Abstract
OBJECTIVE
BI-RADS for mammography and ultrasound subdivides category 4 assessments by likelihood of malignancy into categories 4A (> 2% to ≤ 10%), 4B (> 10% to ≤ 50%), and 4C (> 50% to < 95%). Category 4 is not subdivided for breast MRI because of a paucity of data. The purpose of the present study is to determine the utility of categories 4A, 4B, and 4C for MRI by calculating their positive predictive values (PPVs) and comparing them with BI-RADS–specified rates of malignancy for mammography and ultrasound.
MATERIALS AND METHODS
All screening breast MRI examinations performed from July 1, 2010, through June 30, 2013, were included in this study. We identified in medical records prospectively assigned MRI BI-RADS categories, including category 4 subdivisions, which are used routinely in our practice. Benign versus malignant outcomes were determined by pathologic analysis, findings from 12 months or more clinical or imaging follow-up, or a combination of these methods. Distribution of BI-RADS categories and positive predictive value level 2 (PPV2; based on recommendation for tissue diagnosis) for categories 4 (including its subdivisions) and 5 were calculated.
RESULTS
Of 860 screening breast MRI examinations performed for 566 women (mean age, 47 years), 82 with a BI-RADS category 4 assessment were identified. A total of 18 malignancies were found among 84 category 4 and 5 assessments, for an overall PPV2 of 21.4% (18/84). For category 4 subdivisions, PPV2s were as follows: for category 4A, 2.5% (1/40); for category 4B, 27.6% (8/29); for category 4C, 83.3% (5/6); and for category 4 (not otherwise specified), 28.6% (2/7).
CONCLUSION
Category 4 subdivisions for MRI yielded malignancy rates within BI-RADS–specified ranges, supporting their use for benefits to patient care and more meaningful practice audits.
Keywords: BI-RADS category 4, breast MRI
The American College of Radiology (ACR) BI-RADS is a comprehensive quality assurance tool designed to standardize reporting, reduce confusion regarding breast imaging interpretation and management recommendations, and facilitate outcomes monitoring for mammography, ultrasound, and MRI [1]. The appropriate use of BI-RADS assessment categories and management recommendations for all imaging modalities, including breast MRI, enables a medical practice to perform audits to monitor and improve the quality of patient care [2].
With the use of BI-RADS assessment categories and concordant management recommendations, breast imaging findings with likelihoods of malignancy greater than 2% are deemed suspicious and are recommended for tissue diagnosis. A small percentage of these cases will be assessed as the most suspicious cases (i.e., belonging to BI-RADS assessment category 5, highly suggestive of malignancy), with a likelihood of malignancy of 95% or greater. Most suspicious findings are assigned to BI-RADS assessment category 4, which corresponds to a likelihood of malignancy of between 2% and 95%. Thus, the likelihood of malignancy associated with category 4 is wide, limiting clinical utility because it does not convey stratified levels of the likelihood for cancer.
To better inform patients and providers, facilitate clinical management and radiologic-pathologic concordance, and provide a more meaningful practice audit, it is desirable to stratify BI-RADS assessment category 4 into three subdivisions, each of which has smaller ranges and particular likelihoods of malignancy. The BI-RADS fifth edition recommends that category 4 assessments for mammography and ultrasound be subdivided into the following categories on the basis of likelihood of malignancy: 4A (> 2% to ≤ 10%), 4B (> 10% to ≤ 50%), and 4C (> 50% to < 95%) [1]. Note, however, that the U.S. Food and Drug Administration Mammography Quality Standards Act and Program requires that each mammography report include text corresponding to the overall assessment category (e.g., “suspicious”) [3]. However, assessment category 4 is not currently subdivided for breast MRI because of the paucity of data to guide practice for this modality. Importantly, patients undergoing screening breast MRI typically have a significantly increased risk of malignancy compared with the typical mammography screening population. Screening breast MRI typically is performed for patients with the following characteristics: a genetic mutation that predisposes them to breast cancer, a lifetime risk for breast cancer of 20% or greater on the basis of family history, a history of chest radiation, a personal history of treated breast cancer, or a diagnosis of atypia or lobular carcinoma in situ [4–6].
The purpose of this study is to determine the utility of BI-RADS assessment category 4 subdivisions for breast MRI. We hypothesize that BI-RADS assessment category 4 subdivisions, when used for MRI, will reflect the likelihood of malignancy defined for mammography and ultrasound, despite the high-risk profile of the typical patient population undergoing screening MRI. Specifically, we will calculate the positive predictive values (PPVs) of breast MRI assessment category 4 subdivisions and determine whether they correspond to target ranges for mammography and ultrasound. The ability to use category 4 subdivisions for MRI would have important clinical practice implications, enabling radiologists to provide additional information to inform patients and providers, begin early coordination of care for more suspicious findings, perform radiologic-pathologic correlation, and provide a more meaningful practice audit.
Materials and Methods
All consecutive screening breast MRI examinations performed at the University of Wisconsin Hospitals and Clinics between July 1, 2010, and June 30, 2013, were included in this HIPAA-compliant study. Our institutional review board approved this study and waived the need for informed consent.
Breast MRI Indications
For all breast MRI examinations performed during the study period, we performed a retrospective review of the electronic medical records with the use of electronic medical record software. We identified breast MRI screening examinations, which were defined as MRI examinations of patients without symptoms with a personal or family history of a known genetic mutation, a family history of breast cancer, a personal history of treated breast cancer, a history of chest radiation, or a prior biopsy result indicating the presence of atypia or lobular carcinoma in situ [6]. Of the 1437 breast MRI examinations performed during the study, 532 were categorized as diagnostic and were excluded. Also excluded were 21 unenhanced examinations performed to evaluate the integrity of a silicone implant, 22 non-diagnostic examinations (for reasons including the inability of patients to complete the examination, contrast extravasation or injection failure, and extreme patient motion), and two examinations without a final BI-RADS assessment [6]. Of the remaining 860 screening breast MRI examinations, 82 were assessed as BI-RADS category 4 and comprised the examinations evaluated in this study.
Breast MRI Technique
All study examinations were performed using a 1.5-T MRI scanner (Signa HDxt, GE Healthcare) with a dedicated 7-(Invivo) or 8-(Sentinelle) channel breast coil [6]. A weight-based dose of the gadolinium-based contrast agent gadobenate dimeglumine (0.1 mmol/kg of body weight; MultiHance, Bracco Diagnostics) was administered by power injection at a rate of 2 mL/s, followed by a 20-mL saline flush. Unenhanced sequences included a three-plane localizer, sagittal T2-weighted 2D fast spin-echo with fat saturation, and DWI (b value = 0 and 1000 s/mm2) of each breast. Next, bilateral simultaneous sagittal T1-weighted 3D spoiled turbo gradient-echo imaging (Volume Imaging for Breast Assessment [VIBRANT], GE Healthcare) was performed with and without chemical fat saturation before and after contrast administration. From July 2010 through August 2011, a primarily sagittal imaging protocol was used with an FOV of 16–20 × 16–20 cm, eight contrast-enhanced sequences, and temporal resolution of approximately 70 seconds (frequency by phase-encoding matrix, 256 × 160; slice thickness, 3 mm). Delayed high-resolution axial and sagittal T1-weighted fast spoiled gradient-echo sequences with fat saturation were also obtained with the same sequence specifications but with a frequency by phase-encoding matrix of 288 × 224 and a slice thickness of 2 mm for sagittal images and with a 320 × 256 matrix and a slice thickness of 2 mm for axial images. From August 2011 through August 2013, an axial imaging protocol was performed with three contrast-enhanced sequences, an FOV of 32–36 × 32–36 cm, and a temporal resolution of approximately 180 seconds (matrix, 384 × 384; slice thickness, 1.6 mm). Computer-aided detection software (DynaCAD, Invivo; and Aegis, Hologic) was used for temporal kinetic evaluation and creation of reformatted images, including subtraction and maximum-intensity-projection images.
Breast MRI Interpretation and Data Collection
All breast MRI examinations were prospectively interpreted and reported as part of routine clinical practice according to BI-RADS, in conjunction with the patient’s clinical history and other available breast imaging studies, including prior breast MRI, mammography, and ultrasound examinations [6]. The examinations in our study were interpreted using the BI-RADS fourth edition [7], because the BI-RADS fifth edition was published in 2013 and was introduced into clinical practice in early 2014 [8]. All examinations were prospectively assigned a BI-RADS assessment category by one of nine radiologists who specialized in breast imaging and had 1–20 years of breast MRI experience [6]. All breast imaging radiologists were fellowship trained, with one exception, and that radiologist had more than 20 years of experience interpreting breast MRI examinations. Our practice uses BI-RADS assessment category 4 subdivisions in routine clinical practice. No formal criteria existed regarding which lesion types should be assigned to BI-RADS category 4A, 4B, or 4C. This categorization was done at the discretion of the interpreting radiologist on the basis of individual experience and the known PPV of particular MRI descriptors, as determined from the literature [9]. Tissue diagnosis (typically image-guided biopsy) was recommended for all lesions assessed as BI-RADS categories 4 or 5.
The BI-RADS assessment category indicated on the prospectively interpreted breast MRI report was recorded. Patient age and lesion features (including type and size), as identified in electronic medical records, were retrospectively recorded. Categories for lesion type include a focus, mass, or nonmass enhancement. A focus differs from a mass in that it is too small to be otherwise characterized, and in general it is smaller than 5 mm in size, although BI-RADS does not require that strict size criteria be met [8]. If the MRI examination revealed multiple lesions and thus resulted in the assignment of more than one BI-RADS category, the lesion with the highest-order BI-RADS assessment category was used for analysis. The hierarchy of categories, from highest to lowest order, was as follows: 5 > 4C > 4B > 4A > 0 > 3 > 2 > 1 [6]. Dates of follow-up examinations, biopsy dates and biopsy guidance modality, and biopsy results were also recorded. For biopsy results indicating malignancy, information on histologic subtype, axillary nodal status, and invasive cancer size, as measured on imaging, was collected. Final benign versus malignant outcome was ascertained by pathologic analysis if percutaneous biopsy or surgery was performed. If tissue sampling was not performed, outcomes were determined by the presence or absence of cancer within 365 days of the breast MRI, by means of follow-up imaging and review of the clinical records [6].
Calculations and Statistical Analysis
The proportion of screening breast MRI examinations assigned to each BI-RADS assessment category, including category 4 subdivisions, was calculated. The positive predictive value level 2 (PPV2; based on recommendation for tissue diagnosis) was calculated as the number of cases with a diagnosis of cancer (within 1 year) divided by the number of screening examinations recommended for tissue diagnosis (BI-RADS categories 4, 4A, 4B, 4C, and 5).
The 95% CIs were calculated for the PPV2 values for each BI-RADS assessment category, including category 4 subdivisions. Statistical analysis was performed between each BI-RADS assessment category 4 subdivision with respect to PPV2, with the use of Holm-adjusted p values from the Fisher exact test. A statistically significant difference was denoted by p < 0.05.
Results
Eighty-two screening breast MRI examinations with a BI-RADS category 4 assessment were included in the study. They were obtained from a total of 860 screening breast MRI examinations performed for 566 women with a mean age of 47 years (range, 18–83 years). The clinical indications for screening breast MRI examinations, according to BI-RADS category 4 subdivision, are summarized in Table 1. A family history of breast cancer comprised the largest proportion of indications for both BI-RADS category 4A (53%; 21 of 40 examinations) and BI-RADS category 4B (55%; 16 of 29 examinations). For BI-RADS categories 4C and 4 (not otherwise specified), the most common indication for examination was a personal history of treated breast cancer, comprising three of six BI-RADS category 4C examinations (50%) and four of seven BI-RADS category 4 (not otherwise specified) examinations (57%).
TABLE 1.
Indications for Breast MRI Examination, by BI-RADS Category 4 Subdivision
| Indications for Breast MRI Examination | BI-RADS Category
|
||||
|---|---|---|---|---|---|
| 4A | 4B | 4C | 4 NOS | Total | |
|
| |||||
| Genetic mutation carrier | 8 (20) | 2 (7) | 1 (17) | 1 (14) | 12 (15) |
| Family history of breast cancer | 21 (53) | 16 (55) | 2 (33) | 2 (29) | 41 (50) |
| Personal history of treated breast cancer | 10 (25) | 10 (34) | 3 (50) | 4 (57) | 27 (33) |
| History of chest radiation | 0 | 1 (3) | 0 | 0 | 1 (1) |
| Prior biopsy showing atypia or LCIS | 1 (3) | 0 | 0 | 0 | 1 (1) |
|
| |||||
| Total | 40 (100) | 29 (100) | 6 (100) | 7 (100) | 82 (100) |
Note—Data are number (%) of examinations. NOS = not otherwise specified, LCIS = lobular carcinoma in situ.
Of the 82 BI-RADS category 4 examinations, 40 (48.8%) were BI-RADS category 4A (low suspicion for malignancy), 29 (35.4%) were BI-RADS category 4B (moderate suspicion for malignancy), and six (7.3%) were BI-RADS category 4C (high suspicion for malignancy). Although BI-RADS category 4 is routinely subdivided in our practice, seven examinations (8.5%) were assessed as BI-RADS category 4 (not otherwise specified; suspicious). These results are summarized in Table 2.
TABLE 2.
Likelihood of Malignancy for MRI BI-RADS Category 4 Lesions
| BI-RADS Category | No. of Examinations | No. of Malignancies | PPV2 (%) | 95% CI | Likelihood Range for Mammography and Ultrasound (%)a |
|---|---|---|---|---|---|
|
| |||||
| 4A | 40 | 1 | 2.5b | 0.0–14.7 | > 2 to ≤ 10 |
| 4B | 29 | 8 | 27.6b | 13.4–47.5 | > 10 to ≤ 50 |
| 4C | 6 | 5 | 83.3b | 36.5–99.1 | > 50 to < 95 |
| 4 (NOS) | 7 | 2 | 28.6 | 5.1–69.7 | |
| 5 | 2 | 2 | 100.0 | 19.8–100 | ≥ 95 |
|
| |||||
| Total | 84 | 18 | 21.4 | 13.5–32.0 | |
Note—PPV2 = positive predictive value level 2 (based on recommendation for tissue diagnosis), NOS = not otherwise specified.
Benchmark ranges for likelihood of malignancy for mammography and ultrasound in accordance with American College of Radiology BI-RADS fifth edition [1].
PPV2 values for all three categories are statistically significantly different (p < 0.05) from one another.
One of the 40 BI-RADS category 4A examinations resulted in a diagnosis of cancer, for a PPV2 of 2.5%. Eight of the 29 BI-RADS category 4B examinations resulted in a diagnosis of cancer, for a PPV2 of 27.6%. Five of the six BI-RADS category 4C examinations diagnosed cancer, for a PPV2 of 83.3%. There was a statistically significant difference in PPV values between category 4 subdivisions (for category 4A vs 4B, p = 0.006; for category 4A vs 4C, p < 0.001; for category 4B vs 4C, p = 0.019). Of the seven BI-RADS category 4 examinations for which categories were not further subdivided, two resulted in a diagnosis of cancer, for a PPV2 of 28.6%, which is very similar to the PPV2 of 27.6% noted for BI-RADS category 4B. These results are summarized in Table 2. For context and completeness, we note that the two BI-RADS category 5 screening breast MRI examinations interpreted during the study both indicated cancer, resulting in a PPV2 of 100.0% [6].
The proportions of each type of finding (focus, mass, and nonmass enhancement) in each BI-RADS category 4 subdivision are presented in Table 3. A total of 18 of the 84 examinations assigned BI-RADS assessment category 4 (suspicious) or 5 (highly suggestive of malignancy) showed malignancy. One of these was a previously unsuspected recurrence of a malignant internal mammary lymph node in a woman with a personal history of treated stage IIB invasive ductal carcinoma (estrogen receptor negative, progesterone receptor negative, and ErbB-2 [also known as HER2/neu] positive). Of the remaining 17 cancers found within the breast, 13 were invasive and four were ductal carcinoma in situ alone. The median cancer size was 10 mm, and eight cancers were node negative (61.5%; 8/13). Examples of malignancies assessed using the BI-RADS assessment category 4 subdivisions are shown in Figure 1 (category 4A), Figures 2 and 3 (category 4B), and Figures 4 and 5 (category 4C).
TABLE 3.
Breast MRI Lesion Descriptors, by BI-RADS Category 4 Subdivision
| Descriptor | 4A (n = 40) | 4B (n = 29) | 4C (n = 6) | 4 (NOS) (n = 7) |
|---|---|---|---|---|
|
| ||||
| Focus | 1 (3) | 0 (0) | 0 (0) | 1 (14) |
| Mass | 16 (40) | 15 (52) | 4 (67) | 2 (29) |
| Nonmass enhancement | 23 (58) | 14 (48) | 2 (33) | 4 (57) |
| Mean size (mm), range | 11 (4–37) | 15 (5–50) | 19 (1–28) | 10 (4–22) |
Note—Except where otherwise indicated, data are number (%) of lesions. NOS = not otherwise specified.
Fig. 1.
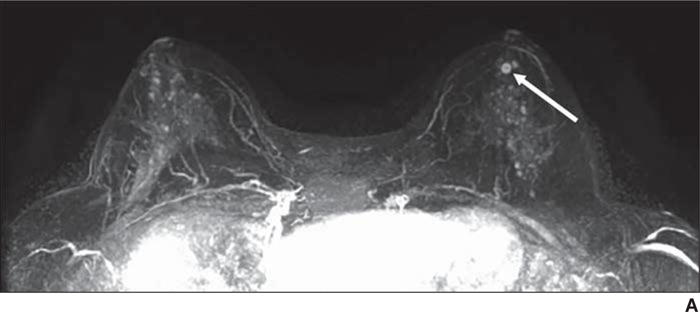
54-year-old woman with family history of breast cancer who presented for high-risk screening breast MRI examination.
A, T1-weighted axial subtraction (early phase contrast-enhanced minus unenhanced) maximum-intensity-projection MR image (A) shows moderate background parenchymal enhancement and mass (arrow) unique from background.
B, Axial early phase contrast-enhanced T1-weighted MR image of left breast shows round mass (arrow) with circumscribed margins and rim enhancement, which was assessed as BI-RADS category 4A.
C, Delayed phase MR image shows plateau enhancement (yellow shading). Biopsy result was intermediate-grade ductal carcinoma in situ.
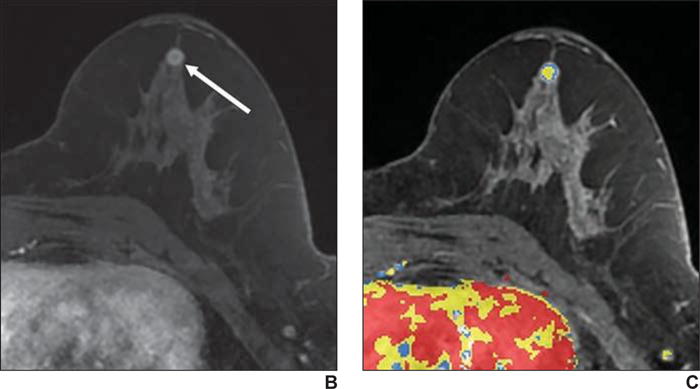
Fig. 2.
59-year-old woman with personal history of treated cancer of right breast who presented for high-risk screening breast MRI examination.
A, T1-weighted subtraction (early phase contrast-enhanced minus unenhanced) maximum-intensity-projection MR image shows minimal background parenchymal enhancement and linear nonmass enhancement (arrows) in right breast.
B, Axial early phase contrast-enhanced T1-weighted MR image of right breast shows clumped nonmass enhancement (arrows) in linear distribution extending posteriorly from prior lumpectomy site, which was assessed as BI-RADS category 4B.
C, Delayed phase MR image shows persistent enhancement (blue shading and arrows). Biopsy result was grade 3 ductal carcinoma in situ.
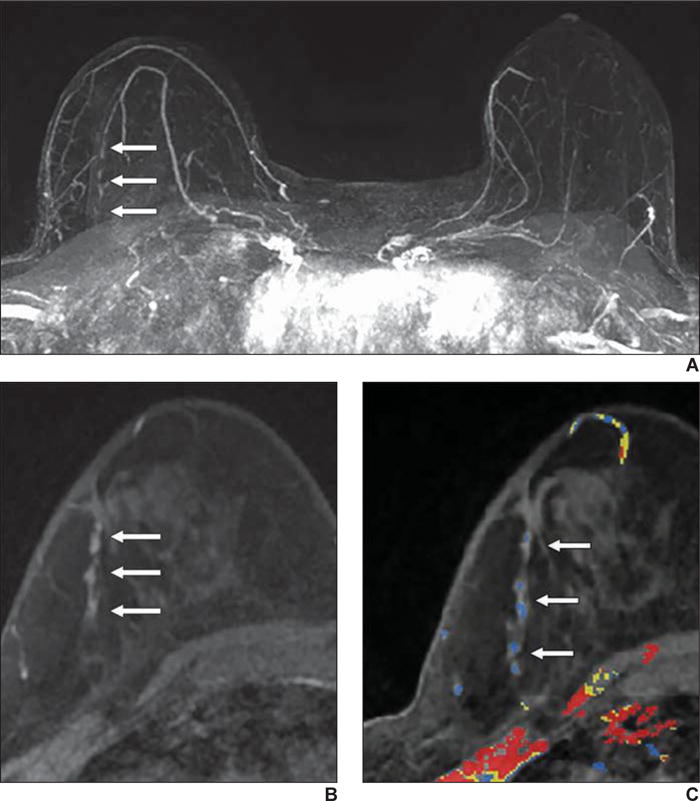
Fig. 3.
62-year-old woman with family history of breast cancer who presented for high-risk screening breast MRI examination.
A, Axial early phase contrast-enhanced T1-weighted MR image of both breasts shows 5-mm irregular mass (arrow) with irregular margins and homogeneous internal enhancement, which was assessed as BI-RADS category 4B.
B, Sagittal early phase contrast-enhanced T1-weighted MR image of left breast shows same 5-mm irregular mass (arrow) shown in A, with irregular margins and homogeneous internal enhancement, which was assessed as BI-RADS category 4B.
C, Delayed phase MR image of left breast shows washout of enhancement (red shading). Biopsy result was grade 2 invasive ductal carcinoma (estrogen receptor positive, progesterone receptor negative, and ErbB-2 [also known as HER2/neu] negative).
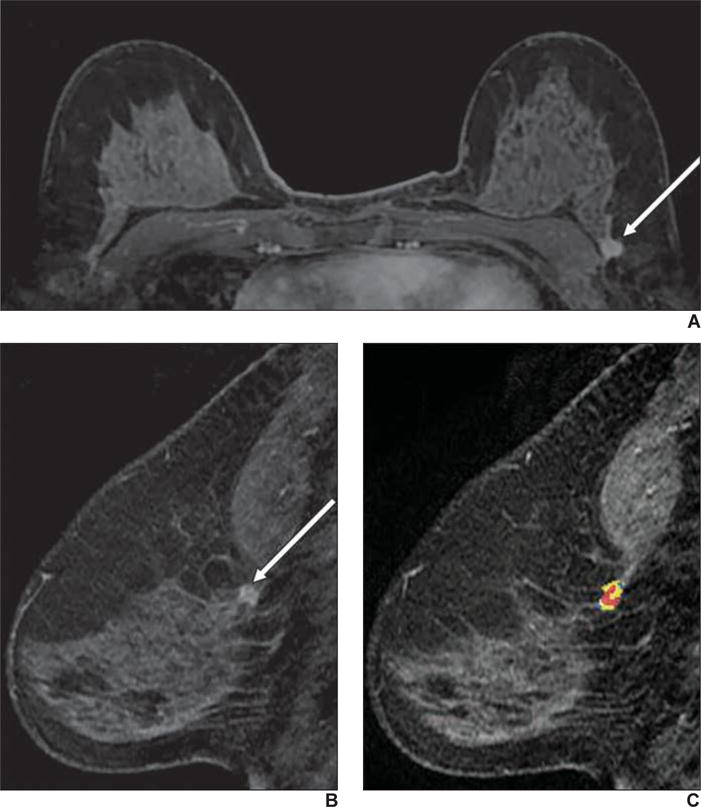
Fig. 4.
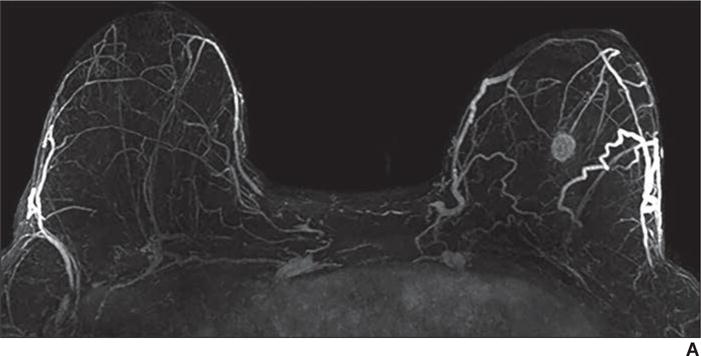
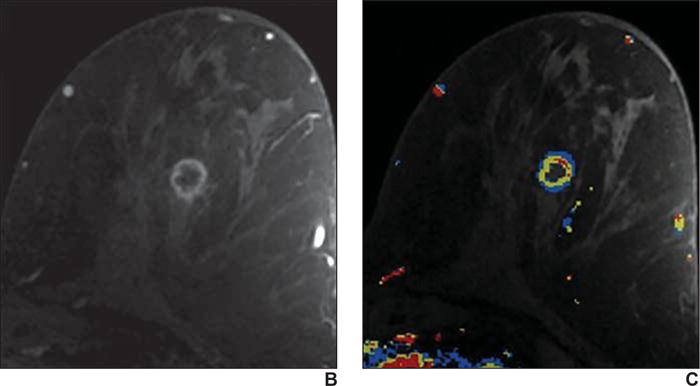
59-year-old woman with personal history of treated cancer of left breast who presented for high-risk screening breast MRI examination.
A, T1-weighted subtraction (early phase contrast-enhanced minus unenhanced) maximum-intensity-projection image shows minimal background parenchymal enhancement and mass in left breast.
B, Axial early phase contrast-enhanced T1-weighted MR image of left breast shows round mass with irregular margin and rim enhancement, which was assessed as BI-RADS category 4C.
C, Delayed phase MR image shows some areas of washout of enhancement (red shading). Biopsy result was grade 3 invasive ductal carcinoma (estrogen receptor negative, progesterone receptor negative, and ErbB-2 [also known as HER2/neu] positive).
Fig. 5.
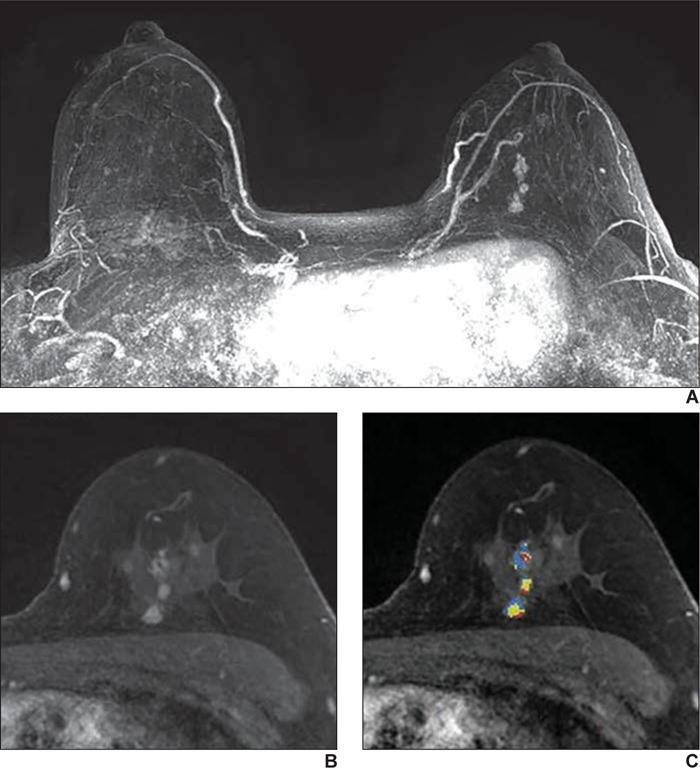
50-year-old woman with known BRCA1 gene mutation who presented for high-risk screening breast MRI examination.
A, T1-weighted subtraction (early phase contrast-enhanced minus unenhanced) maximum-intensity-projection MR image shows minimal background parenchymal enhancement and nonmass enhancement in left breast.
B, Axial early phase contrast-enhanced T1-weighted MR image of left breast shows clumped nonmass enhancement in linear distribution, which was assessed as BI-RADS category 4C.
C, Delayed phase MR image shows some areas of washout of enhancement (red shading). Biopsy result was intermediate grade ductal carcinoma in situ.
Discussion
The purpose of this study was to determine the utility of BI-RADS assessment category 4 subdivisions for breast MRI. We hypothesized that, when used for MRI, BI-RADS assessment category 4 subdivisions would reflect the likelihood of malignancy defined for mammography and ultrasound, despite the high-risk profile of the typical patient population undergoing screening MRI. We calculated the PPV2 of the breast MRI assessment category 4A, 4B, and 4C subdivisions, which are routinely used in our practice. We found that the use of BI-RADS category 4 subdivisions for MRI yielded malignancy rates within the BI-RADS–specified ranges for mammography and ultrasound, including category 4A (> 2% to ≤ 10%), category 4B (> 10% to ≤ 50%), and category 4C (> 50% to < 95%). Our results provide support for the use of subdivisions for breast MRI in routine clinical practice.
For BI-RADS assessment category 4, the range for the likelihood of malignancy is large (> 2% but < 95%). However, multiple studies have shown the feasibility of BI-RADS category 4 subdivisions for mammography and ultrasound [10–12]. Category subdivisions were first proposed in the BI-RADS fourth edition, and the BI-RADS fifth edition encourages use of category 4 subdivisions for mammography and ultrasound on the basis of available data. Although the management recommendation is the same for all category 4 subdivisions (tissue diagnosis), the use of category 4 subdivisions has many potential clinical benefits.
The BI-RADS fifth edition states that assessment category 4 is not currently divided into subcategories for breast MRI [1] because there is a paucity of data showing the feasibility and accuracy of this approach for breast MRI. In addition, it has been unclear how the higher baseline risk of the typical patient undergoing screening breast MRI would affect the likelihood of malignancy predicted by BI-RADS category 4 subdivisions. Thus, even if BI-RADS category 4 subcategorization was possible with MRI, it was unknown whether the ranges for likelihood for malignancy would be the same as those achieved with mammography and ultrasound.
In one retrospective reader study, Maltez de Almeida et al. [13] identified BI-RADS category 4 findings on selected breast MRI examinations. The reviewing radiologists were given a set of instructions to assist with determination of BI-RADS category 4 subdivisions, and the likelihood of malignancy was 15% for category 4A, 37% for category 4B, and 84% for category 4C. Chevrier et al. [14] reviewed the results of MRI-guided biopsies performed between 2005 and 2011 on the basis of findings identified on screening and diagnostic MRI examinations. Using positive predictive value level 3 (PPV3), they found rates of malignancy of 3% for BI-RADS category 4A, 13% for BI-RADS category 4B, 31% for BI-RADS category 4C, and 81% for BI-RADS category 5. Our results dedicated to screening breast MRI and using PPV2s are similar for BI-RADS category 4A (2.5%), but our likelihood of malignancy is higher for BI-RADS categories 4B, 4C, and 5, meeting the ranges set in BI-RADS for mammography and ultrasound.
In the present study, the most common indications for breast MRI examinations with a BI-RADS category 4 finding were a family history of breast cancer (50%; 41/82), a personal history of treated breast cancer (33%; 27/82), and a genetic predisposition to breast cancer (15%; 12/82), as is shown in Table 1. These results are slightly different than those noted in our entire screening practice, where 12% of patients have a genetic predisposition to breast cancer, 43% have a family history of breast cancer, and 42% have a personal history of treated breast cancer [6]. Although examinations resulting in a BI-RADS category 4 finding occur more frequently for patients with a family history of breast cancer than for those with a personal history of breast cancer, we reported a higher PPV3 and cancer yield for patients with a personal history of treated breast cancer than for those with a family history of breast cancer [6], as have other investigators [15–17].
There are many potential benefits of using subdivisions of BI-RADS category 4 assessments for breast MRI. Using subdivisions better informs patient and clinician expectations and can facilitate preparation for the next step in patient management. In our practice, subdivisions have helped expedite care by minimizing the time between biopsy and the clinical appointment for patients who have lesions for which there is a high suspicion of malignancy. In addition, more stratified assessment of the likelihood of malignancy aids radiologic-pathologic correlation and provides a more meaningful practice audit. Finally, subdivision of category 4 facilitates research involving ROC curve analysis.
Further study is warranted to determine which MRI findings are appropriate for each subdivision. In our practice, no formal criteria exist for deciding which category 4 subdivision is appropriate for suspicious findings.
Rather, the interpreting radiologist makes this determination on the basis of his or her training and experience and the known PPVs of particular MRI descriptors [9]. This determination is similar to the current practice for probably benign (category 3) findings on breast MRI, where “the use of category 3 assessment at MRI remains intuitive…” [8]. Despite the lack of guidelines regarding lesion types or features, our results support the feasibility of using category 4 subdivisions in routine clinical care because they complement the ranges specified by BI-RADS for mammography and ultrasound. Establishing formal criteria to guide radiologists in subdividing category 4 is an important area of future research.
Our study has limitations. It was performed at a single site, which is an academic practice with radiologists who subspecialize in breast MRI, most of whom are fellowship trained. Thus, our results may not be generalizable to other practice types or practices with a different spectrum of patients undergoing screening breast MRI. In addition, the sample size is small (82 BI-RADS category 4 examinations were identified from a total of 860 screening breast MRI examinations). The small sample size limited analysis of the differences in our results on the basis of the indication for examination. Finally, we did not differentiate incidence from prevalence screening examinations, and it is unknown whether temporal change in lesion size influences BI-RADS category 4 subdivisions for MRI.
Conclusion
Subdivision of breast MRI BI-RADS assessment category 4 is feasible and stratifies the likelihood of malignancy into the following categories: 4A (> 2% to ≤ 10%), 4B (> 10% to ≤ 50%), and 4C (> 50% to < 95%), meeting the likelihood ranges specified for mammography and ultrasound. This facilitates a more meaningful practice audit and radiologist-pathologic concordance, and it assists patients and clinicians with informed decision making. Future research directions include clarifying which lesion descriptors belong in each subdivision.
Acknowledgments
We thank Scott Hetzel, for his assistance with statistical analysis, and Kristie Guite and Jennifer Rollenhagen, for their help with data acquisition.
R. M. Strigel receives personal and institutional research support from GE Healthcare, and A. M. Fowler and F. Kelcz receive institutional research support from GE Healthcare.
Supported by the Radiological Society of North America Research & Education Foundation and the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Institutes of Health Clinical and Translational Science Award (grant 1 IL1 TR000427) from the National Center for Advancing Transitional Sciences and the National Institutes of Health Big Data to Knowledge (BD2K) Initiative, U54AI117924.
Footnotes
Based on a presentation at the American College of Radiology and Society of Breast Imaging 2015 annual meeting, Orlando, FL.
References
- 1.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 2.Sickles EA, D’Orsi C. ACR BI-RADS follow-up and outcome monitoring. In: D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al., editors. ACR BI-RADS Atlas, Breast Imaging and Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 3.U.S. Food & Drug Administration (FDA) Mammography quality standards act and program. U.S. FDA website; www.fda.gov/Radiation-Emitting-Products/MammographyQualityStandardsAc-tandProgram/Guidance/PolicyGuidanceHelpSys-tem/ucm052100.htm. Accessed March 3, 2016. [Google Scholar]
- 4.Niell BL, Gavenonis SC, Motazedi T, et al. Auditing a breast MRI practice: performance measures for screening and diagnostic breast MRI. J Am Coll Radiol. 2014;11:883–889. doi: 10.1016/j.jacr.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 6.Strigel RM, Rollenhagen J, Burnside ES, et al. Screening breast MRI outcomes in routine clinical practice: comparison with BI-RADS benchmarks. Acad Radiol. 2016 Dec 13; doi: 10.1016/j.acra.2016.10.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda DM, Hylton NM, Kuhl CK, et al. BI-RADS: magnetic resonance imaging. In: D’Orsi CJ, Mendelson EB, Ikeda DM, et al., editors. Breast Imaging Reporting and Data System: ACR BI-RADS— breast imaging atlas. 1st. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 8.Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS Magnetic Resonance Imaging. In: D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al., editors. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 9.Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C. Positive predictive value of BI-RADS MR imaging. Radiology. 2012;264:51–58. doi: 10.1148/radiol.12110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bent CK, Bassett LW, D’Orsi CJ, Sayre JW. Positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. AJR. 2010;194:1378–1383. doi: 10.2214/AJR.09.3423. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology. 2006;239:385–391. doi: 10.1148/radiol.2392042127. [DOI] [PubMed] [Google Scholar]
- 12.Sanders MA, Roland L, Sahoo S. Clinical implications of subcategorizing BI-RADS 4 breast lesions associated with microcalcification: a radiology-pathology correlation study. Breast J. 2010;16:28–31. doi: 10.1111/j.1524-4741.2009.00863.x. [DOI] [PubMed] [Google Scholar]
- 13.Maltez de Almeida JR, Gomes AB, Barros TP, Fahel PE, de Seixas Rocha M. Subcategorization of suspicious breast lesions (BI-RADS category 4) according to MRI criteria: role of dynamic contrast-enhanced and diffusion-weighted imaging. AJR. 2015;205:222–231. doi: 10.2214/AJR.14.13834. [DOI] [PubMed] [Google Scholar]
- 14.Chevrier MC, David J, Khoury ME, Lalonde L, Labelle M, Trop I. Breast biopsies under magnetic resonance imaging guidance: challenges of an essential but imperfect technique. Curr Probl Diagn Radiol. 2016;45:193–204. doi: 10.1067/j.cpradiol.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR. 2010;195:510–516. doi: 10.2214/AJR.09.3573. [DOI] [PubMed] [Google Scholar]
- 16.Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108:djv349. doi: 10.1093/jnci/djv349. [DOI] [PubMed] [Google Scholar]
- 17.Schacht DV, Yamaguchi K, Lai J, Kulkarni K, Sennett CA, Abe H. Importance of a personal history of breast cancer as a risk factor for the development of subsequent breast cancer: results from screening breast MRI. AJR. 2014;202:289–292. doi: 10.2214/AJR.13.11553. [DOI] [PubMed] [Google Scholar]


