Abstract
In many regions of sub-Saharan Africa, both HIV and helminth infections are prevalent. HIV-1 (human immunodeficiency virus type 1) and helminth infections can both compromise immune responses in humans. To determine whether the presence of helminth infection or the treatment of helminth infection alters unstimulated vaccine responses among HIV-1 infected individuals, we conducted two nested serologic studies. Blood samples were collected for HIV disease monitoring and vaccine-specific serologic assays, while stool was evaluated by direct microscopy methods. We compared antibody responses to measles and tetanus vaccines in helminth-infected (Ascaris, Trichuris, hookworm and/or Schistosoma mansoni) and uninfected adults 18 years and older (n = 100). We also compared measles and tetanus antibody responses in Ascaris only-infected adults receiving 400 mg albendazole daily for 3 days (n = 16) vs. placebo (n = 19) in a separate study. In both cohorts, over 70% of participants had measles and tetanus responses above the protective threshold. Prevalence of measles responses were similar between helminth-infected and uninfected individuals (82%, 95% CI: 71–93% vs 72%, 95% CI: 59–85%), as well as log10 tetanus antibody levels (− 0.133 IU/mL vs − 0.190 IU/mL, p > 0.05), and did not differ by helminth species. In the Ascaris-infected cohort, changes in measles responses and tetanus responses did not differ between those who received anthelminthic vs. placebo (p > 0.05 for both). In these studies, neither helminth infection, nor deworming, appeared to affect previously administered vaccine responsiveness in HIV-1 infected, ART naïve, adults in Kenya.
Keywords: Soil-transmitted helminth, HIV, Immunization, Vaccine preventable disease, Co-infection
1. Introduction
While vaccine delivery programs have averted up to 2 to 3 million deaths each year, the burden of vaccine preventable diseases continues to be substantial and an additional 1.5 million deaths are likely avoidable.(World Health Organization, 2016) Immunizations are particularly important in HIV (human immunodeficiency virus) infected individuals, where HIV progressively impairs immune function by depleting CD4 + T-cell populations and cellular immunity, increasing susceptibility to many infectious diseases (Okoye and Picker, 2013). Vaccine responses, including those against measles, wane faster and may be less effective among HIV infected compared to uninfected individuals (Geretti et al., 2008, Geretti and Doyle, 2010, Belaunzaran-Zamudio et al., 2009). Even among populations with high vaccine uptake, HIV infected adults may demonstrate poor vaccine responsiveness. For example, despite relatively high vaccine coverage in many settings, in Brazil only 36% of HIV-1 infected adults had documented immunity to diphtheria and tetanus, while 7% of HIV-1-infected adults in the UKwere measles seronegative (Molton et al., 2010, Ho et al., 2008). As the number of individuals living with HIV continues to increase, optimizing immune responses to commonly administered vaccines is important among HIV infected populations and alternative intervention strategies to improve coverage or efficacy may be needed.
Considerable geographic overlap exists between areas where HIV-1 and helminth infection are prevalent. Of the 22 million Africans estimated to be infected with HIV-1, many are likely co-infected with at least one species of helminth (UNAIDS and WHO, 2009, de Silva et al., 2003). Helminths induce immunomodulatory response in the infected host, which facilitate survival of helminths and establishment of chronic helminth infections (Bethony et al., 2006). Studies of in vitro responses to vaccinations observed decreased Th1 cytokine responses (IL-2, IL-12, and IFN-γ) among helminth infected compared to uninfected individuals, which are some markers of immunosuppression (Elias et al., 2008, Cooper et al., 2001, Sabin et al., 1996). Individuals with heavy Onchocerca volvulus infections had significantly decreased responses to tetanus vaccination compared to those with light or no infection (Cooper et al., 1999).
We sought to determine whether helminth infection with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni alters responses to measles and tetanus vaccines among HIV-1 infected ART (antiretroviral therapy) naïve adults in Kenya. Using two cohorts, we evaluated the seroprevalence of measles and tetanus specific antibodies among HIV-1 infected individuals with and without helminth infections. We also investigated whether deworming modulates vaccine-specific responses among HIV-1 and Ascaris only co-infected individuals, who demonstrated a decrease in IL-10 levels with deworming in a previous study (Blish et al., 2010).
2. Methods
We conducted serologic assays for measles and tetanus specific antibody responses on repository plasma specimens from two previously accrued cohorts involving HIV-1 infected adults. All statistical analyses were performed using Stata 11.2 (StataCorp). Reference to helminth infection in these studies is restricted to the following parasites: Ascaris, Trichuris, hookworm and/or Schistosoma mansoni.
2.1. Study 1: Helminth infected compared to uninfected adults
2.1.1. Study design
A cross-sectional analysis comparing helminth infected and uninfected adults was conducted using 100 previously collected samples from the untreated control arm of a randomized trial evaluating the effect of empiric deworming (400 mg albendazole and 25 mg/kg praziquantel every 3 months) on markers of HIV-1 disease progression in Kenya (Walson et al., 2012). Blood samples were collected between February 2009 and July 2010. We determined a priori that this analysis had 80% power to detect a 31% difference in measles positivity between the helminth infected and uninfected groups, and a mean difference in log10 tetanus response of 0.28 between the helminth infected and uninfected groups.
2.1.2. Ethics statement
All individuals provided written informed consent including use of previously collected samples to evaluate these study-specific outcomes. The trial was approved by the IRB at University of Washington and the Ethical Review Board of the Kenya Medical Research Institute. The trial was registered as NCT00507221 at http://clinicaltrials.gov.
2.1.3. Population
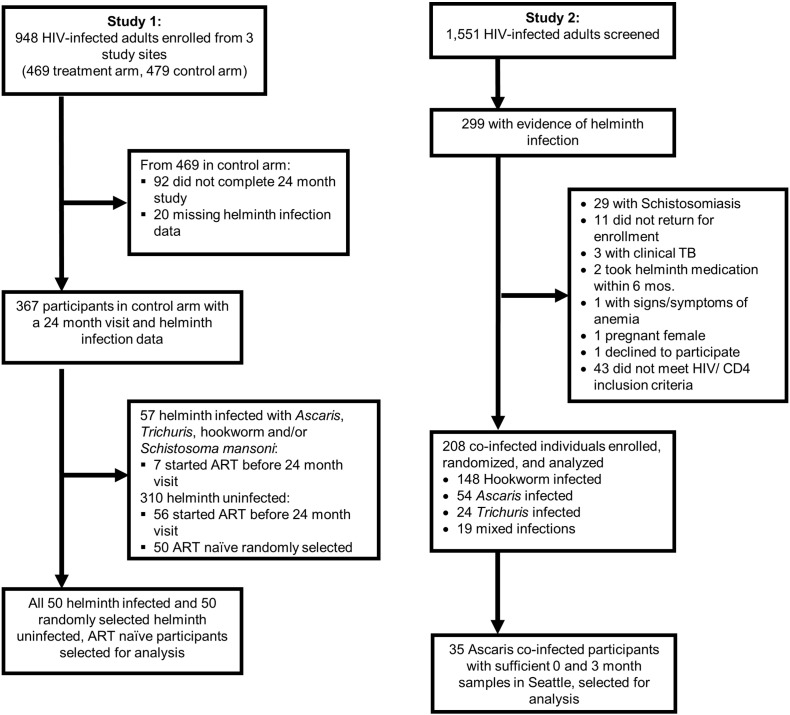
Study participants were enrolled from three sites in Kenya (Kisii Provincial Hospital, Kisumu District Hospital, and Kilifi District Hospital). Participants were older than 18 years, were not pregnant, did not meet criteria for ART initiation based on Kenyan Ministry of Health guidelines, had not used ART in the past, and were willing and able to give written informed consent. At study completion (up to 24 months after enrollment), participants were screened for helminth infection with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni and treated if found to be infected. The nested serologic study included all of the 50 helminth-infected individuals in the control arm (not dewormed), who completed the study and had not started ART. Fifty of the 254 adults not infected with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni, who also completed the study and had not started ART were selected using computer-generated random selection from the same cohort. Time of participant vaccination was not known, though likely occurred in childhood according to the EPI schedule (Fig. 1).
Fig. 1.
Study flow diagram of the 2 studies.
2.1.4. Data collection and statistical analysis
Demographic measures were collected at enrollment. Plasma samples were collected at enrollment and every six months throughout the study to monitor HIV-1 disease. CD4 count was measured every 6 months using a FACSCalibur (Becton Dickinson; Franklin Lakes, NJ, USA), and plasma HIV-1 viral load was measured every 12 months using COBAS Amplicor assay (Roche Molecular Systems Inc., Branchburg, NJ, USA). If viral load was below the lower limit of detection (400 copies/mL) of the assay, half that value was inputed for analysis purposes. Plasma specimens from the final visit (24 months; concurrent with stool helminth testing) were used for vaccine-specific assays. One stool sample also collected at study completion was assessed with 2 slide preparations using direct helminth microscopy, Kato-Katz and formol-ether concentration, by a trained laboratory technologist. Vaccine-specific antibody responses were measured in duplicate and averaged using commercially available ELISA kits (Diagnostic Automation, Inc., Calabasas, CA, Measles: Cat#1408Z; Tetanus: Cat#8900Z). Measles responses were classified as positive, equivocal, or negative based on the manufacturer's protocol, then further dichotomized, including equivocal as negative, and compared by Chi-squared tests. Tetanus responses were log transformed and the association between tetanus response and helminth infection was evaluated by Student's t-test. Analyses were performed comparing any helminth infection (n = 50) to no helminth infection (n = 50), as well as by species-specific helminth infection. Associations between cofactors in Table 1 and helminth infection were assessed using Student's t-tests for continuous variables and Chi-squared tests for dichotomous variables. Also, to evaluate whether positive measles responses (seropositive) or log10 tetanus responses were associated with CD4 count or viral load, logistic and linear regression were used, respectively.
Table 1.
Characteristics of HIV-1-infected participants.
| Frequency (%) or Mean (SD) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Study 1 Helminth infected vs. helminth uninfected |
Study 2 Dewormed vs. placebo treated at baseline |
|||||||
| Uninfected (N = 50) | Infected (N = 50) |
Placebo (N = 19) |
Albendazole (N = 16) | |||||
| Clinical | ||||||||
| Female | 41 | (82%) | 41 | (82%) | 17 | (90%) | 11 | (69%) |
| Age at enrollment (years) | 34.71 | (9.7) | 30.21 | (9.4) | 34.9 | (9.0) | 35.9 | (10.7) |
| Log10 viral load | 4.2 | (1.0) | 4.4 | (0.8) | 4.6 | (0.7) | 4.9 | (1.1) |
| CD4 count (cells/mL3) | 430 | (205) | 441 | (271) | 507 | (203) | 476 | (213) |
| Viral load < 400 copies/mL | 6 | 3 | 0 | 0 | ||||
| Helminth Infection | ||||||||
| Ascaris | 0 | 3 | 19 | 16 | ||||
| Trichuris | 0 | 5 | 0 | 0 | ||||
| Hookworm | 0 | 19 | 0 | 0 | ||||
| Schistosoma | 0 | 15 | 0 | 0 | ||||
| Mixed | 0 | 8 | 0 | 0 | ||||
| None | 50 | 0 | 0 | 0 | ||||
| Helminth Risk Factors | ||||||||
| Primary school education | 30 | (60%) | 28 | (56%) | 14 | (74%) | 12 | (75%) |
| Unemployed | 13 | (26%) | 13 | (26%) | 4 | (21%) | 7 | (44%) |
| Farmer | 10 | (20%) | 11 | (22%) | 9 | (47%) | 3 | (19%) |
| # of children in the home | 2.2 | (1.6) | 2.2 | (1.6) | 1.58 | (1.26) | 2.25 | (1.61) |
| Piped water in home | 4 | (8%) | 3 | (6%) | 1 | (5%) | 4 | (25%) |
| Water source outside home | 42 | (84%) | 40 | (80%) | 6 | (32%) | 7 | (44%) |
| Environmental water source | 4 | (8%) | 7 | (14%) | 12 | (63%) | 5 | (31%) |
| Flush toilet in home | 3 | (6%) | 3 | (6%) | 1 | (5%) | 2 | (13%) |
| Pit latrine outside home | 43 | (86%) | 47 | (94%) | 17 | (90%) | 13 | (81%) |
= significantly different between groups (p < 0.05).
2.2. Study 2: Dewormed compared to placebo treated, Ascaris co-infected individuals
2.2.1. Study design
A nested cohort study of dewormed and placebo treated Ascaris and HIV-1-co-infected individuals was conducted on 35 previously collected samples from a randomized controlled trial evaluating the short-term impact of deworming (400 mg albendazole daily for 3 days) on markers of HIV-1 disease progression in Kenya (Walson et al., 2008). Blood samples were collected between March 2006 and June 2007. Similarly, we determined a priori that this analysis had 80% power to detect a 34% difference in increased measles positivity between dewormed and placebo groups, and a mean difference in log10 tetanus response of 0.49 between dewormed and placebo groups at 3 months.
2.2.2. Ethics statement
All individuals provided written informed consent to participate in the study including use of previously collected samples to evaluate these study-specific outcomes. The trial was independently approved by the IRB at University of Washington and the Ethical Review Board of the Kenya Medical Research Institute. The trial was registered as NCT00130910 at http://clinicaltrials.gov.
2.2.3. Population
Study participants were enrolled from 10 sites across Kenya (including the three sites in Study 1), who were older than 18, not pregnant, did not meet criteria for ART initiation based on Kenyan Ministry of Health guidelines, had not used ART in the past, and were willing and able to give informed consent. Individuals infected with a soil-transmitted helminth treatable by albendazole were randomized to deworming treatment, consisting of 400 mg albendazole given once daily for 3 consecutive days, or placebo with the first dose observed in the clinic, and asked to return in 3 months. At follow-up all participants with one of these helminth infections were given the deworming treatment mentioned above. From the 208 co-infected individuals enrolled in the study, 54 were Ascaris only co-infected, and of those, 35 had sufficient stored sample for analysis after previous studies were conducted (Blish et al., 2010). Again, time of participant vaccination was not known, though likely occurred in childhood according to the EPI schedule (Fig. 1).
2.2.4. Data collection and statistical analysis
Demographic measures were collected at enrollment and plasma samples were collected at baseline and at 3 months of follow-up to monitor HIV-1 disease. CD4 counts were determined using Multiset software on a FACSCalibur machine (Becton Dickinson, USA). Plasma HIV-1 viral loads were quantified using the Gen-Probe HIV-1 viral load assay. One stool sample collected at baseline and at follow-up was assessed with 2 slide preparations using direct helminth microscopy, Kato-Katz and formol-ether concentration, by a trained laboratory technologist. Vaccine-specific antibody responses were measured in duplicate and averaged using commercially available ELISA kits (same as study 1). Positive, equivocal, or negative measles responses were dichotomized, including equivocal as negative. An increase, no change, or decrease in measles seropositivity over 3 months was compared by treatment group using a Chi-squared test. Tetanus responses were log transformed and the association between deworming treatment and tetanus response at 3 months, adjusted for baseline tetanus responses was evaluated using linear regression with robust standard errors (ANCOVA analysis). Associations between cofactors in Table 1 and deworming treatment were assessed using Student's t-tests for continuous variables and Chi-squared tests for dichotomous variables. Intensities of infection with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni was classified by WHO guidelines.(World Health Organization, 2011) Also, to evaluate whether measles seropositivity or log10 tetanus responses at follow-up were associated with CD4 count or viral load at baseline or follow-up, logistic and linear regression were used, respectively.
3. Results
3.1. Study 1: Comparison of measles and tetanus responses in helminth-infected compared to helminth-uninfected HIV-1-infected adults
The 50 HIV-1-infected individuals with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni helminth infection and 50 without helminth infection were comparable for gender, viral load, and CD4 count. The mean CD4 count (cells/mL3) and log10 viral load among individuals with helminths was 441 (SD 271) and 4.4 (SD 0.8) respectively, while among individuals without helminths was 430 (SD 205) and 4.2 (SD 1.0) respectively. Among the individuals with a viral load below 400 copies/mL, 3 had helminths and 6 did not (p = 0.3). However, those with helminth infections were slightly younger (30.2 vs. 34.7 years, p = 0.02) than uninfected individuals. Helminth risk factors were not significantly different between helminth uninfected and helminth infected individuals (Table 1).
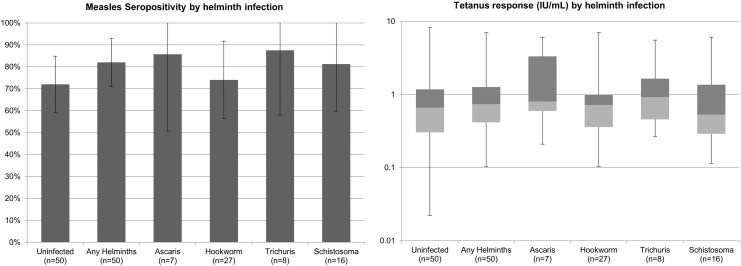
Among individuals without helminth infection, 72% (95% CI: 59–85%) were seropositive for measles, which was similar to the prevalence of measles seropositivity among those with helminth infection (82%, 95% CI: 71–93%, p = 0.24). Rates of measles seropositivity ranged by helminth species: 74% (95% CI: 56–92%) for hookworm, 81% (95% CI: 60–100%) for Schistosoma, 86% (95% CI: 51–100%) for Ascaris and 88% for Trichuris (95% CI: 58–100%). There were no significant differences in measles seropositivity by any helminth infection, nor by species-specific helminth infection (Fig. 2). Log transformed tetanus responses were examined by any helminth infection, as well as species-specific helminth infection. All individuals had a tetanus response above the protective threshold of 0.01 IU/mL (Amanna et al., 2007). Comparing individuals with any helminth infection to helminth uninfected individuals, there was no difference in mean log10 tetanus response (− 0.133 IU/mL vs. − 0.190 IU/mL, p = 0.56). There also was no difference between species-specific helminth infection and no helminth infection in log10 tetanus response when the helminth species were assessed individually (Fig. 2). The 8 patients with mixed helminth infections were included in the species-specific categories because when assessed separately their vaccine responses also did not differ from the helminth uninfected group. Though age was associated with helminth infection and tetanus response, adjusting for age did not change these results. Vaccine responses were not associated with viral load or CD4 count (p > 0.05). Restricting the analysis to those with viral loads of 400 copies/mL or greater did not vary the results.
Fig. 2.
Study 1 – Measles and tetanus antibody responses among helminth infected compared with uninfected individuals with HIV-1 infection. Mean prevalence of measles seropositivity with 95% confidence intervals are shown by helminth infection status. Helminth infected groups were compared to the reference group, helminth uninfected, using Chi-squared test. Tetanus responses are shown as median response with box representing 25% and 75% percentile bounds, and whiskers representing minimum and maximum values by helminth infection status. Log10 tetanus response was used to compare each helminth infected group to the reference group, helminth uninfected, using Student's t-test. p > 0.2 for all comparisons.
3.2. Study 2: Comparison of measles and tetanus responses in dewormed compared to placebo treated, Ascaris and HIV-1 co-infected adults
Among the 35 Ascaris and HIV-1 co-infected individuals, dewormed and placebo recipients were comparable at baseline in gender, age, educational level, viral load and CD4 count before treatment. The mean CD4 count (cells/mL3) and log10 viral load among dewormed recipients was 476 (SD 213) and 4.9 (SD 1.1) respectively, while among placebo recipients was 507 (SD 203) and 4.6 (SD 0.7) respectively. None of the individuals had an undetectable viral load (below 400 copies/mL). The dewormed group had more unemployed individuals, fewer farmers, and more children in the household. More individuals in the placebo group used environmental water sources and pit latrines outside the home. However, none of these differences were statistically significant (Table 1). Intensities of infection were also comparable between groups with 3 heavy infections, 22 moderate infections and 10 light infections.
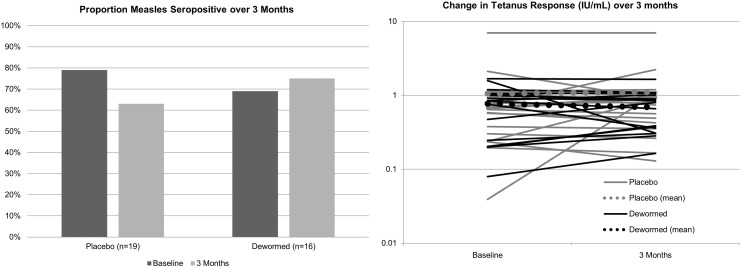
Among HIV-1 and Ascaris co-infected individuals, 3 placebo-treated individuals who were measles seropositive at enrollment reverted to seronegative at follow up, while 1 dewormed individual converted to seropositive during follow-up, though this difference between groups was not significant (p = 0.15). There were no differences in log10 tetanus responses at 3 months both with, and without, adjusting for baseline tetanus responses (adjusted p = 0.39) (Table 2 and Fig. 3). Stratifying by intensity of infection, there was still no association between treatment group and either measles response or tetanus response. In addition, vaccine responses at follow-up were not associated with CD4 count or viral load at baseline or follow-up.
Table 2.
Study 2 – Change in measles and tetanus antibody responses after 3 months among albendazole-dewormed compared to placebo treated, Ascaris and HIV-1 co-infected individuals.
| Dewormed compared to placebo treated after 3 months | |||
|---|---|---|---|
| Measles Responsea | Positive to Negative | No Change | Negative to Positive |
| Placebo | 3 | 16 | 0 |
| Dewormed | 0 | 15 | 1 |
| p-value | 0.15 | ||
| Log10 Tetanus Responseb | Mean Difference | 95% CI | p-value |
| Ascaris-infected | − 0.08 | (− 0.26, 0.11) | 0.39 |
Increase, decrease, or no change in prevalence of measles seropositivity after 3 months among dewormed or placebo treated individuals was compared by chi-squared test (p = 0.15). Log10 tetanus response at 3 months was compared between dewormed and placebo treated individuals, using linear regression with robust standard errors, adjusted for baseline log10 tetanus response (p = 0.39).
Chi2 test.
Linear regression with robust standard errors comparing dewormed to placebo at 3 months, adjusted for baseline.
Fig. 3.
Study 2 – Prevalence of measles seropositivity and mean tetanus antibody levels at baseline and 3 months among dewormed compared to placebo treated, Ascaris and HIV-1 co-infected individuals.
4. Discussion
In this study, infection with Ascaris, Trichuris, hookworm species and/or Schistosoma mansoni was not associated with lower measles and tetanus IgG responses among HIV-1 infected ART naïve adults in Kenya. Most individuals had seropositive measles responses and all had protective levels of tetanus antibody suggesting preservation of vaccine responses despite both helminth and untreated HIV-1 infection. We did not detect a difference in vaccine-specific antibody responses by helminth infection or by species-specific helminth infection. Among Ascaris-infected HIV-1 infected individuals, we observed that measles responses did not increase following deworming compared to placebo. In addition, tetanus antibody levels were universally above the threshold of protection and levels of tetanus antibodies did not differ between dewormed vs. placebo recipients. In both analyses, adjusting for the predictors examined does not change the results. To our knowledge, other studies have not evaluated the association between helminths and vaccine responses specifically among HIV-1 infected adults.
Kenya has had outbreaks of measles infection as recently as 2011, (Mahamud et al., 2013). This underscores the importance of retaining measles immunity during adulthood in settings such as Kenya with episodic measles outbreaks. In this study, most (> 70%) of the HIV-1 and helminth co-infected individuals appear to have adequate immune protection against measles as confirmed by measles seropositivity. The seroprevalence we observed was lower than that seen in a recent study among a similar population of HIV-1 infected adults in Kenya (96%), as well as a study among HIV-1 infected adults in the UK (93%), and comparable to a study in Mexico (76%) (Belaunzaran-Zamudio et al., 2009, Molton et al., 2010, Merkel et al., 2014).
While this study did not reveal an association between vaccine-specific antibody responses and helminth infection with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni, there are several limitations to the analysis that should be noted. First, the participants in these studies represent a more “immune competent” population of HIV infected adults than might be seen in the general population, since these individuals were ART naïve and did not qualify for ART based on the guidelines at the time. This is evident by the mean CD4 count of the included participants. The proportion of individuals who initiated ART was higher in the UK and Mexico studies noted above (81% and 95%, respectively), while all participants in our study and the other Kenyan study were ART naïve. The fact that the participants included in this study may have been more immunocompetent, despite not being on ART may have limited our ability to detect a suppressive effect due to helminth infection.
In addition, it is important to note that these were unstimulated responses. Memory B cells do not actively secrete antibodies until triggered, while plasma cells continually secrete antibody without further stimulation (Amanna and Slifka, 2010). Additionally, antibodies specific to many childhood vaccines are detectable in plasma from adults who received vaccinations in early childhood (Amanna et al., 2007, Amanna and Slifka, 2010). Among healthy individuals, the half-life of tetanus specific serum antibody production is approximately 11 years, while for measles it may be maintained for life (Amanna et al., 2007). Plasma cell secretion in these individuals may persist despite large systemic immune perturbations due to HIV-1 and helminth infection. However, this study cannot speak to the impact of these helminths on stimulated immune cells such as memory B cells. Additionally, the study cannot speak to the impact of these helminths on Th1 cytokine responses because we were unable to look at antigen-specific T cell responses.
Our results also do not exclude a possible association between these helminths and responses to vaccination. Even without circulating antibodies, vaccine-specific memory B cells can provide protective immunity following infection (Amanna and Slifka, 2011). Studies of post-vaccination responses to BCG and an oral cholera vaccine among helminth infected individuals observed improved vaccine responses following albendazole versus placebo prior to vaccination (Elias et al., 2001, Cooper et al., 2000). Additionally, post-vaccination antibody responses to tetanus were lower in Onchocerca-infected compared to helminth uninfected individuals (Cooper et al., 1998). Finally, the effects of HIV-1 infection may outweigh effects of helminth infection on vaccine responses. In a study among 90 HIV-1 infected children, spontaneous seroconversion of measles and tetanus vaccine responses were detected 6 months after ART initiation without revaccination (Farquhar et al., 2009). We did not see a similar effect following deworming with albendazole in our study, though the time intervals differ between studies.
While we did adjust for possible confounders, we may have been unable to detect some potential confounders due to differences in data collection instruments used in both studies that may have resulted in the inability to include all socio-economic data in the analyses. Additionally, these studies had limited power to detect potentially meaningful differences in vaccine responses. We were also unable to evaluate whether these helminths altered recall of vaccine responses since these were previously collected samples from studies that were not designed to study responses to vaccination. This study also only evaluated the impact of a specific group of helminths and other species of helminths might have been present but not identified. Finally, this study relied on direct microscopy methods, which are less sensitive at detecting light intensity infections.
In this analysis, we observed that most HIV-1-infected adults in our cohorts had protective responses to measles and tetanus vaccines, and helminth infection with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni was not associated with differences in pre-existing vaccine-specific antibody responses. Whether helminth infection with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni at the time of vaccination alters responses to vaccination among HIV-1 infected adults is still unclear. Another important question is whether helminth infection with Ascaris, Trichuris, hookworm and/or Schistosoma mansoni alters vaccine responses in HIV-1-infected children, who are more likely to harbor helminth infections compared to adults, often have greater intensities of helminth infection, may receive vaccinations while helminth infected, and have diminished vaccine responses due to HIV-1 infection. Further studies in children are recommended to determine the influence of these helminths and deworming on vaccine efficacy, particularly among HIV-1-infected children who could be easily dewormed through HIV-1 care and treatment services (Gerns et al., 2012).
Acknowledgements
The authors would like to acknowledge the individuals that participated in these two studies.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.parepi.2016.12.003.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
Study 1 data.
Study 2 data.
References
- Amanna I.J., Slifka M.K. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. (IMR912 [pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna I.J., Slifka M.K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011;411:206–215. doi: 10.1016/j.virol.2010.12.016. (S0042-6822(10)00770-1 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna I.J., Carlson N.E., Slifka M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. (357/19/1903 [pii]) [DOI] [PubMed] [Google Scholar]
- Belaunzaran-Zamudio P.F., Garcia-Leon M.L., Wong-Chew R.M., Villasis-Keever A., Cuellar-Rodriguez J., Mosqueda-Gomez J.L., Munoz-Trejo T., Escobedo K., Santos J.I., Ruiz-Palacios G.M., Sierra-Madero J.G. Early loss of measles antibodies after MMR vaccine among HIV-infected adults receiving HAART. Vaccine. 2009;27:7059–7064. doi: 10.1016/j.vaccine.2009.09.063. (S0264-410X(09)01397-8 [pii]) [DOI] [PubMed] [Google Scholar]
- Bethony J., Brooker S., Albonico M., Geiger S.M., Loukas A., Diemert D., Hotez P.J. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. (S0140-6736(06)68653-4 [pii]) [DOI] [PubMed] [Google Scholar]
- Blish C.A., Sangare L., Herrin B.R., Richardson B.A., John-Stewart G., Walson J.L. Changes in plasma cytokines after treatment of ascaris lumbricoides infection in individuals with HIV-1 infection. J. Infect. Dis. 2010;201:1816–1821. doi: 10.1086/652784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.J., Espinel I., Paredes W., Guderian R.H., Nutman T.B. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J. Infect. Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- Cooper P.J., Espinel I., Wieseman M., Paredes W., Espinel M., Guderian R.H., Nutman T.B. Human onchocerciasis and tetanus vaccination: impact on the postvaccination antitetanus antibody response. Infect. Immun. 1999;67:5951–5957. doi: 10.1128/iai.67.11.5951-5957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.J., Chico M.E., Losonsky G., Sandoval C., Espinel I., Sridhara R., Aguilar M., Guevara A., Guderian R.H., Levine M.M., Griffin G.E., Nutman T.B. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 2000;182:1199–1206. doi: 10.1086/315837. (JID000531 [pii]) [DOI] [PubMed] [Google Scholar]
- Cooper P.J., Chico M., Sandoval C., Espinel I., Guevara A., Levine M.M., Griffin G.E., Nutman T.B. Human infection with ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva N.R., Brooker S., Hotez P.J., Montresor A., Engels D., Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. (S1471492203002757 [pii]) [DOI] [PubMed] [Google Scholar]
- Elias D., Wolday D., Akuffo H., Petros B., Bronner U., Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. (cei1446 [pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Britton S., Aseffa A., Engers H., Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. (S0264-410X(08)00540-9 [pii]) [DOI] [PubMed] [Google Scholar]
- Farquhar C., Wamalwa D., Selig S., John-Stewart G., Mabuka J., Majiwa M., Sutton W., Haigwood N., Wariua G., Lohman-Payne B. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre- and post-highly active antiretroviral therapy and revaccination. Pediatr. Infect. Dis. J. 2009;28:295–299. doi: 10.1097/INF.0b013e3181903ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geretti A.M., Doyle T. Immunization for HIV-positive individuals. Curr. Opin. Infect. Dis. 2010;23:32–38. doi: 10.1097/QCO.0b013e328334fec4. [DOI] [PubMed] [Google Scholar]
- Geretti A.M., Brook G., Cameron C., Chadwick D., Heyderman R.S., MacMahon E., Pozniak A., Ramsay M., Schuhwerk M. British HIV Association guidelines for immunization of HIV-infected adults 2008. HIV Med. 2008;9:795–848. doi: 10.1111/j.1468-1293.2008.00637.x. (HIV637 [pii]) [DOI] [PubMed] [Google Scholar]
- Gerns H.L., Sangare L.R., Walson J.L. Integration of deworming into HIV care and treatment: a neglected opportunity. PLoS Negl. Trop. Dis. 2012;6:e1738. doi: 10.1371/journal.pntd.0001738. (PNTD-D-11-00534 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y.L., Enohata T., Lopes M.H., De Sousa Dos S.S. Vaccination in Brazilian HIV-infected adults: a cross-sectional study. AIDS Patient Care STDs. 2008;22:65–70. doi: 10.1089/apc.2007.0059. [DOI] [PubMed] [Google Scholar]
- Mahamud A., Burton A., Hassan M., Ahmed J.A., Wagacha J.B., Spiegel P., Haskew C., Eidex R.B., Shetty S., Cookson S., Navarro-Colorado C., Goodson J.L. Risk factors for measles mortality among hospitalized Somali refugees displaced by famine, Kenya, 2011. Clin. Infect. Dis. 2013;57:e160–e166. doi: 10.1093/cid/cit442. 10.1093/cid/cit442 (cit442 [pii]) [DOI] [PubMed] [Google Scholar]
- Merkel M., Ben-Youssef L., Newman L.P., Gitome V., Gataguta A., Lohman-Payne B., Bosire R., Farquhar C. Seroprevalence of measles IgG among HIV-1-infected and uninfected Kenyan adults. Int. J. Infect. Dis. 2014;19:103–105. doi: 10.1016/j.ijid.2013.10.018. (S1201-9712(13)00348-2 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molton J., Smith C., Chaytor S., Maple P., Brown K., Johnson M., Geretti A.M. Seroprevalence of common vaccine-preventable viral infections in HIV-positive adults. J. Infect. 2010;61:73–80. doi: 10.1016/j.jinf.2010.04.004. (S0163-4453(10)00124-6 [pii]) [DOI] [PubMed] [Google Scholar]
- Okoye A.A., Picker L.J. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol. Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin E.A., Araujo M.I., Carvalho E.M., Pearce E.J. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- UNAIDS, WHO . 2009. AIDS Epidemic Update: November 2009. [Google Scholar]
- Walson J.L., Otieno P.A., Mbuchi M., Richardson B.A., Lohman-Payne B., Macharia S.W., Overbaugh J., Berkley J., Sanders E.J., Chung M.H., John-Stewart G.C. Albendazole treatment of HIV-1 and helminth co-infection: A randomized, double-blind, placebo-controlled trial. AIDS. 2008;22:1601–1609. doi: 10.1097/QAD.0b013e32830a502e. (00002030-200808200-00009 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walson J., Singa B., Sangare L., Naulikha J., Piper B., Richardson B., Otieno P.A., Mbogo L.W., Berkley J.A., John-Stewart G. Empiric deworming to delay HIV disease progression in adults with HIV who are ineligible for initiation of antiretroviral treatment (the HEAT study): a multi-site, randomised trial. Lancet Infect. Dis. 2012;12:925–932. doi: 10.1016/S1473-3099(12)70207-4. (S1473-3099(12)70207-4 [pii]) [DOI] [PubMed] [Google Scholar]
- World Health Organization . second ed. 2011. Helminth Control in School Age Children: A Guide for Managers of Control Programmes. [Google Scholar]
- World Health Organization . 2016. Immunization Coverage: Fact Sheet. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study 1 data.
Study 2 data.