in LLC-PK1 porcine kidney cells. Am. J. Physiol. 1997;272:C810–C817. doi: 10.1152/ajpcell.1997.272.3.C810. [DOI] [PubMed] [Google Scholar]
- 15.Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 16.Fahmy R.G., Khachigian L.M. Antisense egr-1 RNA driven by the CMV promoter is an inhibitor of vascular smooth muscle cell proliferation and regrowth after injury. J. Cell. Biochem. 2002;84:575–582. [PubMed] [Google Scholar]
- 17.Wada Y., Fujimori M., Suzuki J., Tsukioka K., Ito K., Sawa Y., Morishita R., Kaneda Y., Isobe M., Amano J. Egr-1 in vascular smooth muscle cell proliferation in response to allo-antigen. J. Surg. Res. 2003;115:294–302. doi: 10.1016/s0022-4804(03)00213-0. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell A., Dass C.R., Sun L.Q., Khachigian L.M. Inhibition of human breast carcinoma proliferation, migration, chemoinvasion and solid tumour growth by DNAzymes targeting the zinc finger transcription factor EGR-1. Nucleic Acids Res. 2004;32:3065–3069. doi: 10.1093/nar/gkh626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Malak N.A., Mofarrahi M., Mayaki D., Khachigian L.M., Hussain S.N. Early growth response-1 regulates angiopoietin-1-induced endothelial cell proliferation, migration, and differentiation. Arterioscler. Thromb. Vasc. Biol. 2009;29:209–216. doi: 10.1161/ATVBAHA.108.181073. [DOI] [PubMed] [Google Scholar]
- 20.Singleton P.A., Bourguignon L.Y. CD44v10 interaction with rho-kinase (ROK) activates inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ signaling during hyaluronan (HA)-induced endothelial cell migration. Cell Motil. Cytoskelet. 2002;53:293–316. doi: 10.1002/cm.10078. [DOI] [PubMed] [Google Scholar]
- 21.Ng L.C., O’Neill K.G., French D., Airey J.A., Singer C.A., Tian H., Shen X.M., Hume J.R. TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca(2+) entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells. Am. J. Physiol. Cell. Physiol. 2012;303:C1156–C1172. doi: 10.1152/ajpcell.00065.2012. [DOI] [PubMed] [Google Scholar]
- 22.Wang S.J., Peyrollier K., Bourguignon L.Y. The influence of hyaluronan-CD44 interaction on topoisomerase II activity and etoposide cytotoxicity in head and neck cancer. Arch. Otolaryngol. Head. Neck. Surg. 2007;133:281–288. doi: 10.1001/archotol.133.3.281. [DOI] [PubMed] [Google Scholar]
- 23.Thiel G., Mayer S.I., Muller I., Stefano L., Rossler O.G. Egr-1-A Ca(2+)-regulated transcription factor. Cell Calcium. 2010;47:397–403. doi: 10.1016/j.ceca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Taher T.E., Smit L., Griffioen A.W., Schilder-Tol E.J., Borst J., Pals S.T. Signaling through CD44 is mediated by tyrosine kinases. Association with p56lck in T lymphocytes. J. Biol. Chem. 1996;271:2863–2867. doi: 10.1074/jbc.271.5.2863. [DOI] [PubMed] [Google Scholar]
- 25.Xu W., Yuan X., Jung Y.J., Yang Y., Basso A., Rosen N., Chung E.J., Trepel J., Neckers L. The heat shock protein 90 inhibitor geldanamycin and the ErbB inhibitor ZD1839 promote rapid PP1 phosphatase-dependent inactivation of AKT in ErbB2 overexpressing breast cancer cells. Cancer Res. 2003;63:7777–7784. [PubMed] [Google Scholar]
- 26.Khan N., Cisterne A., Devidas M., Shuster J., Hunger S.P., Shaw P.J., Bradstock K.F., Bendall L.J. Expression of CD44, but not CD44v6, predicts relapse in children with B cell progenitor acute lymphoblastic leukemia lacking adverse or favorable genetics. Leuk. Lymphoma. 2008;49:710–718. doi: 10.1080/10428190701861660. [DOI] [PubMed] [Google Scholar]
- 27.Kamazani F.M., Bahoush G.R., Aghaeipour M., Vaeli S., Amirghofran Z. CD44 and CD27 expression pattern in B cell precursor acute lymphoblastic leukemia and its clinical significance. Med. Oncol. 2013;30:359–366. doi: 10.1007/s12032-012-0359-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials
Supplementary figures 1 and 2.