Summary
Anucleate platelets circulate in the blood to facilitate thrombosis and diverse immune functions. Platelet activation leading to clot formation correlates with increased glycogenolysis, glucose uptake, glucose oxidation, and lactic acid production. Simultaneous deletion of glucose transporter (GLUT) 1 and GLUT3 (double knockout (DKO)) specifically in platelets completely abolished glucose uptake. In DKO platelets mitochondrial oxidative metabolism of non-glycolytic substrates such as glutamate, increased. Thrombosis and platelet activation were decreased through impairment at multiple activation nodes including Ca2+ signaling, degranulation, and integrin activation. DKO mice developed thrombocytopenia, secondary to impaired pro-platelet formation from megakaryocytes, and increased platelet clearance resulting from cytosolic calcium overload and calpain activation. Systemic treatment with oligomycin, inhibiting mitochondrial metabolism, induced rapid clearance of platelets with circulating counts dropping to zero in DKO but not wildtype mice, demonstrating an essential role for energy metabolism in platelet viability. Thus substrate metabolism is essential for platelet production, activation and survival.
eTOC blurb
Fidler et al. show that glucose metabolism is essential for platelet production, activation, and clearance. Their findings reveal complementary roles for glycolysis versus mitochondrial metabolism in platelet viability. Blocking both metabolic pathways leads to complete clearance of platelets from the circulation, due to calcium overload and calpain activation.

Introduction
Glucose enters cells via glucose transporters, of which platelets express the facilitative glucose transporter 1 (GLUT1) (Craik, 1995) and glucose transporter 3 (GLUT3) (Heijnen et al., 1997). ~85% of GLUT3 is located in α-granule membranes with ~15% localized in the plasma membrane. Upon degranulation, GLUT3 translocates to the plasma membrane and is believed to account for the increased glucose uptake following activation (Heijnen et al., 1997). Platelet activation also increases glycolysis and lactic acid production (Karpatkin, 1967), glycogenolysis (Scott and Cooper, 1967) and glucose oxidation (Warshaw et al., 1966). In vitro inhibition of these processes blunts platelet activation (Akkerman, 1981; Yamagishi, 2001), while incubation under hyperglycemic conditions potentiates activation in response to ADP, arachidonic acid, thrombin and collagen (Yamagishi, 2001). However, these associations have not been evaluated in vivo.
Although the correlation between activation and increased metabolism is well established, the mechanisms underling these relationships are unknown. Studies of permeabilized platelets, lacking cytosol indicate that ATP is an essential cofactor for Ca2+-induced degranulation (Flaumenhaft, 1999). Administration of the competitive glucose uptake inhibitor 2-deoxyglucose (2-DOG) to platelets suggested that glucose uptake and metabolism plays an important role in platelet aggregation and degranulation. Although these changes correlate with ATP content, a direct relationship between platelet glucose uptake and ATP content remains to be demonstrated (Akkerman, 1979). Conversely, platelets isolated from diabetic patients demonstrate dysfunctional Ca2+ signaling (Li, 2001), although it is unknown if platelet activation in this context arises from altered metabolism.
Mitochondrial respiration has also been linked to platelet activation. Inhibitors of mitochondrial respiration blunt platelet activation (Garcia-Souza and Oliveira, 2014). Upon activation, a subpopulation of platelets demonstrate mitochondrial depolarization and generation of reactive oxygen species (ROS) which facilitates phosphatidylserine (PS) exposure to the outer leaflet of the plasma membrane that promotes platelet procoagulant activity (Garcia-Souza and Oliveira, 2014). In addition to thrombotic functions, platelet mitochondria occupy a central role in the intrinsic apoptosis pathway (Mason et al., 2007). The balance between BCL-XL activity and formation of mitochondrial Bak/Bax pores regulates platelet apoptosis and platelet viability. However, the relationship between mitochondrial metabolism and apoptotic cell death pathways in platelets remain to be clarified. Moreover, mice infected with dengue demonstrate an additional mechanism of platelet clearance that correlated with PS exposure in vivo (Alonzo et al., 2012). Given the diverse mechanisms leading to platelet clearance, the integration of these mechanisms in the regulation of platelet viability and the contribution of platelet metabolism to these processes remains to be clarified.
Given the dearth of information regarding the role of energy metabolism in regulating platelet biology in vivo, we generated mice lacking GLUT1 and GLUT3 specifically in platelets. Analyses of these mice reveal a dynamic interaction between glucose utilization and mitochondrial energy metabolism to maintain platelet energy requirements. Importantly, glucose metabolism is essential for pro-platelet formation from megakaryocytes. Glucose metabolism is essential for agonist mediated Ca2+ signaling, and downstream signaling events leading to platelet activation. We demonstrate a critical role for glycolytic and mitochondrial metabolism in regulating platelet clearance establishing a paradigm whereby impaired platelet energy metabolism undermines calcium homeostasis, leading to increased cytoplasmic Ca2+ that activates a calpain-mediated cell death and clearance pathway.
Results
Glucose metabolism is abolished in DKO platelets
To investigate the contribution of glucose metabolism to platelet function, GLUT1 and GLUT3 were individually or simultaneously deleted from platelets by crossing mice expressing a Pf4 promoter-driven Cre recombinase to mice harboring homozygous floxed GLUT1 and GLUT3 alleles individually or in combination. Immunoblot analysis of platelet protein lysates isolated from GLUT1 single knockout (GLUT1-KO), GLUT3 single knockout (GLUT3-KO) and GLUT1/GLUT3 double knockout (DKO) and their respective littermate controls (Figure 1A) confirmed the absence of the respective proteins. Glucose uptake was measured in control quiescent platelets at ~10 pmoles/1×106 platelets/30 minutes, which after thrombin stimulation increased two-fold. Deletion of GLUT3 reduced basal glucose uptake by ~22% and abolished thrombin stimulated glucose uptake (Figure 1B) illustrating that GLUT3 mediates post-activation glucose uptake. Surprisingly, GLUT1-KO platelets revealed no changes in basal or thrombin-mediated glucose uptake relative to controls. However, deletion of both GLUT1 and GLUT3 completely abolished glucose uptake under basal and thrombin stimulated conditions indicating that GLUT1 and GLUT3 are the biologically relevant glucose transporters in platelets. These data also demonstrate that GLUT1 and GLUT3 have unique, but also overlapping roles in the regulation of platelet glucose uptake.
Figure 1. Glucose metabolism is decreased in DKO platelets.
A. Representative western blot of protein lysates from GLUT1-KO, GLUT3-KO, DKO and respective littermate control platelets. B. [3H] 2-DOG glucose uptake in platelets. C. 13C-Lactic acid production and D. 12C-lactic acid in the presence of 13C-Glucose exclusively in the extracellular media, n=3. E. Seahorse analysis of DKO platelet extracellular acidification rate (ECAR) under non-stimulated and thrombin (IIa) stimulated conditions, n=3. F. Glycogen analysis of platelets normalized to cell number, n=5. Error bars are SEM, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 with respect to control genotype; #P<0.05 with respect to treatment, same genotype. 2-way ANOVA followed by Bonferroni multiple comparison post-hoc test (B, and E)); Student’s t test (C, D, and F).
DKO platelets do not undergo glycolysis. Quantitative analysis of 13C-1,6-glucose flux demonstrated DKO platelets fail to convert exogenous glucose to lactic acid (Figure 1C), whereas controls generated 0.0085pg lactic acid/minute/1×106 platelets. In addition, with 13C-glucose as the exclusive exogenous glucose source, 12C-lactic acid release, which represents the contribution of glycogen to glycolysis, was also abolished (Figure 1D). Qualitative analysis of glycolysis determined by the extracellular acidification rate (ECAR) was markedly repressed in DKO platelets (Figure 1E). Of note, ECAR was not zero in DKO platelets potentially reflecting acidification of the media, by acids other than lactate (TeSlaa and Teitell, 2014). Consistent with studies in human platelets (Karpatkin, 1968), thrombin increased glycolysis by ~2-fold in control murine platelets. In contrast, DKO platelets did not increase glycolysis following thrombin stimulation, or in the presence of inhibitors of mitochondrial metabolism (Figure 1E). Platelets contain large glycogen stores, which can be mobilized upon activation (Scott and Cooper, 1967). Glycogen content in DKO platelets was reduced ~7-fold to levels near the limit of detection (Figure 1F). Thus glucose uptake, and metabolism are largely abolished in DKO platelets.
DKO platelets increase utilization of alternative mitochondrial substrates
In the absence of glucose uptake, alternative metabolic pathways may sustain cellular ATP to maintain cellular viability. DKO platelets displayed a significantly increased ratio of [AMP+ADP]/[ATP] (Figure 2A), suggesting metabolic stress. Consistent with this, freshly isolated DKO platelets demonstrated increased phosphorylated AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC) (Figure 2B). Interestingly, mitochondrial membrane potential was significantly increased in freshly isolated DKO platelets independent of exogenous substrate present in the media (Figure 2C). Seahorse analysis of mitochondrial oxygen consumption rates (OCR) in the presence of glucose, glutamate, and pyruvate indicated that at baseline and under maximal respiration conditions, OCR in DKO platelets were ~3-fold higher than controls (Figure 2D). Increased mitochondrial respiration in DKO reflects increased capacity to utilize mitochondrial substrates. OCR were equivalent in control and DKO platelets with glucose as the sole metabolic substrate, however following addition of glutamate and pyruvate, mitochondrial OCR increased by ~70% in DKO, but was unchanged in controls (Figure 2E). DKO platelets exhibited decreased intracellular lactic acid and relatively increased pyruvate leading to a significantly reduced lactate/pyruvate ratio (Figure 2F), suggesting induction of alternative pathways of pyruvate generation such as the transamination of alanine that requires mitochondrial α-ketoglutarate (Gray et al., 2014). Mitochondrial content and gross structure of DKO platelets were unchanged (Figure 3A and 3B), suggesting a qualitative rather than a quantitative change in mitochondrial function. Thus, absence of glucose metabolism in DKO platelets increased metabolism of alternative mitochondrial substrates.
Figure 2. DKO platelets increase mitochondrial metabolism in response to metabolic stress.
A. Estimation of AMP, ADP to ATP ratio in whole platelet lysates, n=8. B. Western blot analysis of protein lysates from freshly isolated platelets, n=3. C. Mitochondrial membrane potential of platelets incubated in the indicated media for 1 hour, n=3. D. Seahorse analysis of platelet O2 consumption in 25mM glucose + 1mM glutamate + 1mM pyruvate media ±thrombin (IIa), n=3. E. Seahorse analysis of DKO and control platelets under basal conditions in the presence of 25mM glucose ±1mM glutamate and 1mM pyruvate, n=3. F. Ratio of intracellular lactic acid to pyruvic acid, n=6. Error bars are SEM, *P<0.05, **P<0.01, ****P<0.0001 with respect to control genotype; #P<0.05 with respect to treatment, same genotype;(1-way ANOVA followed by Tukey’s multiple comparison post-hoc test (C and E). 2-way ANOVA followed by Bonferroni multiple comparison post-hoc test (D)); Student’s t test (A and F).
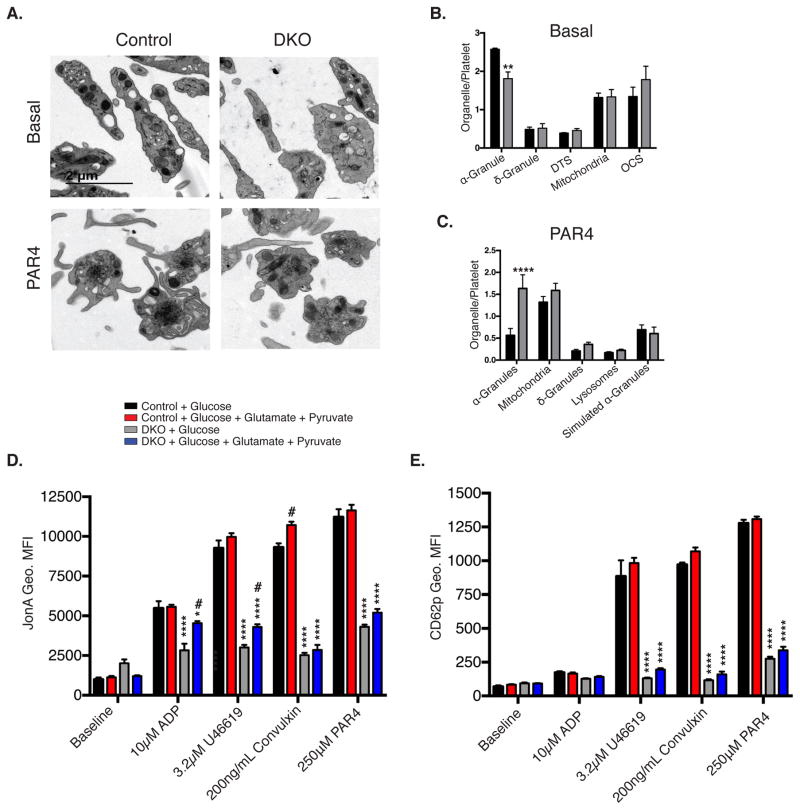
Figure 3. Impaired activation of DKO platelets.
A. Transmission electron microscopy of washed platelets in 5mM glucose media, treated in the presence or absence of 250μM Par4 (scale bar = 2μm). B. Quantification of non-stimulated, n=4, and C. Par4 peptide-stimulated electron micrographs of platelets, n=4. Washed platelets pre-incubated for 30 minutes in the presence of 5mM glucose ±1mM glutamate and 1mM pyruvate were stimulated with the indicated agonist and analyzed for D. GPIIbIIIa activation (JonA geo. MFI), and E. CD62p surface translocation (CD62p Geo. MFI), n=5. Data are mean±SEM, *P<0.05, **P<0.01, ****P<0.0001 with respect to control genotype; #P<0.05 with respect to treatment, same genotype; (2-way ANOVA followed by Bonferroni multiple comparison post-hoc test (B–E)).
Platelet activation is decreased in DKO platelets
DKO platelets exhibited blunted platelet activation. Non-stimulated DKO platelets displayed similar organelle distribution, with the exception of decreased α-granule number (Figure 3A and 3B), which may suggest a biogenesis defect. In addition, following stimulation with 250μM PAR4 peptide, degranulation was impaired in DKO platelets as evidenced by retention of α-granules in platelets (Figure 3A and 3C). In vitro analysis of platelets incubated in 5mM glucose for 1-hour, revealed decreased activation of GPIIbIIIa measured by JonA geo. MFI (Figure 3D), and α-granule degranulation marked by CD62p surface translocation (CD62p geo. MFI) (Figure 3E) in response to PAR4 peptide, the thromboxane receptor agonist U46619, ADP, and convulxin. However, this loss of activation was only partially rescued (ADP and U46619 – JonA) when glutamate and pyruvate was added to the media (Figure 3D and 3E), suggesting a direct link between glucose metabolism and platelet activation, that is not rescued in the presence of alternate mitochondrial substrates in DKO platelets.
Agonist mediated calcium signaling is impaired in DKO platelets
In response to activating stimuli, platelets increase cytoplasmic Ca2+ that activates the Ca2+ sensitive scramblase TMEM16f, to facilitate the translocation of PS to the outer leaflet of the plasma membrane leading to a procoagulant response (Jobe et al., 2008; Van Kruchten, 2012). Although PS is exposed, this procoagulant process is distinct from apoptosis-mediated PS exposure and is not thought to lead to an “eat me” signal that leads to platelet clearance (Van Kruchten, 2012). In response to thrombin plus convulxin, DKO platelets demonstrate impaired exposure of PS to the outer leaflet of the plasma membrane measured by annexin V binding (Figure 4A and S1A–F). Restoring Ca2+ flux by administration of the Ca2+ ionophore ionomycin, completely restored annexin V binding (Figure 4A). In response to thrombin, cytoplasmic Ca2+ flux was blunted in DKO platelets (Figure 4B). Importantly, following the rise in agonist-mediated cytoplasmic Ca2+, control platelets restored cytoplasmic Ca2+ to near basal levels (Figure 4B), whereas levels remained increased in DKO platelets for >10 minutes following stimulation. This inability to export/sequester Ca2+ following stimulation suggests a defect in sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and/or plasma membrane Ca2+-ATPase (PMCA)-mediated Ca2+ transport. Using an independent methodology, we confirmed impairment of platelet Ca2+ mobilization following combined stimulation by thrombin and convulxin of DKO platelets (Figure 4C and 4D). Following thapsigargin treatment to deplete SERCA, DKO platelets failed to restore Ca2+ concentrations to control levels (Figure 4E and 4F), revealing a defect in store operated Ca2+ entry. DKO platelets exhibit impaired GPIIbIIIa activation and CD62p surface translocation following treatment with thrombin plus convulxin, the Ca2+ ionophore ionomycin, or the SERCA inhibitor thapsigargin (Figure 4G and 4H). These data indicate that glucose metabolism regulates PS exposure to the outer leaflet of the plasma membrane via its effect on calcium mobilization, but even when calcium flux is rescued, glucose metabolism mediates additional steps required for integrin activation and α-granule degranulation.
Figure 4. DKO platelets demonstrate decreased thrombosis.
A. Washed platelets in 5mM glucose media treated with the indicated agonist for 15 minutes were monitored for annexin V positivity, n=6. B. Representative tracing of thrombin-mediated cytoplasmic Ca2+ monitored via fluo-4 MFI, using flow cytometry, n=3. C. Fura-2 loaded platelets stimulated with 1U/mL Thrombin + 160ng/mL Convulxin, n=4 D. Change in Ca2+ following administration of thrombin + convulxin, SEM (dashed lines), n=4. E. Fura-2 loaded platelets in 250μM EGTA, treated with thapsigargin (TG), then 1mM Ca2+, n=4. F. Change in Ca2+ following administration of thapsigargin, SEM (dashed lines), n=4. G. Relative GPIIbIIIa activation (relative JonA Geo. MFI) and H. CD62p surface translocation (relative Geo. MFI), following stimulation with the indicated agonist, n≥3. I. Tail-bleeding was assessed by monitoring time to bleeding cessation, n=7. J. Time to occlusion was determined in a 7.5% Ferric chloride induced arterial thrombosis model, n=6. K. Length of survival in a collagen/epinephrine induced pulmonary embolism model, n=12. Data are mean±SEM, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; (2-way ANOVA followed by Bonferroni multiple comparison post-hoc test (A, G, and H)); Students t-test (D, F, I, and J), Log-rank (Mantel-Cox) test (K).
Platelet glucose metabolism is essential for in vivo thrombosis
The contribution of platelet glucose metabolism to thrombosis and hemostasis in vivo is unknown. In a tail-bleeding assay, DKO mice exhibited significantly longer time to bleeding cessation, with many mice failing to stop prior to assay completion (Figure 4I). Although spontaneous bleeding was not observed, hematocrit was modestly yet significantly reduced in untreated DKO mice (Table S1). Arterial thrombosis was also evaluated using 7.5% ferric chloride. DKO mice had significantly increased time to arterial occlusion relative to littermate controls; with many failing to occlude after 20 minutes of observation (Figure 4J). In a collagen/epinephrine-induced pulmonary embolism model, which is dependent on in vivo platelet aggregation, DKO mice demonstrated increased survival (Figure 4K). Thus platelet glucose metabolism is essential for in vivo thrombosis.
DKO mice are thrombocytopenic
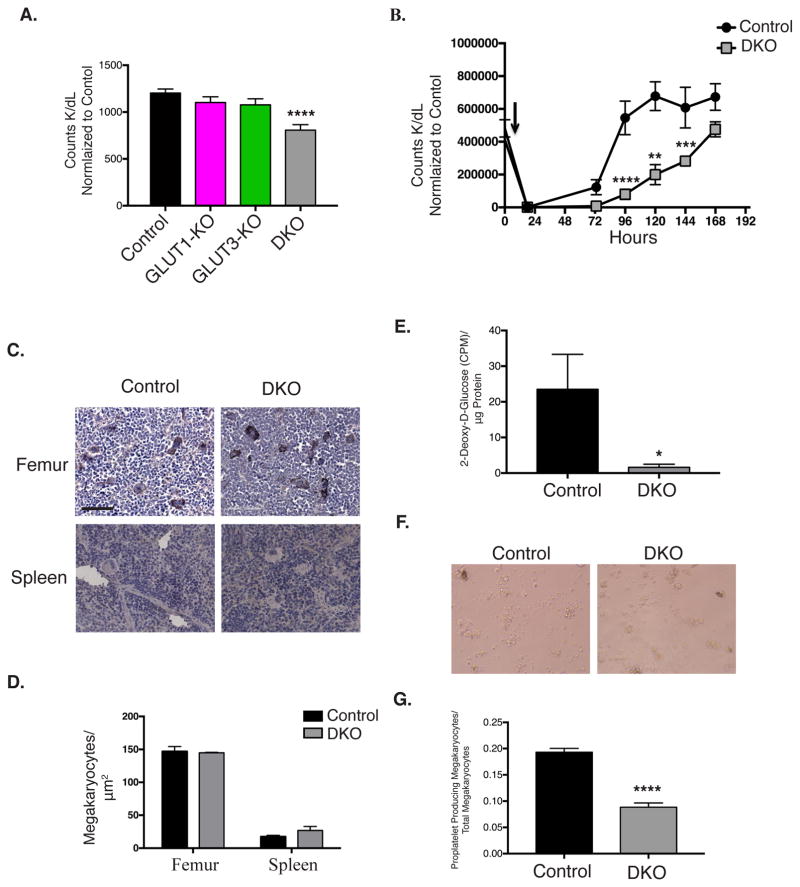
Platelet counts were reduced in DKO mice (Figure 5A, Table S1); however, platelet counts were unchanged in mice with GLUT1 or GLUT3-deficient platelets respectively. To determine if thrombocytopenia resulted from decreased platelet production from megakaryocytes, we administered antibodies to GPIbα that depletes platelets within 18 hours (Figure 5B). In control mice platelet counts completely recovered after 96 hours, whereas DKO mice required 168 hours for platelet recovery, suggesting that megakaryocyte-mediated platelet biogenesis may be impaired under conditions associated with increased platelet consumption. GLUT1 and GLUT3 single knockout mice exhibited no changes in platelet recovery (Figure S2A and S2B). Cross-sectional analysis of megakaryocytes in femurs and spleens of mice under basal conditions revealed no significant change in megakaryocyte number in DKO mice (Figure 5C and 5D). Megakaryocytes cultured from DKO bone marrow displayed significantly reduced GLUT1 and GLUT3 protein content (Figure S2C and S2D), and demonstrated virtually no glucose uptake (Figure 5E). Importantly, the ability of bone marrow derived megakaryocytes, to produce platelets was significantly impaired in DKO cultures (Figure 5F and 5G), even in the presence of glutamate and pyruvate. These observations define an obligate role for glucose metabolism in platelet generation from megakaryocytes.
Figure 5. Platelet production by DKO megakaryocytes is decreased.
A. Platelet counts in whole blood, n=7. B. Platelets were depleted through administration of anti-GP1bα (black arrow), and platelet counts were monitored, n=6. C. Representative image of immunohistochemistry for vWF in femurs, and spleens, from DKO and control mice counterstained with eosin. D. Quantification of megakaryocyte density in femurs, n=4, and spleens, n=3. E. Glucose uptake in cultured megakaryocytes. F. Representative image of megakaryocytes derived from control and DKO bone marrow (scale bar = 60μm). G. Quantification of proplatelet formation by bone marrow derived megakaryocytes, n=5. Data are mean±SEM, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 with respect to control genotype; 1-way ANOVA followed by Tukey’s multiple comparison post-hoc test (A). 2-way ANOVA followed by Tukey’s multiple comparison post-hoc test (B)); Student’s t test (D, E, and G).
Phosphatidylserine exposure is increased in DKO platelets in response to metabolic stress
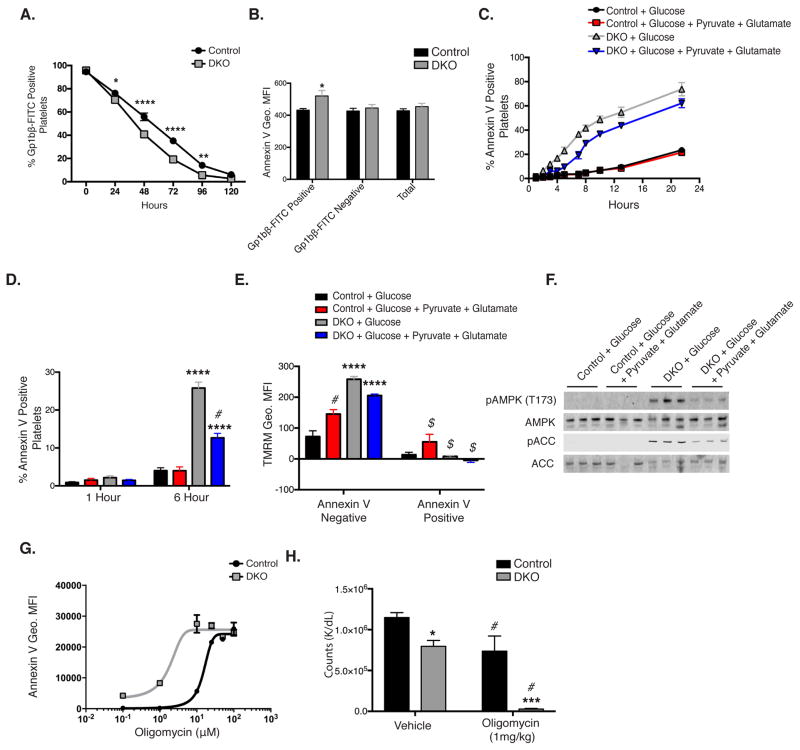
Circulating platelet counts reflect the balance between platelet production and clearance. Therefore we investigated circulating half-life of platelets by administration of a Dylight 488 labeled anti-GP1bβ antibody. GLUT1 and GLUT3 single knockout mice demonstrated no reduction in circulating half-life (Figure S2E and S2F), However, DKO platelets demonstrated significantly reduced circulating half-life compared to controls (Figure 6A), suggesting that increased clearance also contributes to the thrombocytopenia observed. An important mechanism of platelet clearance occurs via protein desialylation, which can be monitored by Ricinus communis aggulutinin I (RCA-1) binding (Li et al., 2015). However, DKO platelets demonstrated no change RCA-1 binding (Figure S3A), rendering this mechanism unlikely. We also ruled out increased autophagy or oxidative stress as mechanisms for increased clearance (Figure S3B, S3C). Platelet clearance can also be potentiated by increasing extracellular exposure of PS on plasma membranes (Alonzo et al., 2012). Therefore we monitored annexin V binding on platelets in diluted whole blood, 72 hours post administration of Dylight 488 labeled anti-GP1bβ antibody. Interestingly, GP1bβ positive platelets, which represent older platelets, displayed increased relative annexin V binding marked by the geo. MFI (Figure 6B), but no significant increase was observed in GP1bβ negative platelets or in the population as a whole, suggesting that as DKO platelets age they increase PS exposure to the outer leaflet of the plasma membrane. In vitro, control platelets incubated in DMEM with 5mM glucose ± 2mM glutamate and 1mM pyruvate had minimal annexin V positive platelets after 22 hours of incubation (Figure 6C). In contrast, after 22 hours, DKO platelets incubated in glucose alone exhibited ~70% annexin V positivity (Figure 6C). Supplementation of the media with the mitochondrial substrates glutamate and pyruvate significantly reduced but did not normalize annexin V binding in DKO platelets (Figure 6C). Due to optimal signal resolution, a 6-hour in vitro incubation time was chosen for subsequent signaling studies. After 6-hours incubation, DKO platelets incubated in glucose alone exhibited ~25% annexin V positivity, while addition of glutamate and pyruvate decreased annexin V binding by half to ~12%. Control platelets regardless of exogenous substrates demonstrated <5% annexin V binding (Figure 6D). Following a 6-hour incubation, annexin V positive platelets displayed mitochondrial depolarization (Figure 6E). Western blot analysis indicated that AMPK and ACC phosphorylation following 6-hour incubation, were significantly increased in DKO platelets incubated in glucose only and addition of glutamate and pyruvate to the media attenuated these changes (Figure 6F). Thus energy deficits as indicated by AMPK activation correlate with increased annexin V positivity. If energy metabolism regulates platelet annexin V binding and clearance, we hypothesized that decreasing platelet energy metabolism would further increased annexin V binding. Thus platelets were treated in vitro with the ATP synthase inhibitor oligomycin. DKO platelets demonstrated increased sensitivity to oligomycin as marked by annexin V geo. MFI (Figure 6G). Furthermore, in vivo intraperitoneal administration of oligomycin at (1mg/kg), a concentration which did not lead to clinical toxicity or weight change (data not shown), reduced circulating platelets counts by ~20% in controls but by ~97% in DKO mice (Figure 6H) after 6 hours. Together these data indicate that platelet metabolic function directly modulates circulating platelet half-life. Oligomycin treatment of controls suggests that glycolytic metabolism might be sufficient to maintain platelet vitality. However, in the absence of glucose transport, oligomycin blocks alternative substrate utilization leading to catastrophic ATP loss, annexin V exposure and nearly complete platelet clearance.
Figure 6. DKO platelets demonstrate increased clearance.
A. Platelets were labeled with anti-Gp1bβ-FITC labeled antibody, then monitored for percent CD41 positive platelets, n=6. B. Whole blood was collected at 72 hours post anti-Gp1bβ-FITC injection, then monitored for annexin V Geo. MFI., n=6 C. Washed platelets incubated in 5mM glucose media ±1mM glutamate and 1mM pyruvate at 37°C with 5% CO2 were monitored for annexin V exposure, n=3. D. Platelet annexin V binding following 6 hour incubation in specified media at 37°C with 5% CO2, n=20. E. Mitochondrial potential of platelets stratified for annexin V positivity, following 6-hour incubation, n=3. F. Western blot analysis of platelet proteins following 6-hour incubation, n=6. G. Annexin V binding in platelets incubated for 1 hour in vitro with oligomycin. H. Mice were injected with 1mg/kg oligomycin or vehicle and monitored for platelet count following 6 hours, n=5. Data are mean±SEM, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 with respect to control genotype; #P<0.05 with respect to treatment, same genotype, $P<0.05 with respect to annexin V negative platelets of equivalent genotype and treatment; 2-way ANOVA followed by Bonferroni multiple comparison post-hoc test (A–E and H)).
Increased platelet clearance in DKO mice is partially mediated by calpain activation
Platelet PS exposure is facilitated by two distinct pathways. The first pathway is agonist-mediated mPTP opening and cytoplasmic Ca2+ activation of the scramblase TMEM16f. The second is through the intrinsic apoptosis pathway, which requires BCL-XL degradation, mitochondrial depolarization, cytochrome c release into the cytosol, and caspase 3 activation (Schoenwaelder et al., 2009). To determine if opening of the mPTP resulted in increased annexin V binding, we co-incubated DKO platelets with cyclosporine A and monitored annexin V binding following 6 hour incubation in vitro (Figure S4A). Cyclosporine A did not alter the time-dependent increase in annexin V positivity. We next considered the intrinsic pathway. Following six hours incubation in vitro, DKO platelets demonstrated no change in BCL-XL protein expression (Figure S4B). No change in sensitivity towards the BH3 mimetic Abt-737 was observed as measured by annexin V binding or caspase 3/7 activity (Figure S4C and S4D). DKO platelets co-treated with the caspase 3 inhibitor Z-DEVD-FMK demonstrated no change in annexin V binding after 6 hours incubation (Figure S4E). Western blot analysis of lysates from DKO platelets incubated in media containing glucose as the sole substrate, did not display caspase 3 activation measured by the formation of a 15kDa and 17kDa cleaved caspase 3 band (Figure 7A). Thus mPTP opening or activation of the intrinsic apoptosis pathway does not appear to facilitate annexin V binding.
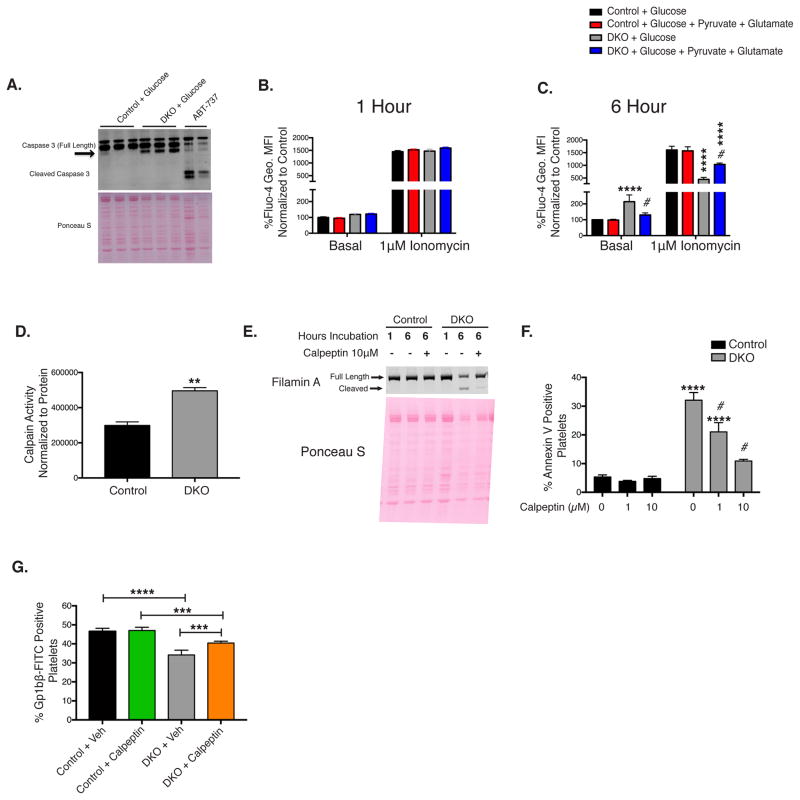
Figure 7. Calpain activation regulates DKO platelet annexin V binding and clearance in vivo.
A. Western blot analysis of caspase 3, black arrow indicates calpain cleavage product. Annexin V negative platelets loaded with Fluo-4 in media with 1mM EGTA were analyzed for basal cytoplasmic Ca2+ concentration and following stimulation with 1μM ionomycin 1 hour (B) and 6 hours post isolation (C), marked by Fluo-4 Geo. MFI, n=6. D. Calpain activity of platelets incubated in glucose media for 6 hours. E. Western blot analysis of Filamin A cleavage, n=3. F. Platelets were incubated in the presence of vehicle (Veh) or calpeptin for 6 hours then analyzed for annexin V binding, n=6. G. Mice were injected with calpeptin (1mg/kg) daily for 7 days prior to injection with anti-Gp1bβ-FITC labeled antibody and calpeptin administration was continued daily until cessation of half-life experiments. Blood was assayed at 55 hours post injection, n=6. Data are mean±SEM, **P<0.01, ***P<0.001, ****P<0.0001 with respect to control genotype; #P<0.05 with respect to treatment, same genotype; Students t-test (D); (1-way ANOVA followed by Tukey’s multiple comparison post-hoc test (G). 2-way ANOVA followed by Bonferroni multiple comparison post-hoc test (B, C, and F)).
Immunoblots revealed a band ~3kDa smaller than procaspase 3 (Figure 7A), which is calpain dependent (Wolf B.B., 1999). Calpain-mediated platelet death has not previously been described, however in other cell types, necrosis is facilitated through calpain activation. Because calpains are Ca2+ dependent proteases, we investigated cytoplasmic Ca2+ concentrations. Following 1-hour incubation, cytoplasmic Ca2+ as well as stored Ca2+ concentrations were unchanged in control and DKO platelets (Figure 7B). However, following 6-hour incubation, DKO platelets incubated in vitro in glucose alone demonstrated increased basal cytoplasmic Ca2+ levels despite decreased stored Ca2+ (Figure 7C), in annexin V negative platelets. Supplementation of the media with glutamate and pyruvate significantly restored cytoplasmic as well as stored Ca2+ levels, indicating metabolic regulation of platelet Ca2+. After 6-hour incubation in vitro, calpain activity was significantly increased in DKO platelets (Figure 7D). Immunoblot analysis of filamin A, a known calpain substrate, revealed increased cleavage compared to controls (Figure 7E). Co-administration of calpeptin to DKO platelets decreased filamin A cleavage (Figure 7E). Interestingly, the calpain inhibitor calpeptin significantly decreased annexin V binding in a dose dependent manner in DKO platelets when co-incubated in vitro for 6 hours (Figure 7F). In vivo treatment of DKO mice with calpeptin, significantly prolonged platelet half-life, relative to vehicle treated DKO mice (monitored 55 hours post Dylight 488 labeled anti-GP1bβ antibody pulse) (Figure 7G And Figure S5). Consistent with previous reports (M. Bonomini, 2004; Wolf B.B., 1999) calpeptin did not reduce agonist or ionophore mediated annexin V exposure (Figure S6), indicating calpeptin acts via a mechanism that is independent of TMEM16f. Thus DKO platelets undergo a necrosis like death, which is Ca2+ and calpain dependent.
Discussion
Several in vitro studies reveal a relationship between glucose metabolism and platelet function (Paterson, 1974; Tang et al., 2011; Yamagishi, 2001), however, the specific roles of glucose transport and utilization in platelet function in vitro and in vivo are incompletely understood. Our studies demonstrate critical roles for glucose metabolism in platelet production, circulating half-life, activation, degranulation and thrombus formation. Moreover, we demonstrate that reduced energy metabolism leads to increased annexin V exposure and platelet clearance through a calpain dependent mechanism.
RNASeq analysis of platelets from mice and humans indicate that GLUT1 and GLUT3 are the only Class I glucose transporters expressed in platelets (Rowley et al., 2011; Shi et al., 2014). Our glucose uptake data indicate that, GLUT1 and GLUT3 exhibit overlapping as well as unique functions. Surprisingly, deletion of GLUT1 alone did not alter glucose uptake, indicating that GLUT3 may compensate for GLUT1; possibly by increased plasma membrane GLUT3 localization or by increased GLUT3 activity. In addition, deletion of GLUT3 alone only slightly decreased basal glucose uptake, but completely abolished agonist mediated glucose uptake. This supports previous studies indicating GLUT3 is primarily located on α-granule membranes and translocates to the plasma membrane when platelets degranulate (Heijnen et al., 1997). The complete loss of glucose uptake in DKO platelets confirms that GLUT1 and GLUT3 represent the biologically relevant glucose transporters in platelets.
In the absence of glucose availability, platelets increase mitochondrial respiration by utilizing alternative mitochondrial substrates. This metabolic plasticity appears to be due to qualitative adaptations in mitochondrial function as evidenced by unchanged mitochondrial number in the face of increased mitochondrial membrane potential and respiration. It is possible that increased mitochondrial respiration and membrane potential increased ATP production. When glutamate and pyruvate were supplemented in the media, AMPK activation was reduced, although not completely normalized. Thus alternative mitochondrial substrates in vivo such as glutamate, lactate, or fatty acids could support platelet metabolism in DKO mice. Even if true, freshly isolated DKO platelets displayed increased AMPK activation relative to controls. Furthermore, in vivo platelet counts were decreased and thrombus formation impaired, indicating that mitochondrial metabolism was insufficient to maintain platelet energy requirements of DKO mice in vivo.
GPIIbIIIa activation, degranulation, and PS exposure to the outer leaflet of the plasma membrane were decreased in DKO platelets. These three markers of platelet activation are facilitated by agonist-mediated activation of IP3 receptors leading to store operated Ca2+ mobilization that increases cytoplasmic Ca2+. Although thrombin and convulxin activate two distinct PLC isoforms, dual agonist stimulation failed to increase Ca2+ flux in DKO platelets. Furthermore DKO platelets exhibited decreased store operated Ca2+ entry, which also contributes to decreased agonist mediated-Ca2+ mobilization. We also demonstrated that treatment with a Ca2+ ionophore restored annexin V binding to control levels, indicating that the reduction in TMEM16f-mediated PS translocation in DKO platelets is secondary to reduced Ca2+ availability. However, in DKO platelets, Ca2+ ionophore treatment, which restored Ca2+ signaling, did not increase GPIIbIIIa activation or platelet degranulation. Agonist-mediated platelet activation was partially rescued with addition of mitochondrial substrates underscoring the energy dependence of these processes. The energetic mechanisms downstream of Ca2+ signaling that lead to GPIIbIIIa activation were not identified. One possibility for impaired integrin activation could be impaired actin remodeling, which serves as a scaffold for integrin activation. Reduced activation of proteins required for degranulation such as the ATPase N-ethylmaleimide-sensitive factor could represent an energy-dependent mechanism for platelet degranulation following Ca2+ restoration. SNAP-23 is a known regulator of degranulation and is a known calpain substrate (Rutledge and Whiteheart, 2002). It is possible that basal calpain activation results in SNAP-23 cleavage, impairing the ability of DKO platelets to degranulate. Although we do not fully understand the mechanism by which glucose regulates platelet activation at these multiple nodes in the activation process, our data unambiguously demonstrates that glucose metabolism is essential for platelet activation and thrombus formation.
DKO mice developed thrombocytopenia, through decreased platelet production and increased platelet clearance. Although megakaryocyte density in the bone marrow was unchanged, the ability of bone marrow derived megakaryocytes to take up glucose was almost completely abolished and their ability to produce platelets was reduced two-fold, demonstrating an important relationship between megakaryocyte glucose metabolism and pro-platelet formation.
Mechanisms regulating platelet clearance are not well understood. Here we demonstrate that energy metabolism plays an essential role in platelet annexin V binding and circulating half-life. Unexpectedly we found that lack of glucose metabolism in vitro led to a rapid translocation of PS to the outer leaflet of the plasma membrane. This PS exposure appears to be energy dependent because addition of mitochondrial substrates to the media normalized AMPK activation and partially rescued this effect, while addition of oligomycin increased annexin V binding. Indeed in vivo we demonstrate that as the glycolysis deficient platelets age they increase annexin V binding, suggesting that although inhibition of metabolism might not be initially detrimental to platelets, but overtime this loss of energy metabolism increases PS exposure to the outer leaflet of the plasma membrane that increases clearance. Together these data indicate that energetically stressed platelets expose PS to the outer leaflet of the plasma membrane.
In vivo, in the absence of glycolysis, platelet counts were significantly reduced and inhibition of mitochondrial function further reduced platelet counts to zero. These findings extend our understanding of the regulation of platelet circulating half-life. Although we cannot rule out additional defects in DKO platelets acquired during biogenesis, we excluded perturbed desialylation and gross perturbations in organelle number and structure with the exception of α-granules. Taken together, we have identified a critical role for platelet metabolism in the regulation of circulating platelet half-life.
This metabolism-mediated clearance is partially dependent on increased cytoplasmic Ca2+ concentrations in parallel with depleted platelet Ca2+ stores, which activates calpain and increases annexin V exposure. In addition, following agonist-mediated Ca2+ flux, DKO platelets were unable to pump Ca2+ out of the cytosol. Therefore we believe that cytoplasmic Ca2+ accumulates in DKO platelets because of reduced ATP availability for SERCA-mediated pumping of Ca2+ into storage organelles, and/or inhibition of the PMCA which pumps Ca2+ into the extracellular space (Varga-Szabo et al., 2009). This energy dependent regulation of cellular Ca2+ homeostasis mirrors similar mechanisms that are implicated in necrosis (Jackson and Schoenwaelder, 2010), but is distinct from the procoagulant necrotic platelet response, because calpeptin although inhibiting spontaneous annexin V exposure did not inhibit agonist or ionophore mediated annexin V exposure. Importantly, calpeptin did not influence the circulating half-life of control platelets. Thus energy-Ca2+-calpain mediated clearance may only occur under specific circumstance such as energetic stress. Therefore we believe this energy-Ca2+-calpain-mediated platelet clearance represents a mechanism by which platelets can undergo necrosis and be cleared from the circulation.
In conclusion, these studies reveal an essential role for glucose metabolism in regulating multiple facets of platelet function including platelet activation, thrombosis, platelet production and clearance from the circulation. Elucidating the fundamental roles of glucose metabolism in platelets provides the conceptual framework to better understand how the extracellular milieu could potentially alter platelet function in metabolic disorders such as diabetes, where dysfunctional Ca2+ signaling is a hallmark of platelet dysfunction.
Experimental Procedures
Animals
All mice were on a C57BL6 background and were housed under standard conditions of temperature and lighting. Pf4 Cre transgenic mice were obtained from Jackson laboratories. GLUT1 floxed mice were generated as previously described (Young et al., 2011). GLUT3 floxed mice were obtained from the trans-NIH Knock-Out Mouse Project repository, Slc2a3tm1c(KOMP)Mbp. LoxP sites were inserted flanking Slc2a3 exon 7 and primers CCAACTTAAACACAATTGCCTGGTG and GGCTCACAATTACCCATAATGA were used for PCR identification of the GLUT3 floxed allele. GLUT3-KO, GLUT1-KO and DKO mice were generated by crossing mice with homozygous GLUT3 floxed alleles to Pf4 Cre transgenic mice. Experiments were conducted on male mice between the ages of 8–14 weeks, unless otherwise noted.
Platelet Isolations
Whole blood was isolated from isoflurane-anesthetized mice through carotid artery cannulation into 1:20 acid-citrate-dextrose (ACD) as previously described (Rowley et al., 2011). When noted, platelets were incubated with Ter119 and CD45 labeled microbeads (Miltenyi Biotec, Auburn CA) and negatively depleted of red blood cells and leukocytes. Following isolation, platelets were allowed to recover for 30 min prior to experimental manipulation. Platelet counts were determined by Cellometer Auto M10 (Nexcelom Bioscience, Lawrence, MA).
Immunoblots
Proteins were isolated by methanol-chloroform precipitation. Protein for GLUT1 and GLUT3 western blots were obtained from CD45 and Ter119 bead depleted platelets by lysis using Ripa buffer. Analysis was normalized to ponceau S staining unless otherwise noted. Protein concentrations were determined by bicinchoninic acid (BCA) analysis. Detailed antibody information can be found in supplemental methods.
Glucose Metabolism
Platelet and megakaryocyte glucose uptake analysis were conducted with 10mM 3H-2-Deoxy-D-Glucose. 13C-glucose flux was determined through incubations of platelets with 25mM 1,6-13C-glucose for up to one hour; 13C-lactic acid was quantified with gas chromatography-mass spectroscopy. Glycogen content was determined in 2×108 CD45 and Ter119 bead depleted platelets using a Glycogen Assay Kit (Cayman Chemical, Ann Arbor, MI). Detailed protocols can be found in the supplemental methods.
Mitochondrial Assays
Platelet bioenergetics were evaluated by Seahorse XF24 Analyzer (Agilent Technologies, Santa Clara, CA) as previously described (Fink et al., 2012). Leukocytes and red blood cells were depleted using Terr119 and CD45 micro beads and data are normalized to platelet counts. Mitochondrial potential was determined by flow cytometric analysis of tetramethylrhodamine methyl ester (TMRM) stained platelets. AMP, ADP, and ATP estimation was determined using an AMP-Glo kit (Promega, Madison WI). Detailed protocols can be found in supplemental methods.
Transmission Electron Microscopy
Washed platelets were incubated in DMEM ±250uM Par4 peptide for 10 minutes at room temperature then fixed with equal volumes 4% glutaraldehyde. Following 30-minute glutaraldehyde incubation, platelets were centrifuged at 120g × 10 minutes, resuspended in 4% glutaraldehyde and processed as previously described (Schwertz et al., 2010). Organelle quantification was performed by an investigator who was blinded to genotype or treatment condition.
Platelet Activation
Freshly washed platelets were incubated in HEPES Tyrode’s (HT) buffer with 5mM glucose ± 1mM pyruvate and 2mM glutamate for 30 minutes. Platelets were then treated with agonists at the indicated concentrations in the presence of JonA-PE, CD62p-FITC, (Emfret Analytics, Germany) and CD41-APC (Ebioscience, San Diego, CA) for 10 minutes at 37° C. Annexin V binding was determined in a similar manner, except staining was conducted for 15 minutes. Samples were analyzed using flow cytometry LSR II (Beckman Dickson, San Jose, CA) gating for CD41 positive events. Detailed protocols can be found in supplemental methods.
Platelet Ca2+ Content
Fluo-4 or Fura-2 were used to determine Ca2+ signaling. Detailed methods for Ca2+ assays can be found in supplemental methods.
In vivo thrombosis
Detailed methods for in vivo thrombosis assays can be found in supplemental methods.
Platelet Regeneration and Half-Life
Baseline platelets counts were determined by flow cytometry counting of diluted whole blood, gated for CD41-APC positive events and normalized to flow-count fluorospheres (BD Bioscience, San Jose, CA). Mice were injected i.v. with 2μg/g anti-GP1bα antibody for depletion or anti-GP1bβ-FITC antibody (Emfret Analytics, Germany) for circulation half-life. Platelet counts were obtained daily.
Megakaryocyte studies
Bone marrow was flushed, filtered and cultured in DMEM with 5mM glucose, glutamate and recombinant thrombopoietin (TPO) for five days. Megakaryocytes were then cultured overnight on fibrinogen coated chamber slides for experiments. In a blinded manner, megakaryocyte-producing platelets were quantified and normalized to total megakaryocytes. Megakaryocyte density in femurs and spleens were determined by immunohistochemistry for von Willebrand factor (vWF). Detailed protocols can be found in supplemental methods.
Oligomycin Treatments
Freshly isolated washed platelets were incubated in 5mM glucose DMEM at 37°C for 1 hour in the presence of oligomycin at concentrations specified. After 45 minutes, samples were stained with annexin V APC for an additional 15 minutes, diluted 1:20 and were analyzed immediately using flow cytometry. In vivo oligomycin administration was performed by injecting 100μL total volume into mice I.P. with 1mg/kg oligomycin in 7% DMSO:Saline. Platelet counts were determined 6 hours later. Animal weight was determined prior to injection and 24 hours post injection.
Calpain Activity and Inhibition
Calpain activity was determined in platelet lysates incubated in 5mM glucose DMEM for 6 hours. After 6 hours, platelets were centrifuged at 13,000g × 5 minutes then lysed in assay buffer and analyzed for calpain activity (ab65308, Abcam, Cambridge MA), which was normalized to protein content. In vitro, calpeptin was co-incubated with platelets incubated in 5mM glucose DMEM for 6 hours and analyzed for annexin V binding. In vivo, calpeptin was administered I.P. at 7mg/kg, in 5% DMSO: Saline (100μL final volume) for 7 days. Mice were then pulsed with Anti-GP1bβ-FITC antibody and calpeptin administration continued. Blood was analyzed daily.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7, or Microsoft office Excel 2011. Data are presented as mean ±SEM. Statistical significance threshold of p<0.05 was utilized.
Supplementary Material
Highlights.
Glucose metabolism is required for megakaryocyte-mediated platelet production.
Platelet metabolism is essential for platelet survival.
Energetically stressed platelets undergo Ca2+-calpain-mediated necrosis and clearance.
Glucose metabolism modulates multiple nodes of platelet activation.
Acknowledgments
We would like to thank Jamie Soto, for Seahorse analyses performed at the University of Iowa metabolic phenotyping core and Kathy Walters for transmission electron microscopy analysis of platelets. The data presented herein were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine core research facility at the University of Iowa. GLUT3 floxed mice were generated by the trans-NIH Knock-Out Mouse Project (KOMP) and obtained from the KOMP Repository (www.komp.org). Targeted ES cells were generated by NIH grants to Velocigene at Regeneron Inc. (U01HG004085) and the CSD Consortium (U01HG004080) which are archived and distributed by the KOMP Repository at UC Davis and CHORI (U42RR024244). RC was funded by T32 DK 007115. DC was funded by R00HL124070. ASW was funded by R01 HL 126547-01. This work was supported by NIH Grant U54 HL112311 to ASW and EDA who are both established investigators of the American Heart Association.
Footnotes
Author Contribution
Conceptualization, EDA, ASW, and TPF; Methodology, EDA, ASW, TPF, and RC; Investigation, TPF, RC, TF, EBA, and ND; Writing-original draft, TPF; Writing-Review and Editing, TPF, EDA, ASW, and RC; Funding Acquisition, EDA and ASW; Resources, EDA and ASW; Supervision DC, EDA, and ASW
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akkerman JWN, Holmsen H. Interrelationship among platelet responses: studies on the burst in proton liberation, lactate production, and oxygen uptake during platelet aggregation and Ca2+ secretion. Blood. 1981;57:956–966. [PubMed] [Google Scholar]
- Akkerman JWN, Holmsen H, Driver AH. Platelet Aggregation and Ca secretion are independent of simultaneous ATP production. FEBS letters. 1979;100:286–290. doi: 10.1016/0014-5793(79)80353-1. [DOI] [PubMed] [Google Scholar]
- Alonzo MT, Lacuesta TL, Dimaano EM, Kurosu T, Suarez LA, Mapua CA, Akeda Y, Matias RR, Kuter DJ, Nagata S, et al. Platelet apoptosis and apoptotic platelet clearance by macrophages in secondary dengue virus infections. J Infect Dis. 2012;205:1321–1329. doi: 10.1093/infdis/jis180. [DOI] [PubMed] [Google Scholar]
- Craik JD, Stewart M, Cheeseman CI. GLUT-3 (Brain-Type) Glucose Transporter Polypeptides in Human Blood Platelets. Thrombosis research. 1995;79:461–469. doi: 10.1016/0049-3848(95)00136-f. [DOI] [PubMed] [Google Scholar]
- Fink BD, Herlein JA, O’Malley Y, Sivitz WI. Endothelial cell and platelet bioenergetics: effect of glucose and nutrient composition. PloS one. 2012;7:e39430. doi: 10.1371/journal.pone.0039430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft RF, Furie B, Furie BC. Alpha-Granule Secretion From Alpha-Toxin Permeavilized, MgATP-Exposed Platelets Is Induced Independently by H+ and Ca2+ Journal of cellular physiology. 1999;179:1–10. doi: 10.1002/(SICI)1097-4652(199904)179:1<1::AID-JCP1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Garcia-Souza LF, Oliveira MF. Mitochondria: biological roles in platelet physiology and pathology. The international journal of biochemistry & cell biology. 2014;50:156–160. doi: 10.1016/j.biocel.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HFG, Oorschot V, Sixma JJ, Slot JW, James DE. Thrombin Stimulates Glucose Transport in Human Platelets via the Translocation of the Glucose Transporter GLUT-3 from alpha-Granules to the Cell Surface. J Cell Biol. 1997;138:323–330. doi: 10.1083/jcb.138.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Schoenwaelder SM. Procoagulant platelets: are they necrotic? Blood. 2010;116:2011–2018. doi: 10.1182/blood-2010-01-261669. [DOI] [PubMed] [Google Scholar]
- Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, Di Paola J. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood. 2008;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Studies on Human Platelet Glycolysis. Effect of Glucose, Cyanide, Insulin, Citrate, and Agglutination and Contraction on Platelet Glycolysis. The Journal of clinical investigation. 1967;46:409–417. doi: 10.1172/JCI105542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S, Langer RM. Biochemical Energetics of Simulated Platelet Plug Formation: Effect of Thrombin, Adenosine Diphosphate, and Epinephrine on Intra- and Extracellular Adenine Nucleotide Kinetics. The Journal of clinical investigation. 1968;47:2158–2168. doi: 10.1172/JCI105902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, van der Wal DE, Zhu G, Xu M, Yougbare I, Ma L, Vadasz B, Carrim N, Grozovsky R, Ruan M, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nature communications. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Woo V, Bose R. Platelet hyperactivity and abnormal Ca2+ homeostasis in diabetes mellitus. American journal of physiology Heart and circulatory physiology. 2001;280:H1480–H1489. doi: 10.1152/ajpheart.2001.280.4.H1480. [DOI] [PubMed] [Google Scholar]
- Bonomini MSD, Amoroso L, Arduin A, Sirolli V. Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. Journal of Thrombosis and Haemostasis. 2004;2:1275–1281. doi: 10.1111/j.1538-7836.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Paterson RA, Heath H, Cranfield T. The Effect of 0-(Beta-hyroxyethyl) Rutoside on Platelet Intermediary metabolism in Normal and streptozotocin. Diabetic Rats Biochemical pharmacology. 1974;23:1591–1597. doi: 10.1016/0006-2952(74)90371-2. [DOI] [PubMed] [Google Scholar]
- Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge TW, Whiteheart SW. SNAP-23 is a target for calpain cleavage in activated platelets. The Journal of biological chemistry. 2002;277:37009–37015. doi: 10.1074/jbc.M204526200. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, O’Reilly LA, Henley KJ, Ono A, Hsiao S, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114:663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- Schwertz H, Koster S, Kahr WH, Michetti N, Kraemer BF, Weitz DA, Blaylock RC, Kraiss LW, Greinacher A, Zimmerman GA, et al. Anucleate platelets generate progeny. Blood. 2010;115:3801–3809. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RB, Cooper LW. Activation of Glycogen Phosphorylase in Blood Platelets. Blood. 1967;30:321–330. [PubMed] [Google Scholar]
- Shi DS, Smith MC, Campbell RA, Zimmerman PW, Franks ZB, Kraemer BF, Machlus KR, Ling J, Kamba P, Schwertz H, et al. Proteasome function is required for platelet production. The Journal of clinical investigation. 2014;124:3757–3766. doi: 10.1172/JCI75247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Stitham J, Gleim S, Di Febbo C, Porreca E, Fava C, Tacconelli S, Capone M, Evangelista V, Levantesi G, et al. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. The Journal of clinical investigation. 2011;121:4462–4476. doi: 10.1172/JCI59291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeSlaa T, Teitell MA. Techniques to monitor glycolysis. Methods Enzymol. 2014;542:91–114. doi: 10.1016/B978-0-12-416618-9.00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kruchten R, Mattheij NJA, Saunders C, Feijge MAH, Swieringa F, Wolfs JLN, Collins PW, Heemskerk JWM, Bevers EM. Both TMEM16F-dependent and TMEM16F-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood. 2012;121:1850–1857. doi: 10.1182/blood-2012-09-454314. [DOI] [PubMed] [Google Scholar]
- Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. Journal of thrombosis and haemostasis: JTH. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- Warshaw AL, Laster L, Shulman NR. The stimulation by thrombin of glucose oxidation in human platelets. The Journal of clinical investigation. 1966;45:1923–1934. doi: 10.1172/JCI105497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf BBGJC, Stennicke HR, Beere Helen, Amarante-Mendes GP, Salvesen GS, Green DR. Calpain Functions in a Caspase-Independent Manner to Promote Apoptosis-Like Events During Platelet Activation. Blood. 1999;94:1683–1692. [PubMed] [Google Scholar]
- Yamagishi S, Edelstein D, Du X, Brownlee M. Hyperglycemia Potentiates Collagen-Induced Platelet Activation Through Mitochondiral Superoxide Overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- Young CD, Lewis AS, Rudolph MC, Ruehle MD, Jackman MR, Yun UJ, Ilkun O, Pereira R, Abel ED, Anderson SM. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PloS one. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.