Abstract
The CD98 heavy chain (CD98hc) regulates virus-induced cell fusion and monocyte fusion, and is involved in amino acid transportation. Here, we examined the role that CD98hc plays in the formation of osteoclasts using CD98hcflox/floxLysM-cre peritoneal macrophages (CD98hc-defect macrophages). Peritoneal macrophages were stimulated with co-cultured with osteoblasts in the presence of 1,25(OH)2 vitamin D3, and thereafter stained with tartrate-resistant acid phosphatase staining solution. The multinucleated osteoclast formation was severely impaired in the peritoneal macrophages isolated from the CD98hc-defect mice compared with those from wild-type mice. CD98hc mediates integrin signaling and amino acid transport through the CD98 light chain (CD98lc). In integrin signaling, suppression of the M-CSF-RANKL-induced phosphorylation of ERK, Akt, JNK and p130Cas were observed at the triggering phase in the CD98h-defect peritoneal macrophages. Moreover, we showed that the general control non-derepressible (GCN) pathway, which was activated by amino acid starvation, was induced by the CD98hc-defect peritoneal macrophages stimulated with RANKL. These results indicate that CD98 plays two important roles in osteoclast formation through integrin signaling and amino acid transport.
Keywords: CD98 heavy chain, Osteoclast formation, RANKL, Integrin signaling, Amino acid transport, Amino acid starvation
Highlights
-
•
The osteoclastogenesis was severely impaired in the CD98hc-defect macrophages.
-
•
CD98hc-defect peritoneal macrophages fall into amino acid starvation, resulting in inducing the general control non-derepressible (GCN) pathway in the osteoclastogenesis.
1. Introduction
Osteoclasts are multinucleated giant cells responsible for bone resorption and are derived from monocytes/macrophages. The receptor activator of the NF-kb ligand (RANKL) plays a critical role in osteoclastogenesis [1], [2]. Previous research has shown that when monocytes/macrophages were incubated with RANKL in the presence of M-CSF, osteoclasts were induced [1], [2].
Our previous study showed that anti-human CD98 heavy chain monoclonal antibodies had the ability to induce multinucleated giant cell formation of blood monocytes, and these giant cells had osteoclastic properties [3]. Thus, theCD98-mediated pathway is considered to be intimately related to osteoclastogenesis. In addition, cross-talk exists between these two pathways RANKL-mediated and CD98-mediated pathways) [4]. CD98-mediated osteoclastogenesis is blocked by the osteoclastogenesis inhibitory factor (OCIF), the suppressive factor of RANKL-mediated osteoclastogenesis, and RANKL-mediated osteoclastogenesis is suppressed by inhibitory monoclonal antibodies directed against human CD98hc [4].
As CD98hc deficiency in mice produced an embryonic lethal phenotype [5], we generated macrophage/neutrophil-specific CD98hc-defect mice. CD98hc molecules were hardly found in the peritoneal macrophages of these CD98hc conditional knock-out mice (CD98hcflox/-LysM-cre mice) [6]. In our previous study, we examined the role that CD98hc plays in the functions of macrophages using these tissue-specific knock-out mice [6]. Macrophage functions, such as antigen-presenting and phagocytic activities, decreased in the CD98hc-defect peritoneal macrophages. Furthermore, IL-4 induced giant cell formation was inhibited in the CD98hc-defect peritoneal macrophages. However, whether the defect of CD98hc influences the activity of osteoclastogenesis remains to be clarified.
To understand the CD98hc function in osteoclastogenesis, we tried to clarify whether the CD98hc deficiency in the macrophages affected osteoclast differentiation. In this study, osteoclast differentiation was induced by two stimulations: RANKL stimulation and co-cultivation with the osteoblast cell line. Osteoclast differentiation induced by RANKL signaling can be divided into three phases: triggering, amplifying and targeting [7]. In this study, expression of various factors was analyzed in each phase. Our results show that multinucleated osteoclast formation is severely suppressed in the peritoneal macrophages isolated from the CD98hc-defect mice.
2. Materials and methods
2.1. Mice
CD98hcflox/flox mice were crossed with LysM-cre knock-in mice to delete the CD98hc gene from the macrophage as per methods previously described [6]. All mice were housed at the National Research Institute for Child Health and Development animal facility, and all experiments were approved by the Institutional Animal Care and Use Committee.
2.2. Isolation of peritoneal macrophages
Peritoneal macrophages collected without any stimulation were purified by incubating collected mouse peritoneal exudate cells with FcR Blocking Reagent (Miltenyi Biotec) at 4 °C for 20 min [8]. The cells were then further incubated with biotinylated anti-mouse F4/80 mAb (eBioscience) at 4 °C for 20 min. After washing, the cells were incubated with streptavidin-Microbeads (Milteyi Biotec) at 4 °C for 20 min [7]. F4/80+ peritoneal macrophages (>90%) were isolated by positive selection using the MACS system (Milteyi Biotec).
2.3. Macrophage adhesion measurement
To complete attachment and spreading using the electric cell-substrate impedance sensing (ECIS) device (Applied Biophysics), two kinds of peritoneal macrophages were cultured in the presence of M-CSF (20 ng/ml) and RANKL (400 ng/ml) on ECIS electrodes (8W10E). Adhesion and cell–cell contact were monitored by measuring impedance for up to 48 h.
2.4. Osteoclast differentiation
Osteoclast differentiation was induced by two stimulations.
-
(1)
Mouse peritoneal macrophages (1×105/well) were cultured in the presence of M-CSF (100 ng/ml) and RANKL (200–400 ng/ml) for 9 days. Osteoclast formation was measured by quantifying cells positively stained for TRAP, and light microscopy was used to identify osteoclasts as TRAP-positive multinuclear (>3 nuclei) cells.
-
(2)
Mouse peritoneal macrophages (5×103–4×104/well) were co-cultured with the mouse osteoblast cell line, UAMS-32, for 7 days. Peritoneal macrophages were cultured on 96 well plates that were preseeded 24 h earlier with UAMS-32 (5×103/well) in the presence of 1,25(OH)2D3(10−8 M). After 3 days, the culture medium and added factors were replenished daily.
2.5. TRAP staining
Fixed cells were incubated for 30 min at 37 °C in acetate buffer containing a-naphthol phasphate at pH 4 with 500 mM tartrate. Next, fast violet was used to visualize the product.
2.6. Pit formation
To examine resorption pit formation, peritoneal macrophages(4×104/well) were cultured for 7 days on dentine slice in 96 well plates preseeded 24 h earlier with UAMS-32 (5×103/well) in the presence of 1,25(OH)2D3(10–8 M). After 3 days, the culture medium and added factors were replenished daily. To quantify resorption lacunae, cells were removed from the bone slices using mechanical agitation, and the bone slices were stained with 1% toluidine blue for 20 min. Resorption pits were observed by microscopy.
2.7. Actin ring
To observe the actin ring, cells were fixed and then washed with 0.2% Triton X-100 PBS and incubated with 0.2 U/ml of rodamine phalloidin (Molecular Probes) for 30 min and washed in PBS. Rhodamine-phalloidin-stained actin rings were visualized using fluorescence microscopy.
2.8. Western blotting
Western blotting was carried out according to the method previously described [6]. Blots were probed using the following primary antibodies: β-actin (A1978, Sigma-Aldrich), CD98 (sc-7094, Santa Cruz), NFATc1 (sc-7294, Santa Cruz), p130Cas (MAB5730, R&D SYSTEMS), Phospho-p130Cas (#4015, Cell Signal), Akt (#4691, Cell Signal), Phospho-Akt (#4668, Cell Signal), ERK (#4695, Cell Signal), Phospho-ERK (#4370, Cell Signal), JNK (#9258, Cell Signal), Phospho-JNK (#9258, Cell Signal), mTOR (#2983, Cell Signal), Phospho-mTOR (#5536, Cell Signal), p70S6K (#2708, Cell Signal), Phospho-p70S6K (#9234, Cell Signal), p38 (#9121, Cell Signal), Phospho-p38 (#4511, Cell Signal), S6 (#2217, Cell Signal), Phospho-S6 (#4858, Cell Signal), Asparagine synthetase (sc-135183, Santa Cruz), eIF2α (#5324, Cell Signal) and Phospho- eIF2α (#3398, Cell Signal).
2.9. Statistical analysis
Data were analyzed using the Student’s two-tailed t-test.
3. Results
3.1. CD98hc deletion in macrophages impairs spreading by stimulation with M-CSF and RANKL
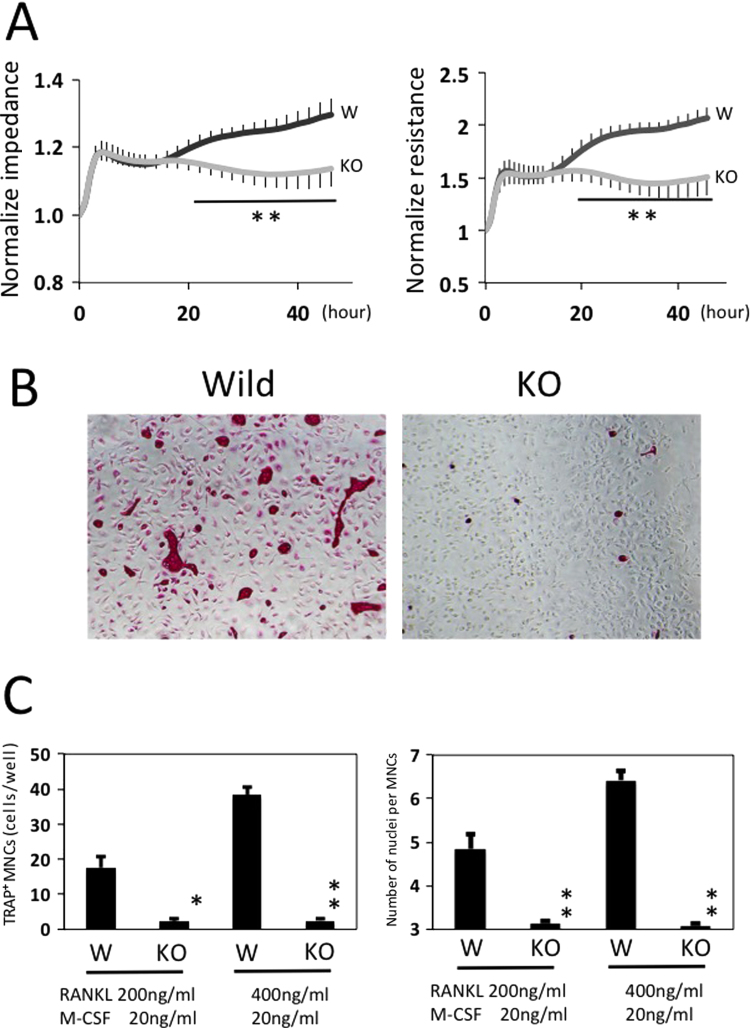
Since CD98hc is linked to/ plays a role in macrophage mobility, we investigated whether the impedance and resistance of residential peritoneal macrophages are affected by defect of CD98hc. First, the impedance and resistance of residential peritoneal macrophages incubated with M-CSF and RANKL were monitored quantitatively and in real-time on ECIS electrodes, in which impedance and resistance represented attachment and spreading respectively. Low attachment abilities between the macrophages derived from CD98hcflox/floxLysM-cre (KO) mice and those from CD98hcflox/flox mice (Wild) were not detected for up to 6 h (Fig. 1A). However, significant differences in spreading were observed from 20 h to 48 h (Fig. 1A).
Fig. 1.
Effect of peritoneal macrophage attachment and spreading by M-CSF and RANKL and effects of CD98hc-defect macrophages on in vitro osteoclastogenesis. (A) ECIS-based attachment and spreading assay of macrophages from CD98hcflox/flox LysM-cre (KO type mice) and CD98hcflox/flox mice (wild-type mice). Peritoneal macrophages were cultured in the presence of M-CSF (20 ng/ml) and RANKL (400 ng/ml) on an electric cell-substrate impedance-sensing electrode, and spreading was monitored by measuring impedance for up to 48 h. Gray line: macrophages from KO-type mice. Black line: macrophages from wild-type mice. Impedance and resistance represent attachment and spreading in macrophages. The experiment was performed in duplicate with 4 mice per group. Representative data are expressed as means + SEM (**p<0.01). (B) In the RANKL system, mouse peritoneal macrophages were stimulated with M-CSF and RANKL for 9 days, followed by TRAP staining. (C) Quantitative analysis of TRAP-positive multinucleated cells and number of nuclei per multinucleated cell in (B). The experiment was performed in duplicate with 5 mice per group. Representative data are expressed as means + SEM (*p<0.05,**p<0.01) .
In our previous study [6], when the macrophages were incubated with MEM supplemented with FBS for 48 h, almost all peritoneal macrophages derived from the CD98hcflox/floxLysM-cre mice displayed a round-shaped morphology, while cells from wild-type mice showed a spindle morphology. In this study, the macrophages were stimulated with M-CSF and RANKL for 48 h, and morphological observation was carried out. Interestingly, almost all peritoneal macrophages derived from CD98hc-defect mice also displayed round-shaped morphologies (data not shown). These data suggest that the defect of CD98hc suppressed the spreading of macrophages stimulated by M-CSF and RANKL.
3.2. CD98hc deletion in macrophages impairs osteoclast formation
To determine the roles of CD98hc in osteoclast differentiation, we first investigated the osteoclastogenesis of peritoneal macrophages stimulated by RANKL. TRAP-positive osteoclasts were generated from peritoneal macrophages derived from wild-type mice in the presence of M-CSF and RANKL, but TRAP-positive osteoclast formation was significantly impaired in macrophages derived from CD98hcflox/floxLysM-cre mice (Fig. 1 B and C).
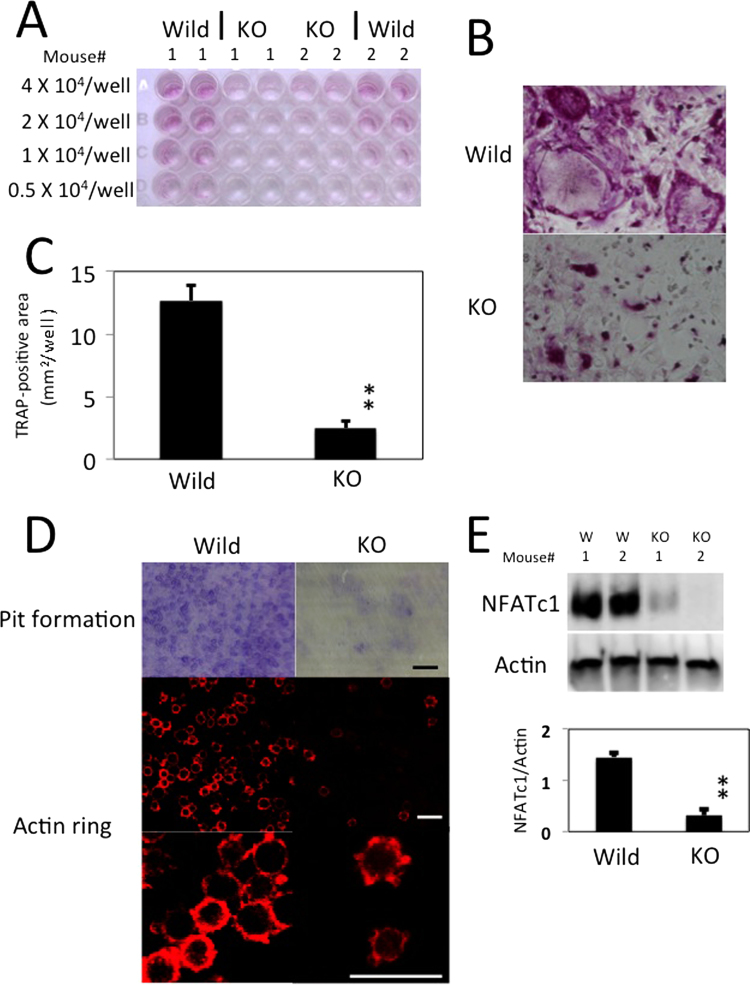
Furthermore, peritoneal macrophages were co-cultured for 7 days with the osteoblast cell line, UAMS-32. The number of osteoclasts was obviously lower in CD98hcflox/floxLysM-cre mice than CD98hcflox/flox mice (Fig. 2A–C). We found a marked difference in actin ring formation and bone resorption between the osteoclasts derived from CD98hcflox/flox mice and CD98hcflox/floxLysM-cre mice (Fig. 2D).
Fig. 2.
CD98hc regulates osteoclast formation in the co-culture system. TRAP-stained osteoclasts in co-culture of UAMS-32 (osteoblasts cell line) and peritoneal macrophages. Peritoneal macrophages (0.5×104–4×104/well) isolated from wild-type and knock-out type mice were co-cultured with UAMS-32 stimulated with VitD3 for 7 days. “W” or “KO” means macrophages obtained from control or CD98hc knockout mice, respectively. (A) TRAP staining was carried out. (B) Higher magnification of wells of 4×104 peritoneal macrophages. (C) TRAP-positive areas in wells of 4×104 peritoneal macrophages were calculated by color extraction system of Hybrid Cell Count (KEYENCE). The experiment was performed in duplicate with 6 mice per group. Representative data are expressed as means + SEM (**p=0.00003). (D) Resorption pit formation and actin ring. (E) NFATc1 protein in wild-type and knock-out type peritoneal macrophages mixed with UAMS-32 were immunoblotted. Actin served as loading control. The experiment was performed with 4 mice per group. Bellow, staining of NFATc1 was normalized to that actin. Data are representative of means + SEM (**p=0.0003).
The essential role of transcription factor NFATc1 in osteoclast differentiation is well established. We therefore examined the expression of NFATc1 at day 7 of culture. Intriguingly, NFATc1 induction was suppressed in the peritoneal macrophages of LysM-cre-mediated CD98hc deletion (Fig. 2E).
3.3. CD98hc deletion in macrophages exacerbates signal transduction related to osteoclast formation
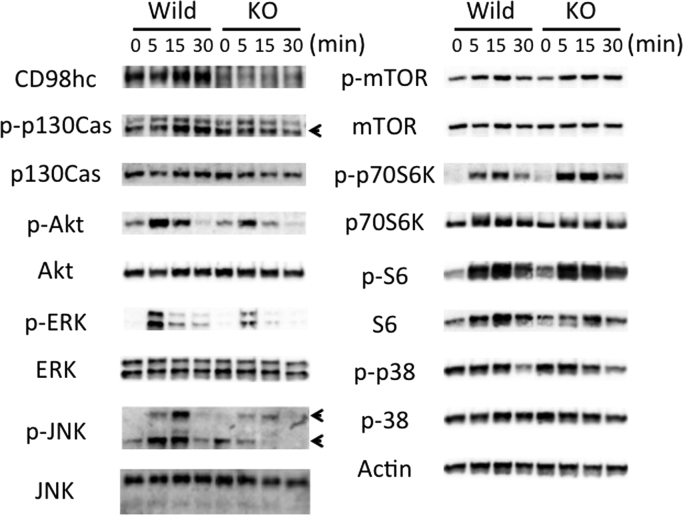
Peritoneal macrophages were cultured at various time points (0–30 min) (triggering phase) after treatment with M-CSF and RANKL. Cell lysates were prepared and probed by immunoblotting with the antibodies listed in the Materials and Methods section. As known well, CD98hc associates with integrin ß subunits, and regulates integrin signaling which governs cell migration. Phosphorylation of p130cas is the initiating step that mediates integrin-dependent Rac activation. As shown in Fig. 3, the phosphorylation p130cas was impaired at the triggering phase in CD98hcflox/floxLysM-cre macrophages. To explore the molecular mechanism underlying the involvement of CD98hc in osteoclast differentiation, we analyzed phosphorylation of downstream signaling molecules of M-CSF and RANKL, such as Akt, ERK, JNK, p70S6K, S6 and p38 at the triggering phase. The activation of Akt, ERK and JNK were significantly decreased in CD98hcflox/floxLysM-cre peritoneal macrophages treated with M-CSF and RANKL (Fig. 3). These results suggest that in the peritoneal macrophage culture system, CD98hc molecules mediate osteoclast differentiation in cooperation with M-CSF and RANKL.
Fig. 3.
Immunoblotting analysis of signal events in CD98hcflox/floxLysM-cre peritoneal macrophages. Peritoneal macrophages were cultured at various time points (0–30 min) (triggering phase) after treatment with M-CSF and RANKL. Cell lysates were prepared and probed by immunoblotting with the antibodies listed in the Materials and Methods section.
3.4. CD98hc deletion causes amino acid deprivation in osteoclast differentiation
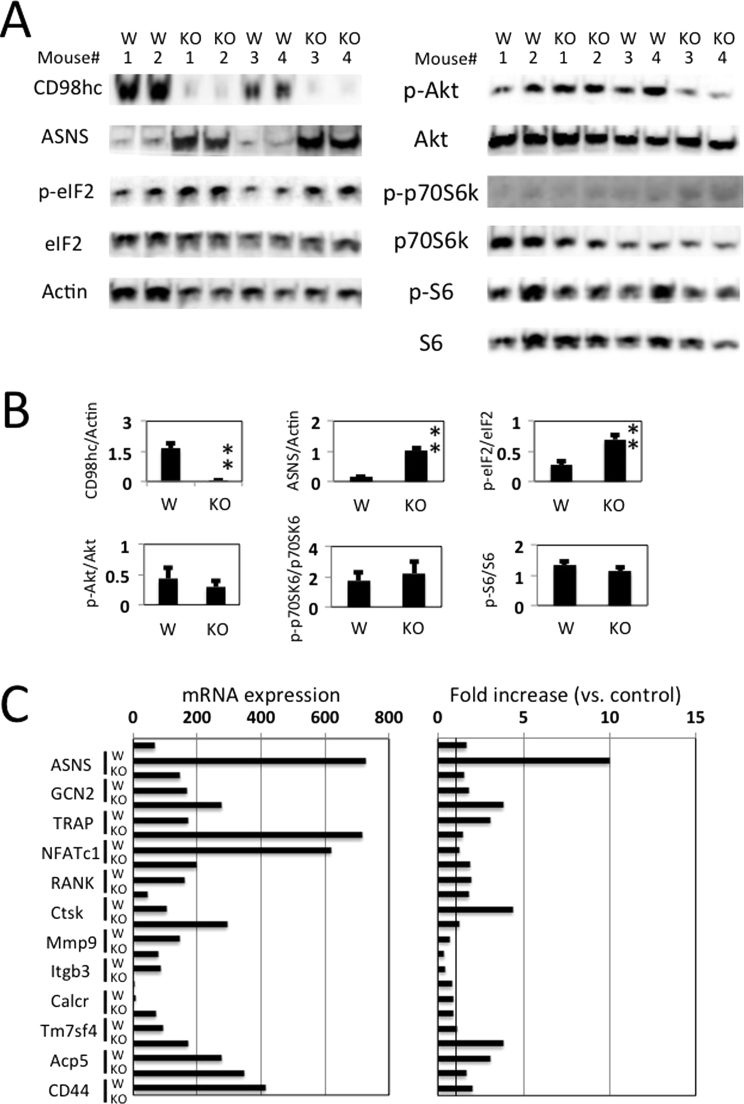
The major amino acid sensing-signaling pathways in mammalian cells are the mammalian target of rapamycin complex 1 (mTORC1) and GCN pathways [9]. The CD98 heterodimer is formed by disulfide bonds between the CD98hc extracellular domain and one of several L-type amino acid transporters (LAT1/Slc7a5, LAT2/Slc7a8, y+LAT1/Slc7a7, y+LAT2/Slc7a6, Asc-1/Slc7a10, xCT/Slc7a11). CD98-mediated amino acid transport is related to mTOR-p70S6K-S6 signaling [10]. To assess whether CD98hc/lc support osteoclast differentiation through amino acid sensing, we analyzed the phosphorylation of mTOR, p70S6K and S6 in peritoneal macrophages stimulated by M-CSF and RANKL for 30 min (at the triggering phase, Fig. 3) or 3 days (at the targeting phase (Fig. 4 A and B). The induction of phosophorylated p70S6K and S6 were similar at the triggering and targeting phases between both groups (Figs. 3 and 4A and B). Furthermore, no difference in mTOR and p38 phosphorylation between both macrophages was observed (Fig. 3). Asparagine synthetase (ASNS) encodes an enzyme involved in asparagine biosynthesis [11], which is induced after amino acid deprivation, low glucose and hypoxia [12], and the ASNS gene is regulated by the GCN2-eIF2-ATF4 pathway [13]. We investigated amino acid deprivation in the process of osteoclast formation. Expression of ASNA at protein level (at the targeting phase, Fig. 4A and B) and its mRNA (at the amplifying phase, Fig. 4C) was enhanced in the CD98 knockout peritoneal macrophages compared to the control macrophages. Induction of phosophorylated eIF2 increased in the CD98hcflox/floxLysM-cre peritoneal macrophages that were treated with M-CSF and RANKL at the targeting phase (Fig. 4A and B).
Fig. 4.
Induction of GCN pathway in CD98hc ablation. (A) GCN and mTORC1 signaling in CD98hcflox/floxLysM-cre peritoneal macrophages. Peritoneal macrophages were cultured for 3 days (targeting phase) after treatment with M-CSF and RANKL. Cell lysates were prepared and probed by immunoblotting with the antibodies listed in the Materials and Methods section. “W” or “KO” means macrophages obtained from control or CD98hc knockout mice, respectively. (B) The experiment was performed with 6 mice per group. The staining density were normalized. Data are representative of means+SEM (**p<0.01). (C) Peritoneal macrophages were cultured for 24 h (amplifying phase) after treatment with M-CSF and RANKL. mRNA transcripts were amalyzed with GeneChip analysis. Left panel: mRNA expression level at 24 h; right panel: the fold increases in the mRNA expression level compared with that in unstimulated cells (control). ASNS: asparagine synthetase, GCN2: general control nonderepressible 2, eukaryotic translation initiation factor 2 alpha kinase 4, TRAP: Tartrate-resistant acid phosphatase, NFATc1: nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1, RANK: Receptor activator of nuclear factor kappa-B, Ctsk: Cathepsin K, Mmp9: Matrix metallopeptidase 9, Itgb3: integrin beta 3, Calcr: calcitonin receptor, Tm7sf4 DC-STAMP: Dendritic Cells (DC)-Specific Transmembrane Protein, Acp5: Acid Phosphatase 5.
As described previously, when macrophages were co-cultured with the osteoblast cell line for 7 days, NFATc1 induction was suppressed in peritoneal macrophages with LysM-cre-mediated CD98hc deletion (Fig. 2E). In addition, peritoneal macrophages were cultured for 24 h (amplifying phase) after treatment with M-CSF and RANKL. mRNA transcripts related to osteoclastogenesis and fusion were analyzed with GeneChip analysis. However, no difference in these mRNAs expression was found at the amplifying phase between the CD98-defect macrophages and the wild-type macrophages (Fig. 4C).
3.5. Trabecular bone was normal in CD98hcflox/floxLysM-cre mice
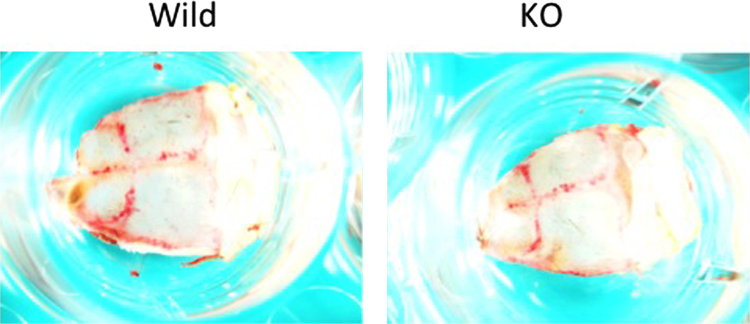
As the multinucleated osteoclast formation was severely impaired in the macrophages isolated from the CD98hc-defect mice, we examined whether CD98hc-defect mice showed the osteopetrosis phenotype. However, µCT analysis of CD98hcflox/floxLysM-cre mice showed tibia of normal size and thickness compared with CD98hcflox/flox mice at 6 weeks of age (data, not shown). Furthermore, TRAP staining of mouse whole calvaria obtained from wild-type and knock-out type mouse (Fig. 5). There was no difference in number of TRAP positive cells between both mice.
Fig. 5.
TRAP staining of mouse whole calvaria obtained from wild-type and knock-out type mouse at 6 weeks of age.
4. Discussion
In this report, we demonstrated that CD98hc deficiency impaired the osteoclast formation induced by M-CSF-RANKL system or co-culture system in vitro. When control peritoneal macrophages derived from wild-type mice were stimulated by RANKL, TRAP-positive osteoclasts were generated. On the other hand, multinucleated osteoclastogenesis was significantly impaired in RANKL-stimulated macrophages derived from CD98hcflox/floxLysM-cre mice. Furthermore, when peritoneal macrophages were co-cultured with the osteoblast cell line(UAMS-32), the number of osteoclasts was lower in the macrophages derived from CD98hc-defect mice than those derived from wild-type mice. Actin ring formation and bone resorption activity were scarcely found in CD98hc–defect macrophages.
Our previous study showed that CD98hc deficiency in mice produced an embryonic lethal phenotype [5]. To investigate the physiological relevance of CD98 function, some researchers generated genetically manipulated mice that exhibited cell-specific downregulation such as ES cells [14], MEF [15], T cells [16], [17], B cells [18], macrophages [5], intestinal epithelial cells [19] and vascular smooth muscle [20]. Transmembrane and cytoplasmic CD98hc are also found to mediate integrin signaling such as FAK [14], [21], PI3-kinase [14], [21] and Erk [18], [22]. On the other hand, amino acid transport is dependent on the extracellular domain of CD98hc, and surface expression of CD98lc is dependent on the presence of CD98hc [23]. Six CD98lc are identified as system L (LAT1/Slc7a5 and LAT2/Slc7a8), system y+L (y+LAT1/Slc7a7 and y+LAT2/Slc7a6), system asc (Asc-1/Slc7a10) and Xc- (xCT/Slc7a11).
In our previous study [6], we examined the role that CD98hc plays in the functions of macrophages using tissue-specific knock-out mice. Macrophage functions, such as antigen-presenting and phagocytic activities, decreased in the CD98hc-defect peritoneal macrophages. Furthermore, when the peritoneal macrophages were stimulated with IL-4 for 4 days, multinucleated giant-cell formation was severely suppressed in the CD98hc-defect peritoneal macrophages, thus showing that CD98hc plays an essential role in macrophage fusion induced by IL4. This study shows that RANKL-induced multinucleated giant-cell formation is also impaired in the CD98hc-defect peritoneal macrophages.
The suppression of the M-CSF-RANKL-induced phosphorylation of ERK, Akt, JNK and p130Cas were observed in the CD98hcflox/floxLysM-cre peritoneal macrophages. In contrast, p-p70S6K and p-S6 expression levels in the peritoneal macrophages from CD98hcflox/floxLysM-cre mice were not changed in macrophages that were stimulated with RANKL and M-CSF at both the triggering and targeting phases. Recently, the import of essential amino acids in CD98hc/LAT1 bidirectional transport was found to regulate a rate-limiting step that activates mTOR [10]. The uptake of essential amino acids also regulates T-cell differentiation and muscle mass [24], [25]. Rapamycin-sensitive mTOR complex 1 in mammalian cells is important for phosphorylation and activation of p70S6K [10]. Intriguingly, our results did not show the inhibition of phosphorylation of mTOR, p70S6K and p-S6 in the CD98hcflox/floxLysM-cre peritoneal macrophages. LAT-1 mRNA was not found in monocytes stimulated with M-CSF and RANKL [4]. It is possible we did not observe the mTOR signal disorder in CD98hcflox/floxLysM-cre peritoneal macrophages due to the tissue distribution of CD98lc. Taken together, the amino acid transports of CD98lc were also impaired by the CD98hc defect, and resulted in amino acid starvation such as GCN pathway activation.
CD98hc plays two important roles in osteoclastogenesis through integrin signaling and amino acid transport in mice. We have demonstrated that CD98 has a significant function in osteoclastogenesis in mice. However, CD98hcflox/floxLysM-cre mice exhibited tibia of normal size and thickness compared with CD98hcflox/flox mice at 6 weeks of age. The role of CD98hc in in vivo osteoclast formation still needs to be clarified, and we aim to further investigate this role using cathepsin K-cre mice in the near future.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.11.023.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi S., Tabata N., Tajima M., Ito M., Tsurudome M., Sudo A., Uchida A., Ito Y. Induction of human osteoclast-like cells by treatment of blood monocytes with anti-fusion regulatory protein-1/CD98 monoclonal antibodies. J. Bone Min. Res. 1998;13:44–49. doi: 10.1359/jbmr.1998.13.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Mori K., Miyamoto N., Higuchi Y., Nanba K., Ito M., Tsurudome M., Nishio M., Kawano M., Uchida A., Ito Y. Cross-talk between RANKL and FRP-1/CD98 Systems: RANKL-mediated osteoclastogenesis is suppressed by an inhibitory anti-CD98 heavy chain mAb and CD98-mediated osteoclastogenesis is suppressed by osteoclastogenesis inhibitory factor. Cell. Immunol. 2001;207:118–126. doi: 10.1006/cimm.2000.1748. [DOI] [PubMed] [Google Scholar]
- 5.Tsumura H., Suzuki N., Saito H., Kawano M., Otake S., Kozuka Y., Komada H., Tsurudome M., Ito Y. The targeted disruption of the CD98 gene results in embryonic lethality. Biochem. Biophys. Res. Commun. 2003;308:847–851. doi: 10.1016/s0006-291x(03)01473-6. [DOI] [PubMed] [Google Scholar]
- 6.Tsumura H., Ito M., Li X.K., Nakamura A., Ohnami N., Fujimoto J., Komada H., Ito Y. The role of CD98hc in mouse macrophage functions. Cell. Immunol. 2012;276:128–134. doi: 10.1016/j.cellimm.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda Y., Matsuo K. Molecular mechanisms of triggering, amplifying and targeting RANK signaling in osteoclasts. World J. Orthop. 2012;3:167–174. doi: 10.5312/wjo.v3.i11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno T., Oboki K., Kajiwara N., Morii E., Aozasa K., Flavell R.A., Okumura K., Saito H., Nakae S. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J. Immunol. 2009;183:7890–7897. doi: 10.4049/jimmunol.0802449. [DOI] [PubMed] [Google Scholar]
- 9.Taylor P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014;99:223S–230S. doi: 10.3945/ajcn.113.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., Myer V.E., MacKeigan J.P., Porter J.A., Wang Y.K., Cantley L.C., Finan P.M., Murphy L.O. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards N.G., Schuster S.M. Mechanistic issues in asparagine synthetase catalysis. Adv. Enzym. Relat. Areas Mol. Biol. 1998;72:145–198. doi: 10.1002/9780470123188.ch5. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian M.N., Butterworth E.A., Kilberg M.S. Asparagine synthetase: regulation by cell stress and involvement in tumor biology. Am. J. Physiol. Endocrinol. Metab. 2013;304:E789–E799. doi: 10.1152/ajpendo.00015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palii S.S., Kays C.E., Deval C., Bruhat A., Fafournoux P., Kilberg M.S. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids. 2009;37:79–88. doi: 10.1007/s00726-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feral C.C., Nishiya N., Fenczik C.A., Stuhlmann H., Slepak M., Ginsberg M.H. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. USA. 2005;102:355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feral C.C., Zijlstra A., Tkachenko E., Prager G., Gardel M.L., Slepak M., Ginsberg M.H. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J. Cell. Biol. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantor J., Slepak M., Ege N., Chang J.T., Ginsberg M.H. Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J. Immunol. 2011;187:851–860. doi: 10.4049/jimmunol.1100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z., Hou J., Chen J., Tsumura H., Ito M., Ito Y., Hu X., Li X.K. Deletion of CD98 heavy chain in T cells results in cardiac allograft acceptance by increasing regulatory T cells. Transplantation. 2012;93:1116–1124. doi: 10.1097/TP.0b013e31824fd7cd. [DOI] [PubMed] [Google Scholar]
- 18.Cantor J., Browne C.D., Ruppert R., Feral C.C., Fassler R., Rickert R.C., Ginsberg M.H. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 2009;10:412–419. doi: 10.1038/ni.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen H.T., Dalmasso G., Torkvist L., Halfvarson J., Yan Y., Laroui H., Shmerling D., Tallone T., D'Amato M., Sitaraman S.V., Merlin D. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J. Clin. Investig. 2011;121:1733–1747. doi: 10.1172/JCI44631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogelstrand P., Feral C.C., Zargham R., Ginsberg M.H. Dependence of proliferative vascular smooth muscle cells on CD98hc (4F2hc, SLC3A2) J. Exp. Med. 2009;206:2397–2406. doi: 10.1084/jem.20082845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poettler M., Unseld M., Braemswig K., Haitel A., Zielinski C.C., Prager G.W. CD98hc (SLC3A2) drives integrin-dependent renal cancer cell behavior. Mol. Cancer. 2013;12:169. doi: 10.1186/1476-4598-12-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulus N., Feral C., Pozzi A., Zent R. CD98 increases renal epithelial cell proliferation by activating MAPKs. PLoS One. 2012;7:e40026. doi: 10.1371/journal.pone.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastroberardino L., Spindler B., Pfeiffer R., Skelly P.J., Loffing J., Shoemaker C.B., Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 24.Fotiadis D., Kanai Y., Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair L.V., Rolf J., Emslie E., Shi Y.B., Taylor P.M., Cantrell D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material