Abstract
Advanced Glycation End Products (AGEs) are toxins that are involved in structural and functional alterations of several organs and tissues, resulting in various pathologies. Several types of AGEs have been described but carboxymethyllysine (CML) is the major antigenic AGE compound. In this study, three different immunogenic carrier proteins (KLH, keyhole limpet hemocyanin; BSA, bovine serum albumin; and HSA, human serum albumin) were modified by glycation. The glycated molecules were used to produce epitope-specific monoclonal antibodies able to recognize the CML domain and to detect uremic toxins in the serum of patients with chronic kidney disease (CKD). A competitive ELISA was standardized in order to quantify CML in the sera of CKD patients. An increase in uremic toxins can compromise the clinical condition of these patients, thus, the detection and quantification of these toxins should contribute to a better management and understanding of this disease.
Keywords: Age, CML, Monoclonal antibody, Chronic kidney disease
Highlights
-
•
We isolated a monoclonal antibody able to recognize carboxymethyl lysine (CML) which is the major antigenic AGE compound.
-
•
A sensitive immunoassay was developed to detect and quantify CML in biological samples.
-
•
A correlation between chronic kidney disease (CKD) grade and the CML concentration deduced from the immunoassay was observed.
1. Introduction
Chronic kidney disease (CKD) is a gradual loss of kidney function over time which is currently considered as one of the most significant medical and health problems. Cases of CKD are growing exponentially, and, together with other chronic and non-transmissible diseases, CKD is responsible for millions of deaths per year [1], [2]. CKD is classically defined by the presence of a renal parenchymal lesion and/or by a diminution in renal function for a period equal to or greater than three months, and by proteinuria. This definition enables the classification of CKD into five stages: stage 1 is the less severe, with a glomerular filtration rate (GFR)≥90 mL/min/1.73 m2, and stage 5 is the most severe, with a GFR <15 mL/min/1.73 m2 (or dialysis) [3]. As a consequence of GFR alteration, there is a progressive retention of a great number of components, which would normally be eliminated by the kidneys under healthy conditions. The increased presence of these molecules and their pathological effects on the body allow to designate them as uremic toxins. The accumulation of these uremic toxins in the circulation characterizes uremic syndrome [4].
To date, more than 150 uremic compounds have been described. Among these, those classified as protein-bound compounds require special attention because they are associated with toxic activities in different biological systems and are difficult to remove by dialysis. Among these toxins, Advanced Glycation End Products (AGEs) are some of the main substances involved in various effects in the body such as vascular lesions and white blood cell and platelet toxicity [4], [5].
AGEs represent a heterogeneous group of substances formed by amino-carbonyl non-enzymatic interactions between reducing sugars or oxidized lipids and proteins, amino phospholipids, or nucleic acids [6]. These complex reactions are initiated with the formation of an unstable Schiff base, generated by the condensation of a reducing sugar carbonyl group, such as glucose, with an amine group, originating from an amino acid that is especially susceptible to the reaction, such as a lysine residue. During AGE formation, different structures are formed, including carboxyethyllysine (CEL), imidazolone, pentosidine, pirraline, and carboxymethyllysine (CML). However, CML is the most abundant modification that occurs by protein glycation [7].
Several studies have shown that CML modifications occur during aging, in oxidative stress, and in the pathogenesis of diseases such as diabetic nephropathy, arteriosclerosis, diabetic retinopathy, hemodialysis-associated amyloidosis, Alzheimer's disease, dysfunctions in bone remodeling, and arterial stiffening [5], [8], [9]. In CKD, the level of AGEs is significantly increased, not only because of increased production of AGEs, but also because of decreased excretion [4].
Considering the importance of conditions related to increased circulating AGEs, detection, quantification, and understanding the cytotoxic effects of AGEs is important. However, these molecules are not frequently titrated, except for glycated hemoglobin (HbA1C), which is quantified in patients with diabetes mellitus [10]. An analysis of the biochemical and immunological properties of AGEs can provide important information about these biomarkers.
Immunological methods may provide several advantages in identifying these compounds, such as fast achievement of results, higher sensitivity, and easier application [11]. Currently, no gold standard method is available for detection and quantification of AGEs. One possible explanation for this is that there is no internationally recognized standard unit of measurement to express AGEs levels, unlike other measurable molecules, which makes the comparison of results between different laboratories extremely difficult. Thus, the development of a specific and sensitive tool for the detection and quantification of AGEs is required to be used in diagnosis in order to understand different processes and also to help patients with appropriate treatment [12].
In order to detect circulating levels of CML in CKD patients, various biochemical and immunological approaches have been employed. The objective of the present study was to produce specific antibody probes that can be used to detect and quantify circulating levels of CML in biological fluids of CKD patients. Moreover, such target-specific antibodies may be employed in in vitro assays as blocker or competitor molecules to promote a greater understanding of the cytotoxic effects of AGEs.
2. Methodology
All the experiments were conducted in accordance with the guidelines of the Ethical Committee in Health of the UFPR (No 39393414.9.0000.0102, CEP/SD), and the Helsinki Declaration of 1975, promulgated by the World Medical Association. In addition, this study was approved by the Animal Ethical Committee of UFPR (No 23,075.087228/2013-74) according to the guidelines of the Brazilian College of Animal Experimentation (COBEA).
2.1. Patients
For the present study, peripheral blood samples were collected from 24 patients from our CKD clinic in Nossa Senhora da Luz Hospital (Curitiba/Brazil). The inclusion criteria were 18 years of age and older, presence of CKD (proteinuria or decreased GFR in 3 consecutive evaluations), and willingness to participate in the study. Exclusion criteria were signs of active inflammation or infectious disease (determined by the absence of clinical signs of acute inflammation), malignancy, prior or current renal replacement therapy, and the use of immunosuppressive drugs. Clinical data were obtained by chart review. All clinical laboratory results recorded from chart review represented values measured in the clinical laboratory of Sugisawa by standard auto analyzer techniques. GFR was measured according to estimating equations developed by the Modification of Diet in Renal Disease (MDRD).
2.2. Collection of samples for in vivo and in vitro studies
On the morning of enrollment in the study, serum was collected, divided into aliquots, and stored at −80 °C. Patients were classified in stages 1–5 according to the National Kidney Foundation criteria [3].
2.3. Glycated protein synthesis
In order to generate CML, protein reactions with glyoxylic acid in the presence of sodium cyanoborohydride were performed as previously described [13], [14]. We synthesized CML-BSA (bovine serum albumin), CML-KLH (keyhole limpet hemocyanin), and CML-HSA (human serum albumin) (All reagents were purchased Sigma-Aldrich, St. Louis, MO). The final protein concentration was determined using the Bradford assay (Bio-Rad, United States).
2.4. Characterization of CML and glycated proteins
The glycated molecules were characterized by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry. SDS-PAGE was performed under reducing conditions, in order to assess the protein profile of each CML-protein. In summary, 10 μg of each sample was electrophoresed in a 10% resolving gel at 100 V for 90 min at room temperature. After, the gels were stained with Coomassie Blue. For mass spectrometry (MS) proteins were directly spotted on to a MALDI target plate with alpha-cyano-4-hydroxycinnamic acid (CHCA) (Sigma-Aldrich, St. Louis, MO) as a matrix (prepared in 50% acetonitrile and 0.1%trifluoroacetic acid). MALDI-TOF analysis was performed using a mass spectrometer MALDI-Tof/Tof Autoflex II (Bruker Daltonics); MS spectra were acquired and analyzed on Flex Analysis 2.0 (Bruker Daltonics) software.
2.5. Development of murine anti-CML antibodies
BALB/C mice (four mice, male, 20 g) were immunized subcutaneously with 20 μg of CML-KLH or CML-BSA antigen as in Becker-Finco et al. [15]. Serum samples were diluted (1:100) and submitted to an ELISA (detailed below).
Animals whose serum analysis led to A490 nm higher than 1.0 for CML-protein and low cross-reactivity to native carrier protein (A490 nm<0.05) received an intravenous boost of 5 μg CML-protein in 100 μL of PBS. Three days later, spleens from hyperimmunized mice were collected and their splenocytes were fused to Sp2/0 myeloma cells for hybridoma generation [15], [16].
Hybridoma supernatants were ELISA-screened using CML-protein and native carrier protein. CML-protein positive hybridomas were subsequently expanded and cloned. One cell line, named mAb 2D6, which was positive for CML-BSA or CML-KLH but negative for BSA or KLH was selected, submitted to additional subclonings leading to clone 2D6G2. Anti-CML monoclonal antibodies (mAbs) were tested using the ELISA described below. mAbs were purified by a single-step immunoaffinity chromatography using Protein A/G Sepharose® 4B fast flow prepared according to the manufacturer's instructions (Sigma-Aldrich).
Analysis of the cDNA encoding mAb 2D6G2 variable heavy (VH) and light (VL) chain domains, sequence analysis, and database search.
Total RNA was isolated from hybridoma 2D6G2. cDNAs encoding the antibody variable domains (Immunoglobulin heavy chain variable, IGHV; Immunoglobulin light chain variable, IGκV) were cloned after RT-PCR using degenerate primers. IGH-For (GAC AGT GGA TAR ACM GAT GG) and IGH-Rev (GAG GTS MAR CTG CAG SAG TCW GG) for mAb 2D6G2 VH amplification, and V-KAPPA For (GGA TAC AGT TGG TGC AGC ATC) and V-KAPPA Rev (GAT ATT GTG CTA ACT CAG TCT) for mAb 2D6G2 light chain variable VL amplification. PCR products were inserted into the cloning vector pGEM-T (Promega A3610) before being sequenced and analyzed according to the protocol previously reported [17].
To compare V-REGION sequences of immunoglobulins, ImMunoGenetTics (IMGT) unique numbering and standards, approved by the World Health Organization (WHO)/International Union of Immunological Societies (IUIS) Nomenclature Subcommittee for Immunoglobulins and T Cell Receptors was used. The Web interface IMGT tools (IMGT/V-QUEST) and databases (IMGT/LIGM-DB and IMDT/3D structure-DB) were also used [18].
3. ELISA protocols
3.1. Direct format
CML-protein (100 µL, 10 µg/mL or an increasing concentration 0.3–40 μg/mL) was immobilized onto 96-well plates (Immunonunc Thermo Fisher Scientific, Rockford, IL) and incubated at 4 °C overnight. Nonspecific binding sites were saturated with 120 μL of 2% casein for 60 min. The plate was then incubated with 100 μL of hybridoma culture supernatant, monoclonal (1 μg/mL) or polyclonal (diluted 1:400) anti-AGE antibody for an additional 60 min at 37 °C.The plate was incubated for an additional 60 min with HRP-conjugated anti-mouse IgG. Finally, 20 μL of the substrate solution (1,2-phenylenediamine dihydrochloride, Sigma-Aldrich, St. Louis, MO) was added to each well for 20 min. The reaction was stopped with 1.0 M sulfuric acid, and the absorbance was measured at 490 nm on a microplate ELISA reader (Bio-Rad 550, Japan).
3.2. Competitive format
96-well plates (Immunonunc, Thermo Fisher Scientific, Rockford, IL) were coated overnight with a solution of CML-protein (100 µL, 10 µg/mL) in carbonate buffer pH 9.6. After blocking (2% casein in PBS) 100 µl of the human sera (CKD patients) diluted 1:10 in presence 0.1 μg/mL of mAb 2D6G2 and incubated for 120 min at 37 °C. Three washing with PBS (pH 7.4) containing 0.1% Tween 20 were performed between each of the intermediate steps. The plate was incubated for an additional 60 min. with HRP-conjugated anti-mouse IgG. Bound antibody was detected as described above. For standard curves, plates were incubated with 0.1 µg/mL of mAb 2D6G2 (100 µL in the presence of CML-HSA at various concentrations (0.4–200 ng/mL)).
3.3. Correlation analysis between CML-HSA concentration and other biological parameters - statistical analysis
CKD patients were distributed between five stages, being stage 1 mild CKD, and 5 severe CKD. All the variants used in the stats analysis (CML-HSA levels, CKD stages and GFR) had a nonparametric distribution, for this reason we used rank correlation coefficient or Spearman’s rho (ρ), that is a test to nonparametric measure of statistical dependence between two variables. It assesses how well the relationship between two variables can be described using a monotonic function. In this way, we performed the correlation of CML levels (ng/dL) and GFR (MDRD) mL/min/1.73 m2 with creatinin, albumin, phosphorous, calcium and glucosis, wich resulted in the correlation represented by ρ and significance represented by P.
Statistical analyses were performed using JMP Windows version 8.1 (SAS Institute Inc., Cary, N.C., USA). Data were analyzed by one-way ANOVA for unpaired data. For multiple pair wise comparisons, a Bonferroni correction was performed to adjust the significance level. Correlations were performed by the Spearman rank test (ρ). Results are expressed as mean±standard error of the mean (SEM). A P value<0.0001 was considered significant.
4. Results
The main clinical and biochemical characteristics of the patient biological samples used in the antibody tests are summarized in Supplemental Table 1. The median GFR was 37 mL/min (ranging from 12 to 107 mL/min). Patients were distributed among CKD stages 1–5 according to the National Kidney Foundation criteria.
4.1. Characterization of CML-proteins
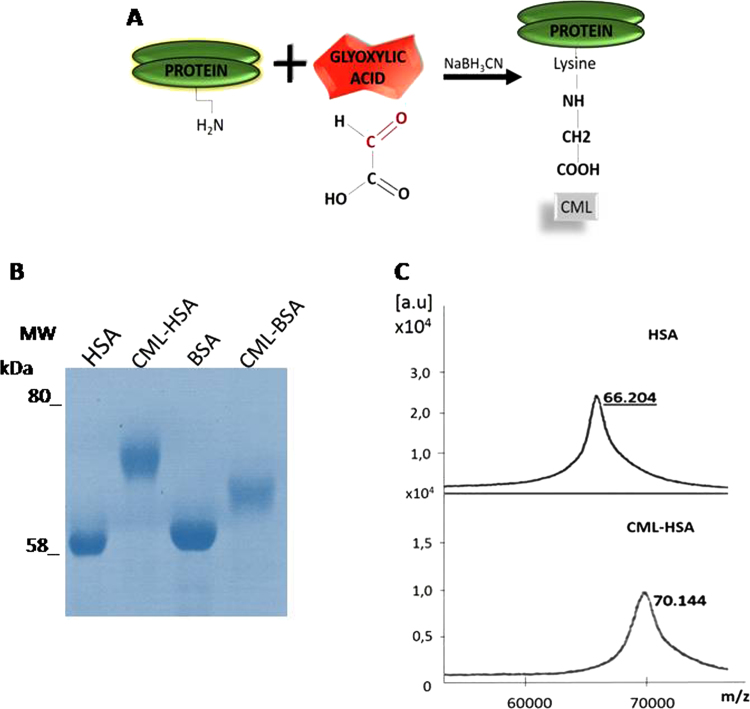
Fig. 1A shows CML-protein synthesis. The difference in electrophoretic mobility indicates glycation of the synthetized samples (Fig. 1B). It was also possible to visualize the molecular mass alteration of HSA (native protein) and CML-protein (glycated protein) by mass spectrometry. The HSA spectrum displayed a peak of 66 kDa and the CML-HSA displayed a 70 kDa peak. The difference in 4 kDa observed by spectroscopy, suggests an average formation of 19 new CML domains per molecules while 59 potential sites of conjugation (Lys residues) have been reported in the sequence of HSA (Gene accession number AAA98797) (Fig. 1C). Following coupling of HSA with glyoxilic acid, no change in solubility neither any formation of aggregates was observed.
Fig. 1.
Molecular characterization of the modified proteins. (A) Schematic drawing of CML-protein formation. (B) The synthesized samples were separated by SDS-PAGE after Coomassie staining. MW, molecular weight marker. (C) Mass spectrometry HSA and CML-HSA by MALDI TOF MS.
4.2. Generation of anti-CML antibody
4.2.1. Characterization of polyclonal antibodies
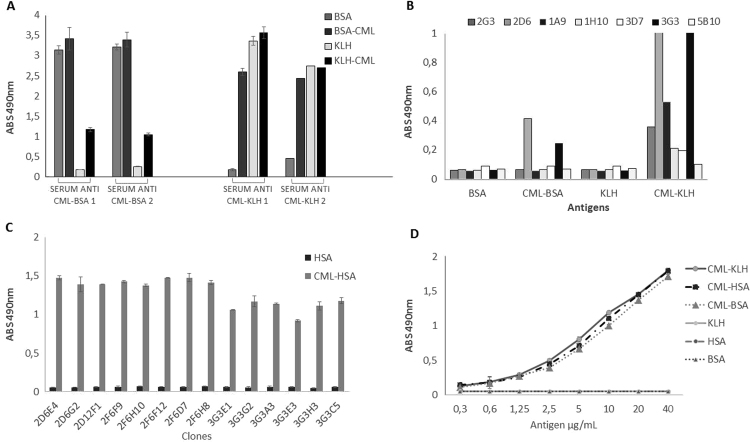
The serum of immunized mice was collected and tested in a direct ELISA. Immunization with glycated protein CML-KLH induced the production of polyclonal antibodies that were highly reactive with CML-BSA but not with native BSA and the signal observed was even higher than the one observed when testing anti CML-BSA polyclonal antibodies against immobilized CML-KLH. These observations are in favor of the production of antibodies specific for the CML domain and directed the choice of an immune serum that presented the lowest reactivity against the native form of the carrier molecule and high reactivity against the glycated protein. The serum from mice immunized with CML-KLH was then used in the process of monoclonal antibody selection and production (Fig. 2A).
Fig. 2.
Reactivity of polyclonal and monoclonal antibodies by ELISA direct. (A) Reactivity of polyclonal antibodies produced against glycated proteins and cross-reactivity against native protein. (B) Reactivity of cell supernatants against glycated and non-glycated proteins. (C) Specificity of monoclonal antibodies against CML-HSA and cross-reactivity to HSA. (D) Dose dependence of mAb 2D6G2 against CML-proteins.
4.3. Characterization of the mAbs
In a direct ELISA, the antibodies evaluated were capable to recognize the CML domain, while no cross-reactivity was observed with the native carrier proteins (Fig. 2B). The hybridoma 2D6 and 3G3 were selected and submitted to successive dilutions for subcloning. Here again, fourteen subclones (8 derived from clone 2D6 and 6 derived from clone 3G3) were evaluated for the recognition of CML-HSA and HSA native form in a direct ELISA. Each subclone secreted antibodies capable of reacting with CML-HSA but not with native HSA coated on microtitration wells (Fig. 2C). ELISA analysis also demonstrated that all subclones derived from hybridoma 2D6 exhibit a higher capacity to secrete specific antibodies as compared to subclones derived from 3G3. Thus, subclone 2D6G2 was selected for purification and characterization. ELISA allowed us to show that antibody secreted by subclone 2D6G2 was able to bind to the CML structural pattern not only in CML-HSA, but also in CML-BSA and CML-KLH, in a dose-dependent manner and this confirmed the sharp antigen-binding specificity of 2D6G2 murine antibody (Fig. 2D).
4.4. Analysis of the cDNA encoding mAb 2D6G2 VH and VL domains
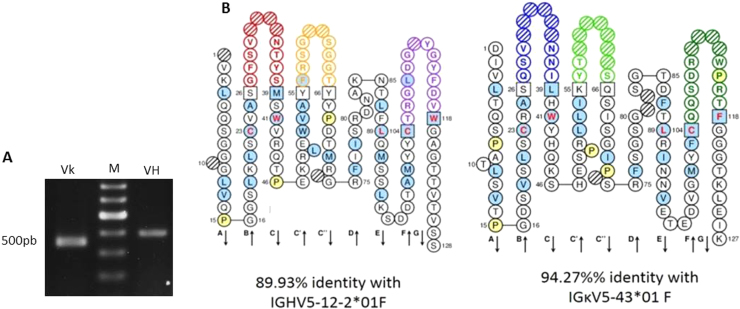
Before carrying any fine functional immunoassays, the cDNA encoding antibody 2D6G2 IGH-V and IGKappa-V domains were amplified by PCR from two distinct preparations of RNA isolated from hybridoma 2D6G2 to ensure correct subcloning. The RT-PCR products, VH and VL, presented bands around 350 bp after agarose gel electrophoresis analysis (Fig. 3A). Sequencing and in silico analysis indicated that 2D6G2 IGH-V and IGKappa-V sequences were unique. Complementary determining regions (CDRs) framework were easily identified. No key conserved residues were mutated in the framework regions, suggesting that the sequences were correctly rearranged and functional. The VH gene belonged to the IGHV5 murine family and showed 90% protein sequence identity with Mus musculus IGHV5-12-2*01, while the VL gene belonged to the IGKV5 family with 94% identity with Mus musculus IGKappaV5-43*01 (Fig. 3B).
Fig. 3.
Cloning of mAb2D6G2 cDNA encoding antibody variable domains. (A) Agarose gel electrophoresis of cDNA encoding IGkV (lane Vk) and IGHV (lane VH) domains. M: 100-bp DNA ladder. (B) “Collier de perles” of mAb 2D6G2 antibody variable domains.
4.5. Characterization of mAb 2D6G2 with the sera of CKD patients
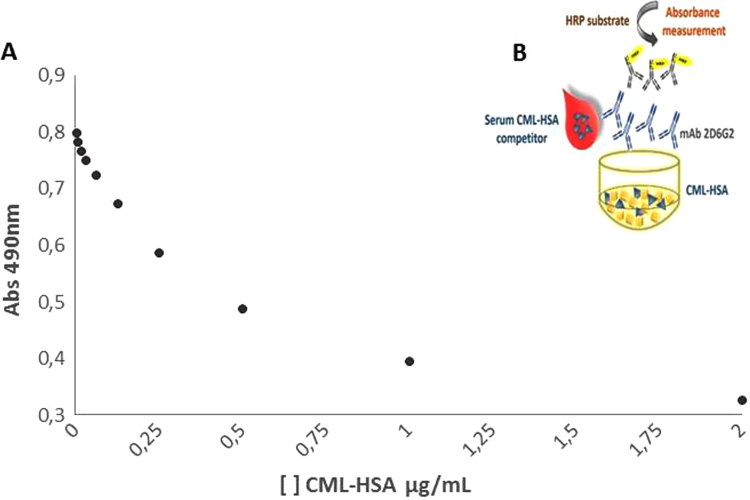
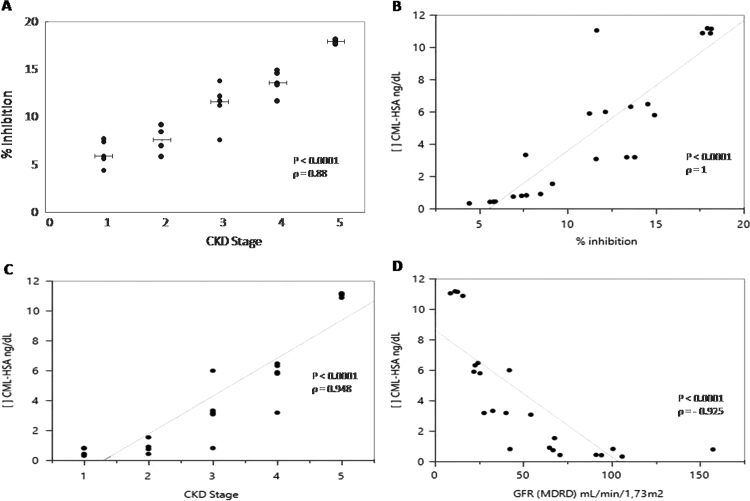
After optimization of several parameters (data not shown), a competitive ELISA was settled (Fig. 4). Serum CML-HSA of patients presented different percentages of inhibition (Fig. 5A). A standard curve of the known concentrations of CML-HSA allowed the quantification of this compound in the CKD sera. The detection limit of CML in this assay was 0.4 ng/dL, an amount equivalent to 5% inhibition (Fig. 5B). In this competitive ELISA, 20% inhibition corresponded to 12 ng/dL CML-HSA, a concentration corresponding to what is observed in patients with more advanced stages of CKD.
Fig. 4.
Detection of synthesized CML-HSA by competitive ELISA using mAb 2D6G2. (A) A standard curve of the known concentrations of CML-HSA. The results are expressed as decreasing absorbance correlated with increasing competitor. (B) Principle of the assay. CML-HSA (10 μg/mL) was immobilized on plate. The sample to be tested (CML-HSA 0.4-200 ng) was mixed with 0.1 μg/mL of mAb 2D6G2 anti-CML antibody. A portion of the mixture (100 μL) was added to the plate prepared above. The reactions were confirmed by adding anti-immunoglobulin.
Fig. 5.
Detection of CKD serum CML levels. (A) Analysis of the competition between the CML-HSA levels in human serum and the immobilized synthesized CML-HSA for binding to mAb 2D6G2. The results are expressed as increasing inhibition correlated with increasing competitor. (B) A curve correlating inhibition and CML-HSA levels. (C) Correlation between CKD stages and CML-HSA concentration. (D) Relationship between serum levels of CML-HSA and GFR. CKD stages were performed following KDOQI Clinical Practice Guidelines for Chronic Kidney Disease.
Using this competitive ELISA, it was possible to correlate CML-HSA serum levels with CKD stages (Fig. 5C) according to Clinical Practice Guidelines for Chronic Kidney Disease. Furthermore, inverse correlation was observed between serum CML-HSA and the GFR (Fig. 5D).
Table 1 summarizes the correlations between CML-HSA concentrations and the biochemical parameters of the biological samples analyzed here. CML-HSA concentration was directly related to CKD stage, as well as to creatinine, phosphor, and glucose. In contrast, a negative correlation was found between serum CML-HSA with GFR and calcium. All analyses indicated P<0.05.
Table 1.
Correlations of CML-HSA levels with CKD stages and GFR, and biochemical characteristics of patients serum.
| CML-HSA (ng/dL) |
GFR (MDRD) mL/min/1.73 m2 |
|||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| CKD stages | 0.95 | <0.0001 | −0.98 | <0.0001 |
| GFR (MDRD) mL/min/1.73 m2 | −0.92 | <0.0001 | NS | NS |
| Creatinine (mg/dL) | 0.91 | <0.0001 | −0.96 | <0.0001 |
| Albumin (g/dL) | −0.36 | NS | 0.37 | NS |
| Phosphor (mg/dL) | 0.45 | <0.02 | −0.46 | <0.024 |
| Calcium (md/dL) | −0.79 | <0.0001 | 0.62 | <0.0012 |
| Glucose | 0.44 | <0.033 | −0.33 | NS |
NS=not significant.
5. Discussion
AGEs synthesis is related to several diseases and their titration in the serum of patients may be of the greatest interest for diagnostic and therapeutic purposes. Today one consider that immunoassays could be valuable approaches to reach this goal but this strategy still suffers from the lack of reliable specific antibodies with applications often limited to immunohistochemical studies and usually no correlation is really observed between serum immunotitration of AGEs and the CKD clinical stage [19], [20], [21]. This may be related to functional characteristics of the antibodies developed so far, which are not suited for sensitive immunotitration [22], [23].
Because CML concentration is increased in serum of patients who have CKD and other diseases such as diabetes with complications including nephropathy, retinopathy, and atherosclerosis its remains important to design sensitive immune titration assays and select immunoprobes able to detect uremic toxins and correlate with clinical aspects [24].
Even if hybridoma technology is conceptually simple, it depends upon complex biological systems and obtaining specific and reliable mAbs is never entirely predictable. This is particularly true when the target is an hapten that is unable to initiate an immune response by itself. Here, CML was conjugated to various carriers to elicit and immune response. KLH, BSA, and HSA were used in the presence of glyoxylic acid and the oxidizing reagent NaCNBH3, which directed the non-enzymatic glycation reaction in order to produce the CML domain. Although EDC, glutaraldehyde and BS3 are conventional coupling reagents, their efficacity remains discussed to produce viable antibodies against CML [25]. Indeed, here the efficiency of direct glycation was clearly demonstrated by SDS-PAGE and confirmed by mass spectrometry analysis, in which differences between the native carriers and CML-carrier conjugates molecular mass were observed. In addition the ELISA screening for cross-reactivity allowed us to select antibodies strictly specific for CML.
mAb 2D6G2 was identified and used as an immunological probe to analyze sera from patients. The competitive ELISA developed here was sensitive with a linear range from 0.4 to 200 ng/dL of CML and a detection limit of 0.4 ng/dL. In addition, the results collected correlated with the CKD profile of patients who suffer from an inadequate clearance of these molecules. All together these observations make the monoclonal antibody 2D6G2 a valuable candidate to design diagnostic kits for the titration of CML protein adducts in unknown samples even if standardization will still be required.
Finally, several strategies could even be considered to further improve the sensitivity of the assay. As a first strategy, antibody 2D6G2 could be biotinylated to amplify the signal [26]. Even more interesting, 2D6G2 could be produced as a recombinant colorimetric tracer in which scFv 2D6G2 is genetically fused to a reporter with a well-defined coupling ratio. This strategy favors the design of homogeneous immunoconjugates and consequently increases assay performances. Besides, additional mutagenesis remains possible to alter the functional properties of both entities leading to variants with increased colorimetric activity or modified antigen selectivity. Recently, Alvarenga et al. [27] proposed to apply recombinant antibody technologies to the production of a bivalent colorimetric immune probe consisting in the V-domains of an antibody fused to a colorimetric tracer such as alkaline phosphatase. The same strategy can be used here because the cDNAs encoding 2D6G2 V-domains have been cloned and sequenced. This precaution was taken to follow the recommendations of researchers who recently pointed out the need to standardize antibodies used in research in order to make reliable binding reagents for all [28].
6. Conclusion
In conclusion, 2D6G2 is a novel monoclonal antibody that allows sensitive detection and titration of CML, the major antigenic AGE structure. Further developments should enable the design of diagnostic assays but also inhibitors of AGEs that could trigger beneficial effects in diabetic complications [9]. Finally, the availability of the sequence makes this antibody available to all researchers for the development of standardized binding reagents [29].
Financial support
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Araucária.
Acknowledgements
We would like to thank Michelle Weber and Rafaela Fogaça for skillful scientific assistance.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.03.011.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
References
- 1.El Nahas M. Cardio-kidney-damage: a unifying concept. Kidney Int. 2010;78:14–18. doi: 10.1038/ki.2010.123. [DOI] [PubMed] [Google Scholar]
- 2.De Lima A.O., Kesrouani S., Gomes R.A. Population screening for chronic kidney disease: a survey involving 38 721 Brazilians. Nephrol. Dial. Transpl. 2012;0:1–4. doi: 10.1093/ndt/gfs063. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation, K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am. J. Kidney Dis. vol. 39, 2013, pp. S1–S266. 〈https://www.kidney.org/sites/default/files/docs/ckd_evaluation_classification_stratification.pdf〉 [PubMed]
- 4.Vanholder R., De Smet R., Glorieux G., Argilés A. European Uremic Toxin Work Group (EUTox). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 5.Vanholder R., Van Laecke S., Glorieux G. What is new in uremic toxicity? PediatrNephrol. 2008;23:1211–1221. doi: 10.1007/s00467-008-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stinghen A.E., Massy Z.A., Vlassara G. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2015 doi: 10.1681/ASN.2014101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.N. Ahmed, Advanced glycation endproducts-role in pathology of diabetic complications, Diabetes Res.Clin. Pract. vol. 67 (1), 2005, pp. 3–21. 〈http://dx.doi.org/10.1016/j.diabres.2004.09.004〉 [DOI] [PubMed]
- 8.Won K.B., Chang H.J., Park S.H. High serum advanced glycation end-products predict coronary artery disease irrespective of arterial stiffness in diabetic patients, Korean. Circ. J. 2012;42:335–340. doi: 10.4070/kcj.2012.42.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlassara H., Striker G.E. Advanced glycation end products in diabetes and diabetic complications. Endocrinol. Metab. Clin. N. Am. 2013;42:697–719. doi: 10.1016/j.ecl.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Jaisson S., Gillery P. Evaluation of nonenzymatic posttranslational modification-derived products as biomarkers of molecular aging of proteins. Clin. Chem. 2010;56:1401–1412. doi: 10.1373/clinchem.2010.145201. [DOI] [PubMed] [Google Scholar]
- 11.Carter P.J. Potent antibody therapeutics by design. Nat. Rev. Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 12.Nagai R., Shirakawa J., Fujiwara Y. Detection of AGEs as markers for carbohydrate metabolism and protein denaturation. J. Clin. Biochem. Nutr. 2014;55:1–6. doi: 10.3164/jcbn.13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.F.G. Njoroge, A.A. Fernandes, V.M. Monnier, Mechanism of formation of the putative advanced glycosylation end product and protein cross-link 2-(2-furoyl)−4(5)-(2-furanyl)−1h-imidazole, J. Biol. Chem. vol. 263, 1988, pp. 10646–10652. 〈http://www.jbc.org/content/263/22/10646.long〉 [PubMed]
- 14.Ikeda K., Higashi T., Sano H. N-(carboxymethyl) lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry. 1996;35:8075–8083. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- 15.Alvarenga L.M., Martins M.S., Moura J.F. Production of monoclonal antibodies capable of neutralizing dermonecrotic activity of Loxosceles intermedia spider venom and their use in a specific immunometric assay. Toxicon. 2003;42:725–731. doi: 10.1016/j.toxicon.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Fields C., O’Connell D., Xiao S. Creation of recombinant antigen binding molecules derived from hibridomas secreting specific antibodies. Nat. Protoc. 2013;8:1125–1148. doi: 10.1038/nprot.2013.057. [DOI] [PubMed] [Google Scholar]
- 17.Becker-Finco A., Costa A.O., Silva S.K. Physiological, morphological, and immunochemical parameters used for the characterization of clinical and environmental isolates of Acanthamoeba. Parasitology. 2012:1–10. doi: 10.1017/S0031182012001746. [DOI] [PubMed] [Google Scholar]
- 18.LeFranc et al. Nucleic Acids Res. vol. 43, 2015. (Database issue):D413-D422 [DOI] [PMC free article] [PubMed]
- 19.Sakata N., Imanaga Y., Meng J. Immunohistochemical localization of different epitopes of advanced glycation end products in human atherosclerotic lesions. Atherosclerosis. 1998;141:61–75. doi: 10.1016/s0021-9150(98)00149-x. [DOI] [PubMed] [Google Scholar]
- 20.H. Makino, K. Shikata, K. Hironaka, et al., Ultrastructure of nonenzymatically glycated mesangial matrix in diabetic nephropathy, Kidney Int. vol. 48, 1995, pp. 517–526. PMID: 7564121 (PubMed - indexed for MEDLINE) [DOI] [PubMed]
- 21.Koito W., Araki T., Horiuchi S., Nagai R. Conventional Antibody against Nε-(Carboxymethyl)Lysine (CML) Shows Cross-Reaction to Nε-(Carboxyethyl)Lysine (CEL): immunochemical quantification of CML with a specific antibody. J. Biochem. 2004;136:831–837. doi: 10.1093/jb/mvh193. [DOI] [PubMed] [Google Scholar]
- 22.Schleicher E.D., Wagner E., Nerlich A.G. Increased accumulation of the glycoxidation product N(episilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J. Clin. Invest. 1997;9:457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai R., Fujiwara Y., Mera K. Immunochemicaldetectionof Nε-(carboxyethyl)lysineusing a specificantibody. J. Immunol. Methods. 2008;332:112–120. doi: 10.1016/j.jim.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 24.T.P. Degenhardt, S.R. Thorpe, J.W. Baynes, Chemical modification of proteins by methylglyoxal, Cell. Mol. Biol. vol. 44, 1998, pp. 1139–1145. PMID: 9846896 (PubMed - indexed for MEDLINE) [PubMed]
- 25.Mera K., Nagai M., Brock J.W. Glutaraldehyde is an effective cross-linker for production of antibodies against advanced glycation end-products. J. Immunol. Methods. 2008;20(334):82–90. doi: 10.1016/j.jim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 26.S. Sarkar, X.I. Tang, D. Das, et al., A bispecific antibody based assay shows potential for detecting tuberculosis in resource constrained laboratory settings, PLoS One vol. 7 (2), 2012 pp. e32340. 〈http://dx.doi.org/10.1371/journal.pone.0032340〉 [DOI] [PMC free article] [PubMed]
- 27.Alvarenga L.M., Muzard J., Ledreux A. Colorimetric engineered immunoprobe for the detection and quantification of microcystins. J. Immunol. Methods. 2014;406:124–130. doi: 10.1016/j.jim.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Bradbury A.R., Plückthun A. Getting to reproducible antibodies: the rationale for sequenced recombinant characterized reagents. Protein Eng. Des. Sel. 2015;10:303–305. doi: 10.1093/protein/gzv051. [DOI] [PubMed] [Google Scholar]
- 29.Bradbury A., Plückthun A. Reproducibility: standardize antibodies used in research. Nature. 2015;518:27–29. doi: 10.1038/518027a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material