Abstract
Methodological limitations of prior studies have prevented progress in the treatment of patients with borderline resectable pancreatic adenocarcinoma. Shortcomings have included the absence of staging and treatment standards and pre-existing biases with regard to the use of neoadjuvant therapy and the role of vascular resection at pancreatectomy. In this manuscript, we will review limitations of studies of borderline resectable PDAC reported to date, highlight important controversies related to this disease stage, emphasize the research infrastructure necessary for its future study, and present a recently-approved Intergroup pilot study (Alliance A0201101) that will provide a foundation upon which subsequent well-designed clinical trials can be performed.
INTRODUCTION
Defining borderline resectable pancreatic ductal adenocarcinoma (PDAC) as a unique entity worthy of investigation is rational based on five clinical observations: 1) complete resection of the primary tumor and regional lymph nodes is mandatory for long-term survival1, 2) the incidence of margin-negative resection following surgery de novo decreases with increasing involvement of the superior mesenteric vein-portal vein (SMV-PV) and superior mesenteric artery (SMA)2–4, 3) resection of the SMV-PV and hepatic artery—but not the SMA—at pancreatectomy is associated with acceptable outcomes5–7, 4) “downstaging” of locally advanced cancers is rare following the administration of conventional cytotoxic agents8, and 5) chemotherapy and chemoradiation may be used to select patients with favorable physiology and tumor biology who may benefit from aggressive operations. Within this context, borderline resectable tumors are best conceptualized as those that involve the mesenteric vasculature to a limited extent, and for which resection, while possible, would likely be compromised by positive surgical margins in the absence of preoperative therapy.
Recognition of borderline resectable PDAC as a unique clinical entity is critical, both for optimal patient care, as well as for the proper evaluation of novel treatment regimens in clinical trials. Unfortunately, methodological limitations and biases associated with prior studies have impeded progress and have contributed to confusion. In this manuscript, we review limitations of studies of borderline resectable PDAC reported to date, emphasize the research infrastructure necessary for its future study, and present a recently approved Intergroup pilot study that will provide a foundation upon which subsequent well-designed clinical trials can be performed.
HISTORICAL CONTEXT
A collection of radiographic criteria characterizing a subset of non-metastatic PDAC tumors with anatomic features intermediate between resectable and unresectable was first described in 20019. This evolved from the recognition that a margin-negative resection was critical to long-term survival and that venous resection at pancreatectomy could be performed safely and could enhance the ability to achieve a margin-negative resection in cases where this had previously been considered impossible10. Simultaneously, the potential benefits of neoadjuvant therapy with regard to both patient selection and margin sterilization were emerging11. These advances reduced the clinical gap between resectable cancers, for which R0 resection as primary therapy was considered potentially curative, and unresectable cancers, for which palliative nonsurgical therapies were historically favored. By 2006, the National Comprehensive Cancer Network (NCCN) adopted the term “borderline resectable” to describe localized cancers thought to be at high risk for margin-positive resection and treatment failure when treated primarily with surgery, and for which the administration of neoadjuvant therapy was logical.
To our knowledge, 23 studies that report outcomes of patients with tumors labeled as borderline resectable and who were treated with chemotherapy and/or chemoradiation prior to surgery have been published (TABLE 1). These studies primarily represent retrospective, single-institution series or small prospective studies of patients with locally advanced disease in which patients with borderline resectable cancers were included as a subset. Only two prospective trials of neoadjuvant therapy for borderline resectable PDAC have previously been reported9, 12. Just one of these was a multi-institutional trial and it closed prematurely almost a decade ago for reasons including the absence of a well-defined study population and the absence of standardized therapeutic algorithms12. Since then, a universally accepted radiographic definition of borderline resectable PDAC has not been established. Likewise, preoperative assessment, disease staging, surgical decision-making and technique, and integration of chemotherapy and chemoradiation into the care of these patients have not been standardized13.
Table 1.
Prior studies of borderline resectable pancreatic cancer
| AuthorRef | Year | Study Centers/ Type |
Staging Definitions Used |
Stages Studied |
Neoadjuvant Regimen |
Indication for Surgery |
Response Criteria Reported |
Resection Rate Reported |
Vascular Resection Rate Reported |
Survival Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Leone27 | 2012 | S/1 | Other | LA BLR | CTX-CXRT | No progression | RECIST | Yes | No | Yes |
| Lee28 | 2012 | S/1 | NCCN* 2008 | LA BLR | CTX | No progression | RECIST | Yes | Yes | No |
| Hosein29 | 2012 | S/2 | AHPBA | LA BLR PR | CTX | Radiographically resectable | NR | Yes | No | No |
| Katz21 | 2012 | S/2 | AHPBA MDACC | BLR | Various | No progression | RECIST Stage | Yes | Yes | Yes |
| Barugola30 | 2012 | S/3 | Other | LA BLR | Various | No progression | RECIST | N/A | No | No |
| Kang31 | 2012 | S/3 | AHPBA | BLR | CXRT | No progression | NR | N/A | Yes | No |
| Chuong32 | 2011 | S/3 | Other | BLR | CTX-CXRT | Significant radiographic response | WHO RECIST | N/A | No | No |
| Stokes33 | 2011 | S/2 | MDACC | BLR | CXRT | No progression | NR | Yes | Yes | No |
| Arvold34 | 2011 | S/2 | Other | LA BLR | Various | NR | NR | Yes | No | No |
| Sahora35 | 2011 | S/1 | AHPBA | LA BLR | CTX | Partial response and at least stable disease | RECIST | Yes | No | No |
| Sahora36 | 2011 | S/1 | AHPBA | LA BLR | CTX | Borderline resectable or unresectable with improved PS or CA 19–9 | RECIST | No | No | No |
| Takahashi37 | 2011 | S/3 | NCCN* 2010 | LA BLR | Various | NR | NR | No | No | No |
| Chun38 | 2010 | S/3 | Other | BLR | Various | NR | NR | N/A | Yes | Yes |
| McClaine24 | 2010 | S/2 | Other | BLR | Various | Response | NR | Yes | Yes | No |
| Landry12 | 2010 | S/4 | Other | BLR | Various | NR | RECIST | Yes | No | Yes |
| Turrini39 | 2009 | S/2 | MDACC | BLR | CXRT | NR | RECIST | No | No | No |
| Satoi40 | 2009 | S/2 | NCCN* 2005 | PR BLR | CXRT | NR | NR | Yes | No | No |
| Brown41 | 2008 | S/3 | NR | BLR | CXRT-CTX | No progression | NR | N/A | Yes | Yes |
| Small42 | 2008 | S/1 | NCCN | BLR LA | CXRT | Resectable | RECIST | Yes | No | Yes |
| Massucco43 | 2006 | S/2 | Other | BLR LA | Various | Downstaging or stable disease with normal CA 19–9 | RECIST | Yes | No | No |
| Pipas44 | 2005 | S/1 | Other | PR BLR LA | CTX-CXRT | Resectable or borderline resectable | RECIST | Yes | No | No |
| Zimmermann45 | 2004 | S/1 | Other | BLR LA | CXRT | No progression | NR | Yes | No | Yes |
| Mehta9 | 2001 | S/1 | Other | BLR | CXRT | No progression | RECIST | Yes | No | Yes |
Studies included are those published in English that included patients treated with neoadjuvant therapy for borderline resectable PDAC (using any definition) and specifically provided data for borderline resectable patients independently.
Study Centers/Type: S, single-center; M, multi-center; 1, prospective, single-arm; 2, retrospective, observational; 3, retrospective, only resected patients; 4, prospective, randomized
Stages Studied: PR, potentially resectable; BLR, borderline resectable; LA, locally advanced
Staging Definitions Used: NCCN, National Comprehensive Cancer Network; MDACC, MD Anderson Cancer Center; AHPBA, Americas Hepatopancreatobiliary Association/Society for Surgery of the Gastrointestinal Tract/Society of Surgical Oncology
Neoadjuvant Regimen: CTX, chemotherapy; CXRT, chemoradiation
NR, not reported
N/A, not applicable because only resected patients were included in the study
The NCCN criteria have changed over the years. The date of publication of the NCCN criteria used is included, when reported
INTERGROUP TRIAL
Within this historical context, multi-institutional study of borderline resectable PDAC is justified given the following considerations. First, no standard therapeutic approach has been established for patients with this disease stage. Second, although a significant number of patients with borderline resectable PDAC exist nationwide, the number treated at most institutions is small. Third, the application of both neoadjuvant therapy and vascular resection for patients with localized PDAC has increased, indicating a growing acceptance of these two historically controversial treatment modalities; the recent publication of national “consensus statements” suggests interest in this unique patient population14, 15.
The Alliance for Clinical Trials in Oncology (Alliance), in cooperation with the Southwest Oncology Group, Eastern Cooperative Oncology Group and Radiation Therapy Oncology Group, recently obtained NCI approval to conduct a multi-institutional treatment trial for patients with borderline resectable PDAC (Alliance A021101). Opening in early 2013, this single-arm pilot study will evaluate the survival outcomes and toxicity rates associated with four cycles of mFOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, leucovorin 400 mg/m2, 5-fluorouracil 2,400 mg/m2) followed by external beam radiation therapy to 50.4 Gy with capecitabine (825 mg/m2) administered prior to surgery (FIGURE 1). More importantly, however, it will evaluate the feasibility of conducting future multi-institutional trials of borderline resectable PDAC and will establish a research infrastructure upon which those future trials can be based. This will include standardization and quality control of the following elements.
Figure 1.

Study schema from intergroup trial (Alliance Trial #A021101). BLR PDAC, borderline resectable pancreatic adenocarcinoma; mFOLFIRINOX, modified FOLFIRINOX; EBRT, external beam radiation therapy; CAPE, capecitabine; GEM, gemcitabine.
Definition
A uniformly accepted set of criteria that define patients with borderline resectable PDAC does not exist. The two most commonly cited definitions are those proposed by the MD Anderson group and the Americas Hepatopancreatobiliary Association (AHPBA)/Society for Surgery of the Alimentary Tract (SSAT)/Society of Surgical Oncology (SSO, and modified by the NCCN) (TABLE 2)15–17. Both criteria differentiate borderline resectable from unresectable cancers on the basis of radiographic evidence for limited SMA involvement (predicted radiographically by a tumor-SMA interface less than 180 degrees3) that would allow resection of the tumor without resection of the artery because pancreatectomy with concomitant resection and reconstruction of the SMA has generally been found to be futile6, 7.
Table 2.
Comparison of Americas Hepatopancreaticobiliary Association/Society for Surgery of the Alimentary Tract/Society of Surgical Oncology (AHPBA/SSO/SSAT), MD Anderson, National Comprehensive Cancer Network (NCCN) and Intergroup radiographic definitions of borderline resectable pancreatic cancer.
| AHPBA/SSAT/SSO12 | MD Anderson5,8 | NCCN 201217* | Intergroup Trial | |
|---|---|---|---|---|
| SMV-PV | Abutmenta, encasement b or occlusion | Occlusion | Abutment with impingement or narrowing | Interface between tumor and vessel measuring 180° or greater of the circumference of the vessel wall, and/or reconstructable¥ occlusion |
| SMA | Abutment | Abutment | Abutment | Interface between tumor and vessel measuring less than 180° of the circumference of the vessel wall |
| CHA | Abutment or short-segment encasement | Abutment or short-segment encasement | Abutment or short-segment encasement | Reconstructable¥, short-segment interface between tumor and vessel of any degree |
| Celiac Trunk | No abutment or encasement | Abutment | No abutment or encasement | Interface between tumor and vessel measuring less than 180° of the circumference of the vessel wall |
Abbreviations: SMV, superior mesenteric vein; PV, portal vein; SMA, superior mesenteric artery; CHA, common hepatic artery.
The NCCN criteria have changed over the years. The most recent criteria (2.2012) are included.
Defined as tumor-vessel interface less than 180 degrees of vascular circumference.
Defined as tumor-vessel interface at least 180 degrees of vascular circumference.
Normal vein or artery proximal and distal to the site of suggested tumor-vessel involvement suitable for vascular reconstruction
The two classifications differ primarily in the extent to which radiographic evidence of tumor involvement of the superior mesenteric vein-portal vein (SMV-PV) discriminates borderline resectable primary tumors from resectable ones. The MD Anderson group, which favors the use of neoadjuvant chemoradiation for both resectable and borderline resectable cancers, considers venous occlusion to represent the cutoff; tumors that radiographically abut (< 180 degree tumor-vessel interface) or encase (≥ 180 degree interface) the SMV-PV are considered resectable. In contrast, the AHPBA/SSAT/SSO considers venous abutment the cutoff; all tumors with any degree of abutment or encasement of the SMV-PV are considered borderline resectable4.
Increasing evidence suggests that the radiographic indicator of venous involvement that best describes tumors at high risk of margin-positive resection among those which are not clearly unresectable lies somewhere between venous abutment and outright occlusion. Tumors that approach the right lateral aspect of the SMV-PV can typically be resected with negative margins, while tumors that infiltrate to the left lateral aspect of the vein have a higher likelihood of margin-positive resection whether or not concomitant venous resection is performed. In an early study of patients who all underwent venous resection at pancreatectomy, Ishikawa found that patients with bilateral narrowing or long-segment abutment of the SMV-PV on preoperative angiograms had a poorer prognosis than patients with radiographically normal, shifted, or unilaterally (right) narrowed veins, suggesting that these patients had more aggressive disease biology and/or a higher rate of positive margins2. More recently, Nakao confirmed these findings and investigators from the Central Pancreas Consortium showed that margin-negative resection of tumors with minimal radiographic evidence for SMV-PV involvement could be performed without the need for neoadjuvant therapy4.
The differences between the AHPBA/SSAT/SSO and MD Anderson classifications notwithstanding, over half of prior studies that included patients with borderline resectable disease did not use either definition. Indeed, the radiographic criteria used to define a group of tumors labeled “borderline resectable” often vary between or even within institutions, and reflect preconceptions about the results of surgery for patients with PDAC, as well as deep biases with regard to the performance of vascular resection at pancreatectomy and the role of neoadjuvant therapy rather than objective data.
The ideal definition for borderline resectable PDAC should be free of subjective terminology (e.g. impingement, abutment), could be applied using routine, axial pancreatic protocol CT images, and should be reproducible. Based on the data available, and within the context of a cooperative group setting, we have defined borderline resectable PDAC radiographically as localized cancers with one or more of the following (Figure 2):
An interface between the primary tumor and SMV-PV measuring 180° or greater of the circumference of the vein wall, and/or
Short-segment occlusion of the SMV-PV with normal vein above and below the level of obstruction that is amenable to resection and venous reconstruction, and/or
Short-segment interface (of any degree) between tumor and hepatic artery with normal artery proximal and distal to the interface that is amenable to resection and arterial reconstruction, and/or
An interface between the tumor and SMA or celiac trunk measuring less than 180° of the circumference of the artery wall.
Figure 2.
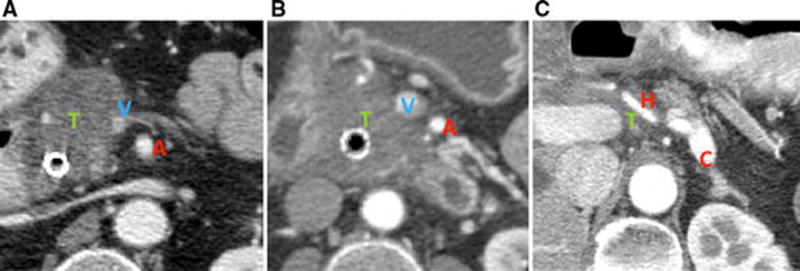
Radiographic findings consistent with borderline resectable PDAC. A) An interface between the primary tumor and the superior mesenteric vein measuring at least 180° of the circumference of the vessel wall; B) An interface between the tumor and superior mesenteric artery measuring less than 180° of the circumference of the vessel wall; C) Circumferential interface between the tumor and the common hepatic artery. T, tumor; A, superior mesenteric artery; V, superior mesenteric vein; H, common hepatic artery; C, celiac trunk.
Study Populations
Conversion of unresectable cancers with significant involvement of the SMA or aorta to resectable ones is a distinctly uncommon event. In a study of 87 patients with well-staged, unresectable tumors, Kim identified only one patient who was able to undergo curative resection following preoperative therapy8. Thus, trials evaluating the effects of novel preoperative regimens for borderline resectable PDAC would be negatively biased by the enrollment of patients with unresectable cancers whose tumors are unlikely to ever be resectable despite preoperative therapy. Borderline resectable cancers should be studied independently. In over half of prior trials, however, patients with borderline resectable cancers were studied together with patients considered to have locally advanced disease.
The Intergroup trial incorporates a pre-registration phase during which a real-time, centralized review of the staging CT images of all patients will be performed by a dedicated gastrointestinal radiologist prior to final enrollment. This mechanism will ensure enrollment of similarly staged patients who each meet well-defined radiographic criteria.
Metrics of Therapeutic Response
Recent concensus has established that patients with borderline resectable cancers should be treated with neoadjuvant chemotherapy and chemoradiation either on- or off-protocol18, but the true effects of these therapies are unknown. Theoretically, preoperative therapy can improve patient selection for surgery, downstage the primary tumor to allow margin-negative resection of advanced disease, and yield longer durations of survival.
A complete understanding of the role of preoperative therapy has remained elusive, however, because the outcome metrics reported in prior studies are biased by variability in the regimens used, response criteria applied, and indications and surgical techniques for resection following treatment. Resection rate, for example, has been routinely reported as a primary outcome measure of studies of borderline resectable PDAC, and has been used as a principal metric of comparison of the effects of different preoperative regimens19, 20. This metric, however, is a subjective one that reflects biases not only in pre- and post-treatment staging, but also in preoperative and intraoperative decision-making. Comparatively few studies have reported unbiased measures of treatment response such as Response Evaluation Criteria in Solid Tumors (RECIST); change in primary tumor diameter or radiographic stage following treatment; or even durations of survival.
The conclusions of an oft-cited meta-analysis of neoadjuvant therapy illustrate the pitfalls of using resection rate as a meaningful comparator19. The authors found that 33% of patients with unresectable PDAC in the analysis were resected following neoadjuvant therapy, and concluded that one-third of unresectable tumors can be converted to resectable ones with neoadjuvant therapy. However, 53% of the studies included in the meta-analysis did not state the criteria used to stage disease, only 40% reported the criteria used to measure treatment response, and most did not report the indications for surgery. The high resection rate reported likely reflected less “downstaging” by neoadjuvant therapy than an artifact of variability in staging and criteria used to indicate operative intervention. Indeed, in a recent analysis of patients treated at MD Anderson that used objective radiographic response criteria, only 12% of 129 patients with borderline resectable cancers had a reduction in size sufficient to meet the definition of a RECIST response following neoadjuvant therapy, and the tumor of only one patient was downstaged to meet a radiographic definition of potentially resectable21. Nonetheless, R0 resection was performed in 66% of patients. Vascular resection was required at 60% of all resections.
Objective outcome metrics must be defined and reported to allow meaningful comparisons of the effects of future novel preoperative treatment regimens. In the Intergroup trial, the diameter and radiographic stage of each patient’s primary tumor will be documented prior to therapy, between chemotherapy and chemoradiation, and prior to surgery. Response will be characterized by RECIST and a change in radiographic stage. Survival of the entire group and the resected group will be reported independently.
Indications for Surgery and Technical Considerations
The criteria used to select patients for surgery and the use of vascular resection at pancreatectomy may both effect outcomes of patients with borderline resectable PDAC. However, these critical elements of surgery—the only therapeutic component proven to be potentially curative—have been among the least standardized in previous trials of any stage of disease22. Indeed, no concensus yet exists with regard to the indications for surgical resection following neoadjuvant therapy, and controversy continues to surround the use of vascular resection at pancreatectomy. This variability may explain the wide range in both rates of resection (24 – 100%) and vascular resection (31 – 73%) reported in prior studies.
In the Intergroup trial, attempted resection is mandatory for all patients with preserved performance status in the absence of radiographic evidence for cancer progression. Venous resection and hepatic arterial resection is advocated, when necessary, to obtain negative margins, but SMA resection is prohibited because survival is poor when SMA resection is performed6. This protocol requirement is based upon an assumption that enrolling multidisciplinary groups will include surgeons skilled and willing to perform vascular reconstructions when indicated. In this context, using the anatomic criteria discussed above, margin-negative resection may be expected in a significant proportion of patients without SMA resection, even when abutment of the SMA is observed radiographically3, 23, 24. This reflects the concept that the tumor-SMA interface can be ‘sterilized’ in the absence of radiographic changes.
Other Clinical Areas
Attention to these methodological issues that are particularly germane to the study of borderline resectable disease does not minimize the need for standardization and quality control of all other trial components as should be required in the multi-institutional study of any stage of PDAC. For example, specific guidelines for chemotherapy drug and dose adjustments should be in place, and radiation therapy plans should be centrally reviewed prior to initiation of treatment25. Similarly, uniform histopathologic review of the surgical specimen at each participating center is essential to accurately determine the influence of preoperative therapy upon tumor response22, 26. Mechanisms to standardize these components are incorporated into the Intergroup trial.
CONCLUSION
Variability in patients studied, therapeutic algorithms employed, and metrics reported may introduce heterogeneity in the reported outcomes of patients with borderline resectable PDAC treated with preoperative therapy. Rigorous standards of clinical trial design incorporated into trials of other disease stages must therefore be adopted in all future studies of borderline resectable PDAC. The Intergroup trial should serve as a paradigm for such investigations.
Synopsis.
No multiinstitutional trial of preoperative therapy for patients with borderline resectable pancreatic cancer has yet been completed. Herein we highlight the research infrastructure necessary to conduct such a trial and present a recently-approved Intergroup pilot study (Alliance Trial A0201101).
Acknowledgments
Funding: Matthew HG Katz is supported in part by the Alliance for Clinical Trials in Oncology 2012 Clinical Scholar Award
Bibliography
- 1.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2006;10(9):1199–210. doi: 10.1016/j.gassur.2006.08.018. discussion 210–1. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa O, Ohigashi H, Imaoka S, et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Annals of surgery. 1992;215(3):231–6. doi: 10.1097/00000658-199203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR. American journal of roentgenology. 1997;168(6):1439–43. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 4.Nakao A, Kanzaki A, Fujii T, et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Annals of surgery. 2012;255(1):103–8. doi: 10.1097/SLA.0b013e318237872e. [DOI] [PubMed] [Google Scholar]
- 5.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2004;8(8):935–49. doi: 10.1016/j.gassur.2004.09.046. discussion 49–50. [DOI] [PubMed] [Google Scholar]
- 6.Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Annals of surgery. 2011;254(6):882–93. doi: 10.1097/SLA.0b013e31823ac299. [DOI] [PubMed] [Google Scholar]
- 7.Stitzenberg KB, Watson JC, Roberts A, et al. Survival after pancreatectomy with major arterial resection and reconstruction. Annals of surgical oncology. 2008;15(5):1399–406. doi: 10.1245/s10434-008-9844-y. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2002;6(5):763–9. doi: 10.1016/s1091-255x(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 9.Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2001;5(1):27–35. doi: 10.1016/s1091-255x(01)80010-x. [DOI] [PubMed] [Google Scholar]
- 10.Allema JH, Reinders ME, van Gulik TM, et al. Portal vein resection in patients undergoing pancreatoduodenectomy for carcinoma of the pancreatic head. The British journal of surgery. 1994;81(11):1642–6. doi: 10.1002/bjs.1800811126. [DOI] [PubMed] [Google Scholar]
- 11.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(3):928–37. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 12.Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101(7):587–92. doi: 10.1002/jso.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MH, Pisters PW, Lee JE, Fleming JB. Borderline resectable pancreatic cancer: what have we learned and where do we go from here? Annals of surgical oncology. 2011;18(3):608–10. doi: 10.1245/s10434-010-1460-y. [DOI] [PubMed] [Google Scholar]
- 14.Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Annals of surgical oncology. 2009;16(7):1751–6. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 15.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Annals of surgical oncology. 2009;16(7):1727–33. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 16.Loyer EM, David CL, Dubrow RA, Evans DB, Charnsangavej C. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdominal imaging. 1996;21(3):202–6. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 17.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10(6):703–13. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Annals of surgical oncology. 2009;16(7):1736–44. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 19.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS medicine. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morganti AG, Massaccesi M, La Torre G, et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Annals of surgical oncology. 2010;17(1):194–205. doi: 10.1245/s10434-009-0762-4. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012 doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 22.Katz MH, Merchant NB, Brower S, et al. Standardization of surgical and pathologic variables is needed in multicenter trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Annals of surgical oncology. 2011;18(2):337–44. doi: 10.1245/s10434-010-1282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012;16(1):68–78. doi: 10.1007/s11605-011-1748-7. discussion 78–9. [DOI] [PubMed] [Google Scholar]
- 24.McClaine RJ, Lowy AM, Sussman JJ, Schmulewitz N, Grisell DL, Ahmad SA. Neoadjuvant therapy may lead to successful surgical resection and improved survival in patients with borderline resectable pancreatic cancer. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2010;12(1):73–9. doi: 10.1111/j.1477-2574.2009.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. International journal of radiation oncology, biology, physics. 2012;82(2):809–16. doi: 10.1016/j.ijrobp.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbeke CS, Menon KV. Variability in reporting resection margin status in pancreatic cancer. Annals of surgery. 2008;247(4):716–7. doi: 10.1097/SLA.0b013e31816a7077. [DOI] [PubMed] [Google Scholar]
- 27.Leone F, Gatti M, Massucco P, et al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: A single institutional experience. Cancer. 2012 doi: 10.1002/cncr.27736. [DOI] [PubMed] [Google Scholar]
- 28.Lee JL, Kim SC, Kim JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery. 2012 doi: 10.1016/j.surg.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC cancer. 2012;12(1):199. doi: 10.1186/1471-2407-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barugola G, Partelli S, Crippa S, et al. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. American journal of surgery. 2012;203(2):132–9. doi: 10.1016/j.amjsurg.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Kang CM, Chung YE, Park JY, et al. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012;16(3):509–17. doi: 10.1007/s11605-011-1784-3. [DOI] [PubMed] [Google Scholar]
- 32.Chuong MD, Hayman TJ, Patel MR, et al. Comparison of 1-, 2-, and 3-Dimensional Tumor Response Assessment After Neoadjuvant GTX-RT in Borderline-Resectable Pancreatic Cancer. Gastrointestinal cancer research : GCR. 2011;4(4):128–34. [PMC free article] [PubMed] [Google Scholar]
- 33.Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Annals of surgical oncology. 2011;18(3):619–27. doi: 10.1245/s10434-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 34.Arvold ND, Niemierko A, Mamon HJ, Fernandez-del Castillo C, Hong TS. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. International journal of radiation oncology, biology, physics. 2011;80(5):1383–90. doi: 10.1016/j.ijrobp.2010.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahora K, Kuehrer I, Schindl M, Koelblinger C, Goetzinger P, Gnant M. NeoGemTax: gemcitabine and docetaxel as neoadjuvant treatment for locally advanced nonmetastasized pancreatic cancer. World journal of surgery. 2011;35(7):1580–9. doi: 10.1007/s00268-011-1113-8. [DOI] [PubMed] [Google Scholar]
- 36.Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149(3):311–20. doi: 10.1016/j.surg.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Kinoshita T, Konishi M, et al. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. Journal of hepato-biliary-pancreatic sciences. 2011;18(4):567–74. doi: 10.1007/s00534-011-0371-z. [DOI] [PubMed] [Google Scholar]
- 38.Chun YS, Milestone BN, Watson JC, et al. Defining venous involvement in borderline resectable pancreatic cancer. Annals of surgical oncology. 2010;17(11):2832–8. doi: 10.1245/s10434-010-1284-9. [DOI] [PubMed] [Google Scholar]
- 39.Turrini O, Viret F, Moureau-Zabotto L, et al. Neoadjuvant chemoradiation and pancreaticoduodenectomy for initially locally advanced head pancreatic adenocarcinoma. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35(12):1306–11. doi: 10.1016/j.ejso.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Satoi S, Yanagimoto H, Toyokawa H, et al. Surgical results after preoperative chemoradiation therapy for patients with pancreatic cancer. Pancreas. 2009;38(3):282–8. doi: 10.1097/MPA.0b013e31819438c3. [DOI] [PubMed] [Google Scholar]
- 41.Brown KM, Siripurapu V, Davidson M, et al. Chemoradiation followed by chemotherapy before resection for borderline pancreatic adenocarcinoma. American journal of surgery. 2008;195(3):318–21. doi: 10.1016/j.amjsurg.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Small W, Jr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(6):942–7. doi: 10.1200/JCO.2007.13.9014. [DOI] [PubMed] [Google Scholar]
- 43.Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Annals of surgical oncology. 2006;13(9):1201–8. doi: 10.1245/s10434-006-9032-x. [DOI] [PubMed] [Google Scholar]
- 44.Pipas JM, Barth RJ, Jr, Zaki B, et al. Docetaxel/Gemcitabine followed by gemcitabine and external beam radiotherapy in patients with pancreatic adenocarcinoma. Annals of surgical oncology. 2005;12(12):995–1004. doi: 10.1245/ASO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann FB, Schuhmacher C, Lersch C, et al. Sequential and/or concurrent hypofractionated radiotherapy and concurrent chemotherapy in neoadjuvant treatment of advanced adenocarcinoma of the pancreas. Outcome and patterns of failure. Hepato-gastroenterology. 2004;51(60):1842–6. [PubMed] [Google Scholar]


