Abstract
We recently reported a new UV-curable polyurethane-methacrylate (PUMA) resin that has excellent qualities as a disposable microfluidic substrate for clinical diagnostic applications. This article discusses strategies to improve the production yield of PUMA chips that contain dense and high-aspect-ratio features, which presents unique challenges in demolding and bonding steps. These fabrication improvements were deployed to produce a microfiltration device that contained closely spaced and high-aspect-ratio columns, suitable for retaining and concentrating cells or beads from a highly diluted suspension.
Introduction
Polydimethylsiloxane (PDMS) has been an attractive substrate for the fabrication of disposable microfluidic devices; chief among its advantages include the ease of fabrication and its elastomeric nature, which permits facile on-chip valving.1–4 However, casting high-aspect-ratio relief features or low-aspect-ratio microchannels is highly challenging in elastomeric PDMS: due to a low shear modulus, frequently microstructures buckle under their own weight5–7 and interfacial adhesion,8 microchannels become pinched off from a sagging ceiling,5,7,9,10 or apertures expand under increased operating pressure.11,12 Efforts to address these mechanical integrity issues include the introduction of harder microfluidic susbstrates such as h-PDMS (“hard” PDMS),13,14 and UV-casting of thermoset polyester (TPE)1,15 or commercial optical adhesives, which includes Norland 6316 or blends of polyacrylate.17
We recently reported a new UV-curable polyurethane-meth-acrylate (PUMA) resin that is non-elastomeric and has excellent qualities as a disposable microfluidic substrate, especially for clinical diagnostic applications.18 This PUMA substrate is transparent optically, resistant to biofouling, compatible with many chemicals encountered in microfluidic applications, curable to a typical thickness (about the thickness of glass slides), bondable to form enclosed devices easily, and capable of generating comparable electroosmotic flow — without surface modification — as a fused-silica capillary. Certified by the supplier as United States Pharmacopeia (USP) Class VI-compliant, this PUMA resin has been tested thoroughly for its chemical inertness, working temperature, biocompatibility, and sterilizability — all qualities necessary for manufacturing medical diagnostic devices.
In this article, we focus on the back-end steps — demolding, bonding, and interconnecting to external fluidic delivery — of producing a PUMA chip. During demolding, high-aspect-ratio microstructures are prone to shear-induced damage, whereas during bonding, they are prone to compression-related damage. Losses during these two steps must not be convoluted with the yield of UV-casting, which is highly consistent once the UV dosage and the thickness of the resin is properly optimized. We have devoted a great deal of effort to troubleshoot the demolding and bonding steps, and developed techniques to eliminate inconsistencies and inadvertent damages to the replicated microstructures. The result is an increased quality control and improvement in yield. These techniques can be easily adapted for commercial scale production.
Materials and methods
Polydimethylsiloxane (PDMS) molds were prepared according to procedures described previously18 from a molding master that was prepared by deep-reactive ion etching (DRIE) of silicon wafer and subsequently silanized with (tridecafluoro-1,1,2,2-tet-rahydrooctyl)trichlorosilane overnight. PUMA resin (Dymax 140-M, Torrington, CT) was dispensed to 3-mm thickness onto the PDMS mold, then covered with a sheet of cellophane tacked to a clear polypropylene backing (8-mil thick) to prevent oxygen inhibition of the cross-linking reaction (Fig. 1A). Aclar sheets (Honeywell, Morristown, NJ), which is a polychloro-trifluoro-ethylene (PCTFE) polymer containing no plasticizer, may be used in lieu of cellophane in critical applications. To form fluidic reservoirs or holes for external connection, PTFE posts (3 mm (D) × 3 mm (H)) were embedded in the PUMA resin before curing. The entire assembly was placed in a high-intensity UV source (ADAC Cure Zone 2 UV Flood Light Source, fitted with a 400W metal halide lamp, providing nominally 80 mW/cm2 at 365 nm) for 80 sec (expose through resin side), followed by an additional 40 sec (expose through mold). Once released from the mold, PUMA substrate was conformally bonded to another PUMA-coated (cured) glass coverslip with gentle mechanical pressure. This conformal bond was converted to permanent bond by placing the PUMA chip under the UV flood source for an additional 10 min.
Fig. 1.

(A) Material layout during molding of PUMA chip. 1: PDMS mold; 2: PUMA resin; 3: PTFE posts; 4: polypropylene sheet; 5: cellophane (or Aclar). (B) Two methods of interfacing PUMA chip with external tubing: 1: PUMA chip; 2: barb elbow connector; 3: polyurethane tubing; 4: additional resin to seal leak (Dymax 186M); 5: PTFE tubing; 6: polyolefin heat-shrink; 7: retaining ring.
Between each replication, the PDMS molds were sonicated in isopropanol and H2O and baked at 75 °C for at least 15 min.
Results and discussion
Fluidic interconnect
Fig. 1B shows two examples of interfacing a PUMA chip for external fluidic delivery. The left side of Fig. 1B illustrates the use of a barbed elbow connector. The elbow connector was inserted into a thick-wall polyurethane (PU) tubing (1/8-in outer diameter (OD), 1/16-in inner diameter (ID)), which served as a mechanical anchor against shear. The PU tubing was then inserted into a 1/8-in hole, formed either by embedding PTFE posts or laser cutting, in the PUMA substrate and additional resin (Dymax 186M) was dispensed around the junction and cured for 2–4 min. A tubing (1/16″ ID) was attached to the external end of the barbed elbow connector; this design offers facile attachment or detachment of an external tubing.
The right side of Fig. 1B illustrates interfacing a 1/16-in OD (or of equivalent dimensions as PE100 tubing from Becton Dickinson) PTFE tubing with a PUMA chip. We found that conventional polyethylene (PE) tubing (e.g. PE100), which is commonly used to interface with PDMS-based microfluidic devices, did not work well with PUMA chips, because (1) polyethylene surfaces are resistant to adhesive bonding, and (2) highly elastic tubings collapse easily when pulled in the longitudinal direction, causing delamination from the adhesive. The best tubing among those evaluated was the 1/16-in OD PTFE tubing. Although it is nearly impossible to chemically bond to PTFE tubings, that can be circumvented by covering the external surface with a polyolefin heat-shrink. Then the PTFE tubing may be inserted either directly into a 1/16-in diameter hole and secured with additional resin, or into a 1/8-in hole with a supplemental PU tubing (1/8-in OD) as a shear anchor, and lastly secured with additional resin.
For creating small through-holes in PUMA, we found laser-cutting to be more convenient than embedding and then removing PTFE posts. Sharpened punches do not produce clean holes in PUMA as they do in PDMS. We had tested an Epilog Helix CO2 laser-cutting system (45W; laser made by Coherent) which is commonly used as a computer-controlled souvenir or sign engraver. We noticed two minor issues with laser-cutting: (1) sputtering of melted plastic could damage structures or block channels; and (2) rastering rate, laser power, and spot focus need to be fine-tuned to avoid excessive melting. The sputtering problem should be readily addressable with a protective mask (e.g. cellophane or Scotch tape) on top of critical features.
Comparison with PDMS Chips
Cured PUMA resin has a Shore hardness of D 60, which is significantly harder than the elastomeric PDMS (Shore A 50 for Dow Corning’s Sylgard 184). For free standing, mechanically fragile features (in particular unsupported tall vertical columns or whiskers), PDMS cannot be used as the material of fabrication because of low shear modulus;6 the features would simply lean and topple over under gravity.
Fig. 2 shows an example of features that can be fabricated in PUMA but not PDMS. Fig. 2A shows a scanning electron microscopy (SEM) image of a replica in PUMA resin; this test pattern for replication consists of densely spaced vertical columns alternating with solid walls. The feature height was ~40 μm and the aspect ratio of the vertical columns was ~3.5. A 180° bend was incorporated in the design to help troubleshooting if there were directional issues in either the replication or demolding process. As evident in Fig. 2A, the columns produced in PUMA had a sharp vertical profile with no evidence of leaning.
Fig. 2.

Scanning electron microscopy images of (A) PUMA replica of an array of closely spaced high-aspect-ratio columns. (B) DRIE-Si master that is opposite in polarity as (A), and (C) PDMS replica made from DRIE-Si master in (B).
Fig. 2B shows a SEM image of a silicon master produced using deep-reactive ion etching (DRIE). This master had an inverse polarity (i.e. relief becomes recess) and was intended for replicating features in PDMS in the same polarity as Fig. 2A.
Fig. 2C shows the PDMS molded from the silicon master in Fig. 2B. One immediately notices that even though the PDMS columns were of the same height as the curved walls, which indicates a successful replication, the columns could not support their own weight and thus leaned over.
Demolding process
We found that for low-aspect-ratio (H/W<1) features the cured PUMA resin could be released from the PDMS mold either by (1) peeling the mold slightly away from the cured resin or (2) wedging a scalpel between the resin and the mold to gently lift up the resin. Here, the odds of damaging the relief features during releasing was very low. For high-aspect-ratio features, however, especially those that are mechanically fragile due to lack of support, proper release from the mold plays a pivotal role in the chip yield.
To curb inadvertent motion in the shear plane of the features during the demolding process, we devised a simple pulling station to separate the cured resin from the PDMS mold. By accurately controlling the direction and the speed of separation, damage to microstructures was greatly minimized.
Fig. 3 shows the schematic of the pulling station. It was based on a Dremel Workstation 220-01 assembly, which was intended to be a table-top drill press. The Workstation featured a spring-loaded lever that controlled the vertical translation along a shaft; upon releasing the lever, the upper mount translated upward until hitting a stop, ensuring that the chip is pulled 180-degrees away from the PDMS mold. A 1-in diameter vinyl suction cup was secured to the upper mount for attachment to the PUMA chip, and a second vinyl suction cup for attachment to the PDMS mold was immobilized to a metal base. Through holes (1/16-in diameter) were drilled at the base of the suction cups for connecting to a diaphragm vacuum pump.
Fig. 3.

A custom-designed pulling station for releasing a PUMA chip from a PDMS mold. 1: standard Dremel Workstation components (gray outline); 2: 1-in diameter vinyl suction cup; 3: a base suction cup; 4: metal base to secure the base suction cup.
After UV curing, the PUMA-PDMS assembly was placed on the base suction cup and the vacuum pump was turned on. The base suction cup held the PDMS mold in place while the upper suction cup was slowly brought down to contact the transparent polypropylene cover on top of the cured resin. The touch-down speed should be sufficiently slow such that minimal downward force was exerted on the resin. Once the vacuum gauge on the pump dropped from atmospheric pressure to the ultimate pressure of the pump, indicating that a good vacuum seal was achieved between the upper suction cup and the polypropylene cover, the spring-loaded lever was released to pull apart the resin and the mold.
We noticed the following in designing the pulling station: (1) the upper suction cup and the base suction cup must be properly aligned to distribute forces evenly, and (2) all parts must be securely fastened to avoid inadvertent vibration or motion in the horizontal directions (shear plane of the microstructures). The speed of release (faster the better) also helped to reduce defects.
Fig. 4 shows the improvement in demolding offered by the pulling station. Fig. 4A is an image of a PUMA replica taken under a stereoscope (same pattern as Fig. 2A), released from the mold without the assistance of the pulling station. Two types of defects were evident: (1) wavy walls, and (2) irregular vertical column spacings. The waviness of the wall is generally attributable to improper cleaning of the PDMS mold between replication runs, which increases the adhesion between the mold and the resin. Consequently it is not possible to lift apart without some horizontal motion to counter the adhesive force. Fresh, unused PDMS molds did not exhibit this problem. Rigorous sonication of PDMS molds in isopropanol and water between replications greatly reduced the incidents of defective curved walls. While this cleaning requirement lengthens the cycle time, disruption in production can be minimized by parallel processing and stockpiling enough PDMS molds in advance, such that while some molds are in use, some are being cleaned.
Fig. 4.

(A) Defects commonly observed when producing high-aspect-ratio structures. 1: wavy wall, usually a result of inadequately cleaned PDMS mold; 2: irregular column spacing, usually due to inadvertent mechanical damage during demolding. (B) SEM image of damaged high-aspect-ratio columns (pulling station not used during demolding). (C) Perfectly released high-aspect-ratio structures (pulling station used).
Fig. 4B shows a SEM image of the vertical columns that would have been deemed “irregular” under stereoscope inspection. The irregularity came from the columns leaning against each other, upsetting the periodic spacings. Although PUMA is significantly harder than PDMS, at this scale, the features are mechanically fragile. Fig. 4C shows a stereoscope image of a perfectly released PUMA replica using the pulling station. The spacing between the vertical columns was periodic as designed (no irregular dark spots).
We also tried several surface modification processes on PDMS to improve the demolding process, including plasma oxidation, silanization with (tridecafluoro-1,1,2,2-tetrahydrooctyl)tri-chlorosilane, and coating of surfactants such as n-dodecyl-beta-D-maltoside (DDM),19 Gransurf 71, and Gransurf 77. These surface modification techniques did not improve the demolding process; in the case of silanization, the silanized PDMS surface became too hydrophobic for uncured PUMA resin to wet properly. We also exploited the differences in thermal expansion (e.g. either quick freeze to −80 °C or heat): thermal treatment caused warping of PDMS in a direction opposite from the cured resin, but the cured resin also globally conformed to the warped PDMS. The result was a warped PUMA resin, rendering the subsequent conformal seal to a planar substrate impossible.
Bonding
Fig. 5 shows several methods that may be used to form enclosed PUMA microchannels. Since PUMA is a thermoplastic, heat is an effective way to form a permanent bond between the micro-channel substrate and the lid.
Fig. 5.
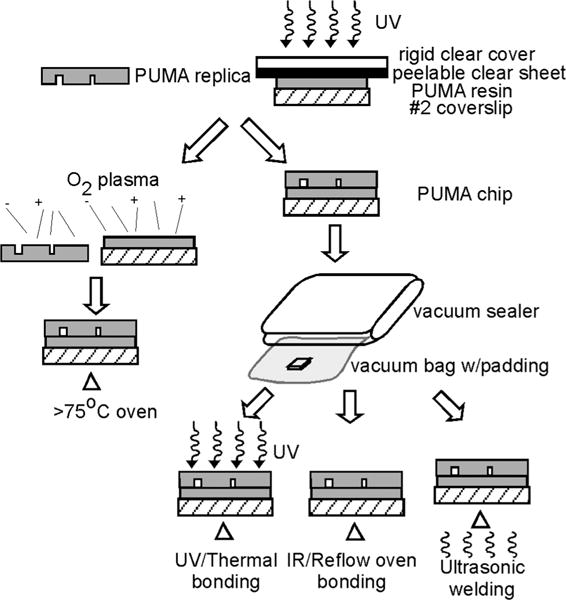
Methods of bonding PUMA chips to form enclosed channels. A PUMA chip, consisting of a piece of a PUMA replica and a PUMA-coated coverslip, can be bonded either by using oxygen plasma, followed by extended baking (left), or conformally bonded using a vacuum sealer, followed by UV/thermal bonding, IR bonding, or ultrasonic welding (right).
Because of the rigidity of the substrate, conformal seal of PUMA is not as simple as that of PDMS; care must be taken to avoid trappping air bubbles. In addition, excessive pressure could damage the microstructures. Our preferred method was to place the chip in a plastic bag, use a vacuum sealer that is commercially sold as a kitchen appliance to pull a vacuum on the bag, and rely on the collapsing bag to apply a compressive force evenly on the chip to form the conformal seal. Plastic bags intended for vacuum seal often have ridges to reduce premature collapsing of the bag before air is completely evacuated; these ridges can leave imprints on the PUMA substrate. We avoided the ridge imprint by lining the vacuum bag with lint-free cloth. The chip was removed from the plastic bag once >80% of the area was conformally sealed.
Following conformal seal, the enclosed chips were placed in the UV source to bond for 10–15 min. The intense UV and heat from the source caused softening of the PUMA substrate and the conformal seal became a permanent bond during the reflow process. The reflow did not lead to distortion of microstructures as long as no pressure was applied above the chip while it was still soft. Once the chip cooled, the permanent seal was capable of withstanding sustained high flow rate (>1 ml/min) at high pressure (20–40 psi); we routinely observed that the microscope coverslip (No. 2), which constituted the bottom surface of the chip, fractured before the permanent seal failed. This bonding method is our method of choice; however, other bonding techniques also may be used, which we describe briefly below.
Oxygen plasma may be used to enhance the conformal seal. After 15 minutes of oxygen plasma the conformal contact was improved: less air bubbles were trapped and the area of seal increased. However, manual elimination of air bubbles was still required because the sealing area usually was nowhere near 100% as typically witnessed between PDMS and glass. Following oxygen plasma, a permanent bond could be formed after the enclosed chip was placed in a 75 °C oven for two days; however, using this procedure, the frequency of seal failure during experiments was higher than with the chips produced using the UV-bonding method described above.
Alternate solventless-bonding methods that bear more resemblance to commercial production of thermoplastics may also be used. For example, programmable infrared oven, which provides fast ramping of temperature and is frequently used to reflow solder in circuit-board fabrication, should provide a more reliable temperature control than a UV lamp. Ultrasonic welding, which is a common technique for joining thermoplastics, may also be used provided the operating condition is properly optimized to reduce microstructure damage from local melting.
Bioanalytical applications
One motivation that drove our development of PUMA substrate was the need to fabricate a dense array of high-aspect-ratio slits for applications in microfiltration. Fig. 6 shows microscope images in which a dense packing of cells (Fig. 6A) and beads (Fig. 6B) were retained by an array of vertical columns produced in PUMA. In both experiments the distance between the columns was 8 mm and the height of the column was 40 mm. In Fig. 6A, a dilute suspension of fixed cancer cells (MCF-7 cells fixed in 4% paraformaldehyde for 15 min) was flowed through the chip at 0.3 ml/min. Such a microfluidic filter may serve to complement existing grid-based manual haemacytometer for clinical diagnostic use, because the ability to concentrate cells into a small area reduces the need to scan through many fields of view; this should allow for a more accurate and rapid enumeration of cells from a highly diluted suspension. In Fig. 6B, a solution of 15mm-diameter beads was used to pack a reaction zone in flow channel. This capability to pack beads closely in a microchannel may also find broad use, such as in affinity purification (e.g. where the beads were conjugated with antibodies) or in size-exclusion chromatography. For all such microfiltration-based applications, it is imperative to be able to fabricate the filtration elements with high yield, because replication failure of even a single column will result in the failure of the entire chip. This article shows that PUMA possesses the material property for fabricating such demanding microfluidic systems, provided that care is taken and that the described microfabrication procedure is followed.
Fig. 6.
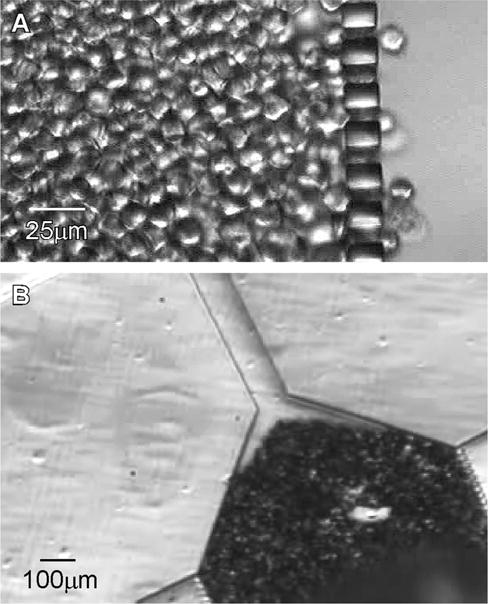
(A) Concentration and retention of MCF-7 (breast cancer) cells by high-aspect-ratio PUMA columns. Nominal flow rate was 0.3 ml/min (from left to right); cells were fixed in 4% paraformaldehyde for 15 min. (B) Close-packing of 15mm beads by high-aspect-ratio PUMA columns.
Conclusion
PUMA is a highly promising substrate for commercial production of microfluidic chips for clinical diagnostic applications. Because PUMA is a non-elastomeric substrate, extra care must be taken to avoid damaging high-aspect-ratio microstructures during demolding or during bonding to form an enclosed microfluidic device. The UV-curing process of PUMA resin is highly robust; however, improper demolding or bonding can significantly reduce the chip yield. We showed that a pulling station to release PUMA from PDMS mold minimized the motion in the shear plane of the microstructures; high-aspect-ratio microstructures can be perfectly replicated even in a high-density array, such as those used in our microfiltration chip. To avoid excessive compressive forces during conformal seal, a vacuum sealer should be used to remove the air between the PUMA replica and the bottom surface of the chip, while utilizing a collapsing plastic bag to exert a gentle yet even compressive force. Once conformal seal has been established, various bonding strategies can be used to convert this conformal seal to a permanent bond, including the use of a UV lamp to further cure and heat the chip, a process that offers high yield and a strong bond. The ability of PUMA to replicate high-aspect-ratio microstructure should find use for a wide range of analytical applications, and we believe PUMA will complement existing substrates in the production of disposable microfluidic devices, especially those that will be used in a clinical setting.
Acknowledgments
We are grateful to Washington State’s Life Sciences Discovery Fund (LSDF), the National Institutes of Health (EB005197), and the Keck Foundation for the support of this work.
References
- 1.Fiorini GS, Lorenz RM, Kuo JS, Chiu DT. Anal Chem. 2004;76:4697–4704. doi: 10.1021/ac0498922. [DOI] [PubMed] [Google Scholar]
- 2.McDonald JC, Whitesides GM. Acc Chem Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 3.Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 4.Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 5.Bietsch A, Michel B. J Appl Phys. 2000;88:4310–4318. [Google Scholar]
- 6.Delamarche E, Schmid H, Michel B, Biebuyck H. Adv Mater. 1997;9:741–746. [Google Scholar]
- 7.Hui CY, Jagota A, Lin YY, Kramer EJ. Langmuir. 2002;18:1394–1407. [Google Scholar]
- 8.Roca-Cusachs P, Rico F, Martinez E, Toset J, Farre R, Navajas D. Langmuir. 2005;21:5542–5548. doi: 10.1021/la046931w. [DOI] [PubMed] [Google Scholar]
- 9.Decre MMJ, Timmermans PHM, van der Sluis O, Schroeders R. Langmuir. 2005;21:7971–7978. doi: 10.1021/la050709p. [DOI] [PubMed] [Google Scholar]
- 10.Hsia KJ, Huang Y, Menard E, Park JU, Zhou W, Rogers J, Fulton JM. Appl Phys Lett. 2005;86:154106. [Google Scholar]
- 11.Gervais T, El-Ali J, Gunther A, Jensen KF. Lab Chip. 2006;6:500–507. doi: 10.1039/b513524a. [DOI] [PubMed] [Google Scholar]
- 12.Holden MA, Kumar S, Beskok A, Cremer PS. J Micromech Microeng. 2003;13:412–418. [Google Scholar]
- 13.Odom TW, Love JC, Wolfe DB, Paul KE, Whitesides GM. Langmuir. 2002;18:5314–5320. [Google Scholar]
- 14.Schmid H, Michel B. Macromolecules. 2000;33:3042–3049. [Google Scholar]
- 15.Fiorini GS, Yim M, Jeffries GDM, Schiro PG, Mutch SA, Lorenz RM, Chiu DT. Lab Chip. 2007;7:923–926. doi: 10.1039/b702548c. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Yang Y, Kim M, Nam SW, Lee KM, Lee NY, Kim YS, Park S. Adv Funct Mater. 2007;17:3493–3498. [Google Scholar]
- 17.Zhou WX, Chan-Park MB. Lab Chip. 2005;5:512–518. doi: 10.1039/b419330j. [DOI] [PubMed] [Google Scholar]
- 18.Kuo JS, Ng L, Yen GS, Lorenz RM, Schiro PG, Edgar JS, Zhao Y, Lim DSW, Allen PB, Jeffries GDM, Chiu DT. Lab Chip. 2009;9:870–876. doi: 10.1039/b818873d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang B, Wu HK, Kim S, Zare RN. Lab Chip. 2005;5:1005–1007. doi: 10.1039/b509251e. [DOI] [PubMed] [Google Scholar]


