Abstract
Recent evidence demonstrates that new events are learned in the context of their relationships to existing memories. Within the hippocampus and medial prefrontal cortex, related memories are represented by integrated codes that connect events experienced at different times and places. Integrated codes form the basis of spatial, temporal, and conceptual maps of experience. These maps represent information that goes beyond direct experience and support generalization behaviors that require knowledge be used in new ways. The degree to which an individual memory is integrated into a coherent map is determined by its spatial, temporal, and conceptual proximity to existing knowledge. Integration is observed over a wide range of scales, suggesting that memories contain information about both broad and fine-grained contexts.
Introduction
Although episodic memory research often focuses on representation of discrete events, memories can extend beyond direct experience by connecting information encountered at different times or places [1, 2]. For instance, learning your way around a new town involves gradually learning about landmarks and their relative positions. You might learn about one set of landmarks on one trip through the town and an overlapping set on a different trip. An effective way to represent the paths taken on both trips is through a common map that represents the relationships between landmarks experienced at different times. A similar process can support forming maps of social relationships [3, 4]; for example, meeting a woman and her son, then later meeting the woman’s husband, one can infer that the man and the boy are father and son.
These types of knowledge structures, known as cognitive maps [5], are thought to rely on memory integration. Memory integration is a dynamic process wherein new events interact with existing knowledge [1]. For memory integration to occur, a new event that overlaps with prior experience (e.g., meeting the husband) must trigger reactivation of a prior related episode (initially meeting the woman and her son); new information may then be integrated into the reactivated memory (connecting the boy with his father in memory) [1, 6]. As a result of integration, the two events are represented with overlapping neural codes, in which elements common to both events act as nodes linking the two memories [7, 8]. Such links allow representation of information beyond direct experience and can support flexible behaviors [1], such as taking a shortcut between places in a new town.
Memory integration is thought to be supported by bidirectional interactions between hippocampus and medial prefrontal cortex (mPFC) [9, 10]. Reactivation of related memories during new events is mediated by hippocampal pattern completion processes that allow for reinstatement of entire memory traces from overlapping input [11, 12]. Medial PFC, which is thought to represent mental models that guide behavior [13, 14], may further bias hippocampal pattern completion to the most relevant prior knowledge [9]. Hippocampus then signals deviations between current events and reactivated memories, triggering memory updating [1, 15, 16]. Finally, new content is integrated with existing mental models via hippocampus—mPFC interactions [17–19]. During integration, mPFC biases hippocampal encoding processes to emphasize representation of features common to multiple events [20, 21], resulting in cognitive maps that use overlapping neural codes to represent the relationships among discrete learning episodes [22, 23]. Here, we review recent evidence that hippocampus—mPFC memory integration mechanisms support the formation of cognitive maps across different domains of experience, including space, time, and concepts. Hippocampus and mPFC may organize information about spatial, temporal, and conceptual relationships in similar ways, allowing for the formation of flexible knowledge about many different aspects of the world. Furthermore, memory integration occurs simultaneously at different spatial, temporal, and conceptual scales, which may facilitate learning about both detailed features and broader contextual attributes that are shared across events.
Integration of spatial experience
The seminal work of Tolman first showed that memories of recently traveled routes are combined with memories of previously traveled routes to create an integrated map of the environment [5]. More recent evidence indicates that representations of past and present spatial trajectories are simultaneously active within hippocampus during learning [24–26], providing an opportunity for links to be formed among different spatial experiences in an environment. Reinstatement of behaviorally relevant memories within hippocampus during spatial learning may be particularly dependent on top-down influence from mPFC [10]. When rodents make decisions that depend on spatial context, mPFC drives hippocampal responses [9], suggesting that mPFC coordinates reinstatement of hippocampal memory representations that are most relevant to ongoing experience [27]. Increased hippocampal—mPFC coupling is also observed during spatial memory retrieval in humans [28, 29], and such coupling has been further shown to support individuals’ ability to connect past and present experience [17, 18, 30].
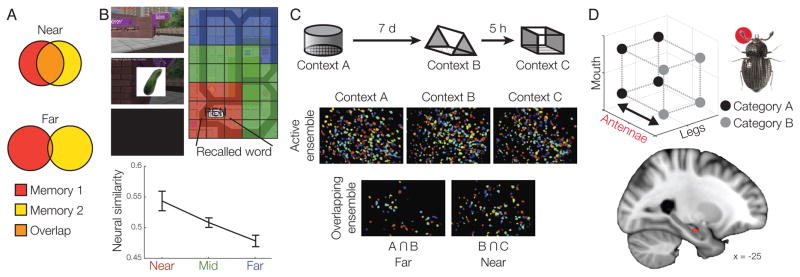
Memory integration mechanisms may cause events (e.g., encountering landmarks) experienced at different times within the same spatial environment to be represented by overlapping neural responses within hippocampus. Evidence from rodents has revealed highly structured hippocampal population codes, wherein responses are similar for objects that share spatial context or position information [33] as well as for locations that share relationships [34]. To represent cognitive maps, the similarity between hippocampal representations of events experienced within an environment should scale with their distance from one another in the environment. Events experienced close together in space should be represented by highly overlapping hippocampal populations, while events experienced in more distant locations should evoke less similar hippocampal responses (Fig. 1A). Human electrophysiological data shows that when participants recall objects learned within a virtual environment, hippocampal place cell activity is highest for locations closest to an object’s learned location, dropping off with increasing distance from the object (Fig. 1B) [31]. Moreover, human neuroimaging data indicate that hippocampal patterns for individual objects experienced in different locations within a virtual town scale with individual’s subjective memory for their distance in space [35]; objects judged as more spatially proximal evoke more similar hippocampal activation patterns after learning.
Figure 1.
(A) The overlap in representations of different memories reflects the distance between their spatial, temporal, and conceptual attributes, with memories nearer to one another resulting in more overlapping representations. (B) Ensemble place-cell activity in human hippocampus reflects the spatial distance between memories. Participants learned the locations of objects in a virtual environment, then verbally recalled the objects. Ensemble activity during object recall was most similar to place-cell activity for locations near to where the object was learned. Adapted from Ref. [31]. (C) In mice, memories of different cage contexts are represented by overlapping neural ensembles in hippocampal subfield CA1 when exposure to the cages is separated by a shorter delay of 5 h, but do not show significant overlap when separated by a longer interval of 7 d. Adapted from Ref. [32]. (D) Model-based fMRI data in humans demonstrates that item representations within hippocampus reflect conceptual similarity. The representation of three distinct features of categorized items was estimated based on patterns of activity. After learning, hippocampal representations emphasize features that are diagnostic for the category, causing items from the same conceptual category (e.g., the beetles with thin antennae represented by black dots) to become representationally more similar, and dissimilar to items from a different category (the beetles with thick antennae represented by the gray dots). Adapted from Ref. [20].
Collectively, these findings indicate that reactivation of prior spatial experience during new learning results in the formation of integrated spatial maps within the hippocampus. Importantly, such maps include information not only about directly traveled paths, but also routes through the environment that can be inferred from relationships among those traveled [36–38]. When directly experienced routes to a goal are blocked, the integrated hippocampal map can thus support flexible navigation via novel, alternate paths [39–41]. The mPFC may resolve interference by biasing the hippocampus to retrieve the behaviorally relevant route [42, 43]. Reactivation of integrated spatial maps also facilitates new learning. Work in rodents has shown that integrated spatial maps provide a framework for rapid learning of new object-location associations in the same environment [44, 45]. Reactivation of hippocampal spatial maps also facilitates learning in new environments that share properties with existing maps [24], thus allowing for generalization of spatial experience to novel contexts.
The physical similarity between environments or events within an environment may be an important boundary condition that determines which events are combined into a coherent cognitive map of space. A new spatial experience that shares many features with an existing memory may be more likely to trigger reactivation of the related memory than an event that shares fewer overlapping features. As a result, increased physical similarity among spatial experiences may be associated with an increasing likelihood of integration. Consistent with this idea, population responses within the CA1 subfield of the rodent hippocampus scale linearly with the number of spatial features shared across environments [46]; the greater the number of shared features, the greater the overlap in the CA1 population response. However, the integration boundaries may be different across hippocampal subfields [47]. In contrast to CA1, CA3 population responses overlap only when environments are highly similar, suggesting that the CA3 subfield has a higher threshold for integration [46]. One possibility for future study is to explore whether different hippocampal subregions support different levels of memory integration that represent the similarities among events in terms of whether they share fine details or global characteristics.
Representation of temporal context through memory integration
Similar to maps of space, memory integration in hippocampal and mPFC networks may code the temporal context of events by representing temporally proximal events with overlapping neural ensembles (Fig. 1C). Lesions to the hippocampus in rodents and humans impair the ability to extract temporal regularities from the environment and remember the sequential order of events [48, 49], suggesting a critical role for the hippocampus in temporal integration. Recent evidence suggests that temporal proximity affects memory integration at a range of timescales, which involve distinct mechanisms [50]. Memory integration at time scales on the order of seconds may be organized by “time cells” in the hippocampus and mPFC, which respond at specific temporal intervals during task performance [51, 52]. Computational modeling suggests that, like place cells, time cells may provide a map for organizing sequences of memories [53]. Integration of new memories into an existing temporal map may facilitate learning about the relative timing of overlapping sequences of events [54]. For example, if a musician learns to play a song, then later learns a new introduction to the song, memories of these sub-sequences may be integrated to form a complete song timeline.
Human neuroimaging data has shown that hippocampal [55–57] and mPFC [58] activation patterns reflect the temporal organization of events that occur seconds apart. In these studies, hippocampal and mPFC activation patterns are more similar for items that are seen in immediate succession to one another [55, 56, 59]. Similar to maps of space, the degree of similarity in these regions scales with the temporal distance among items [35, 56, 58] and, in the case of hippocampus, is related to individuals’ subjective estimates of temporal proximity [35, 56]. In rodents, the temporal context of individual items in a sequence can be decoded from CA1 ensembles, and sequence coding at the single cell and ensemble level is correlated with sequence memory [60]. Neuroimaging has also revealed coding of sequential position information within human hippocampus that relates to memory performance [61].
Notably, integration of memories at longer timescales, on the order of minutes [62] as well as days and weeks (Fig. 2C) [63], is also observed within hippocampus. Memory integration across longer temporal intervals may result from a different mechanism than that supported by time cells. Temporal proximity at longer time scales may promote integration through a memory tagging and allocation mechanism, whereby neurons and synapses recruited to represent a recent episode are more readily reactivated during new events that occur within hours of the original episode [32, 50, 64]. Recruitment of the same neural ensembles through tagging and allocation would thus result in overlapping population codes for events that occur within minutes and hours of one another as observed in CA1 of rodents (Fig. 1C) [32] and CA1 and mPFC in humans [16, 22]. Similarly, neurogenesis in the dentate gyrus of hippocampus may affect memory allocation on longer scales of days and weeks, linking memories encoded at similar times while avoiding interference between temporally distant events in memory [65]. Through these different mechanisms, hippocampus and mPFC may come to represent the temporal context of individual events at different time scales, from fine (e.g., position in a short sequence of events) to broad (e.g., whether an event happened one week or four weeks ago).
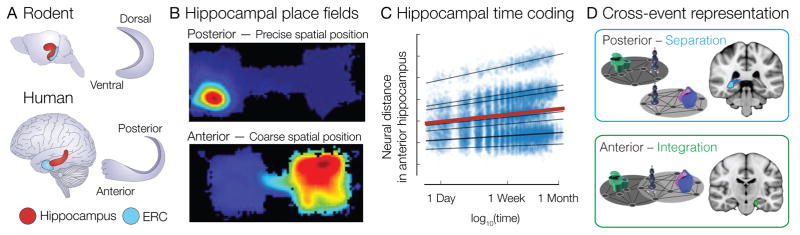
Figure 2.
(A) Illustration of the hippocampal long axis in rodent and human. Ventral and dorsal hippocampus in rodent correspond to anterior and posterior hippocampus, respectively, in human. ERC: entorhinal cortex. Adapted from Ref. [78]. (B) Hippocampal place fields differ in size along the hippocampal long axis; place fields in anterior hippocampus are larger than those observed in posterior hippocampus. Smaller place fields in posterior hippocampus may support coding of detailed event information (such as precise spatial position), whereas larger place fields in anterior hippocampus may integrate information across spatial positions to support coding of global context information. Adpated from Ref. [79]. (C) Anterior hippocampus represents memories for real-world events more similarly when they occur closer together in time. The temporal organization of memories within anterior hippocampus is even apparent at long time scales, such as one week and one month. Adapted from Ref. [63]. (D) Memory elements that share a common association with an overlapping item are represented differently within anterior and posterior hippocampus. Anterior hippocampal activation patterns become more similar for elements that share relationships, reflecting integration of overlapping events. In contrast, posterior hippocampal activation patterns for those same elements become more dissimilar after learning, reflecting the formation of orthogonal representations for overlapping events. Adapted from Ref. [22].
In addition to providing a map of temporal context, integration may support flexible generalization of information across episodes that share temporal context. For instance, a study examining hippocampal ensemble responses in rodents found that memories that are encoded close together in time (within 5 h) are represented with overlapping populations of CA1 neurons, with the amount of overlap predicting the degree of fear responding that generalized across the two memories (Fig. 1C) [32]. However, there may be important boundary conditions on the degree of integration and generalization observed across longer time intervals. Although reactivation of related memories has been found to promote integration [17], a recent study found that memories encoded on different days were less likely to be integrated compared to memories encoded on the same day, even when controlling for the amount of reactivation [66]. Future work is needed to determine precisely how different mechanisms for temporal coding facilitate generalization of information across episodes.
Integrated maps of conceptual space
Emerging evidence suggests that the same mechanisms that support integration of spatial and temporal information may also play a key role in our ability to acquire concepts [67]. Concepts organize our experiences by highlighting shared features and allow for meaningful generalizations in novel situations. Acquiring new concepts requires extracting information across multiple individual learning experiences to learn both what features are common to concept exemplars and what features differentiate between concepts. As a result of learning, concept representations emphasize diagnostic features for a given concept, rather than the overall perceptual similarity of individual stimuli (Fig. 1D). When presented with a stimulus to be assigned to a concept, hippocampus—mPFC interactions may trigger reactivation of similar, concept-relevant learning experiences. Medial PFC also influences hippocampal encoding by compressing memory representations to emphasize features that capture commonalities across events [21], consistent with its hypothesized role in forming mental models of latent structures that are not directly observable [13, 14, 68]. The retrieved memories may then be used to predict a concept label for the current experience. The outcome of that prediction may then lead to integration of the new exemplar into an existing concept or formation of a new concept representation. In this way, learning concepts requires similar computations and representations as learning about the spatial and temporal regularities of the environment [67].
Electrophysiological data from human patients indicates that hippocampal neurons show high selectivity to the conceptual rather than the perceptual features of events [69]. Moreover, hippocampal responses scale with the conceptual novelty of experiences [70, 71], and such signals may be critical to concept formation and updating [72, 73]. Further evidence for the role of the hippocampus and mPFC in formation of conceptual maps comes from a set of recent studies that use representational approaches to neuroimaging data analysis [20, 74, 75]. These studies have shown that the similarity of hippocampal [20, 75] and mPFC [74] activation patterns between individual concept exemplars scales with their distance in a learned conceptual space; exemplars of the same concept evoke more similar activation patterns in hippocampus and mPFC and are distinct from exemplars of different concepts (Fig. 1D). In one case, the conceptual organization observed in medial temporal lobe and mPFC regions followed a grid-like organization that is commonly observed during spatial navigation and imagery [74], providing a direct link between spatial and conceptual representation within these regions. Additional evidence suggests that hippocampal—mPFC interactions are critical when conceptual knowledge is applied to new situations with the same underlying conceptual structure [9, 76]. Collectively, these results indicate that individual memories are integrated through hippocampal—mPFC interactions to create conceptual knowledge that supports flexible decisions when confronted with new stimuli or environments.
The scale of integration differs between hippocampal subregions
The evidence reviewed thus far indicates that integration of spatial, temporal, and conceptual relationships may occur at a variety of scales from fine-grained to broad. Different scales of integration may be linked to the function of different hippocampal subregions. For instance, the spatial remapping properties of CA1 and CA3 noted above [46] suggest that CA1 may integrate information across a broader set of contexts than CA3, which forms overlapping representations for only highly similar environments. Computational modeling further indicates that CA1, but not CA3, representations support extraction of temporal and associative regularities from the environment and enable inference about the relationships among episodes [47]. Human and rodent data have also exclusively linked memory integration processes to CA1 [16, 32]. The connectivity of CA1 may explain its privileged role in memory integration. It receives input about incoming sensory information from entorhinal cortex simultaneously with memory-driven expectations derived from CA3 pattern completion processes [77]; networks of CA1 cells that represent new content and existing memories may therefore become linked due to their coactivation within CA1.
Differences in representational scaling have also been observed along the long axis (ventral—dorsal in rodents, anterior—posterior in humans) of hippocampus (Fig. 2A) [22, 23]. Place cells in anterior hippocampus have larger receptive fields than in posterior hippocampus (Fig. 2B) [78–80]. Place field gradients along the hippocampus axis may thus support integration of experiences at different spatial, temporal, and conceptual scales. For instance, large place fields in anterior hippocampus are well suited for integrating events that occur at different positions within the same environment, while smaller place fields in posterior hippocampus allow distinct coding of events experienced in the same environment [81]. It is possible that temporal representations may vary in scale along the hippocampal long axis in a similar manner to spatial representations, with larger timescales represented in anterior hippocampus and smaller timescales in posterior hippocampus [82]. Consistent with this hypothesis, anterior hippocampus is more likely to demonstrate integrated codes for overlapping events that are separated by long time intervals than posterior hippocampus (Fig. 2C,D) [22, 23, 63]. The different representational capacities of anterior and posterior hippocampus may thus simultaneously support behaviors that rely on different levels of memory detail [83], with posterior hippocampus supporting fine judgments relating individual memory elements and anterior hippocampus supporting abstractions across broader experiential scales. There is also evidence that anterior and posterior mPFC subregions exhibit different levels of integration that mirror functional differences along the hippocampal long axis [22], but more work is needed to determine whether human mPFC subregions are functionally distinct.
Conclusions
Much of our knowledge is derived by forming links across the individual events we experience. The findings reviewed here indicate that structured knowledge about the spatial, temporal, and conceptual relationships among events arises from memory integration processes supported by the hippocampus and mPFC. By forming overlapping memory codes that integrate information acquired at different times and places, our memories extend beyond what we directly experience and can be deployed flexibly to support behavior in new situations.
However, several open questions remain for future research on memory integration. For instance, what are the boundary conditions that determine when representations of overlapping memories are integrated rather than separated, and how might we manipulate the formation of integrated codes of experience? While initial findings suggest that the strength of existing knowledge [18], the degree of memory reactivation during encoding [17], the magnitude of memory-based prediction errors [15], and task demands [84] all impact the likelihood of integration, more work is needed to understand the full complement of conditions that influence how we represent related episodes in memory. As noted above, different memory circuits (e.g., anterior and posterior hippocampus) may integrate information at different scales. It remains to be tested whether these circuits play functionally different roles in memory, with fine-scaled representation supporting decisions about relationships among detailed event elements and integration at broader scales supporting more abstract knowledge about the conceptual relationships among events. The fact that hippocampus and mPFC continue to mature through the third decade of life [85, 86] further suggests that memory integration may have a prolonged developmental timecourse that extends through adolescence, with corresponding impacts on spatial, temporal, and conceptual learning abilities. In reviewing the current state of knowledge on memory integration, we hope to inspire future work on the rich set of questions that remain.
Highlights.
Individual memories can become integrated through overlapping neural codes
Spatial, temporal, and conceptual proximity promote memory integration
Integration supports the formation of maps of space, time, and concepts
Integrated codes in hippocampus and prefrontal cortex support flexible memory use
Different scales of integration may be supported by distinct hippocampal subregions
Acknowledgments
This work was supported by the National Institute of Mental Health (R01 MH100121 to A.R.P.), the National Institute of Child Health and Human Development (R21 HD083785 to A.R.P.), and the National Institute of Neurological Disorders and Stroke (F32 NS098808 to K.R.S.) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlichting ML, Preston AR. Memory integration: neural mechanisms and implications for behavior. Curr Opin Behavioral Sciences. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York: Oxford University Press; 2001. [Google Scholar]
- 3.Tavares RM, et al. A Map for Social Navigation in the Human Brain. Neuron. 2015;87(1):231–43. doi: 10.1016/j.neuron.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez JE, et al. Knowledge of social affliations biases economic decisions. PLos One. 2016;11(7):e0159918. doi: 10.1371/journal.pone.0159918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55(4):189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 6.Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: building memories to navigate future decisions. Front Hum Neurosci. 2012;6:70. doi: 10.3389/fnhum.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokose J, et al. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science. 2017;355(6323):398–403. doi: 10.1126/science.aal2690. [DOI] [PubMed] [Google Scholar]
- 8.Eichenbaum H, et al. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23(2):209–26. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 9*.Place R, et al. Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci. 2016;19(8):992–4. doi: 10.1038/nn.4327. In this study, rats were trained on a set of contextual rules that determined which object was associated with a reward in a given spatial environment. Simultaneous recording of hippocampal and PFC neurons indicated a bidirectional flow of information between the structures, with the directional flow changing depending on the phase of the task. Upon a rat’s entry into a spatial context, information about the context flowed from hippocampus to PFC; when the rats sampled the object, the information flow was reversed, with PFC controlling responses in hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23(17):R764–73. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 12.Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60(2):378–89. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RC, et al. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81(2):267–79. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan SC, Niv Y, Norman KA. A Probability Distribution over Latent Causes, in the Orbitofrontal Cortex. J Neurosci. 2016;36(30):7817–28. doi: 10.1523/JNEUROSCI.0659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long NM, Lee H, Kuhl BA. Hippocampal Mismatch Signals Are Modulated by the Strength of Neural Predictions and Their Similarity to Outcomes. J Neurosci. 2016;36(50):12677–12687. doi: 10.1523/JNEUROSCI.1850-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlichting ML, Zeithamova D, Preston AR. CA1 subfield contributions to memory integration and inference. Hippocampus. 2014;24(10):1248–60. doi: 10.1002/hipo.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75(1):168–79. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlichting ML, Preston AR. Hippocampal-medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiol Learn Mem. 2016;134:91–106. doi: 10.1016/j.nlm.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kesteren MT, et al. How schema and novelty augment memory formation. Trends Neurosci. 2012;35(4):211–9. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 20*.Mack ML, Love BC, Preston AR. Dynamic updating of hippocampal object representations reflects new conceptual knowledge. Proc Natl Acad Sci U S A. 2016;113(46):13203–13208. doi: 10.1073/pnas.1614048113. In this study, subjects first learned to categorize a set of multidimensional objects into one of two categories before learning to categorize the same set of objects in a new, orthogonal category structure. A computational model was used to quantify the similarity of individual object representations learned within the context of the changing problems. Using the model-based similarity matrices, the authors then searched for brain regions that showed the predicted conceptual structure. This model-based representational similarity analysis revealed that anterior hippocampal activation patterns reflect the conceptual similarities among items and are rapidly updated to reflect learning of new concepts through interactions with mPFC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack ML, Preston AR, Love BC. Medial prefrontal cortex compresses concept representations through learning. International Workshop on Pattern Recognition in Neuroimaging (PRNI); 2017; Toronto, CA. [Google Scholar]
- 22**.Schlichting ML, Mumford JA, Preston AR. Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat Commun. 2015;6:8151. doi: 10.1038/ncomms9151. In this study, subjects learned overlapping pairs of object images (AB and BC, for which B refer to a common object shared across pairs). Both prior to and after learning, subjects viewed the individual objects during fMRI scanning. Using representational similarity analysis, the authors showed that anterior hippocampal activation patterns evoked by indirectly related objects (A and C) became more similar after learning, consistent with integration of the overlapping pairs. In contrast, posterior hippocampal activation patterns for indirectly related objects became more dissimilar after learning, reflecting orthogonalization of overlapping memory content. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collin SH, Milivojevic B, Doeller CF. Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci. 2015;18(11):1562–4. doi: 10.1038/nn.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12(7):913–8. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catanese J, et al. Retrospectively and prospectively modulated hippocampal place responses are differentially distributed along a common path in a continuous T-maze. J Neurosci. 2014;34(39):13163–9. doi: 10.1523/JNEUROSCI.0819-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27(45):12176–89. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadhav SP, et al. Coordinated Excitation and Inhibition of Prefrontal Ensembles during Awake Hippocampal Sharp-Wave Ripple Events. Neuron. 2016;90(1):113–27. doi: 10.1016/j.neuron.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan R, et al. Medial prefrontal theta phase coupling during spatial memory retrieval. Hippocampus. 2014;24(6):656–65. doi: 10.1002/hipo.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown TI, et al. Prospective representation of navigational goals in the human hippocampus. Science. 2016;352(6291):1323–6. doi: 10.1126/science.aaf0784. [DOI] [PubMed] [Google Scholar]
- 30.Backus AR, et al. Hippocampal-Prefrontal Theta Oscillations Support Memory Integration. Curr Biol. 2016;26(4):450–7. doi: 10.1016/j.cub.2015.12.048. [DOI] [PubMed] [Google Scholar]
- 31.Miller JF, et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013;342(6162):1111–4. doi: 10.1126/science.1244056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Cai DJ, et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534(7605):115–8. doi: 10.1038/nature17955. This study used calcium imaging and immunohistochemistry to show that memories of two distinct spatial contexts were represented by overlapping populations of CA1 neurons when those contexts were experienced on the same day, but were represented by non-overlapping CA1 populations when experienced one week apart. Furthermore, when the representations of the contexts overlapped in CA1, greater generalization of fear responses was observed across the two spatial contexts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenzie S, et al. Hippocampal Representation of Related and Opposing Memories Develop within Distinct, Hierarchically Organized Neural Schemas. Neuron. 2014;83(1):202–15. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer AC, et al. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci. 2010;30(35):11586–604. doi: 10.1523/JNEUROSCI.0926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Deuker L, et al. An event map of memory space in the hippocampus. Elife. 2016;5 doi: 10.7554/eLife.16534. In this fMRI study, subjects navigated through a virtual town, learning the locations of objects within the town and their relative positions. After the navigation task, subjects performed a memory test in which they judged how close together two objects appeared in space and time. Representational similarity analysis of hippocampal responses to the individual objects prior to and after navigation indicated that hippocampal activation patterns were more similar for the objects that were later judged as close together in space and time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta AS, et al. Hippocampal replay is not a simple function of experience. Neuron. 2010;65(5):695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497(7447):74–9. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schinazi VR, et al. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus. 2013;23(6):515–28. doi: 10.1002/hipo.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvernhe A, Save E, Poucet B. Local remapping of place cell firing in the Tolman detour task. Eur J Neurosci. 2011;33(9):1696–705. doi: 10.1111/j.1460-9568.2011.07653.x. [DOI] [PubMed] [Google Scholar]
- 40.Howard LR, et al. The hippocampus and entorhinal cortex encode the path and Euclidean distances to goals during navigation. Curr Biol. 2014;24(12):1331–40. doi: 10.1016/j.cub.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiers HJ, Gilbert SJ. Solving the detour problem in navigation: a model of prefrontal and hippocampal interactions. Front Hum Neurosci. 2015;9:125. doi: 10.3389/fnhum.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito HT, et al. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522(7554):50–5. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan R, et al. Medial Prefrontal-Medial Temporal Theta Phase Coupling in Dynamic Spatial Imagery. J Cogn Neurosci. 2017;29(3):507–519. doi: 10.1162/jocn_a_01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tse D, et al. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 45.Tse D, et al. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333(6044):891–5. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- 46.Leutgeb S, et al. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–8. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 47.Schapiro AC, et al. Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Philos Trans R Soc Lond B Biol Sci. 2017;372(1711) doi: 10.1098/rstb.2016.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schapiro AC, et al. The necessity of the medial temporal lobe for statistical learning. J Cogn Neurosci. 2014;26(8):1736–47. doi: 10.1162/jocn_a_00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5(5):458–62. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogerson T, et al. Synaptic tagging during memory allocation. Nat Rev Neurosci. 2014;15(3):157–69. doi: 10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macdonald CJ, et al. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–49. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiganj Z, et al. Sequential firing codes for time in rodent medial prefrontal cortex. Cereb Cortex. 2016:1–9. doi: 10.1093/cercor/bhw336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard MW, Eichenbaum H. The hippocampus, time, and memory across scales. J Exp Psychol Gen. 2013;142(4):1211–30. doi: 10.1037/a0033621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howard MW, et al. A distributed representation of internal time. Psychol Rev. 2015;122(1):24–53. doi: 10.1037/a0037840. [DOI] [PubMed] [Google Scholar]
- 55.Schapiro AC, Kustner LV, Turk-Browne NB. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr Biol. 2012;22(17):1622–7. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ezzyat Y, Davachi L. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81(5):1179–89. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guarino KF, et al. Development of medial prefrontal cortex is related to statistical learning and inference. Annual meeting of the Society for Neuroscience; 2015; Chicago, IL. [Google Scholar]
- 58.Garvert MM, Dolan RJ, Behrens TE. A map of abstract relational knowledge in the human hippocampal-entorhinal cortex. Elife. 2017:6. doi: 10.7554/eLife.17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schapiro AC, et al. Neural representations of events arise from temporal community structure. Nat Neurosci. 2013;16(4):486–92. doi: 10.1038/nn.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Allen TA, et al. Nonspatial Sequence Coding in CA1 Neurons. J Neurosci. 2016;36(5):1547–63. doi: 10.1523/JNEUROSCI.2874-15.2016. CA1 responses were recorded as rats performed a non-spatial sequence task. Rats were presented repeated sequences of odors and had to identify deviations in sequence presentations. The temporal context of individual odor presentations could be decoded from the activity of CA1 ensembles, and sequence coding at the single cell and ensemble level was correlated with the rats’ ability to successfully detect when odor presentations occurred in the predicted order or were out of sequence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsieh LT, et al. Hippocampal activity patterns carry information about objects in temporal context. Neuron. 2014;81(5):1165–78. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobs NS, et al. Critical role of the hippocampus in memory for elapsed time. J Neurosci. 2013;33(34):13888–93. doi: 10.1523/JNEUROSCI.1733-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielson DM, et al. Human hippocampus represents space and time during retrieval of real-world memories. Proc Natl Acad Sci U S A. 2015;112(35):11078–83. doi: 10.1073/pnas.1507104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rashid AJ, et al. Competition between engrams influences fear memory formation and recall. Science. 2016;353(6297):383–7. doi: 10.1126/science.aaf0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–7. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 66.Zeithamova D, Preston AR. Temporal Proximity Promotes Integration of Overlapping Events. J Cogn Neurosci. 2017:1–13. doi: 10.1162/jocn_a_01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mack ML, Love BC, Preston AR. Building concepts one episode at a time: The hippocampus and concept formation. Neurosci Lett. doi: 10.1016/j.neulet.2017.07.061. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wikenheiser AM, Schoenbaum G. Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci. 2016;17(8):513–23. doi: 10.1038/nrn.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quian Quiroga R. Neuronal codes for visual perception and memory. Neuropsychologia. 2016;83:227–41. doi: 10.1016/j.neuropsychologia.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 70.Reggev N, Bein O, Maril A. Distinct Neural Suppression and Encoding Effects for Conceptual Novelty and Familiarity. J Cogn Neurosci. 2016;28(10):1455–70. doi: 10.1162/jocn_a_00994. [DOI] [PubMed] [Google Scholar]
- 71.Poppenk J, et al. Why is the meaning of a sentence better remembered than its form? An fMRI study on the role of novelty-encoding processes. Hippocampus. 2008;18(9):909–18. doi: 10.1002/hipo.20453. [DOI] [PubMed] [Google Scholar]
- 72.Davis T, Love BC, Preston AR. Learning the exception to the rule: model-based FMRI reveals specialized representations for surprising category members. Cereb Cortex. 2012;22(2):260–73. doi: 10.1093/cercor/bhr036. [DOI] [PubMed] [Google Scholar]
- 73.Davis T, Love BC, Preston AR. Striatal and hippocampal entropy and recognition signals in category learning: simultaneous processes revealed by model-based fMRI. J Exp Psychol Learn Mem Cogn. 2012;38(4):821–39. doi: 10.1037/a0027865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.Constantinescu AO, O’Reilly JX, Behrens TE. Organizing conceptual knowledge in humans with a gridlike code. Science. 2016;352(6292):1464–8. doi: 10.1126/science.aaf0941. Humans were trained on a two-dimensional conceptual space. During fMRI scanning, subjects navigated through the conceptual space by viewing the objects. The authors found that medial temporal lobe and mPFC responses evoked by the stimuli were hexagonally organized, similar to the hexagonal grid patterns observed in these regions during physical navigation. These findings suggest that the same underlying representational structure may support the formation of maps of space and concepts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis T, et al. Global neural pattern similarity as a common basis for categorization and recognition memory. J Neurosci. 2014;34(22):7472–84. doi: 10.1523/JNEUROSCI.3376-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumaran D, et al. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63(6):889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11(5):578–98. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- 78.Strange BA, et al. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–69. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 79.Komorowski RW, et al. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci. 2013;33(18):8079–87. doi: 10.1523/JNEUROSCI.5458-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 81.Keinath AT, et al. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus. 2014;24(12):1533–48. doi: 10.1002/hipo.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long LL, Bunce JG, Chrobak JJ. Theta variation and spatiotemporal scaling along the septotemporal axis of the hippocampus. Front Syst Neurosci. 2015;9:37. doi: 10.3389/fnsys.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poppenk J, et al. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–40. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Richter FR, Chanales AJ, Kuhl BA. Predicting the integration of overlapping memories by decoding mnemonic processing states during learning. Neuroimage. 2016;124(Pt A):323–35. doi: 10.1016/j.neuroimage.2015.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murty VP, Calabro F, Luna B. The role of experience in adolescent cognitive development: Integration of executive, memory, and mesolimbic systems. Neurosci Biobehav Rev. 2016;70:46–58. doi: 10.1016/j.neubiorev.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee JK, Ekstrom AD, Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. Neuroimage. 2014;94:162–71. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]