Abstract
Selective MSCs differentiation protocol into pancreatic beta cells was conducted in the present study using exendin-4 and TGF-beta. Differentiated and undifferentiated MSCs were assessed in experimental type I diabetes in rats. Ninety female white albino rats were included in the study and divided equally (n=15/group) into 6 groups: healthy control, healthy control rats received acellular tissue culture medium, diabetic rats, diabetic rats received acellular tissue culture medium, diabetic rats received undifferentiated MSCs and diabetic rats received differentiated MSCs. Therapeutic efficacy of undifferentiated versus differentiated MSCs was evaluated via assessment of quantitative gene expressions of insulin1, insulin 2, Smad-2, Smad-3, PDX-1, PAX-4, neuroD. Blood glucose and insulin hormone levels were also assessed. Results showed that quantitative gene expressions of all studied genes showed significant decrease in diabetic rat groups. Use of undifferentiated and differentiated MSCs led to a significant elevation of expression levels of all genes with more superior effect with differentiated MSCs except smad-2 gene. As regards insulin hormone levels, use of either undifferentiated or differentiated MSCs led to a significant elevation of its levels with more therapeutic effect with differentiated MSCs. Blood glucose levels were significantly decreased with both undifferentiated and differentiated MSCs in comparison to diabetic groups but its levels were normalized 2 months after injection of differentiated MSCs. In conclusion, use of undifferentiated or differentiated MSCs exhibited significant therapeutic potentials in experimental type I diabetes in rats with more significant therapeutic effect with the use of differentiated MSCs.
Keywords: MSCs, Experimental diabetes, TGF-beta, Exendin-4
Highlights
-
•
Differentiated MSCs exhibited significant therapeutic potentials in type I diabetes.
-
•
TGF-beta1 and exendin-4 enhance MSCs differentiation into pancreatic beta cells.
-
•
Pancreatic lineage is evaluated by gene expressions of insulin-1, insulin-2.
-
•
Pancreatic differentiation is evaluated by expressions of PDX-1, PAX-4 and NeuroD.
-
•
Differentiated MSCs have more therapeutic potentials than undifferentiated MSCs.
1. Introduction
Type I diabetes mellitus (DM) is a prevalent disease affecting millions of people with serious morbid complications. Efficient glycemic control with exogenous insulin therapy is the cornerstone of treatment which imposes a great burden to most patients. Isolated beta cells or whole intact pancreas transplantation is an alternative treatment for patients with type I DM. However, the shortages of cadaveric pancreas and the need to immune-suppressive drugs are limiting factors [15].
Recent progress in regenerative therapies has focused attention on generation of surrogate β cells from mesenchymal stem cells derived adult tissues [4]. Bone marrow-derived mesenchymal stem cells (BM-MSCs) are multipotent that have efficient trans-differentiation potentials into mesodermal, endodermal, and ectodermal lineages [7,13]. BM-MSCs are good sources for generation of large numbers of autologous β cells circumventing the major limitations of cadaveric organs availability and allogenic rejection [11], [2].
Exendine-4 is a novel insulinotropic peptide and a long acting analog of glucagon-like peptide-1 (GLP-1). It interacts with endocrine pancreatic islet GLP-1 receptors, inducing a stimulatory effect on insulin secretion. Exogenous exendin-4 has shown to induce insulin-positive/endocrine differentiation in pancreatic exocrine AR42J cells [14], [21].
The co-application of exogenous exendin-4 and, specifically, low-dose exogenous transforming growth factorβ-1 (TGFβ-1) led to a dramatic 20-fold increase in insulin mRNA levels, supporting a novel synergistic and codependent relationship between exendin-4 signaling and TGF-isoform signaling [20]. Exogenous TGF-beta1 and exendin-4 each individually enhanced both insulin and glucagon differentiation dose-dependently. However, when combined there was an additive effect to a 4.5-fold increase in insulin-positive differentiation [18]. The pancreatic lineage differentiation was evaluated via assessment of gene expressions of insulin-1, insulin-2, PDX-1, PAX-4 and NeuroD [12].
The present study was conducted to assess the therapeutic efficacy of undifferentiated versus differentiated mesenchymal stem cells in experimental type I diabetes mellitus in rats. Selective differentiation protocol using TGF-beta and exendin-4 was implemented.
2. Materials and methods
2.1. Preparation of BM-derived MSCs
Bone marrow was isolated and propagated according to the standard described method [1]. Bone marrow was harvested by flushing the tibiae and femurs of 6-week-old white albino rats with Dulbecco's modified Eagle's medium (DMEM, GIBCO/BRL) supplemented with 10% fetal bovine serum (GIBCO/BRL). Nucleated cells were isolated with a density gradient [Ficoll/Paque (Pharmacia)] and resuspended in complete culture medium supplemented with 1% penicillin–streptomycin (GIBCO/BRL). Cells were incubated at 37 °C in 5% humidified CO2 for 12–14 days as primary culture or upon formation of large colonies. When large colonies developed (80–90% confluence), cultures were washed twice with phosphate buffer saline (PBS) and the cells were trypsinized with 0.25% trypsin in 1 mM EDTA (GIBCO/BRL) for 5 min at 37 °C. After centrifugation, cells were resuspended with serum-supplemented medium and incubated in 50 cm2 culture flask (Falcon). Cells were identified as being MSCs by their morphology, adherence, and their power to differentiate into osteocytes [9] and chondrocytes [16].
2.2. BM-derived MSCs differentiation into islet like cell clusters (ICC)
MSCs at passage 2 was induced with L-DMEM (4.5 mmol/L), glucose 5%, FBS, nicotinamide (10 mmol/L) and β-meracptoethanol (2.5 mmol/L) for 24 h. Re-induction was conducted for 10 h with the same culture medium without FBS and with L-DMEM (25 mmol/L). Maintenance of ICC was conducted for 3 weeks with L-DMEM (25 mmol/L), glucose 5%, without FBS, nicotinamide (10 mmol/L), β-meracptoethanol (2.5 mmol/L), exendin-4 (10 pmol/L) and TGFβ-1 (100 pmol/L). Smad2 gene expression was assessed in the undifferentiated and in the differentiated MSCs in vitro.
2.3. Labeling stem cells with PKH26
Undifferentiated and differentiated MSCs cells were labeled with PKH26 according to the manufacturer's recommendations, (Sigma, Saint Louis, Missouri, USA). The procedure involved use of 2 mL staining dye containing final concentrations of 2×10–6 M of PKH26 and 1×107 cells/mL. PKH26 is a fluorescent labeling dye. Labeling occurs by partitioning of the lipophilic dye into cell membranes. Labeling intensity is a function of both dye concentration and cell concentration and is not saturable. Incubation of the cell/dye suspension for 1–5 min with periodic mixing was conducted. The staining was stopped by adding an equal volume (2 mL) of a protein solution (e.g., 1% BSA) and incubation for 1 min to allow binding of excess dye. Visualization of stained cells was conducted using Nikon's SMZ25 (USA) fluorescent microscopy using PE filter for PKH26.
Cells were injected intravenously into rat tail vein. After one month, pancreatic tissue was examined with a fluorescence microscope to detect the cells stained with PKH26.
2.4. Preparation of animal groups
The present study was conducted on ninety female rats inbred strain (Cux1: HEL1) of matched age and weight (6 months to 1 year and 120−150 gm). Animals were inbred in the experimental animal unit, Faculty of Medicine, Cairo University. Rats were maintained according to the standard guidelines of Institutional Animal Care and Use Committee and after Institutional Review Board approval. Animals were fed a semi-purified diet that contained (gm/kg): 200 casein, 555 sucrose, 100 cellulose, 100 fat blends, 35 vitamin mix, and 35 mineral. Diabetes type I was induced in the relevant rat groups using a single intra-peritoneal injection of streptozotocin dissolved in 0.1 M sodium citrate buffer, pH 4.5, at a dose of 50 mg/kg. Induction of diabetes was confirmed by assessment of blood glucose after 72 h of streptozotocin injection. Rats with blood glucose above 200 mg/dL were considered to be diabetic.
Animals were divided equally into the following groups:
Group 1: Included 15 normal healthy rats that received the standard diet i.e. negative control group.
Group 2: Included normal healthy rats 15 rats that received the standard diet and acellular tissue culture medium.
Group 3: Included 15 rats that received streptozotosin to induce type I diabetes mellitus. i.e. diabetic control (positive control group).
Group 4: Included 15 diabetic rats received acellular tissue culture media.
Group 5: Included 15 diabetic rats that received undifferentiated MSCs stained with PKH26 red flouresence (3×106 cells intravenously, once) [2].
Group 6: Included 15 diabetic rats that received selectively differentiated MSCs into pancreatic progenitor cells stained with PKH26 red flouresence (102−103 cells intravenously, once) [7].
Three rats died in the control diabetic group and 5 rats died in the control diabetic injected with acellular group. All rats were sacrificed at the end of the third month. Blood samples were collected at the first, second and the third month to assess blood glucose and insulin levels by ELISA.
2.5. Real-time quantitative analysis for insulin1, insulin2, Smad-2, Smad-3, PDX-1, PAX-4, neuroD and GAPDH genes in pancreatic tissues
Total RNA was extracted from pancreatic tissue homogenate using RNeasy purification reagent (Qiagen, Valencia, CA). cDNA was generated from 5 μg of total RNA using RT reagent kit (Applied biosystems, catalog number 4306736). Real-time qPCR amplification and analysis were performed using SYBR® Green PCR Master Mix Reagents Kit (Catalog Number 4309155) and Applied Biosystem Instrument with software version 3.1 (StepOne™, USA). The qPCR assay with the primer sets were optimized at the annealing temperature (Table 1).
Table 1.
PCR primers of insulin 1, insulin 2, smad-2, smad-3, PDX-1, PAX4, and Neuro-D.
| NM_019129.3 Rattus norvegicus insulin 1 (Ins1) | 5′- CCAAGTCCCGTCGTGAAGT–3′ | |
| 5′- GGTGCAGCACTGATCCACAA–3′ | ||
| NM_019130.2 Rattus norvegicus insulin 2 (Ins2) | 5′- GTGACCAGCTACAGTCGGAA-3′ | |
| 5′-GCTTCCACCAAGTGAGAACCA -3′ | ||
| NM_001277450.1 Rattus norvegicus SMAD family member 2 (Smad2) | 5′-AGCCGCCCGAAGGGTA-3′ | |
| 5′-CAAGACTGGTGTCTCCACCC-3′ | ||
| NM_013095.3 Rattus norvegicus SMAD family member 3 (Smad3) | 5′-GTTAAAAGCGAAGTTCGGGCG-3′ | |
| 5′-CTTGGTGTTCACGTTCTGCG-3′ | ||
| NM_022852.3 Rattus norvegicus pancreatic and duodenal homeobox 1 (Pdx1) | 5′- GCACAAGAGCCAGTTGGGTA-3′ | |
| 5′- ATTGTCCTCAGTTGGGAGCC-3′ | ||
| NM_031799.1 Rattus norvegicus paired box 4 (Pax4) | 5′- GACGGTCTCAGCAGTGTGAA-3′ | |
| 5′- GGGGACTAGGAAGAGCTGGA-3′ | ||
| NM_019218.2 Rattus norvegicus neuronal differentiation 1 (Neurod1) | 5′- AATCATACAGCGAGAGCGGG-3′ | |
| 5′- GCTGGGACAAACCTTTGCAG-3′ | ||
| NM_017008.4 Rattus norvegicus glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 5′-GTTACCAGGGCTGCCTTCTC-3′ | |
| 5′- GATGGTGATGGGTTTCCCGT-3′ | ||
2.6. Analysis of pancreatic tissue pathology
Pancreatic tissue samples were collected into PBS and fixed overnight in 40 g/L paraformaldehyde in PBS at 4 °C. Serial 5-μm sections of the pancreatic tissues were stained with hematoxylin and eosin (HE) and were examined histo-pathologically.
2.7. Statistical analysis
Data were coded and entered using the statistical package SPSS version 16. Data was summarized using mean and standard deviation. Comparisons between groups were done using analysis of variance (ANOVA) with multiple comparisons post hoc test in normally distributed quantitative variables while nonparametric Kruscal-Wallis test and Mann-Whitney test were used for non-normally distributed quantitative variables by pearson correlation. P values less than 0.05 were considered as statistically significant.
3. Results
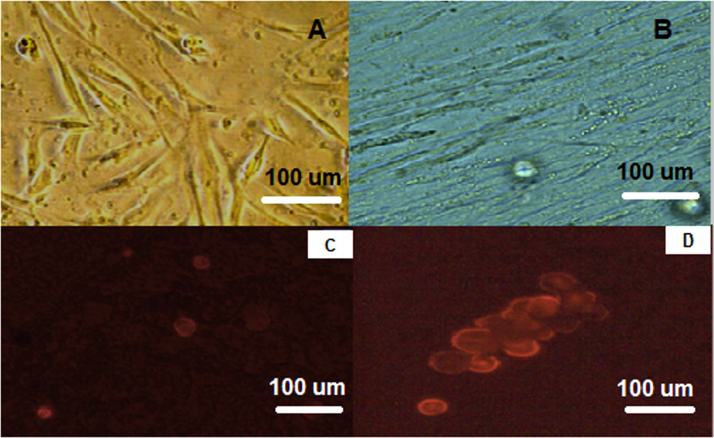
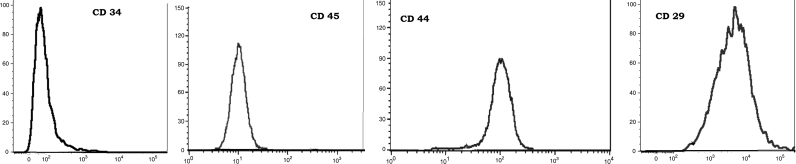
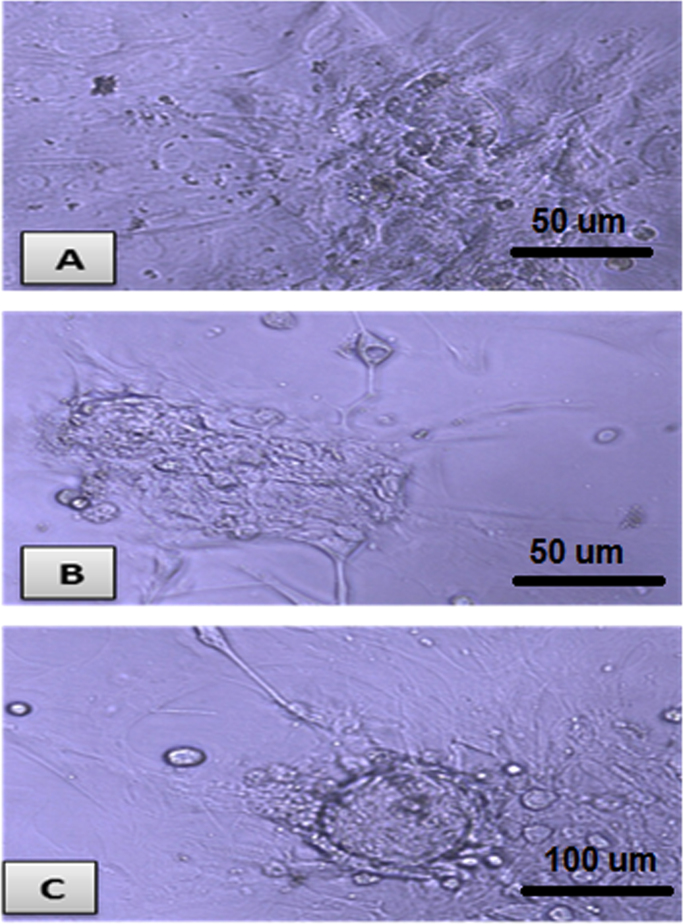
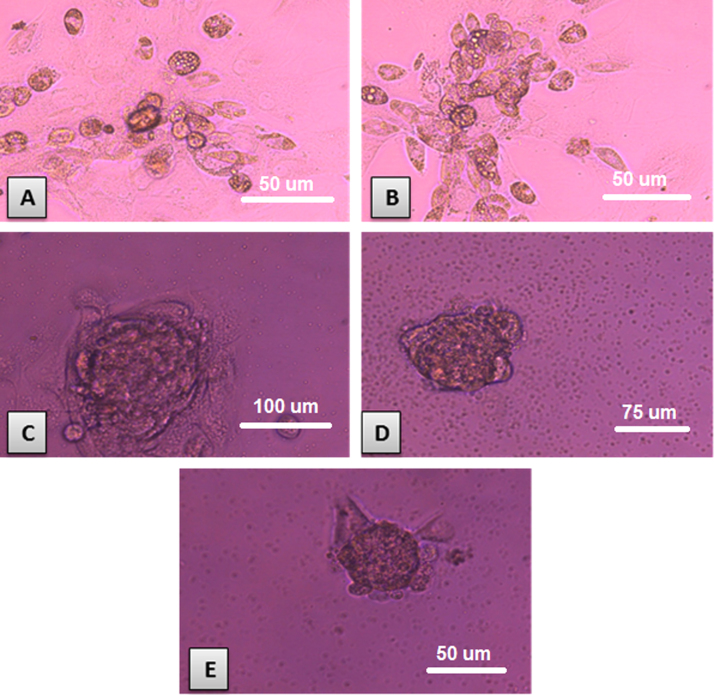
Isolated and cultured MSCs were identified by their characteristic fibroblast shape after 1 and 2 weeks (Fig. 1A and B). Mesenchymal stem cells labeled with PKH26 fluorescent dye detected in the pancreatic tissue, confirming homing into the injured pancreatic tissues (Figs. 1C and D). Flow-cytometric characterization revealed that MSCs were uniformly positive for CD29, and CD44 and negative for CD34 and CD45 (Fig. 2). Morphological change of unstained and stained (dithizone stain; DTZ) rat bone marrow MSCs during differentiation showed that MSCs started to form cell clusters after 10 days of differentiation, collected cell clusters after 20 days and well defined cell clusters with spheroid configuration were formed after 25 days of differentiation (Fig. 3, Fig. 4).
Fig. 1.
A: Spindle shaped MSCs at one week culture, B: MSCs at 2 weeks culture. C: Homing of PKH26 fluorescent labeled undifferentiated MSCs in rat pancreas. D: PKH26 fluorescent labeled differentiated MSCs into pancreatic like cells in rat pancreas.
Fig. 2.
Flow-cytometric characterization analysis of bone marrow- derived MSCs. Cells were uniformly positive for CD29, and CD44 and negative for CD34 and CD45.
Fig. 3.
Unstained rat bone marrow MSCs during differentiation. A: MSCs started to form cell clusters after 10 days of differentiation (X200). B: collected cell clusters after 20 days of differentiation (X200). C: well defined cell clusters with spheroid configuration were formed after 25 days of differentiation (X200).
Fig. 4.
Rat bone marrow MSCs during differentiation stained with DTZ: A: MSCs started to form cell clusters after 10 days of differentiation (X200). B: collected cell clusters after 20 days of differentiation (X200). C, D, and E: well defined cell clusters with spheroid configuration were formed after 25 days of differentiation (X200).
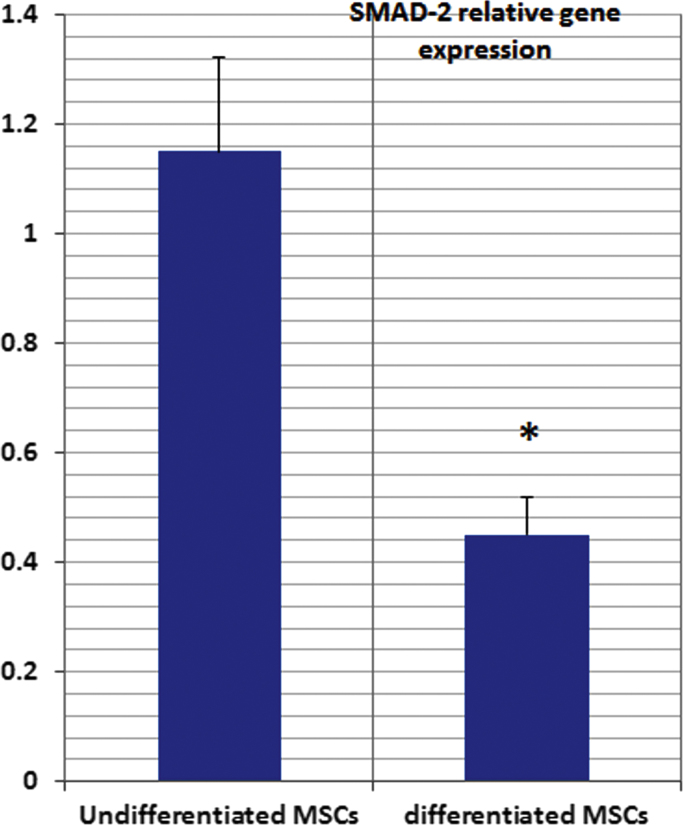
Smad-2 gene expression in cultured MSCs showed significant increase in the undifferentiated MSCs versus the differentiated MSCs in culture. (Fig. 5).
Fig. 5.
Smad2 relative gene expression in the undifferentiated and the differentiated MSCs in vitro.
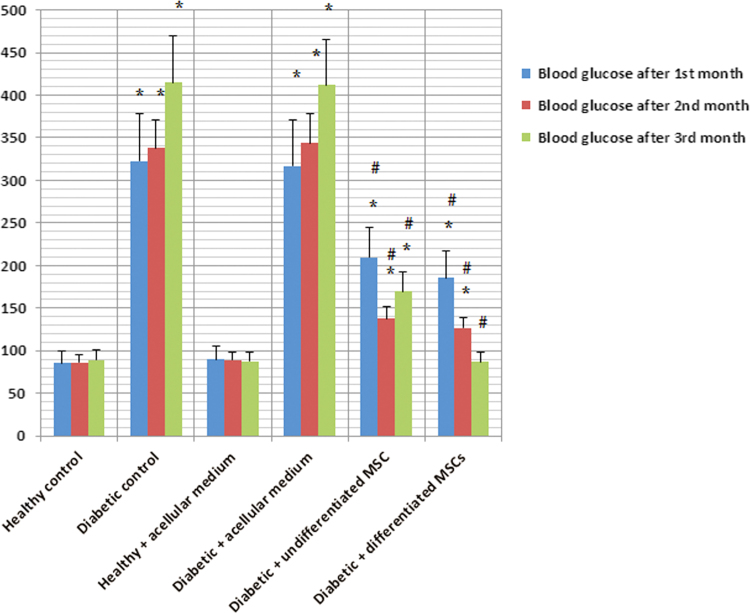
As regards blood glucose levels, use of either undifferentiated or differentiated MSCs exhibited significant decrease in blood glucose levels as compared to diabetic rats, whereas, the levels were still higher than healthy control animals except two months after injection of differentiated MSCs where blood glucose levels were normalized (Table 2 and Fig. 6).
Table 2.
Blood glucose levels (reference range 90–130 mg/dL) in all studied rat groups expressed as mean±SD.
| Healthy control | Diabetic control | Healthy+acellular medium | Diabetic+acellular medium | Diabetic+undifferentiated MSCs | Diabetic+differentiated MSCs | |
|---|---|---|---|---|---|---|
| Blood glucose levels after 1st month | 85.6±4.9 | 322.9±23.1 | 90.2±6.7 | 317±25.8 | 209.8±19.6 | 185.4±15.2 |
| Blood glucose levels after 2nd month | 86.6±5.7 | 337.8±19.6 | 89.2±5.9 | 344±21.9 | 137.6±16.8 | 126.6±17.1 |
| Blood glucose levels after 3rd month | 89.2±5.4 | 415.2±31.2 | 87.6±6.3 | 412±32.4 | 170.1±18.4 | 86.9±6.2 |
Fig. 6.
Blood glucose levels (mg/dL) in the studied rat groups after 1st, 2nd and 3rd months.
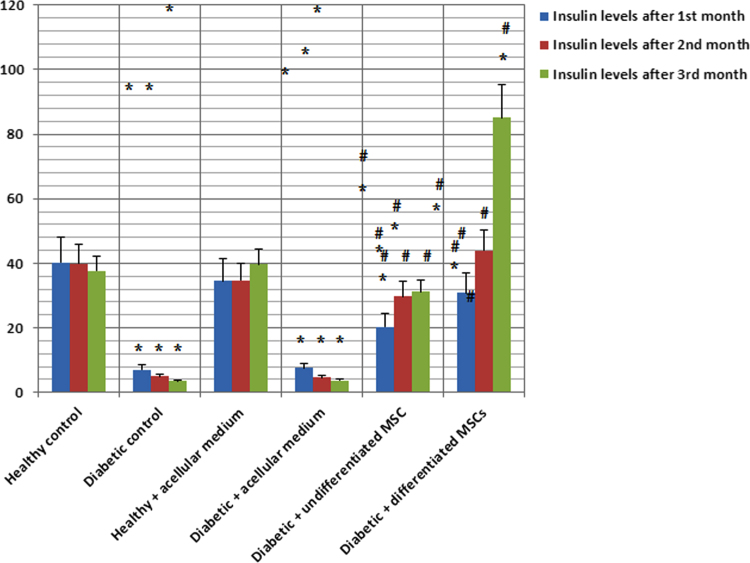
Insulin levels were significantly decreased in diabetic rat groups. Use of either undifferentiated or differentiated MSCs led to a significant increase and normalization in insulin levels except after the 1st month of injection of undifferentiated MSCs (Table 3 and Fig. 7).
Table 3.
Insulin levels (30–39 µU/mL) in all studied rat groups expressed as mean±SD.
| Healthy control | Diabetic control | Healthy+acellular medium | Diabetic+acellular medium | Diabetic+undifferentiated MSCs | Diabetic+differentiated MSCs | |
|---|---|---|---|---|---|---|
| Insulin levels after 1st month | 40.2±2.9 | 7±0.5 | 34.5±2.8 | 7.5±0.4 | 20.3±2.1 | 30.8±2.7 |
| Insulin levels after 2nd month | 39.9±3.0 | 4.9±0.4 | 34.7±2.9 | 4.6±0.4 | 29.8±2.7 | 43.9±3.2 |
| Insulin levels after 3rd month | 37.7±3.1 | 3.5±0.5 | 39.6±3.0 | 3.6±0.5 | 31.1±3.2 | 85.1±11.3 |
Fig. 7.
Insulin hormone levels (IU/mL) in the studied rat groups after 1st, 2nd and 3rd months.
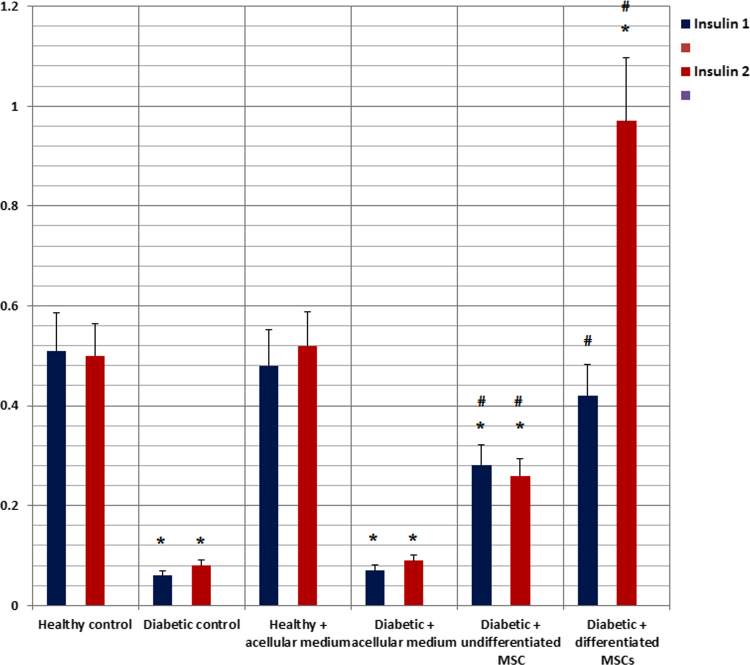
Insulin 1 and Insulin 2 gene expressions showed significant decrease in diabetic rat groups. Use of undifferentiated MSCs led to a significant elevation in insulin genes expression but their levels were still lower than control group. Use of differentiated MSCs led to a significant elevation and normalization of insulin genes expression as compared in control rat group (Fig. 8).
Fig. 8.
Relative gene expression of insulin-1 and insulin-2 in the pancreatic tissues of the studied rat groups.
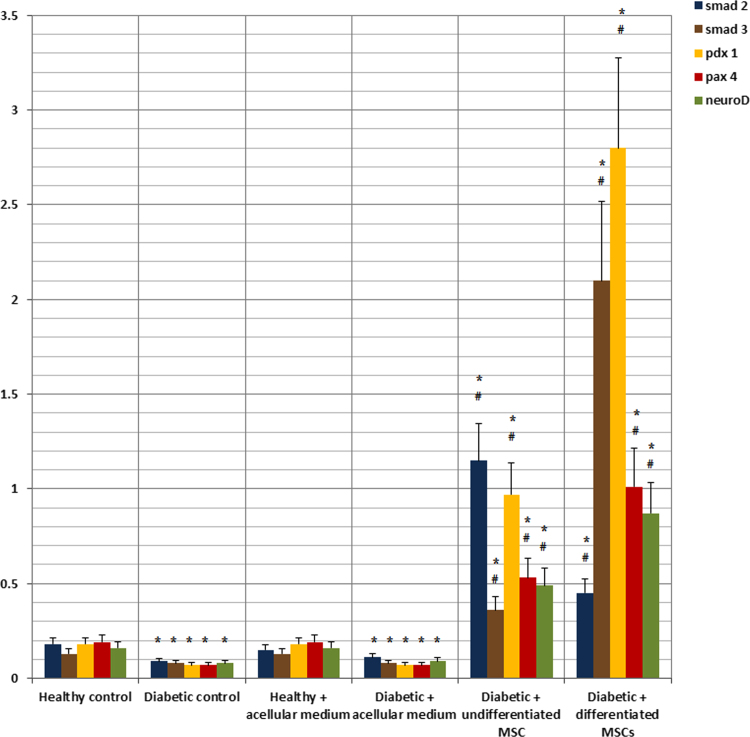
As regards SMAD-2, SMAD-3, PDX-1, PAX-4 and NeuroD gene expressions, their levels showed significant decrease in diabetic rat groups. Use of undifferentiated and differentiated MSCs led to a significant elevation of expression levels of all genes with more superior effect with differentiated MSCs (Fig. 9).
Fig. 9.
Relative gene expression of smad2, smad3, PDX-1, PAX-4 and neuroD in the pancreatic tissues of the studied rat groups.
4. Discussion
As a paradigm for stem cell therapy, we have studied the combined use of exendin-4 and TGFβ1 to induce selective lineage differentiation of MSCs into pancreatic progenitor beta cells. In the in vitro study, results showed that gene expression levels of smad2 were significantly elevated in the undifferentiated MSCs. However, in the differentiated MSCs there was significant down regulation of smad2 gene expression and upregulation of smad3 gene expression. The same pattern of genes expression was observed in the in vivo study for smad2 and smad3.
Yew et al. [20] stated that the acquisition of a β-cell phenotype, including insulin mRNA and protein expression, islet amyloid polypeptide (IAPP) expression, and pdx-1 expression, was dependent on smad2 pro-endocrine differentiation in the initial stages of differentiation followed by smad3 induced maturation and expression of β-cell–specific markers such as PDX1 [20], [6]. Based on these data, it appears that smad3 may support maturation of β-cells and inhibition of proliferation but only after smad2 has initiated a pro-endocrine differentiation program.
Results of the present study showed that use of either undifferentiated or differentiated MSCs led to a significant increase in insulin 1, insulin 2 genes, insulin hormone levels and genetic markers of beta cell maturation as PDX-1, PAX-4 and neurogenin D. Differentiated MSCs was superior to undifferentiated MSCs regarding all of the studied parameters. Similar findings were reported by other studies. Yew et al. [20], [21] reported that exendin-4 induce insulin-positive/endocrine differentiation in rat AR42J pancreatic epithelial cells. Insulin-positive differentiation was initiated by exendin-4 which induced down-regulation of smad2 and up-regulation of smad3 gene expression. The latter appears to be dependent on endogenous transforming growth factor (TGF)-β release by the AR42J cells and could be a mechanism to promote β-cell maturation. The co-application of exogenous exendin-4 and low-dose exogenous TGF-β1 led to a dramatic 20-fold increase in insulin gene expression, supporting a novel codependent synergistic relationship between exendin-4 signaling and TGF-β isoform signaling. More recently, Tamaki et al. [17] stated that glucagon-like peptide 1 (GLP-1) and its mimetic compound, exendin-4, exert their functions through multiple pathways, acting on the brain to induce satiety and potentiating glucose-stimulated insulin secretion. In addition, GLP-1 also has proliferative and anti-apoptotic effects on rodent beta-cells. Not only regulating replication and apoptosis of pre-existing b-cells, GLP-1 and exendin-4 also induce neogenesis of b-cells both in vivo and in vitro. Moreover, Zhou et al. [23] studied the effects of exendin-4 (Ex-4) on MSC. The authors found that after treatment with Ex-4, MSC displayed a higher proliferative capacity, increased expression of C-X-C motif receptor 4 (CXCR4) and an enhanced migration response. Moreover, Ex-4 preserved mitochondrial function through scavenging ROS and balancing the expression of anti- and pro-apoptotic proteins, leading to increased cell survival. Moreover, the authors proved that the PI3K/Akt pathway is partly responsible for Ex-4-mediated MSC growth, mobilization and survival. These findings provide an attractive method of maximizing the effectiveness of MSC-based therapies in clinical applications.
Our findings agreed with previous studies that showed the efficient protocol used in this study for trans-differentiation of bone marrow-derived MSCs into beta cells. The differentiated cells expressed nestin, pancreatic duodenal homeobox-1 (PDX-1), Neurogenin3, Pax4, insulin and glucagon [3], [5], [10].
PDX-1 gene is a transcriptional activator of several genes, including insulin, somatostatin, glucokinase, islet amyloid polypeptide, and glucose transporter type 2. The encoded nuclear protein is involved in the early development of the pancreas and plays a major role in glucose-dependent regulation of insulin gene expression. Defects in this gene are a cause of pancreatic agenesis, which can lead to early-onset insulin-dependent diabetes mellitus (NIDDM), as well as maturity onset diabetes of the young type 4 (MODY4) [19]. PDX-1 is a master switch gene that control gene expression cascade of all other transcription factors responsible for endocrine neogenesis [22]. These facts could explain our results that demonstrated significant elevation of PDX-1 gene expression and other pancreatic genes: PAX-4, insulin-1, insulin2 and neuroD with the use of MSCs in diabetic rats [8].
5. Conclusion
Use of undifferentiated or differentiated MSCs exhibited significant therapeutic potentials in experimental type I diabetes in rats with more significant therapeutic value with the use of differentiated MSCs. Further studies are recommended for use of differentiated MSCs in experimental diabetes with long term follow up to ensure safety and efficacy for justification of clinical trials.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.02.001.
Contributor Information
M.A. Wassef, Email: yosra_wassef@yahoo.com.
H. Fouad, Email: hanan.fouad@kasralainy.edu.eg.
D. Sabry, Email: dinnasabry69@yahoo.com.
N. Afifi, Email: talaatabdaziz@yahoo.co.uk.
A.M. Abbas, Email: abbas_moh648@yahoo.com.
W. Mostafa, Email: wagih-92mostafa@hotmail.com.
S.H. Ahmed, Email: dr.sahar2010@hotmail.com.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Abdel Aziz M.T., Atta H.M., Mahfouz S., Fouad H.H., Roshdy N.K., Ahmed H.H., Rashed L.A., Sabry D., Hassouna A.A., Hasan N.M. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin. Biochem. 2007;40:893–899. doi: 10.1016/j.clinbiochem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Abdel Aziz M.T., El-Asmar M.F., Haidara M., Atta H.M., Roshdy N.K., Rashed L.A., Sabry D., Youssef M.A., Abdel Aziz A.T., Moustafa M. Effect of bone marrow-derived mesenchymal stem cells on cardiovascular complications in diabetic rats. Med. Sci. Monit. 2008;14:BR249–BR255. [PubMed] [Google Scholar]
- 3.Bhonde R.R., Sheshadri P., Sharma S., Kumar A. Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors. Int. J. Biochem. Cell Biol. 2014;46:90–102. doi: 10.1016/j.biocel.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Calne R.Y., Gan S.U., Lee K.O. Stem cell and gene therapies for diabetes mellitus. Nat. Rev. Endocrinol. 2010;6:173–177. doi: 10.1038/nrendo.2009.276. [DOI] [PubMed] [Google Scholar]
- 5.Dave S. Mesenchymal stem cells derived in vitro trans-differentiated insulin-producing cells: a new approach to treat type 1 diabetes. Adv. Biomed. Res. 2014;3:266. doi: 10.4103/2277-9175.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Gohary Y., Tulachan S., Wiersch J., Guo P., Welsh C., Prasadan K., Paredes J., Shiota C., Xiao X., Wada Y., Diaz M., Gittes G. A Smad signaling network regulates islet cell proliferation. Diabetes. 2014;63:224–236. doi: 10.2337/db13-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabr M.M., Zakaria M.M., Refaie A.F., Khater S.M., Ashamallah S.A., Ismail A.M., El-Badri N., Ghoneim M.A. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed. Res. Int. 2014;2014:832736. doi: 10.1155/2014/832736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasa R., Mrejen C., Lynn F.C., Skewes-Cox P., Sanchez L., Yang K.Y., Lin C.H., Gomis R., German M.S. Induction of pancreatic islet cell differentiation by the neurogenin-neuroD cascade. Differentiation. 2008;76:381–391. doi: 10.1111/j.1432-0436.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal N., Haynesworth S., Caplan A., Bruder S.P. Osteogenic differentiation of purified, culture- expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 10.Jian R.L., Mao L.B., Xu Y., Li X.F., Wang F.P., Luo X.G., Zhou H., He H.P., Wang N., Zhang T.C. Generation of insulin-producing cells from C3H10T1/2 mesenchymal progenitor cells. Gene. 2015;562:107–116. doi: 10.1016/j.gene.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 11.Khorsandi L., Nejad-Dehbashi F., Ahangarpour A., Hashemitabar M. Three-dimensional differentiation of bone marrow-derived mesenchymal stem cells into insulin-producing cells. Tissue Cell. 2015;47:66–72. doi: 10.1016/j.tice.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Marappagounder D., Somasundaram I., Dorairaj S., Sankaran R.J. Differentiation of mesenchymal stem cells derived from human bone marrow and subcutaneous adipose tissue into pancreatic islet-like clusters in vitro. Cell. Mol. Biol. Lett. 2013;18:75–88. doi: 10.2478/s11658-012-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittenger M.F1., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 14.Ren L., Chen L., Qi H., Li F., Gong F. In vitro differentiation of human adipose tissue-derived stem cells into islet-like clusters promoted by islet neogenesis-associated protein pentadecapeptide. Cells Tissues Organs. 2014;199:329–341. doi: 10.1159/000362500. [DOI] [PubMed] [Google Scholar]
- 15.Ryan E.A., Paty B.W., Senior P.A., Bigam D., Alfadhli E., Kneteman N.M., Lakey J.R., Shapiro A.M. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 16.Seo M.S., Jeong Y.H., Park J.R., Park S.B., Rho K.H., Kim H.S., Yu K.R., Lee S.H., Jung J.W., Lee Y.S., Kang K.S. Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells. J. Vet. Sci. 2009;10:181–187. doi: 10.4142/jvs.2009.10.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamaki M., Fujitani Y., Uchida T., Hirose T., Kawamori R., Watada H. Combination treatment of db/db mice with exendin-4 and gastrin preserves β-cell mass by stimulating β-cell growth and differentiation. J. Diabetes Invest. 2010;1:172–183. doi: 10.1111/j.2040-1124.2010.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tei E., Mehta S., Tulachan S.S., Yew H., Hembree M., Preuett B., Snyder C.L., Yamataka A., Miyano T., Gittes G.K. Synergistic endocrine induction by GLP-1 and TGF-beta in the developing pancreas. Pancreas. 2005;31:138–141. doi: 10.1097/01.mpa.0000172566.70619.58. [DOI] [PubMed] [Google Scholar]
- 19.Vishwakarma S.K., Rahamathulla S., Bardia A., Tiwari S.K., Srinivas G., Raj A., Tripura C., Sandhya A., Habeeb M.A., Khan A.A., Pande G., Reddy K.P., Reddy P.Y. In vitro quantitative and relative gene expression analysis of pancreatic transcription factors Pdx-1, Ngn-3, Is1-1, Pax-4, Pax-6 and Nkx-6.1 in trans-differentiated human hepatic progenitors. J. Diabetes Invest. 2014;5:492–500. doi: 10.1111/jdi.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yew K.H., Prasadan K.L., Preuett B.L., Hembree M.J., McFall C.R., Benjes C.L., Crowley A.R., Sharp S.L., Li Z., Tulachan S.S., Mehta S.S., Gittes G.K. Interplay of glucagon-like peptide-1 and transforming growth factor-beta signaling in insulin-positive differentiation of AR42J cells. Diabetes. 2004;53:2824–2835. doi: 10.2337/diabetes.53.11.2824. [DOI] [PubMed] [Google Scholar]
- 21.Yew K.H., Hembree M., Prasadan K., Preuett B., McFall C., Benjes C., Crowley A., Sharp S., Tulachan S., Mehta S., Tei E., Gittes G. Cross-talk between bone morphogenetic protein and transforming growth factor-beta signaling is essential for exendin-4-induced insulin-positive differentiation of AR42J cells. J. Biol. Chem. 2005;280:32209–32217. doi: 10.1074/jbc.M505465200. [DOI] [PubMed] [Google Scholar]
- 22.Zaret K.S. Genetic programming of liver and pancreas progenitors: lessons for stem-celldifferentiation. Nat. Rev. Genet. 2008;9:329–340. doi: 10.1038/nrg2318. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H., Li D., Shi C., Xin T., Yang J., Zhou Y., Hu S., Tian F., Wang J., Chen Y. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci. Rep. 2015;5:12898. doi: 10.1038/srep12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material