Abstract
Introduction
Anticoagulation is a common treatment modality in patients with pulmonary arterial hypertension (PAH). Further studies are needed to appropriately assess the risk / benefit ratio of anticoagulation, particularly in PAH patients receiving PAH-specific therapies.
Aims
We use observational long-term data on PAH patients treated with subcutaneous (SQ) treprostinil from a large open-label study. Patients were followed for up to four years. The use of warfarin and bleeding events were recorded.
Results
At total of 860 patients (age [mean ± SD] 46 ± 15 years, 76% female, 83% Caucasian, 49% idiopathic PAH, and 76% NYHA functional class III) were included. All patients received SQ treprostinil (15% also other PH-therapies) and 590 (69%) received warfarin during the study. The proportions of women, African-American and IPAH patients were higher in the group receiving warfarin. A higher proportion of patients with congenital heart disease and porto-pulmonary hypertension did not receive warfarin. There were no differences in unadjusted long-term survival between PAH patients receiving warfarin or not (log-rank test, p value=0.69), even when only considering idiopathic PAH (p=0.32). In addition, no difference was found in adjusted long-term survival both in PAH (p=0.84) and idiopathic PAH patients (p=0.44) based on the use of warfarin. Furthermore, no survival difference based on the use of warfarin were noted between propensity score matched PAH patients (p=0.37).
Conclusions
Long-term anticoagulation with warfarin was not associated with any significant effect on survival in PAH or idiopathic PAH patients treated with SQ treprostinil.
Keywords: Pulmonary arterial hypertension, treprostinil, anticoagulation, warfarin, survival
Introduction
Pulmonary Arterial Hypertension (PAH) is a progressive pulmonary vascular condition that can lead to right ventricular failure and premature death (3). The characteristic increase in pulmonary vascular resistance (PVR) results from the combination of smooth muscle and endothelial cell proliferation, vasoconstriction, fibrosis, and in-situ thrombosis (4). Over the last three decades, 10 therapeutic agents have received Food and Drug Administration (FDA) approval for use in PAH (5, 6).
Supportive therapies such as oxygen, diuretics and anticoagulants are commonly used in patients with PAH. The rationale to use anticoagulants derives from post-mortem studies of patients with idiopathic PAH (IPAH) that demonstrated in-situ thrombosis (7–9). In addition, there is evidence of abnormalities of the platelet activation, coagulation and fibrinolytic pathways in IPAH patients (10–13)(14, 15). These findings, coupled with evidence from retrospective studies demonstrating a benefit of anticoagulation, have justified the use of oral anticoagulation in PAH patients.
There are no randomized controlled studies that evaluate the effectiveness and risks of oral anticoagulation in patients with PAH (16). Furthermore, limited information exists on the impact of oral anticoagulants in patients with PAH receiving disease-specific therapies. This is striking because some coagulation abnormalities in PAH patients can be improved with PAH-specific therapies (14, 17–20). Retrospective studies, mostly done before the availability of PAH-specific therapies, suggest that oral anticoagulants increase survival in PAH (8, 21, 22). More contemporary studies corroborated that warfarin use was associated with improved survival (23), particularly at one year (24). Two systematic reviews (one with meta-analysis) revealed that anticoagulation decreases mortality risk in PAH patients (25, 26). The Comparative Prospective Registry of Newly Initiated Therapies for pulmonary hypertension (COMPERA) registry reported that the use of oral anticoagulants was associated with a three-year survival benefit only in patients with IPAH (27). However, results from Johnson et al (28) and the REVEAL registry (29) have recently challenged these findings, questioning the effectiveness of anticoagulation in patients with PAH—even in patients with IPAH.
Studies have shown that chronic treatment of PAH with parenteral prostanoids produces a measurable and consistent anti-platelet effect (30, 31) and may normalize the coagulation/fibrinolysis balance observed in PAH patients (17); factors which could weaken the beneficial effects and increase the bleeding risks of anticoagulation. Therefore, it remains unclear whether PAH patients treated with prostacyclin analogues experience any additional benefit from anticoagulation that outweighs the bleeding risks. Given than a randomized study assessing the risk and benefit of warfarin in PAH patients is unlikely to occur, retrospective studies using data from well-structured patient registries and modern statistical methodologies are the best next alternative. In the present study, our aim was two-fold: 1) whether warfarin reduces mortality and 2) if warfarin is associated with a higher risk of bleeding in a large cohort of PAH patients treated with subcutaneous treprostinil (32).
Methods
Patients and study design
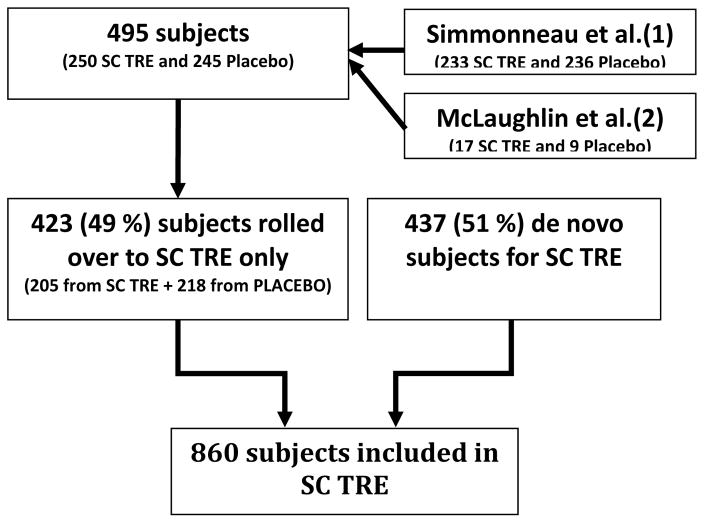
We used the long-term data from an open-label extension study of PAH patients treated with subcutaneous treprostinil (32). Subjects were eligible de novo or rolled over from either a placebo-controlled pilot study or a large placebo-controlled randomized study (open label extension) (1, 2) (Figure 1). De novo patients were eligible if they had PAH (idiopathic, associated with connective tissue disease (CTD), congenital heart disease (CHD), portal hypertension, or human immunodeficiency virus (HIV)) and were in New York Heart Association (NYHA) functional class II, III and IV. Patients needed to be ≥ 8 year of age, walk 50–450 m in a six-minute walk test (6MWT) and have a mean PAP (mPAP) ≥ 25 mmHg, pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg and pulmonary vascular resistance (PVR) > 3 Wood units. Placebo-controlled studies (1, 2) had similar inclusion criteria but excluded patients with porto-pulmonary hypertension or HIV-associated PAH.
Figure 1. Flowchart of the study patients.
Abbreviations: SC TRE: subcutaneous treprostinil study.
The objective of the open-label study was to assess the impact of subcutaneous treprostinil on long-term outcomes in PAH. Data were collected from 06/25/1998 to 12/1/2003. Patients were followed for up to four years. The end of the study was defined by death, transplantation, intolerable side effects, or the initiation of alternative prostacyclin analogues. A total of 354 (41 %) subjects remained on the study and 506 (59%) prematurely discontinued it for a variety of reasons (32). Subcutaneous treprostinil was administered for one, two, and three years to 538 (63%), 312 (36%) and 135 (16%) patients with a mean dose of 26, 36 and 42 ng/kg/min, respectively (32). All patients initially received subcutaneous treprostinil as monotherapy; however, other PAH-specific therapies could be added based on the criteria of the treating physician. In fact, bosentan was initiated in 105 (12%) and sildenafil in 25 (3%) of the patients. Furthermore, 97 patients were transitioned off subcutaneous treprostinil to an alternative prostacyclin class drug and one patient had inhaled iloprost added for a few days (32). Importantly, warfarin as well as other background PAH therapies were administered at the discretion of the treating physician. Compliance with anticoagulation was recorded. The level of anticoagulation (international normalized ratio (INR)) was not routinely collected.
Statistical analysis
Baseline parameters were obtained at the start of treprostinil administration in the open label study. All PAH patients treated with treprostinil were included in the analysis. Continuous variables were summarized using mean ± standard deviation. We compared numerical variables using Wilcoxon Rank Sum Test and categorical variables with Chi-square test or Fisher’s exact test, when appropriate. Survival was assessed using Kaplan-Meier methodology, from the start of subcutaneous treprostinil (index date) to death or data cut-off. Patients were censored if they underwent transplantation, discontinued the study drug or were lost to follow-up. Survival distributions between groups were tested with Logrank statistics. Cox proportional-hazards regression was used to compare survival between groups, adjusted for variables of interest, i.e. age, NYHA functional class, PAH etiology, mPAP, PAWP and PVR. Results of Cox regression are given as hazard ratio with 95% confidence interval. Data imputation was performed when described using Markov chain Monte Carlo. We used propensity score to reduce bias due to the imbalance of measured variables associated with outcomes, between the groups of interest (those who received warfarin and those that did not) (33). Propensity score was generated using logistic regression and including the variables age, gender, race, PAH etiology, baseline height, weight, NYHA functional class, initiation of bosentan or sildenafil during follow-up, right atrial (RA) pressure, mPAP, PAWP, PVR and cardiac output. Analyses were performed for all the PAH groups and for the patients with IPAH. We also performed sensitivity analyses testing survival based on groups matched on newly generated propensity scores, including 3 subgroups a) patients in whom the date of PAH diagnosis was available, b) subjects that receive warfarin throughout the course of the study (without stops) and c) individuals who were followed during the entire course of the study or died. Adverse events were recorded through the study. All p-values are two-tailed and a value of < 0.05 was considered statistically significant. The statistical analyses were performed using the Statistical Analysis System (SAS) 9.3, SAS Institute Inc., Cary, NC, USA.
Results
Patient characteristics
We included 860 patients of whom 425 (49%) had IPAH. The mean age was 46 ±15 years and 653 (76 %) patients were female. Most of the patients were in NYHA functional class II or III (n=782, 91%). Functional and hemodynamic characteristics are shown in table 1. A total of 590 (69%) patients received warfarin during the study and 270 (31%) did not. Baseline characteristics of these two groups are presented in table 1. The proportions of women, African-American race and IPAH patients were higher in those individuals receiving warfarin. A higher proportion of patients with congenital heart disease and porto-pulmonary hypertension did not receive warfarin. NYHA functional class and most of hemodynamic determinations were similar between the groups; however, the mPAP was higher in subjects who did not receive warfarin. When only considering patients with IPAH (n=425) we noted no significant demographic differences in the group that received warfarin (n=311, 73%) versus the group that did not (n=114, 27%) (Supplemental Table 1).
Table 1.
Baseline Characteristics of Entire Cohort
| Overall n (%) / mean ± SD |
No warfarin n (%) / mean ± SD |
Warfarin n (%) / mean ± SD |
p | |
|---|---|---|---|---|
| n | 860 | 270 (31) | 590 (69) | |
| Age (years) | 45.8 ± 15.3 | 44.5 ± 14.0 | 46.3 ± 15.8 | 0.12 |
| Female gender | 653 (76) | 189 (70) | 464 (79) | 0.01 |
| Race | ||||
| -White | 711 (83) | 220 (82) | 491 (83) | <0.01 (Ex) |
| -African-American | 48 (6) | 8 (3) | 40 (7) | |
| -Hispanic | 63 (7) | 32 (12) | 31 (5) | |
| Etiology of PAH | ||||
| -Idiopathic | 425 (49) | 114 (42) | 311 (53) | <0.01 |
| -SCL associated | 100 (12) | 21 (8) | 79 (13) | |
| -Other CTD | 66 (8) | 12 (5) | 54 (9) | |
| -CHD associated | 177 (21) | 73 (27) | 104 (18) | |
| -PoPH | 43 (5) | 34 (13) | 9 (2) | |
| -Other | 49 (6) | 16 (6) | 33 (6) | |
| Time from PH diagnosis to enrollment (months)* | 30.2 ± 55.6 Median: 10.9 |
37.8 ± 68.7 Median: 12.1 |
27.1 ± 48.9 Median: 10.2 |
0.17 |
| Initiation of bosentan or sildenafil during follow-up | 60 (7) | 21 (8) | 39 (7) | 0.53 |
| 6-MWD (m) | 335 ± 85 | 326 ± 79 | 336 ± 87 | 0.81 |
| NYHA functional class | ||||
| -II | 128 (15) | 48 (18) | 80 (14) | 0.23 |
| -III | 654 (76) | 196 (73) | 458 (78) | |
| -IV | 78 (9) | 26 (10) | 52 (9) | |
| Baseline Hemodynamics | ||||
| -RA (mmHg) | 10.0 ± 5.8 | 9.5 ± 5.7 | 10.2 ± 5.8 | 0.09 |
| -mPAP (mmHg) | 59.3 ± 15.5 | 62.9 ± 17.9 | 57.9 ± 14.2 | <0.01 |
| -PAWP (mmHg) | 9.5 ± 3.6 | 9.1 ± 3.5 | 9.7 ± 3.6 | 0.06 |
| -CO (L/min) | 4.1 ± 1.6 | 4.3 ± 1.6 | 4.1 ± 1.5 | 0.33 |
| -PVR (Wood U) | 13.6 ± 6.6 | 13.9 ± 7.3 | 13.4 ± 6.4 | 0.68 |
Abbreviations: 6-MWD: six-minute walk distance, CHD: congenital heart disease, CO: cardiac output measured by thermodilution, CTD: connective tissue disease, Ex: Fisher exact test, mPAP: mean pulmonary artery pressure, NYHA: New York Heart Association, PAWP: pulmonary artery wedge pressure, PoPH: portopulmonary hypertension, PVR: pulmonary vascular resistance, RA: right atrium, SCL: scleroderma.
Data only available in 733 patients (215 patients for the group on no warfarin and 518 for the group on warfarin).
Warfarin treatment
Of the 590 patients in the warfarin group, 370 (63 %) were on warfarin for the full duration of the subject’s participation. Baseline characteristics of these subjects are presented in Supplemental Table 2. A total of 489 patients had no stops (complete cessation of warfarin treatment) recorded in their warfarin administration and received it for a mean ± SD (range) of 544 ± 405 (3 – 1,579) days. Of note, it is possible that patients began treatment with warfarin after being enrolled in the study. For example, a patient started warfarin one year after beginning treatment with treprostinil; therefore in this case the patient did not receive treatment for the full duration of the study but had no stops (once the medication was initiated) during the remainder of the study time. Eighty-five patients had one stop in warfarin administration and received it for 734 ± 495 (38 – 2,219) days. Twelve patients had two recorded stops in warfarin and received it for 988 ± 508 (221 – 1,956) days. Four patients had ≥ 3 stops. Supplemental Table 3 demonstrates PAH patients who remained throughout the study and in which treatment was not prematurely discontinued.
Impact of Warfarin treatment
Survival analysis
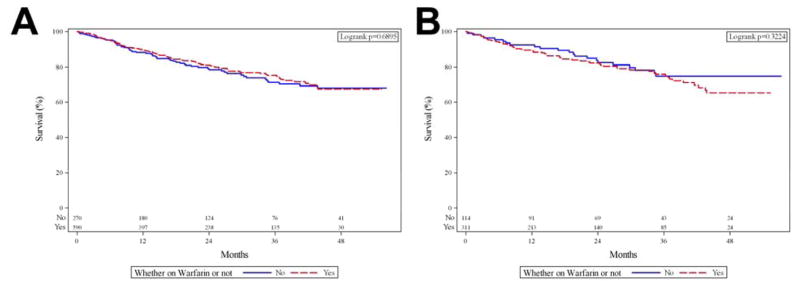
The Kaplan-Meier survival analysis for PAH patients receiving warfarin versus those not receiving warfarin is presented in Figure 2, panel A; with the start point being entry into the trial. No significant differences were noted between the groups (Logrank p=0.69). When the analysis was adjusted by age, NYHA functional class, PAH etiology, mPAP, PAWP and PVR, we noted no significant difference in survival based on the use or not of warfarin (n=621, HR: 0.96 (95% CI: 0.63–1.45), p=0.84). When missing data was imputed in the previous model, the use of warfarin had no significant impact on survival (n=860, p=0.45). A sensitivity analysis on PAH patients who received warfarin throughout the study without stops (n=370) demonstrated a similar survival (Supplemental Figure 1).
Figure 2. Kaplan-Meier survival analysis for PAH patients who received and not received warfarin.
Panel A includes all PAH patients (n=860), log-rank test p=0.69. Panel B includes only patients with IPAH (n=425), log-rank test p=0.32. Patients at risk for each time period are shown at the bottom of the figure.
When solely considering the patients with IPAH (n=425), we found no significant difference in survival by log-rank test (Figure 2, panel B). When adjusted by age, NYHA functional class, mPAP, PAWP and PVR, the treatment with warfarin was not significantly associated with survival (n=324, HR: 1.26 (95%CI: 0.70–2.26), p=0.44). Similar results were observed when imputing missing variables in the last model (n=425, p=0.21). In the subgroup of patients with CTD-PAH, the use of warfarin did not significantly impact survival (Log-rank test (p=0.07) and Cox survival analysis adjusted for the same variables of interest (HR: 0.76 (95%CI: 0.35–1.66), p=0.49)).
Propensity score matching
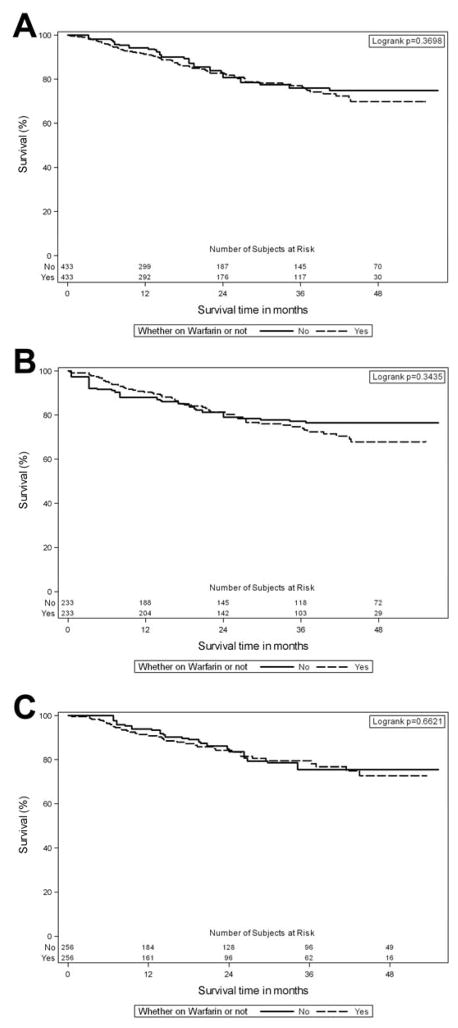
Due to missing values, 433 out of 590 PAH patients on warfarin had a propensity score. Based on the propensity score, we matched 433 PAH patients who received warfarin with patients drawn from the group who did not receive this treatment, using matching with replacement to balance the groups (i.e., a single control may appear several times to match different cases). After propensity score matching, baseline characteristics were balanced except for RA pressure (Supplemental Table 4). The use of warfarin had no significant impact on survival (Logrank p=0.37, Figure 2, panel A). After imputing missing data, we generated propensity scores for all PAH patients who received warfarin (n=590). This group was then matched with patients drawn from the PAH group not on warfarin. After imputation and propensity score matching, baseline characteristics were balanced (Supplemental Table 5). Similarly, the use of warfarin had no significant impact on survival (Log rank p=0.72).
We performed three sensitivity analyses. The first analysis consisted of a propensity score matching including time from diagnosis as an additional variable in the model. This analysis matched 384 patients treated with warfarin since the date at diagnosis was missing in 127 cases of the entire cohort. After matching, groups were well balanced, except for a significant difference in RA pressure (9 ± 5 and 10 ± 5 mmHg for the groups on no warfarin and warfarin, respectively (p=0.01)). The time from PAH diagnosis was also similar between the groups (34 ± 73 (median 11) versus 28 ± 48 (median 11) months for the groups on no warfarin and warfarin, respectively (p=0.56). Importantly, no significant survival differences were noted between the groups (Logrank test p=0.53, Supplemental Figure 2).
The second analysis involved a propensity score matching comprising patients who received warfarin through the course of the study (n=256 in each group). All variables were well balanced between the groups except of RA pressure (10 ± 6 and 11 ± 6 for the groups on no warfarin and warfarin, respectively). No survival differences were noted between the no warfarin and warfarin groups (Logrank p=0.66, Figure 2B). The last sensitivity analysis matched the propensity score in patients who stayed throughout the entire course of the study or died (we excluded patients that prematurely dropped out of the study). For this analysis, we included 233 patients in each group. The 2 groups were well balanced including RA pressure (p=0.16). Similarly, no survival differences were noted between the no warfarin and warfarin groups (Logrank p=0.34, Figure 2C).
Adverse effects of warfarin treatment
Hemorrhagic events that were not related to the infusion site were not ostensibly related to the dose of treprostinil (Table 2). Only two events were severe, leading to hospitalization: gastrointestinal hemorrhage and epistaxis. Thrombocytopenia was attributed to treprostinil treatment in six (0.7%) patients with one related hospitalization and two considered a severe event.
Table 2.
Hemorrhagic events in patient receiving warfarin or not
| Group | Warfarin (n=590) | No Warfarin (n=270) | ||||
|---|---|---|---|---|---|---|
| Severity | Mild | Moderate | Severe | Mild | Moderate | Severe |
| Type | n1(%) | n(%) | n(%) | n(%) | n(%) | n(%) |
| Gastrointestinal2 | 12(2%) | 9(2%) | 1(0%) | 2(1%) | 2(1%) | 2(1%) |
| Genitourinary3 | 7(1%) | 7(1%) | 2(0%) | 5(2%) | 4(1%) | 0(0%) |
| Respiratory4 | 7(1%) | 11(2%) | 5(1%) | 13(5%) | 6(2%) | 5(2%) |
| Neurologic 5 | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| ENT6 | 41(7%) | 19(3%) | 2(0%) | 10(4%) | 7(3%) | 5(2%) |
| Soft Tissue7 | 129(22%) | 40(7%) | 6(1%) | 40(15%) | 10(4%) | 1(0%) |
n in each cell indicates the number of patients having certain adverse event by severity; % is calculated using the total number of patients in each group as the denominator,
melena, rectal hemorrhage, gastrointestinal bleeding, hemorrhagic gastritis, hematemesis, and bloody diarrhea,
hematuria and menorrhagia,
hemoptysis,
cerebral hemorrhage,
gingival hemorrhage, epistaxis, eye hemorrhage,
ecchymosis, injection site hemorrhage, infusion site bleed/bruise.
Abbreviations: ENT: ear, nose and throat.
Discussion
The present study builds upon the growing body of literature that warfarin does not appear to provide a survival advantage in patients with PAH. Using long-term data from an open-label study on PAH patients treated with subcutaneous treprostinil (32), we found that patients receiving anticoagulation during the study had similar survival to those individuals who were not receiving this treatment. This observation held true even when we adjusted our analyses for different variables of interest or matched groups using propensity scores. In fact, the use of warfarin was not significantly associated with survival in the subgroup of patients with IPAH, individuals that received warfarin during the entire course of the study or remained in the study without early discontinuation.
In our study, 590 (69%) patients with PAH received warfarin. The proportion of subjects receiving warfarin was higher in women and patients with IPAH. Warfarin use was less frequent in individuals with PAH associated with congenital heart disease or porto-pulmonary hypertension. Anticoagulation in patients with PAH is largely driven by composite information from other large-scale retrospective analyses. The COMPERA registry (27) included 1,283 consecutive adult patients with newly diagnosed PAH between July 2007 and April 2013. A total of 738 (57.5%) patients received anticoagulation but only two percent of the patients received prostacyclin analogues. Three-year survival was better in IPAH patients that received anticoagulation versus those that never received it. Conversely, anticoagulants did not demonstrate a significant survival benefit in patients with other forms of PAH and potentially a trend towards increased mortality. In our analysis, we did not notice a survival difference based on the use of warfarin, both in the overall cohort or in the subjects with IPAH. It should be noted that our results are primarily based on patients with NYHA class II and III since limited patients were available in other groups to draw valid conclusions.
The benefit of anticoagulation in patients with PAH was recently examined in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL). Preston et al (29) assessed the effect of warfarin on survival of patients with IPAH and systemic sclerosis-associated pulmonary arterial hypertension (SSc-PAH). A total of 187 patients receiving warfarin were matched 1:1 with patients never on warfarin. They found no significant survival advantage in any of these two groups of PAH patients when compared to matched controls, corroborating the results from our study. Furthermore, they found that long-term warfarin use in SSc-PAH patients was associated with poorer survival than patients not receiving warfarin, even after adjusting for confounders.
The available evidence shows that warfarin is not beneficial in patients with SSc-PAH (27–29). In patients with IPAH who received parenteral prostacyclin analogues the use of warfarin is likely not beneficial. This is supported by our results and the fact that one of the reasons for the discordant findings between the COMPERA and REVEAL studies might be the higher use of parenteral prostacyclin analogues in the latter (46% versus 2%, respectively). The lack of effect of warfarin in patients on parenteral prostacyclin analogue therapy could be due to the fact that prostacyclin analogues have antiplatelet effects (34–36), decrease plasma levels of tissue plasminogen activator and plasminogen activator inhibitor-1(18) and normalize plasma markers of endothelial cell injury (14).
A systemic review of 12 non-randomized studies evaluating survival in patients with IPAH, anorexigen-induced PAH, and CTD-PAH treated with anticoagulation demonstrated a mortality benefit in IPAH in 6 of 12 studies. The authors noted that earlier studies tended to show a benefit, but many of these studies were limited by cohort heterogeneity, small sample size, and patient and treatment selection biases. More recent studies, concluded that anticoagulation may not be beneficial, particularly in patients with CTD-PAH, as noted in our study.
Results of this study should not be extrapolated to anticoagulants other than warfarin. It is possible that direct coagulation factor inhibitors such as apixaban, rivaroxaban, edoxaban, and dabigatran may alter the risk/benefit ratio when used in PAH patients. Unlike warfarin, these drugs are fixed dose, have a predictable anticoagulant effect that eliminates the need for routine monitoring, and have few drug or dietary interactions. Limited research has been performed in PAH with these novel anticoagulants. Delbeck et al showed that the chronic use of rivaroxaban reduced systolic RV pressure increase and prevented RV hypertrophy in a monocrotaline pulmonary hypertension rat model (37).
Anticoagulation is also not without side effects, given reported bleeding rates of 5.4 and 19 per 100 patient-years in IPAH and CTD associated-PAH, respectively (38); which is higher than the reported risk in as chronic thromboembolic PH (2.4 per 100 patient-years) (38). The risk of bleeding is increased in patients with systemic sclerosis (39); in fact one study found a 70% probability that warfarin provides no significant benefit or might be harmful in these patients (28). Ogawa et al reported frequent hemorrhagic complications in IPAH patients treated with anticoagulation and epoprostenol (40) suggesting that this combination of therapy may increase the bleeding risk. In the present study we did not observe a higher incidence of major bleeding complication in patients receiving warfarin; however, we did note a higher proportion of soft tissue bleeding at the injection site.
The European Respiratory Society/European Society of Cardiology Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension (2015) (41) mention that anticoagulation may be considered in idiopathic, heritable or anorexigen-induced PAH (class of recommendation IIb and level of evidence C). Furthermore, they express that PAH patients on intravenous long-term prostacyclin analogues generally receive anticoagulation in the absence of contraindications due to the additional risk of catheter-associated thrombosis (41). In their 2009 expert consensus, the American College of Cardiology Foundation recommended warfarin anticoagulation in PAH patients on continuous intravenous therapy, in the absence of contraindications (42). The present study suggests that oral warfarin does not appear to provide a survival advantage in PAH patients receiving subcutaneous treprostinil therapy. This finding is important given that many practitioners commonly prescribe warfarin to these patients.
Limitations of the present study include a) the lack of randomization based on the use of anticoagulation, b) the time on target INR was not recorded, c) reasons for giving or withholding anticoagulation were not available, d) we only included PAH patients treated with treprostinil and e) the criteria used to decide not to anticoagulate patients was not stated and the target INR levels were not recorded. However, a systematic review and meta-regression of 67 studies totaling 50,208 patients followed for 57,155 patient-years demonstrated that the INR is in the therapeutic range for an average of 64 % of the time (43). We believe that this percentage could be used as a reference point for our study. It remains unclear whether a more strict INR control, leading to longer times in the therapeutic INR range could have demonstrated a survival benefit; nevertheless, our results reflect the effect of warfarin as used in real-world clinical practice. Despite these limitations, our study is certainly important, since most PH centers continue to use anticoagulation for IPAH patients treated with prostacyclin analogues (44), an approach that might not be necessary. In the absence of a randomized control trial, observational studies using propensity score matching to balance the study groups are the best contemporary alternative to fill this gap in knowledge.
Conclusion
Long-term use of warfarin was not associated with survival in PAH or idiopathic PAH patients treated with subcutaneous treprostinil.
Supplementary Material
Figure 3. Kaplan-Meier survival analysis after propensity score matching for PAH patients who received and not received warfarin.
Patients at risk for each period are shown at the bottom of the figures. Panel A includes patients after propensity score matching, panel B comprises patients in whom warfarin was given through the study and panel C omits individuals that prematurely dropped out of the study.
Acknowledgments
We appreciate the contributions of all the investigators involved in obtaining the data. The data was provided by United Therapeutics Study Data Query Program.
Funding sources: A.R.T. was supported by CTSA KL2 [Grant # TR000440] (A.R.T.) and R01HL130307.
Abbreviations
- 6WMD
Six-minute walk distance
- COMPERA
Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension
- CTD
Connective tissue disease
- ERA
Endothelin receptor antagonists
- INR
International Normalized Ratio
- IPAH
Idiopathic pulmonary hypertension
- mPAP
Mean pulmonary artery pressure
- MID
Minimal important difference
- NIH
National Institute of Health
- NYHA
New York Heart Association
- PAH
Pulmonary arterial hypertension
- PAI-1
plasminogen activator inhibitor-1
- PAWP
Pulmonary artery wedge pressure
- PH
Pulmonary hypertension
- PHIRST
Pulmonary Arterial Hypertension and Response to Tadalafil
- PVR
Pulmonary vascular resistance
- PDE-5I
Phosphodiesterase type 5 inhibitors
- REVEAL
Registry to Evaluate Early and Long-term PAH Disease Management
- SSc-PAH
Systemic sclerosis associated pulmonary arterial hypertension
- t-PA
Tissue Plasminogen Activator
- WHO
World Health Organization
Footnotes
Contributions of authors:
Mona Ascha BS: Participated in critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Xuan Zhou MS: Participated in the statistical analysis, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Youlan Rao PhD: Participated in the statistical analysis, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Omar A. Minai MD: Participated in the conception and design of the study, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Adriano R. Tonelli MD: Participated in the design of the study, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. Dr Tonelli is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Conflict of interest statements:
Mona Ascha BS: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Xuan Zhou MS: The author is an employee of United Therapeutics, the company who conducted and analyzed these clinical trials.
Youlan Rao PhD: The author is an employee of United Therapeutics, the company who conducted and analyzed these clinical trials.
Omar A. Minai MD: The author is a member of the scientific advisory board of Actelion, Gilead, and Bayer and a member of the speakers bureau of Actelion, Gilead, United Therapeutics, and Bayer.
Adriano R. Tonelli MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Contributor Information
Mona Ascha, Case Western Reserve University School of Medicine. Cleveland, OH, USA.
Xuan Zhou, United Therapeutics Corporation, Research Triangle Park, NC, USA.
Youlan Rao, Senior Biostatistician, United Therapeutics Corporation, Research Triangle Park, NC, USA.
Omar A. Minai, Staff, Pulmonary and Critical Care, Southside Regional Medical Center, Petersburg, VA, USA.
Adriano R. Tonelli, Staff, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
References
- 1.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ Treprostinil Study G. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. American journal of respiratory and critical care medicine. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Gaine SP, Barst RJ, Oudiz RJ, Bourge RC, Frost A, Robbins IM, Tapson VF, McGoon MD, Badesch DB, Sigman J, Roscigno R, Blackburn SD, Arneson C, Rubin LJ, Rich S. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol. 2003;41:293–299. doi: 10.1097/00005344-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:365–369. doi: 10.1164/rccm.201209-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) The European respiratory journal. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 7.Wagenvoort CA. Lung biopsy specimens in the evaluation of pulmonary vascular disease. Chest. 1980;77:614–625. doi: 10.1378/chest.77.5.614. [DOI] [PubMed] [Google Scholar]
- 8.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580–587. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 9.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989;80:1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 10.Sauler M, Fares WH, Trow TK. Standard nonspecific therapies in the management of pulmonary arterial hypertension. Clin Chest Med. 2013;34:799–810. doi: 10.1016/j.ccm.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Herve P, Humbert M, Sitbon O, Parent F, Nunes H, Legal C, Garcia G, Simonneau G. Pathobiology of pulmonary hypertension. The role of platelets and thrombosis. Clin Chest Med. 2001;22:451–458. doi: 10.1016/s0272-5231(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Sosada M, Fabel H. Plasma coagulation profiles in patients with severe primary pulmonary hypertension. Eur Respir J. 1998;12:1446–1449. doi: 10.1183/09031936.98.12061446. [DOI] [PubMed] [Google Scholar]
- 13.Huber K, Beckmann R, Frank H, Kneussl M, Mlczoch J, Binder BR. Fibrinogen, t-PA, and PAI-1 plasma levels in patients with pulmonary hypertension. Am J Respir Crit Care Med. 1994;150:929–933. doi: 10.1164/ajrccm.150.4.7921465. [DOI] [PubMed] [Google Scholar]
- 14.Friedman R, Mears JG, Barst RJ. Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hypertension. Circulation. 1997;96:2782–2784. doi: 10.1161/01.cir.96.9.2782. [DOI] [PubMed] [Google Scholar]
- 15.Roldan T, Landzberg MJ, Deicicchi DJ, Atay JK, Waxman AB. Anticoagulation in patients with pulmonary arterial hypertension: An update on current knowledge. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015 doi: 10.1016/j.healun.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Ezedunukwe IR, Enuh H, Nfonoyim J, Enuh CU. Anticoagulation therapy versus placebo for pulmonary hypertension. Cochrane Database Syst Rev. 2014;6:CD010695. doi: 10.1002/14651858.CD010695.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopec G, Moertl D, Steiner S, Stepien E, Mikolajczyk T, Podolec J, Waligora M, Stepniewski J, Tomkiewicz-Pajak L, Guzik T, Podolec P. Markers of thrombogenesis and fibrinolysis and their relation to inflammation and endothelial activation in patients with idiopathic pulmonary arterial hypertension. PLoS One. 2013;8:e82628. doi: 10.1371/journal.pone.0082628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer-Neumann C, Brenot F, Wolf M, Peynaud-Debayle E, Duroux P, Meyer D, Angles-Cano E, Simonneau G. Continuous infusion of prostacyclin decreases plasma levels of t-PA and PAI-1 in primary pulmonary hypertension. Thromb Haemost. 1995;73:735–736. [PubMed] [Google Scholar]
- 19.Veyradier A, Nishikubo T, Humbert M, Wolf M, Sitbon O, Simonneau G, Girma JP, Meyer D. Improvement of von Willebrand factor proteolysis after prostacyclin infusion in severe pulmonary arterial hypertension. Circulation. 2000;102:2460–2462. doi: 10.1161/01.cir.102.20.2460. [DOI] [PubMed] [Google Scholar]
- 20.Sakamaki F, Kyotani S, Nagaya N, Sato N, Oya H, Satoh T, Nakanishi N. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation. 2000;102:2720–2725. doi: 10.1161/01.cir.102.22.2720. [DOI] [PubMed] [Google Scholar]
- 21.Frank H, Mlczoch J, Huber K, Schuster E, Gurtner HP, Kneussl M. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714–721. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- 22.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 23.Kawut SM, Horn EM, Berekashvili KK, Garofano RP, Goldsmith RL, Widlitz AC, Rosenzweig EB, Kerstein D, Barst RJ. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95:199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Saeed W, Tiawari N, Sardar MR, Salamon JN, Rab R, Iqbal MM, Zolty R. Effect of warfarin on long term pulmonary arterial hypertension (PAH) mortality: change of facts? Circulation. 2011;124:A16034. [Google Scholar]
- 25.Caldeira D, Loureiro MJ, Costa J, Pinto FJ, Ferreira JJ. Oral anticoagulation for pulmonary arterial hypertension: systematic review and meta-analysis. Can J Cardiol. 2014;30:879–887. doi: 10.1016/j.cjca.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SR, Mehta S, Granton JT. Anticoagulation in pulmonary arterial hypertension: a qualitative systematic review. Eur Respir J. 2006;28:999–1004. doi: 10.1183/09031936.06.00015206. [DOI] [PubMed] [Google Scholar]
- 27.Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Lange TJ, Behr J, Klose H, Claussen M, Ewert R, Opitz CF, Vizza CD, Scelsi L, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Coghlan G, Pepke-Zaba J, Schulz U, Gorenflo M, Pittrow D, Hoeper MM. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) Circulation. 2014;129:57–65. doi: 10.1161/CIRCULATIONAHA.113.004526. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SR, Granton JT, Tomlinson GA, Grosbein HA, Le T, Lee P, Seary ME, Hawker GA, Feldman BM. Warfarin in systemic sclerosis-associated and idiopathic pulmonary arterial hypertension. A Bayesian approach to evaluating treatment for uncommon disease. J Rheumatol. 2012;39:276–285. doi: 10.3899/jrheum.110765. [DOI] [PubMed] [Google Scholar]
- 29.Preston IR, Roberts KE, Miller DP, Sen GP, Selej M, Benton WW, Hill NS, Farber HW. Effect of Warfarin Treatment on Survival of Patients With Pulmonary Arterial Hypertension (PAH) in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) Circulation. 2015;132:2403–2411. doi: 10.1161/CIRCULATIONAHA.115.018435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato H, Takashima T, Kishikawa H, Emura S, Ohmori K. Effect of beraprost sodium, a stable prostaglandin I2 analogue, on platelet aggregation in diabetes mellitus. Int J Clin Pharmacol Res. 1996;16:99–102. [PubMed] [Google Scholar]
- 31.Darius H, Hossmann V, Schror K. Antiplatelet effects of intravenous iloprost in patients with peripheral arterial obliterative disease. A placebo-controlled dose-response study. Klin Wochenschr. 1986;64:545–551. doi: 10.1007/BF01735317. [DOI] [PubMed] [Google Scholar]
- 32.Barst RJ, Galie N, Naeije R, Simonneau G, Jeffs R, Arneson C, Rubin LJ. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28:1195–1203. doi: 10.1183/09031936.06.00044406. [DOI] [PubMed] [Google Scholar]
- 33.Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, Fremes SE. A Review of Propensity-Score Methods and Their Use in Cardiovascular Research. Can J Cardiol. 2016;32:259–265. doi: 10.1016/j.cjca.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Chin KM, Channick RN, de Lemos JA, Kim NH, Torres F, Rubin LJ. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135:130–136. doi: 10.1378/chest.08-1323. [DOI] [PubMed] [Google Scholar]
- 35.Yardumian DA, Machin SJ. Altered platelet function in patients on continuous infusions of epoprostenol. Lancet. 1984;1:1357. doi: 10.1016/s0140-6736(84)91855-5. [DOI] [PubMed] [Google Scholar]
- 36.Sinzinger H, Horsch AK, Silberbauer K. The behaviour of various platelet function tests during long-term prostacyclin infusion in patients with peripheral vascular disease. Thromb Haemost. 1983;50:885–887. [PubMed] [Google Scholar]
- 37.Delbeck M, Nickel KF, Perzborn E, Ellinghaus P, Strassburger J, Kast R, Laux V, Schafer S, Schermuly RT, von Degenfeld G. A role for coagulation factor Xa in experimental pulmonary arterial hypertension. Cardiovasc Res. 2011;92:159–168. doi: 10.1093/cvr/cvr168. [DOI] [PubMed] [Google Scholar]
- 38.Henkens IR, Hazenoot T, Boonstra A, Huisman MV, Vonk-Noordegraaf A. Major bleeding with vitamin K antagonist anticoagulants in pulmonary hypertension. Eur Respir J. 2013;41:872–878. doi: 10.1183/09031936.00039212. [DOI] [PubMed] [Google Scholar]
- 39.Duchini A, Sessoms SL. Gastrointestinal hemorrhage in patients with systemic sclerosis and CREST syndrome. Am J Gastroenterol. 1998;93:1453–1456. doi: 10.1111/j.1572-0241.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa A, Matsubara H, Fujio H, Miyaji K, Nakamura K, Morita H, Saito H, Kusano KF, Emori T, Date H, Ohe T. Risk of alveolar hemorrhage in patients with primary pulmonary hypertension--anticoagulation and epoprostenol therapy. Circ J. 2005;69:216–220. doi: 10.1253/circj.69.216. [DOI] [PubMed] [Google Scholar]
- 41.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G Guidelines ESCCfP. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc. and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 43.van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129:1155–1166. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 44.Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Lange TJ, Behr J, Klose H, Claussen M, Ewert R, Opitz CF, Vizza CD, Scelsi L, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Coghlan G, Pepke-Zaba J, Schulz U, Gorenflo M, Pittrow D, Hoeper MM. Response to letters regarding article, “Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA)”. Circulation. 2014;130:e110–112. doi: 10.1161/CIRCULATIONAHA.114.010921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.