Abstract
Dual-probe fluorescence in situ hybridization (D-FISH) is a widely accepted method to determine the gene amplification status of human epidermal growth factor receptor 2 (Her-2). In 2013, the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) updated the guidelines on the Her-2 testing for invasive breast cancer (BCa). The interpretation criteria for D-FISH changed accordingly. In this study, we compared the Her-2 FISH statuses based on the 2013 and 2007 ASCO/CAP guidelines in 1931 cases of BCa with Her-2 D-FISH testing at our hospital. We analyzed the clinicopathologic features of cases with equivocal results by the 2013 ASCO/CAP guidelines. Although the guideline update significantly improved the detection rate of Her-2 amplification, it also significantly increased the rate of equivocal results, posing a dilemma for clinical management. The equivocal results had good reproducibility. The distribution of D-FISH equivocal cases did not correlate with Her-2 status by immunohistochemistry, suggesting that Her-2 D-FISH equivocality may not reflect Her-2 overexpression. Compared with Her-2 negative cases by D-FISH, Her-2 D-FISH equivocal cases had higher Ki-67 expression, higher histological grade, more frequent lymph node metastasis, and lower estrogen receptor α expression, indicating a group of BCa with worse prognosis. The clinical significance of Her-2 equivocal results by D-FISH warrants further investigation.
Keywords: breast cancer, Her-2, Dual-probe fluorescence in situ hybridization (D-FISH), equivocal, the 2013 ASCO/CAP guidelines
Introduction
Human epidermal growth factor receptor 2 (Her-2) status is of predictive and prognostic importance in breast carcinoma. Overexpression of Her-2 and/or amplification of Her-2/neu gene are associated with poorer prognosis [1]. Her-2 testing is essential in selecting patients for treatment with trastuzumab and other Her-2 targeted therapies. Her-2 positivity appears to be associated with relative resistance to endocrine therapies [2]. Her-2 status also appears to be predictive for response to chemotherapeutic agents [3–5]. Dual-probe fluorescence in situ hybridization (D-FISH) is an established method to determine the gene amplification status of Her-2. The number of Her-2 signals, the number of signals determined by a control probe to the centromeric portion of chromosome 17 (CEP17) and their ratio were the three parameters for Her-2 FISH interpretation [6]. In 2007, a guideline was developed by American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) to improve the accuracy of Her-2 testing in invasive breast cancer (BCa). For D-FISH, Her-2 amplification was defined by Her-2/CEP17 ratio and a tumor with Her-2/CEP17 ratio of 1.8–2.2 was classified as equivocal according to the 2007 guidelines [7]. In 2013, the ASCO/CAP guidelines were updated. For D-FISH, the most notable change was that Her-2 interpretation also took the average number of Her-2 signals per cell into account. The cutoff Her-2/CEP17 ratio was defined as 2.0. Cases with Her-2/CEP17 ratio >= 2.0, or cases with Her-2/CEP17 ratio < 2.0 and the average number of Her-2 signals >= 6 was classified as positive; and cases with Her-2/CEP17 ratio < 2.0 and the average number of Her-2 signals per cell >= 4 and <6 was classified as equivocal [8].
Although the 2013 ASCO/CAP guidelines significantly improved the detection rate of Her-2 amplification, equivocal results have increased significantly, which poses a dilemma for clinical management. In this study, we compared the Her-2 D-FISH results based on the 2013 and 2007 ASCO/CAP guidelines, and analyzed the clinicopathologic features of cases with equivocal results by the 2013 ASCO/CAP guidelines.
Materials and Methods
Ethics statement
All BCa samples were collected with written consent from the patients prior to participation in the study. The protocols for collection and analysis of the samples were approved by the Institutional Review Board of Tianjin Medical University Cancer Institute and Hospital, in accordance with the revision of the Declaration of Helsinki.
Human BCa specimens
At our institution, all invasive breast carcinomas are routinely tested for Her-2 expression by immunohistochemistry (IHC), and cases showing equivocal (2+) staining by IHC require reflex testing by Her-2 D-FISH. Her-2 D-FISH was also performed on some Her-2 negative (1+) or Her-2 positive (3+) cases by IHC to further verify Her-2 status per specific requests by treating physicians for various reasons. We retrieved all invasive breast carcinoma specimens with Her-2 D-FISH testing at our institution from March 2014 to June 2015. Most of the cases were Her-2 equivocal (2+) by IHC. In order to gain a better understanding of correlation between Her-2 IHC and FISH results, we also performed Her-2 D-FISH on 255 consecutive cases of invasive BCa from all patients who underwent surgical excision from January to February 2014 at our institution regardless of Her-2 results by IHC. Tissue sections were independently reviewed by two pathologists to confirm the diagnosis using the WHO criteria and the histologic grade of invasive carcinoma. Patients received neoadjuvant chemotherapy were excluded.
Immunohistochemistry (IHC)
IHC for Her-2 was performed with BenchMark XT Automated IHC/ISH slide staining system (VENTANA) using anti Her-2/neu antibody (clone number 4B5, VENTANA). Each case was scored independently according to the 2013 ASCO/CAP Guidelines [8] by two pathologists. IHC for estrogen receptor α (ERα) was performed using monoclonal rabbit anti-human ERα antibody (clone # EP1, Dako) and evaluated by two pathologists independently according to the ASCO/CAP guidelines [9]. IHC for Ki67 was performed using monoclonal rabbit anti-human Ki67 antibody (clone # SP6, Lab Vision Corporation) and evaluated by two pathologists independently recording the proportion of positive tumor cells [10–11].
Her-2 D-FISH
Four μm deparaffinized tissue sections were used for Her-2 gene amplification testing using the US Food and Drug Administration–approved Vysis PathVysion probe set (Abbott Diagnostics), according to the protocol recommended by the manufacturer. The entire slides were scanned using a NIKON 90i fluorescence microscope (NIKON, Japan) with a triple-pass filter band (DAPI/green/orange). A total of 20 cells of invasive carcinoma with optimal nuclear signals were randomly selected in 2–4 separate fields for evaluation. Signals of Her-2 and CEP17 were counted manually according to the specification of the kit. Her-2 gene amplification status of each case was scored according to the 2007 [7] and 2013 [8] ASCO/CAP guidelines respectively. In cases with equivocal results, a repeat counting of additional 20 cells in another 2–4 separate fields was performed. All the equivocal cases by D-FISH according to the 2007 and 2013 ASCO/CAP guidelines remained equivocal in the repeat counting. Examples of Her-2 negative, equivocal and positive cases by D-FISH according to the 2013 ASCO/CAP guidelines are shown in Figure 1.
Figure 1.
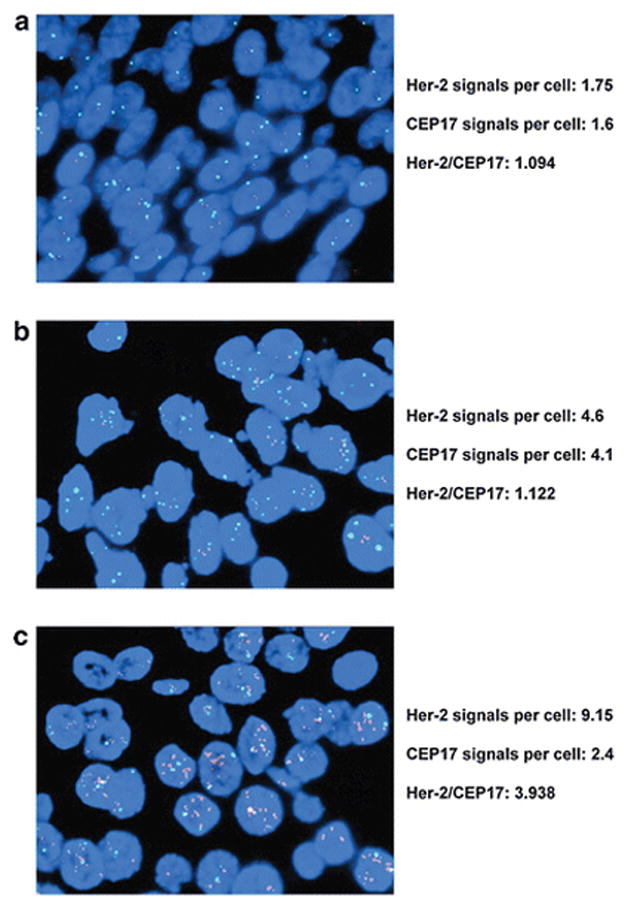
Examples of Her-2 negative (a), equivocal (b) and positive (c) by dual-probe fluorescent in situ hybridization according to the 2013 ASCO/CAP guidelines. Her-2: red signals. CEP17: green signals.
Statistic analysis
All analyses were performed using SPSS software (version 13.0). The agreement between the two analyses was analyzed using kappa test, and the discrepancy significance was analyzed using 2-sided Mcnemar test. ERα status, Ki67 index, histological grade, lymph node status and pTNM stage between two groups were compared using non-parametric Mann-Whitney U test. ERα status and Ki67 index of three or more groups were compared using the Kruskal-Wallis test. Histological grade, lymph node status and pTNM stage of three or more groups were compared using the Jonckheere-Terpstra test. The correlation between equivocal D-FISH results and Her-2 IHC results were analyzed using non-parametric spearman correlation test.
Results
1. Analysis of invasive BCa with reflex Her-2 D-FISH test from March 2014 to June 2015
1.1 Her-2 D-FISH according to the 2007 and 2013 guidelines and the correlation between Her-2 D-FISH equivocal status and Her-2 IHC results (Table 1)
Table 1.
Results of Her-2 D-FISH assay per the 2013 and 2007 ASCO/CAP guidelines in 1676 cases of BCa with Her-2 IHC analysis
| IHC | ASCO 2013 | Negative versus Others | Equivocal versus Others | Positive versus Others | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Negative | Equivocal | Positive | Total | Mcnemar | Kappa | Mcnemar | Kappa | Mcnemar | Kappa | |||
| 1+ | ASCO 2007 | Negative | 101(85.6) | 12(10.2) | 2(1.7) | 115(97.5) | P<0.001 | P<0.001 | P=0.003 | P=0.735 | ||
| Equivocal | 0(0.0) | 0(0.0) | 1(0.8) | 1(0.8) | ||||||||
| Positive | 0(0.0) | 0(0.0) | 2(1.7) | 2(1.7) | ||||||||
| Total | 101(85.6) | 12(10.2) | 5(4.2) | 118(100.0) | ||||||||
| 2+ | ASCO 2007 | Negative | 711(56.2) | 116(9.2) | 5(0.4) | 832(65.7) | P<0.001 | P=0.063 | ||||
| Equivocal | 10(0.8) | 4(0.3) | 4(0.3) | 18(1.4) | ||||||||
| Positive | 0(0.0) | 0(0.0) | 416(32.9) | 416(32.9) | ||||||||
| Total | 721(57.0) | 120(9.5) | 425(33.6) | 1266(100.0) | ||||||||
| 3+ | ASCO 2007 | Negative | 5(1.7) | 4(1.4) | 18(6.2) | 27(9.2) | P=0.077 | P=0.089 | P<0.001 | P<0.001 | ||
| Equivocal | 2(0.7) | 1(0.3) | 10(3.4) | 13(4.5) | ||||||||
| Positive | 0(0.0) | 0(0.0) | 252(86.3) | 252(86.3) | ||||||||
| Total | 7(2.4) | 5(1.7) | 280(95.9) | 292(100.0) | ||||||||
Her-2 D-FISH: Her-2 dual-probe fluorescent in situ hybridization
BCa: Breast Cancer
IHC: Immunohistochemistry
A total of 1676 cases of invasive carcinoma with reflex Her-2 D-FISH testing between March 2014 and June 2015 were identified, including 118 cases with Her-2 1+ by IHC, 1266 cases with Her-2 2+, and 292 cases with Her-2 3+, according to the 2013 ASCO/CAP guidelines.
Among the 292 Her-2 3+ cases, Her-2 D-FISH was positive in 95.9% and 86.3% of the cases respectively by 2013 and 2007 ASCO/CAP guidelines (P<0.001). Among the 118 Her-2 1+ cases, Her-2 D-FISH was negative in 85.6% and 97.5% of the cases respectively by 2013 and 2007 ASCO/CAP guidelines (P<0.001).
There was a higher rate of Her-2 D-FISH equivocal results by the 2013 ASCO/CAP guidelines among the 118 Her-2 IHC 1+ cases and 1266 Her-2 IHC 2+ cases (10.2% and 9.5% respectively) than by the 2007 ASCO/CAP guidelines (0.8% and 1.4% respectively) (P=0.003 and P<0.001, respectively). There was no agreement in equivocal results by the 2013 and 2007 ASCO/CAP guidelines in both Her-2 1+ and Her-2 2+ groups (P=0.735 and P=0.063 respectively).
Although the rate of Her-2 equivocal results by D-FISH among the 292 IHC 3+ cases by the 2013 ASCO/CAP guidelines was lower than that by the 2007 ASCO/CAP guidelines (1.7% versus 4.5%), the difference was not statistically significant (P=0.077).
The findings showed the equivocal results by Her-2 D-FISH in Her-2 IHC 1+ and 2+ cases were increased, but not in Her-2 IHC 3+ cases using the 2013 ASCO/CAP guidelines. The equivocal cases by 2013 and 2007 ASCO/CAP guidelines are not statistically concordant.
1.2 Clinicopathologic characteristics of the invasive BCas with Her-2 equivocal results by D-FISH by the 2013 ASCO/CAP guidelines
ERα status, Ki67 index and histological grade of these invasive BCas with Her-2 equivocal results were compared among the Her-2 IHC 1+, 2+ and 3+ groups, and no significant differences were identified (P=0.587, P=0.547 and P=0.401 respectively). However, in the Her-2 IHC 1+ and 2+ subgroups, their Ki67 index (for IHC 1+, P=0.002; for IHC 2+, P<0.001) and histological grade (for IHC 1+, P=0.022; for IHC 2+, P<0.001) were significantly higher than those with Her-2 negative results by D-FISH, while their ERα expression was significantly lower (for IHC 1+, P=0.043; for IHC 2+, P=0.015).
2. Data analysis of 255 consecutive cases of invasive BCa with Her-2 D-FISH test from January to February 2014 (Detailed information of each cases were shown in Table S1)
2.1 Comparison between Her-2 D-FISH results by 2013 and 2007 ASCO/CAP guidelines (Table 2)
Table 2.
Comparison of Her-2 D-FISH assay per the 2013 and 2007 ASCO/CAP guidelines in 255 consecutive cases of BCa
| ASCO 2013 | Equivocal versus Others | Positive versus Others | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Negative | Equivocal | Positive | Total | Mcnemar | Kappa | Mcnemar | Kappa | ||
| ASCO 2007 | Negative | 166(65.1) | 31(12.2) | 7(2.7) | 204(80.0) | P<0.001 | P=0.132 | P=0.002 | P<0.001 |
| Equivocal | 1(0.4) | 2(0.8) | 3(1.2) | 6(2.4) | |||||
| Positive | 0(0.0) | 0(0.0) | 45(17.6) | 45(17.6) | |||||
| Total | 167(65.5) | 33(12.9) | 55(21.6) | 255(100.0) | |||||
Her-2 D-FISH: Her-2 dual-probe fluorescent in situ hybridization
BCa: Breast Cancer
The positive rate by Her-2 D-FISH according to the 2013 ASCO/CAP guidelines was significantly higher than that by the 2007 ASCO/CAP guidelines (21.6% versus 17.6%, P=0.002). The equivocal rate by Her-2 D-FISH was also significantly higher according to the 2013 ASCO/CAP guidelines (12.9% versus 2.4%, P<0.001). There was no agreement between these two interpretation methods (P=0.132).
2.2 The distribution of equivocal D-FISH results by the 2013 ASCO/CAP guidelines in the patients with different Her-2 IHC results
Her-2 expression evaluated by IHC was scored as 0 in 30 cases, 1+ in 103 cases, 2+ in 87 cases, and 3+ in 35 cases, according to the 2013 ASCO/CAP guidelines. In Her-2 D-FISH analysis, we defined cases with Her-2/CEP17 ratio >= 2.0 and the average number of Her-2 signals per cell >= 4 and <6 as “low Her-2 copy positive” (LCP) that are classified as gene amplified according to the 2013 ASCO/CAP guidelines. There were no cases with Her-2/CEP17 ratio >= 2.0 and the average number of Her-2 signals per cell < 4 in our cohort. We found the distribution of LCP cases positively correlated with Her-2 status by IHC (P<0.001), while the distribution of Her-2 D-FISH equivocal cases did not (P=0.096, Table 3). In addition, the average number of Her-2 signals per cell in the 17 LCP cases were significantly higher than that in the 33 Her-2 D-FISH equivocal cases (P=0.025 by Mann-Whitney U Test), while the average number of CEP17 signals in LCP were significantly lower than that in Her-2 D-FISH equivocal cases (P<0.001 by Mann-Whitney U Test). There was no agreement between the 87 cases of Her-2 IHC 2+ (IHC equivocal) and the 33 cases of D-FISH equivocal by the 2013 ASCO/CAP guidelines (P=0.771, Table 4).
Table 3.
Distribution of Her-2 D-FISH equivocal cases and “low copy positive” cases per the ASCO/CAP 2013 guidelines corresponding to IHC assessment
| HER-2 IHC | |||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 1+ | 2+ | 3+ | ||
| A1 | Yes | 6(20.0) | 14(13.6) | 12(13.8) | 1(2.9) |
| No | 24(80.0) | 89(86.4) | 75(86.2) | 34(97.1) | |
| Total | 30(100.0) | 103(100.0) | 87(100.0) | 35(100.0) | |
| rs | −0.104 | ||||
| P value | 0.096 | ||||
| B2 | Yes | 0(0.0) | 2(1.9) | 6(6.9) | 9(25.7) |
| No | 30(100.0) | 101(98.1) | 81(93.1) | 26(74.3) | |
| Total | 30(100.0) | 103(100.0) | 87(100.0) | 35(100.0) | |
| rs | 0.270 | ||||
| P value | <0.001 | ||||
Equivocal cases by dual-probe FISH according to the 2013 ASCO/CAP guidelines, i.e. cases with the Her-2/CEP17 ratio < 2.0 and the average number of Her-2 signals per cell >= 4 and <6
“low Her-2 copy positive (LCP)”, i.e. cases with the Her-2/CEP17 ratio >= 2.0 and the average number of Her-2 signals per cell >= 4 and <6.
Her-2 D-FISH: Her-2 dual-probe fluorescent in situ hybridization
IHC: Immunohistochemistry
Table 4.
Comparison of Her-2 equivocal results by IHC and by D-FISH per the 2013 ASCO/CAP guidelines
| IHC (ASCO 2013) | Equivocal versus Others | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Negative (0&1+) | Equivocal( 2+) | Positive (3+) | Total | Mcnemar | Kappa | ||
| FISH (ASCO 2013) | Negative | 108(42.4) | 58(22.7) | 1(0.4) | 167(65.5) | P<0.001 | P=0.771 |
| Equivocal | 20(7.8) | 12(4.7) | 1(0.4) | 33(12.9) | |||
| Positive | 5(2.0) | 17(6.7) | 33(12.9) | 55(21.6) | |||
| Total | 133(52.2) | 87(34.1) | 35(13.7) | 255(100.0) | |||
Her-2 D-FISH: Her-2 dual-probe fluorescent in situ hybridization
IHC: Immunohistochemistry
The Her-2 statuses by IHC in Her-2 D-FISH equivocal group was not significantly different from those in Her-2 D-FISH negative group (P=0.854 by Mann-Whitney U Test, Figure 2), while it was significantly lower than those in Her-2 D-FISH positive group (P<0.001 by Mann-Whitney U Test, Figure 2).
Figure 2.
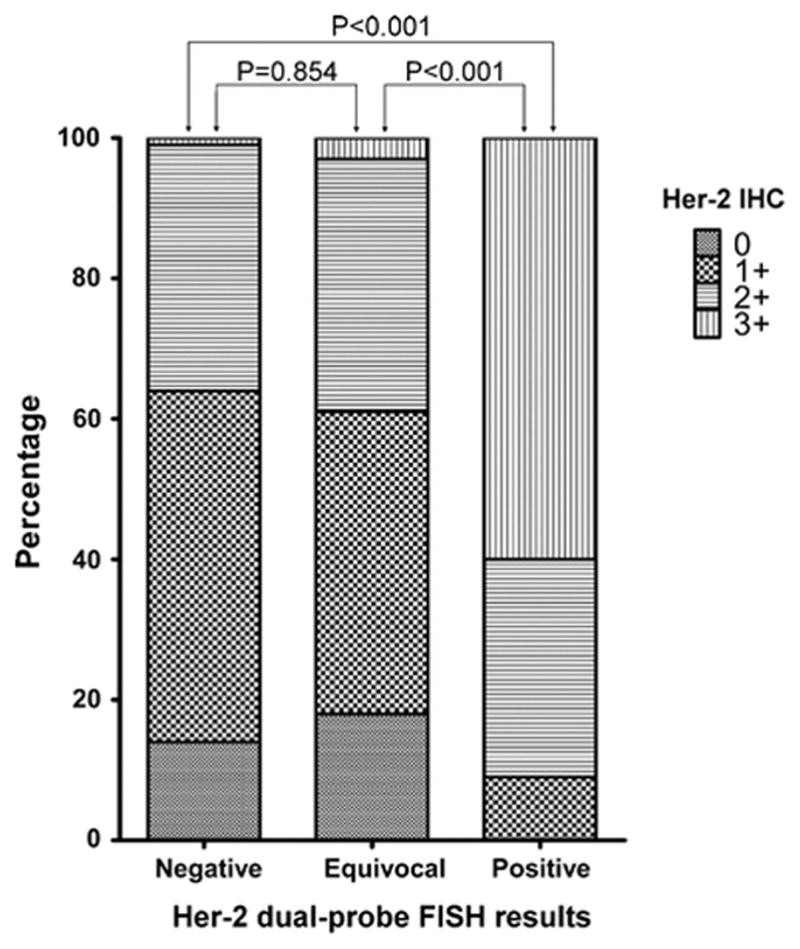
Results of Her-2 immunohistochemistry in dual-probe Her-2 fluorescent in situ hybridization negative, equivocal, and positive groups according to the 2013 ASCO/CAP guidelines in 255 consecutive invasive breast cancer cases
2.3 Clinicopathologic characteristics of the consecutive cases with Her-2 equivocal results by D-FISH by the 2013 ASCO/CAP guidelines
We compared the ERα status, Ki67 index, histological grade, pTNM stage and lymph node status of the consecutive invasive BCa cases with Her-2 equivocal results by D-FISH in Her-2 0, 1+ and 2+ groups, and no significant differences were identified among them (P=0.430, P=0.535, P=0.829, P=0.499 and P=0.460 respectively). Only 1 of the 35 Her-2 IHC 3+ cases was equivocal by D-FISH, which was not included in the analysis. Meanwhile, the Ki67 index, histological grade, and lymph node metastasis status of the patients with Her-2 equivocal results by D-FISH were significantly higher than those with Her-2 D-FISH negative results, while ERα expression was significantly lower (figure 3).
Figure 3.
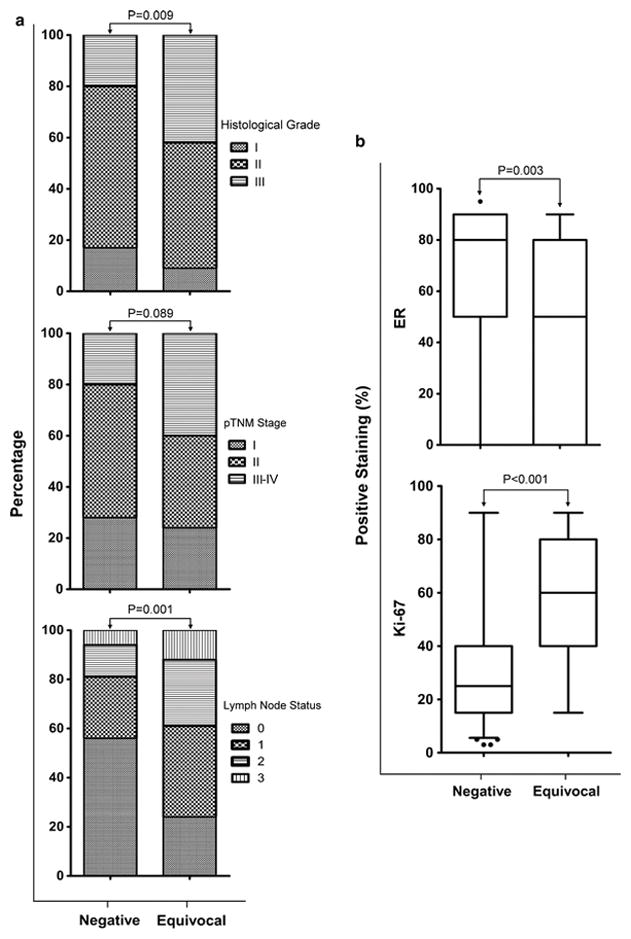
Histological grade, pTNM stage and lymph node status (a), ERα status and Ki67 index (b) of consecutive invasive breast cancer cases in dual-probe Her-2 fluorescent in situ hybridization negative and equivocal groups according to the 2013 ASCO/CAP guidelines
2.4 Reproducibility of the equivocal results
For the 33 cases identified as equivocal by D-FISH by the 2013 ASCO/CAP guidelines, test was repeated in each case at alternative tissue samples (a different block of the primary tumor or the lymph node metastasis). Thirty-two of the 33 cases remained equivocal. One cases became negative when tested in an alternative block, and then equivocal again in the third specimen.
Discussion
In our experience, when using the 2013 ASCO/CAP guidelines, the positive rate by Her-2 D-FISH was significantly higher than that using the 2007 ASCO/CAP guidelines, which may identify more patients potentially benefit from Her-2-targeted drugs. Several recent studies reported similar results [12–15]. The fact that Her-2 IHC 3+ cases (IHC positive) had higher positive rate by Her-2 D-FISH confirms the advantage of using the 2013 ASCO/CAP guidelines. If Her-2 signals per cell are >=6, assay should be interpreted as positive regardless of the Her-2/CEP17 ratio [8], according to the 2013 ASCO/CAP guidelines. In comparing to the results according to the ASCO/CAP 2007 guidelines, the positive rate of D-FISH detection was increased from 86.3% to 95.9% in the group of 292 Her-2 IHC 3+ cases, and 21 of 28 additional D-FISH positive cases were with Her-2 signals per cell >= 6 and Her-2/CEP17 ratio <2.0. In the group of 255 consecutive cases, the positive rate of D-FISH detection was similarly increased from 17.6% to 21.6%, and 8 of 10 additional positive cases were with Her-2 signals per cell >= 6 and Her-2/CEP17 ratio <2.0. Our data suggest that the increase in Her-2 D-FISH positive rate according to the 2013 ASCO/CAP guidelines were mainly due to the reclassification of cases with Her-2 signals per cell >= 6 and Her-2/CEP17 ratio <2.0.
Per the ASCO/CAP 2007 guidelines, a tumor with Her-2/CEP17 ratio of 1.8–2.2 was classified as equivocal by D-FISH assay. However, the 2013 ASCO/CAP guidelines change the criteria for D-FISH equivocal cases into tumor with Her-2/CEP17 ratio <2.0 and the average number of Her-2 signals per cell >= 4 but <6. The difference of the guidelines regarding Her-2 D-FISH equivocal cases is obvious. Here we used the kappa test to analyze the concordance of the equivocal results read by different criteria. It was found there was no concordance in the equivocal results, confirming the above impression. Equivocal results have increased significantly since the adoption of the 2013 ASCO/CAP guidelines for D-FISH, which poses a dilemma for making treatment decisions. In our study, the equivocal cases by D-FISH accounted for 12.9% in the group of 255 consecutive BCa and 9.5% in 1266 Her-2 IHC 2+ cases. A recent study also showed that the percentage of Her-2 D-FISH equivocal cases was as high as 9.4% according to the ASCO/CAP 2013 guidelines [16], similar to our results. Several other studies also demonstarted a significant increase in the D-FISH equivocal cases after the ASCO/CAP 2013 guideline update [12,14,15,17,18]. In addition, our assay revealed the interpretation of D-FISH equivocal results with good reproducibility. In our view, the following findings are worth of emphasizing: First, the distribution of equivocal cases by Her-2 D-FISH did not correlate with Her-2 status by IHC. Second, there was no equivocality concordance between IHC and D-FISH assays. Third, Her-2 expression by IHC in Her-2 D-FISH equivocal group was not significantly different from that in Her-2 D-FISH negative group, while significantly lower than that in Her-2 D-FISH positive group. These results suggest that her-2 D-FISH equivocality do not reflect Her-2 protein over-expression. Since trastuzumab and the antibody-drug conjugate adotrastuzumab emtansine [T-DM1] [19] target the Her-2 protein over-expressed cells, we consider D-FISH equivocal cases are compatible with D-FISH negative cases in regarding to the targeted therapy, and may not benefit from these treatments.
In Her-2 D-FISH analysis, Her-2/CEP17 ratio >= 2.0 and the average number of Her-2 signals per cell >= 4 and < 6 are classified as gene amplified according to the 2013 ASCO/CAP guidelines. However, the clinical significance and the therapeutic correlation of these BCas with low gene copy numbers are not well studied yet. We defined the group as “low Her-2 copy positive” (LCP) tumor and further explored it. The distribution of LCP cases was positively correlated with the Her-2 status by IHC, while that of D-FISH equivocal cases was not. The higher average number of Her-2 signals may partly contribute to the positive correlation between Her-2 protein expression and the occurrence of LCP, however other factors that upregulate Her-2 expression cannot be excluded. Although the increase of the CEP17 copy numbers in D-FISH equivocal cases do not frequently represent Chromosome 17 polysomy [20], we speculated that amplified Her-2 gene may be linked to CEP17 and limit its expression. However, in LCP the amplified regions are more accurate and hence the Her-2 gene is easily expressed. D-FISH could identify more patients who have potential to benefit from Her-2-targeted drugs than single-probe FISH. In Her-2 single-probe FISH, the cases with average Her-2 copy number>=4.0 and <6.0 signals per cell were classified as equivocal per the 2013 ASCO/CAP guidelines that actually include D-FISH equivocal cases and LCP cases.
Although we do not have outcome data pertinent to the prognosis of Her-2 D-FISH equivocal patients who received trastuzumab with those who did not, our results questioned the benefit of trastusumab in these patients in comparison to Her-2 D-FISH negative patients. Multiplex ligation-dependent probe amplification (MLPA) may be of support in reassessing Her-2 gene status of D-FISH equivocal cases. A recent report suggested that MLPA could rule out Her-2 amplification in 75% of ISH-evaluated Her-2-equivocal carcinomas [14]. Her-2 mRNA expression analysis by quantitative reverse transcription-PCR (qRT-PCR) may also be of help to determine the status of Her-2 [21]. Her-2 D-FISH equivocal cases determined by the ASCO/CAP 2013 guidelines always have an increase in CEP17 copy number and hence a <2.0 Her-2/CEP17 ratio. Using probes to Smith-Magenis syndrome (SMS), retinoic acid receptor alpha (RARA), and tumor protein p53 (TP53) genes instead of CEP17 representing chromosome 17 are an effective way to determine the true Her-2 amplification status in patients with polysomy 17 [20]. However, a recent study suggested that this strategy may cause over-grading of Her-2 status in tumors [15]. As mentioned earlier, since the therapeutic targets of trastuzumab and the antibody-drug conjugate adotrastuzumab emtansine [T-DM1] [19] are the Her-2 protein over-expressed cells, it is reasonable to suspect that the findings on genome level or transcriptional level including D-FISH may not completely override the Her-2 immunohistochemical results. Although Her-2 gene amplification is highly correlated with Her-2 protein over-expression in BCa cells, complex factors in regulating gene expression on transcriptional and translational level can not be ignored, especially in D-FISH equivocal cases. In trial N9831, the hazard ratio (HR) was 1.11 (95% CI 0.36–3.43) in the 219 cases that had a Her-2/CEP17 ratio ≥ 2, but were IHC ≤ 2+, suggesting an apparent lack of a disease-free survival (DFS) benefit from trastuzumab [8]. Although some cases with Her-2 over-expression but do not reach IHC 3+ may not have any detectable Her-2 gene amplification, they may benefit from trastuzumab therapy. It is therefore meaningful to establish a supplemental scoring method for IHC 2+ that will separate patients who may benefit from trastuzumab from those who may not. Different Her-2 targeting drugs may involve different mechanisms, such as pertuzumab inhibits the dimerization of Her-2 with other human epidermal growth factor receptors, while lapatinib, a dual tyrosine kinase inhibitor, interrupts the Her-2/neu and epidermal growth factor receptor (EGFR) pathways. These drugs target Her-2 down-stream signaling instead of Her-2 protein expression cells, in which accurate assessment of the Her-2 gene amplification status may be important, especially for those patients with Her-2 equivocality by both IHC and D-FISH.
Compared with Her-2 negative cases, Her-2 D-FISH equivocal cases were significantly associated with some worse prognostic factors, such as higher Ki-67 index, higher histological grade, more frequent lymph node metastasis, and lower ERα expression.
In summary, this study identifies that the 2013 new guidelines significantly improves the detection rate of Her-2 amplification in BCa, while significantly increasing the number of equivocal results. The Her-2 D-FISH equivocality doesn’t correlate with Her-2 overexpression, although it is associated with worse prognostic factors. Whether the Her-2 D-FISH equivocal cases are of independent clinical significance, or whether they may benefit from Her-2 targeted therapies warrants further investigation.
Supplementary Material
Detailed features of 255 consecutive cases of invasive breast cancer with dual-probe Her-2 fluorescent in situ hybridization test from January to February 2014
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81302292, 30930038, 81302294, 81272358, 81172531, and 81202101); National Natural Science Foundation of Tianjin City, China (15JCQNJC45300).
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the Her-2/neu protooncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 2.Konecny G, Pauletti G, Pegram M, et al. Quantitative association between Her-2/neu and steroid hormone receptors in hormone receptorpositive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 3.Menard S, Valagussa P, Pilotti S, et al. Response to cyclophosphamide, methotrexate, and fluorouracil in lymph node-positive breast cancer according to HER2 overexpression and other tumor biologic variables. J Clin Oncol. 2001;19:329–335. doi: 10.1200/JCO.2001.19.2.329. [DOI] [PubMed] [Google Scholar]
- 4.Konecny GE, Thomssen C, Luck HJ, et al. Her-2/neu gene amplification and response to paclitaxel in patients with metastatic breast cancer. J Natl Cancer Inst. 2004;96:1141–1151. doi: 10.1093/jnci/djh198. [DOI] [PubMed] [Google Scholar]
- 5.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 6.Fukushige S, Matsubara K, Yoshida M, et al. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol. 1986;6:955–958. doi: 10.1128/mcb.6.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 9.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long TH, Lawce H, Durum C, et al. The New Equivocal: Changes to HER2 FISH Results When Applying the 2013 ASCO/CAP Guidelines. Am J Clin Pathol. 2015;144:253–262. doi: 10.1309/AJCP3Q9WFOQTKUVV. [DOI] [PubMed] [Google Scholar]
- 13.Pu X, Shi J, Li Z, et al. Comparison of the 2007 and 2013 ASCO/CAP evaluation systems for HER2 amplification in breast cancer. Pathol Res Pract. 2015;211:421–425. doi: 10.1016/j.prp.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Sapino A, Maletta F, Verdun di Cantogno L, et al. Gene status in HER2 equivocal breast carcinomas: impact of distinct recommendations and contribution of a polymerase chain reaction-based method. Oncologist. 2014;19:1118–1126. doi: 10.1634/theoncologist.2014-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang MH, Kim EJ, Kim HJ, et al. Assessment of HER2 status in invasive breast cancers with increased centromere 17 copy number. Breast Cancer Res Treat. 2015;153:67–77. doi: 10.1007/s10549-015-3522-0. [DOI] [PubMed] [Google Scholar]
- 16.Bethune GC, Veldhuijzen van Zanten D, MacIntosh RF, et al. Impact of the 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 (HER2) testing of invasive breast carcinoma: a focus on tumours assessed as ‘equivocal’ for HER2 gene amplification by fluorescence in-situ hybridization. Histopathology. 2015;67:880–887. doi: 10.1111/his.12723. [DOI] [PubMed] [Google Scholar]
- 17.Muller KE, Marotti JD, Memoli VA, et al. Impact of the 2013 ASCO/CAP HER2 Guideline Updates at an Academic Medical Center That Performs Primary HER2 FISH Testing: Increase in Equivocal Results and Utility of Reflex Immunohistochemistry. Am J Clin Pathol. 2015;144:247–252. doi: 10.1309/AJCPE5NCHWPSMR5D. [DOI] [PubMed] [Google Scholar]
- 18.Lim TH, Lim AS, Thike AA, et al. Implications of the Updated 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations on Human Epidermal Growth Factor Receptor 2 Gene Testing Using Immunohistochemistry and Fluorescence In Situ Hybridization for Breast Cancer. Arch Pathol Lab Med. 2016;140:140–147. doi: 10.5858/arpa.2015-0108-OA. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tse CH, Hwang HC, Goldstein LC, et al. Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J Clin Oncol. 2011;29:4168–4174. doi: 10.1200/JCO.2011.36.0107. [DOI] [PubMed] [Google Scholar]
- 21.Pazhoomand R, Keyhani E, Banan M, et al. Detection of HER2 status in breast cancer: comparison of current methods with MLPA and real-time RT-PCR. Asian Pac J Cancer Prev. 2013;14:7621–7628. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed features of 255 consecutive cases of invasive breast cancer with dual-probe Her-2 fluorescent in situ hybridization test from January to February 2014


