Abstract
The consequences of UVB and UVA irradiation on hatch rate, mortality, and malformation were studied in embryonic zebrafish (Danio rerio). The use of zebrafish embryos has expanded from traditional developmental models to diverse studies, including many techniques utilizing light exposure. To characterize useful indicators of photodamage, the responses and threshold limits of UV radiation as a function of embryonic stage and fish source were evaluated. Significant differences in UVB susceptibility were observed in embryos at 3, 6–7, 12, and 24 h post-fertilization (hpf), with the 1000-cell stage (3 hpf) having greatest tolerance to UVB. Embryos derived from zebrafish raised in outdoor ponds were more tolerant to UVB than were embryos from laboratory-raised fish. Combinations of UVB and UVA exposure were used to confirm the presence of a competent photorepair system in zebrafish that could return otherwise malformed embryos to a normal phenotype. Overall, embryonic zebrafish had large tolerances (LD50 of 850 J/cm2) to UVA, confirming their suitability for photoactivation and photorepair studies.
Keywords: Danio rerio, Ultraviolet radiation, UVA, Embryo, Photorepair
1. Introduction
Zebrafish (Danio rerio) have been widely used as model organisms for studying various aspects of developmental biology because of their small size, fecundity, and because zebrafish embryos undergo rapid development in vitro, which facilitates morphological monitoring (reviewed by [1,2]). Over the past decade, these attributes have extended the use of this model fish to a wide variety of fields in biomedical research, including functional genomics, environmental and high-throughput toxicology screening [3,4] and specific human diseases [5]. Zebrafish embryos and juveniles are particularly useful for imaging and photoactivation studies, because they do not require in utero development, can be whole-mounted, and are optically transparent, allowing visualization of specific cells, tissues, and organs (reviewed by [6]).
Several optical techniques are applied in zebrafish to understand and control metabolic and genetic processes. Microinjection and subsequent photoactivation of caged fluorophores have enabled fate mapping of cells as they migrate and differentiate in the developing embryo [7]. More recently, larger caged molecules such as mRNA have been used to control gene expression with light exposure in developing embryos [8,9]. Excitation of calcium indicators (e.g. Fura-2, DNA stains (e.g., 4′,6-diamidino-2-phenylindole (DAPI))) and newer photoactivatable fluorescent proteins also requires exposure of zebrafish embryos to near-ultraviolet (UV) light [10]. While zebrafish have been widely used in these and other photoactivation studies, their tolerances have not been established for wavelengths of UV light that are known to cause photodamage in other systems.
Ultraviolet light is generally classified into three categories: UVA (400–315 nm), UVB (315–280 nm), and UVC (280–100 nm), and has long been known to cause adverse effects to aquatic organisms (reviewed by [11]). The discovery of the “ozone hole” over Antarctica [12] has stimulated much of the recent research on the biological effects of UV radiation in aquatic environments. Most of the current research focuses on bacteria [13], marine phytoplankton [14], zooplankton, and marine fishes [15]. Comparatively few studies have addressed the UV effects on freshwater fishes, and fewer specifically in zebrafish. The impact of ambient and sub-ambient UVB exposure on oxidative stress and mortality in embryonic and young zebrafish has been explored [16]. In addition to environmental exposures, many laboratory studies are using near-UV and UV light as tools to understand and control biological processes in cells, tissues, and organisms. Zebrafish morphology has been monitored in response to UVB or exposure to p53-acting drugs as a model screening system for anticancer compounds [4]. UVB light is known to activate p53 apoptotic pathways, whereas the role of UVA is more linked to reactive oxygen species, and can depend on cell type [17]. Thresholds of UV light sensitivity have been established for cell cultures as part of dermatological and in vitro photoactivation studies [18,19]. Analogous in vivo studies in zebrafish to determine dose–response to UV exposure as a function of developmental stage in embryonic zebrafish has not been explored.
Because many strains and sources exist for zebrafish, significant differences in UV toxicities could exist among lines and also within lines as a function of developmental stage. Embryos of various stages are commonly UV-irradiated in studies tracing cell lineage, and these stages might have different UV tolerances. One fundamental recurring question in embryonic photobiology among different organisms is: when does the occurrence of UV protective mechanisms first appear? Pigmentation from UV-absorbing chromophores is a common injury-prevention strategy seen in developing embryos, and pigment cells in zebrafish can be identified within 24 h of development. Post-exposure mechanisms for UV repair include nucleotide excision repair and photorepair of DNA (reviewed by [20]). Photorepair refers to the specific light-induced activity of photolyase that cleaves cyclopyrimidine dimers (CPDs) in DNA and is commonly seen in marine plankton, bacteria, some fishes and amphibians. To date, there are limited data pertaining to photorepair in zebrafish. Zebrafish embryos were shown to possess 6-4 photolyase expression patterns that vary with UV exposure, and their ability to tolerate UV was linked with circadian rhythms, where incubation in total darkness or constant light reduced survival rates [21].
In this study, the effects of UVA and UVB light on zebrafish embryos at various stages were studied and the thresholds and nature of light-induced embryonic phenotypes were characterized. Hatch and mortality rates, as well as the presence of gross malformations in development, were used to assess stage and source differences in UV responses. Additionally, the presence and fidelity of photorepair was evaluated in zebrafish embryos exposed to UVB followed by UVA exposure.
2. Materials and methods
2.1. Fish husbandry and embryo collection
Wild-type zebrafish were purchased from EkkWill Waterlife Resources, Gibsonton, FL (hereafter referred to as “pond-raised”) or obtained from the zebrafish breeding colony in the LSU Department of Biological Sciences (“laboratory-raised”) in October 2005. These “laboratory-raised” fish comprised fish from the AB line, those obtained from a local pet store in Baton Rouge, and those from the AB line that were crossed with the pet store fish. During a 2-week conditioning period, and also during and after light exposure experiments, healthy fish and embryos were raised and kept at standard laboratory conditions of 28 °C on a 14 h light: 10 h dark photoperiod [22] in a recirculating system. The fish were fed three times daily with either the zebrafish diet (Zeigler) or live artemia (Aquatic Habitats, Apopka Florida). Embryos were collected from group spawns or paired spawns, and were rinsed several times in embryo medium [22] containing penicillin–streptomycin (0.05%). Embryos at various developmental stages were used for experiments with an emphasis on middle gastrula stage embryos (6–7 h post-fertilization, hpf). Unless otherwise specified in the experiments detailing differences in fish source, all embryos used were collected from the pond-raised fish.
2.2. Ultraviolet radiation exposure
Ultraviolet radiation B (UVB) was supplied with a high performance transilluminator (TFM-20, UVP Inc., Upland, CA), which provided an irradiance of 5.19 mW/cm2 at 302 nm (818-ST-UV detector and 1815-C optical power meter, Newport Corporation, Irvine, CA). The broad output in the UVB region (shown in Supplemental Data, Fig. S1) matches the absorbance spectra of the many targets affecting cellular processes, such as DNA, cell membranes, and proteins [23], and in particular, the CPDs that are corrected in photorepair [24]. Ultraviolet radiation A (UVA) was supplied with a high intensity (GreenSpot 100-Watt) super pressure mercury lamp with a 5 × 1000 mmlight guide (American Ultraviolet, Lebanon, IN), which provided an irradiance at 4.2 cm of 0.705 W/cm2 at 365 nm with short bandpass and infrared filters. The short bandpass filter used (SWP-2502U-400; Lambda Research Optics, CA) has a cutoff at 400 nm and transmittance greater than 90% at 365 nm to select for common UVA photoactivating wavelengths. A heat-absorbing filter was also used to absorb potential infrared emittance (Schott KG-2; Germany), having a transmittance of greater than 85% at 365 nm and less than 10% at 1100 nm. A spectral characterization of the UVA light source indicated that ~60% fell within the range of 365 ± 8 nm (USB2000 spectrometer, Ocean Optics, Dudedin, FL). The output of this UVA source is similar to that known to activate photolyases across various terrestrial and aquatic organisms’ systems [24], with the exception that longer wavelength (e.g., photosynthetically active radiation) contribution here was minimal. The UVA source was chosen primarily for its ability to photoactivate caged compounds, where longer wavelengths are not as effective [25]. Wavelengths greater than 370 nm can effectively stimulate photorepair, yet the specific action spectra for these proteins in zebrafish are unknown. The closest known long wave absorbers in zebrafish are cyptochromes, which show only weak photolyase activity [26] and are thought to play larger roles in circadian rhythm [27].
Embryos were exposed to UVA in the bottom of a 35-mm diameter cell culture dish containing 3 ml of embryo medium. UVB exposures were administrated from bottom-up while UVA exposures were administered from top-down at a distance of 4.2 cm from the fiber to the bottom of the dish.
2.3. Viability criteria
Hatch, mortality, and malformation were used as evaluation criteria for the assessment of UV effects. Percent hatch was calculated as the number of embryos hatched within 5 d after fertilization divided by the total number of embryos. Percent mortality was calculated as the cumulative mortality of embryos within 5 d. Cessation of heartbeat and circulation were used as end points for mortality. Classification of malformations was based on comparison to control groups using criteria for gross changes in zebrafish development [1,3] similar to other UV-malformation studies in other fish species [28]. Larvae designated as being malformed typically had mild twisting or kinked spinal deformities and slightly enlarged pericardial sacs. Severe malformation was characterized by significant spinal deformities and grossly enlarged pericardial sacs. Percent total malformation (or severe malformation) was calculated as the number of embryos having any deformities (or severe deformities) after hatch divided by the total number of embryos surviving at 5 d. For treatment groups with 100% mortality before hatch, percent total and severe malformation were considered to be 100%. Dishes were examined daily for developmental progress, hatch, mortality, and malformation. Dead embryos were removed, and embryo media was replaced.
2.4. UV exposure during mid-gastrula stage of development
To determine UVB sensitivities, 6–7 hpf embryos were exposed to UVB for 0, 1, 3, 5, and 10 min at a dosage of 0.31 J/cm2 per min. This experiment was replicated twice, each with 40 embryos cultured in two Petri dishes (20 embryos per dish). For UVA dose–responses, embryos were exposed to UVA for 0, 8, 10, 12, 15, 18, and 20 min at a dosage of 42.3 J/cm2 per min. This experiment was replicated three times, each with 20 embryos per treatment. After treatment, embryos were incubated in the dark for the first 20 h, followed by exposure to a regular 14 h light: 10 h dark photoperiod.
2.5. UVA photorepair after exposure to UVB
To evaluate whether UVA radiation could repair the damage caused by UVB, embryos were exposed to UVB at 0.93 and 1.56 J/cm2 (corresponding to 3- and 5-min exposures), and followed immediately with UVA exposure of 1, 3, 5, 8, and 10 min at the dosage of 42.3 J/cm2 per min. Controls included embryos exposed to UVB but without subsequent UVA irradiation, and embryos without any UV irradiation. For each control group, 20 embryos were incubated in the dark for the first 20 h (referred to as “dark control”), and another 20 embryos were incubated in the light (referred to as “light control”) with regular photoperiod. All experiments were replicated a minimum of three times each with 20 embryos per treatment.
2.6. Assessment of UVB exposure on embryos at various stages of development
Embryos were exposed to 0.93 J/cm2 of UVB light at the following time points in development: 3, 6–7, 12, and 24 hpf. This type of experiment was replicated four times, each with 20 embryos per treatment. After treatment, embryos were incubated in the dark for the first 20 h, followed by a regular photoperiod described in Section 2.5.
2.7. Comparison of animal source on UVB sensitivity and UVA photorepair
UVB sensitivities were evaluated in embryos collected from adult laboratory-raised zebrafish as described for the pond-raised fish in Section 2.4. This experiment was replicated three times with 15 embryos per treatment. Photorepair was evaluated between embryos collected from the pond-raised and laboratory-raised animals by exposure to either UVB (0.93 J/cm2) alone or UVB (0.93 J/cm2) followed immediately by UVA (211.5 J/cm2). This experiment was replicated three times with 20 embryos per treatment. After treatment, embryos were incubated with regular photoperiods as described in Section 2.5.
2.8. Data analysis
To analyze dose–responses, the concentration causing 50% mortality (LD50) was calculated with logistic regression (Logit model). For other experiments, data were analyzed using one-way and two-way (fixed model) analysis of variance (ANOVA) (SAS 9.0, SAS Institute Inc., Cary, NC, USA). When a significant difference (α = 0.05) was observed among treatments, Tukey’s Honestly Significant Difference Procedure was used for pair-wise comparisons. Results were presented as means ± SD, and probability values of P < 0.05 were considered to be significant. Percentage data for hatch, mortality, malformation, and severe malformation were arcsine-square root transformed prior to analysis.
3. Results
3.1. Morphological phenotypes observed in embryos subjected to UV radiation
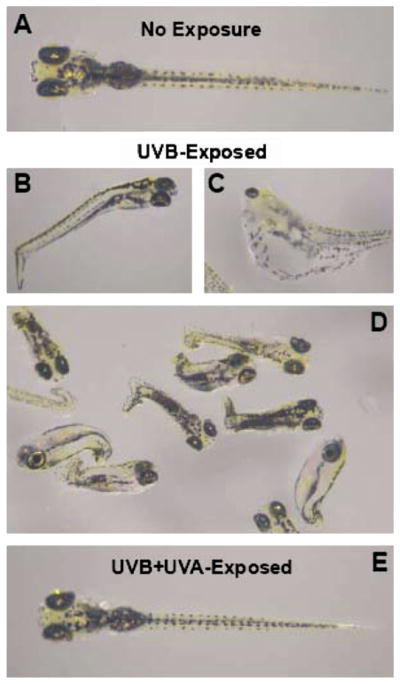
Zebrafish embryos typically exhibited spinal deformities within 5 d following fertilization as a result of being exposed to UVB or large UVA light doses at the mid-gastrula stage. In general, increasing doses of UVB radiation produced minor spinal deformities (Fig. 1B), enlarged pericardial sacs (Fig. 1C), and severe spinal curling or twisting (Fig. 1D). Embryos exposed to high doses of UVA typically showed minor spinal deformities (similar to Fig. 1B) and somewhat enlarged pericardial sacs (similar to Fig. 1C). Furthermore, these morphological changes were present only in the highest doses of UVA that were tested (detailed below). Embryos first exposed to 0.93 J/cm2 of UVB followed by (211.5 J/cm2) of UVA radiation appeared to have completely recovered, showing morphology (Fig. 1E) similar to the control treatments which did not receive UV irradiation (Fig. 1A).
Fig. 1.
Typical morphology of zebrafish embryos before and after UV exposure: (A) control embryo without UV exposure; consequences of high UVB doses such as (B) minor spinal bending, (C) enlarged pericardial sacs and (D) severe spinal deformities; (E) embryo exposed to (0.93 J/cm2) UVB followed immediately with (211.5 J/cm2) UVA showing normal appearance.
3.2. Effect of UV exposure during the mid-gastrula stage of development
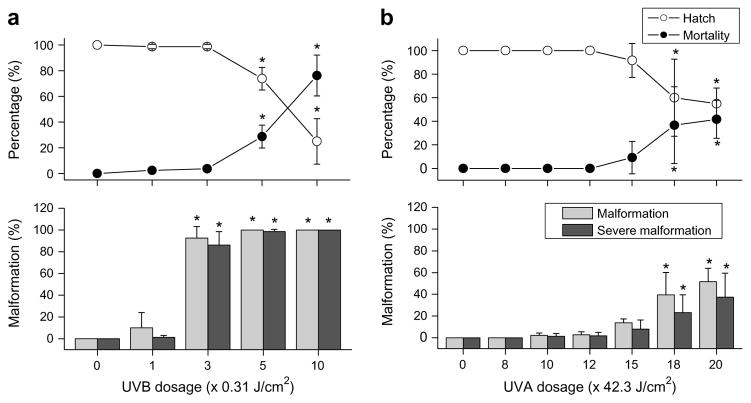
Decreases in hatch rate, and increases in mortality and malformations of embryos from pond-raised fish (6–7 hpf) were observed with increasing UVB exposure (Fig. 2a). Embryos exposed to 3.11 J/cm2 of UVB radiation had significantly lower percent hatch rates (25 ± 18%) and higher mortality rates (76 ± 16%) than did the lower doses (P < 0.05). Significantly lower percent hatch and higher mortality were also observed with the dose of 1.56 J/cm2 when compared with controls. Although there were no significant differences in percent hatch and mortality among doses of 0, 0.31, and 0.93 J/cm2, embryos did exhibit malformations at the dose of 0.93 J/cm2, yielding significantly higher deformity (total 93 ± 11%; severe: 86 ± 12%) when compared to controls not exposed to UVB radiation (0 ± 0%; P < 0.05). However, there were no significant differences in the occurrence of malformations between 0.31 J/cm2 and the controls. Doses above 0.93 J/cm2 did not significantly increase malformation, as all of the embryos were malformed at that dose, but doses above this did significantly increase mortality. The calculated LD50 of UVB radiation for embryos exposed at the mid-gastrula stage was 2.32 J/cm2.
Fig. 2.
Dose–responses of UVB and UVA exposures in mid-gastrula zebrafish embryos. Percent hatch, mortality, malformation, and severe malformation of gastrulated zebrafish embryos within 5 d of exposure to UVB for 0, 1, 3, 5, and 10 min at a dosage of 0.31 J/cm2 per min (a), and UVA for 0, 8, 10, 12, 15, 18, and 20 min at the dosage of 42.3 J/cm2 per min (b). Asterisks indicate significant difference (P < 0.05) from control treatments receiving no UV exposure.
Embryos exposed to UVA at the mid-gastrula stage tolerated UVA doses as high as 635 J/cm2 without significant loss in percent hatch and mortality when compared to the controls (Fig. 2b). Decreased hatch and increased mortality along with the occurrence of malformations were not evident until the exposure dose was increased to 761 J/cm2. A further increase in UVA dose to 846 J/cm2 did not produce lower percent hatch, higher mortality, or higher incidence of malformation. The calculated LD50 of UVA radiation for embryos exposed at the mid-gastrula stage was 855.3 J/cm2.
3.3. UVA photorepair in embryos exposed to UVB during the mid-gastrula stage
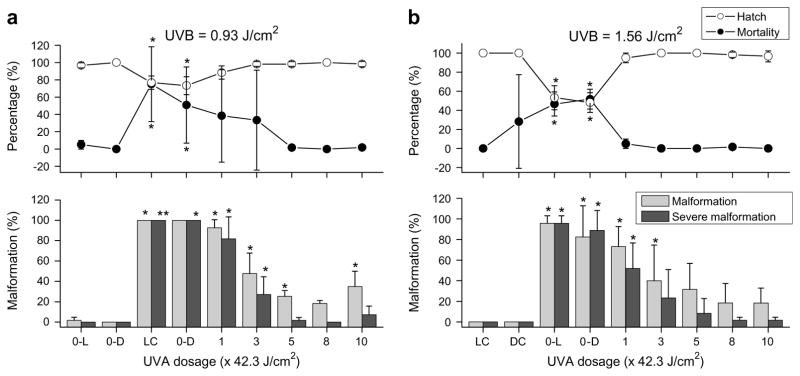
For embryos exposed to UVB at 0.93 J/cm2 (Fig. 3a), percent hatch (e.g., 73 ± 10% for the dark incubation) was significantly lower in embryos exposed to UVB radiation alone than in those with subsequent UVA radiation or those in unexposed controls, while percent mortality showed the opposite. Malformation rates arising from exposure to this UVB dose alone were 100% for both dark and light incubation, but they decreased rapidly with increased UVA radiation doses. In particular, a subsequent UVA radiation at 338 J/cm2 reduced the total malformation to 18 ± 3%, and severe malformation to 0 ± 0%, which were not significantly different from the control treatments, i.e., exposures occurring without UV radiation (P > 0.05). However, UVA exposure at 423 J/cm2 resulted in a significantly higher percent total malformation, but not an increase in severe malformation. As for incubation methods, there were no significant differences in percent hatch, mortality, or malformation between dark and ambient light incubation for controls without UV radiation, or for controls with UVB radiation alone.
Fig. 3.
UVA-stimulated photorepair in UVB-exposed zebrafish embryos. Percent hatch, mortality, malformation, and severe malformation of gastrulated zebrafish embryos within 5 d of exposure to UVB at 0.93 J/cm2 (a) and 1.56 J/cm2 (b) followed immediately with UVA exposure of 1, 3, 5, 8, and 10 min at the dosage of 42.3 J/cm2 per min. Controls exposed to UVB without subsequent UVA irradiation were incubated in the light (0-L) or dark (0-D) for the first 20 h, and controls without UV irradiation were incubated in light (LC) or dark (DC). Asterisks indicate significant difference (P < 0.05) from LC or DC treatments.
At a higher dose of UVB (1.56 J/cm2, Fig. 3b), similar trends were evident. In embryos exposed to UVB alone, percent hatch was lower than that of the unexposed controls. Moreover, when UVB exposure was coupled with UVA exposure, percent hatch rates were restored to control hatch rates. Mortality was greatly increased by the UVB exposure, but when the UVB exposure was coupled with UVA exposure, the mortality rates were reduced significantly (from 52% to <2%). Malformations in embryos exposed to UVB alone was above 80%, but decreased when embryos were first exposed to UVB and subsequently with UVA radiation. There was no significant difference in malformation (both total and severe) in embryos between the control groups (0 ± 0%) and those provided with subsequent UVA radiation at and above 211.5 J/cm2 (P > 0.05). For control exposures, except for percent mortality of controls without UV radiation, there were no significant differences in percent hatch, mortality, or malformation between dark and ambient light incubation.
3.4. UVB sensitivity is dependent upon embryonic developmental stage
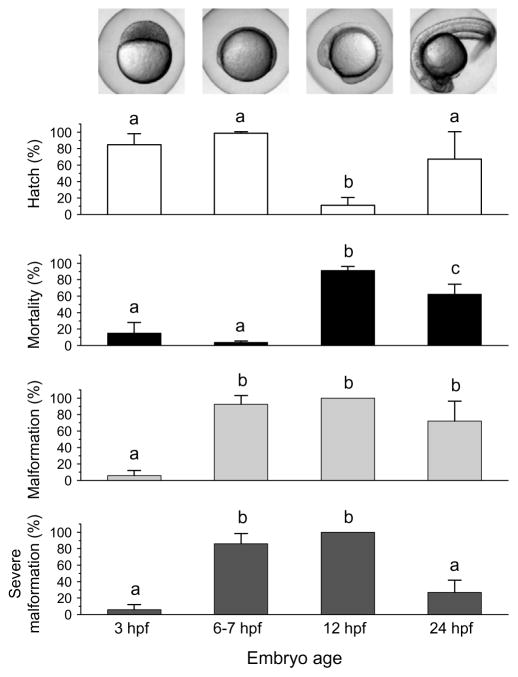
Embryos were exposed to different doses of UVB light at different times during development including: 3 hpf (~1000 cell stage), 6–7 hpf (early to mid-gastrula stage), 12 hpf (5–7 somite stage) and 24 hpf. Similar doses of UVB were used as those presented in the dose–response curves shown in Fig. 2. For a dose of 0.93 J/cm2, the embryos at the 12 hpf stage showed significantly lower (P < 0.05) hatch rates and higher mortality than did embryos exposed at other times during development (Fig. 4). Embryos exposed at 3 hpf exhibited only minimal occurrences of malformations (total or severe, 6 ± 6%). However, when embryos were exposed at 12 hpf, 100% of the embryos exhibited both types of malformation. At 24 hpf, the 0.93 J/cm2UVB exposure resulted in lesser incidence of malformations than those embryos exposed at 6–7 and 12 hpf, respectively. When embryos were exposed to doses of UVB higher than 0.93 J/cm2, the same pattern was observed. The embryos exposed at 12 and 24 hpf exhibited higher rates of mortality than the embryos exposed at 3 hpf. (See supplemental data for UVB dose–responses across developmental stages.)
Fig. 4.
Effect of developmental stage on UV tolerance in zebrafish embryos. Percent hatch, mortality, malformation (light gray bars), and severe malformation (dark gray bars) of zebrafish embryos at 3, 6–7, 12, and 24 h after fertilization within 5 d of exposure to UVB at 0.93 J/cm2. Inset images show typical morphologies of the developing embryos at these times. Comparisons amongst embryo ages were made, with bars sharing the same letter indicating no significant difference from each other (P > 0.05).
3.5. Animal source influences both UVB toxicity and UVA photorepair
Responses of embryos from pond-raised fish to UVB radiation were compared to those of embryos from laboratory-raised fish (Fig. 5, with Fig. 2 data included). There were lower hatch rates and higher mortality in laboratory embryos than pond-derived embryos throughout the dose–response curve, although significant differences (P < 0.05) occurred only with UVB doses of 1.56 J/cm2 or greater. In addition, embryos from pond-fish were able to tolerate UVB exposures as high as 3.11 J/cm2. For malformation, there were no significant differences between these two sources (P > 0.05). However, the calculated LD50 of UVB radiation for laboratory embryos was 0.63 J/cm2, which is lower than that of pond-derived embryos (2.32 J/cm2, see above).
Fig. 5.
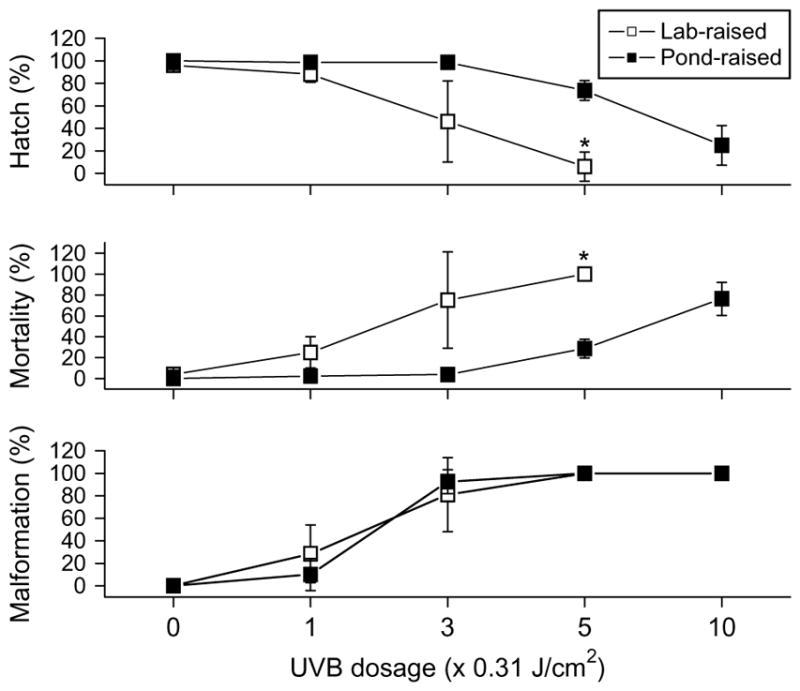
Effect of animal source on UV tolerance in zebrafish embryos. Comparisons of percent hatch, mortality, and malformation of embryos collected from pond-raised (filled symbols) and laboratory-raised (open symbols) zebrafish within 5 d of exposure to UVB for 0, 1, 3, 5, and 10 min at the dosage of 0.31 J/cm2 per min. The exposure occurred at the mid-gastrula stage of development. Asterisks indicate significant difference (P < 0.05) of laboratory-raised embryo responses from pond-raised embryo responses at that exposure.
A dose of 0.93 J/cm2UVB radiation induced severe deformations in embryos from both pond-raised and laboratory-raised zebrafish (Fig. 6, 97 ± 5% vs. 90 ± 17%, P = 0.550). Both sources of zebrafish had similar photorepair responses to this dose of UVB, with no occurrences of severe malformations after exposure to UVA (0 ± 0%). These results indicate a competent photorepair system is present in embryos obtained from both the pond-raised and laboratory-raised fish, even though the embryos obtained from laboratory-raised zebrafish were less tolerant to UVB exposure.
Fig. 6.
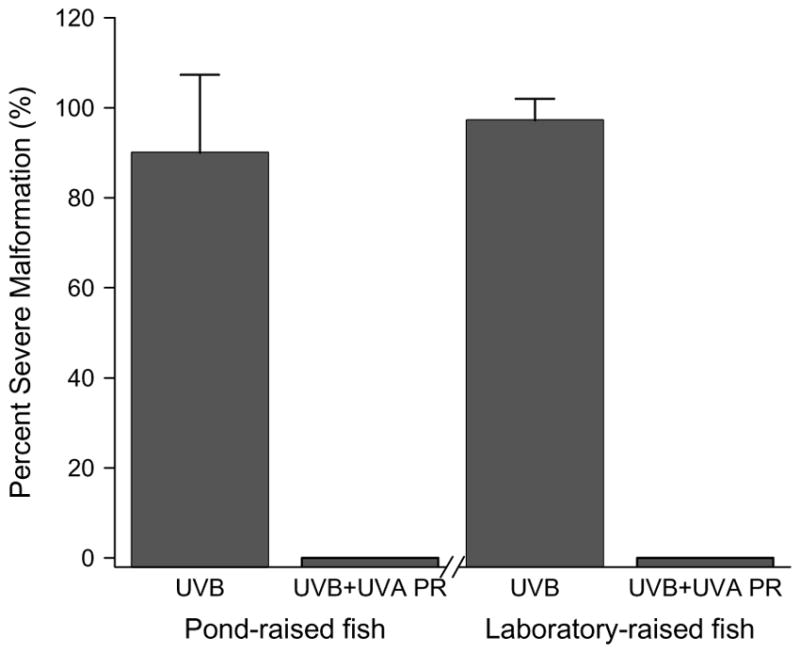
Effect of animal source on photorepair ability in zebrafish embryos. Comparisons of percent severe malformations arising in embryos collected from pond- and laboratory-raised zebrafish exposed to UVB (0.93 J/cm2) alone or UVB (0.93 J/cm2) followed immediately by UVA (211.5 J/cm2). The exposure occurred at the mid-gastrula stage of development.
4. Discussion
Zebrafish have become a major model organism for studying embryonic development, physiology and disease. While many of these studies use light for imaging and other optical methods, tolerance thresholds to UV light have not been established, nor have clear indicators of malformation been identified as photodamage markers. In most UVA photoactivation studies utilizing zebrafish, dose–responses to light are not characterized, although these wavelengths are commonly used to photocleave caged compounds, excite and photobleach fluorophores, or continuously monitor fluorescent protein expression [7–10]. While the effects of UVA may be more subtle than those of UVB and UVC due to less direct absorption by biological targets, they can nonetheless have impacts via the generation of oxidative radical species [19]. In addition to the more common UVA photoactivation methods, UVB exposures have also been applied in zebrafish to study antioxidant status, p53 signaling, and targeted mutagenesis [4,16,17]. Thus we systematically characterized UVB and UVA sensitivities of zebrafish embryos to identify thresholds limits for such studies utilizing these light exposures.
4.1. Zebrafish embryo morphological responses to UV
Morphological monitoring of developing zebrafish embryos for gross photodamage is simple and informative. As shown in Fig. 1, phenotypic responses such as slight curvature or pronounced kinking of the spine can be easily detected in developing embryos. The predominant spinal deformities associated with UVB radiation may relate to the exposure position. For embryos exposed during the mid-gastrula stage, one side of the blastoderm is thicker than the other and thus may differentially expose lateral tissues depending on orientation. The thinner side marks the site of the future dorsal surface of the embryo [29]. Because we did not orient embryos, future studies controlling the exposure position could confirm this possibility. However, given the large malformation rates at the doses of UVB tested, there may not be a significant effect of orientation on resulting developmental responses.
In addition to the observation of mortality (indicated by heartbeat cessation), the occurrence of larvae hatching from the egg can be used as an indicator of UV-induced photodamage. The frequency of hatching and mortality are not reciprocal, as there were larvae that hatched but died prior to 5 d. Similar representation of zebrafish hatch and mortality rates is seen in toxicological studies [30,31]. The time of hatching in zebrafish embryos can vary within the third day after fertilization and thus is not a precise staging index for development [1]. Nonetheless, lag times in hatch onset beyond the third day can be indicative of developmental delays and toxicities. At the UVB doses tested here, a significant delay in hatch onset was not observed. However, for UVA exposures, a difference in the percentage of hatched embryos at 3, 4, and 5 d following fertilization was observed as a function of dose (Table 1). Doses large enough to cause a measurable delay in development must also be less than the threshold for lethality. This fact is particularly noticeable when comparing the UVB and UVA hatch times, as most of the UVB doses either had no effect or were lethal in nature, preventing an observation of delayed hatching. The dependence of delayed development on ultimate survival may also play a role in these experiments. For instance, it is plausible that because UVA exposure could create a delay in development, the lag time could allow recovery processes (e.g., photorepair) to occur that increase survival rates. This UVA-induced delay could be a result of decreased metabolic rates, similar to the responses observed in adult cichlid fish (Cichlasoma nigrofasciatum) after exposure to varying UVA doses [32].
Table 1.
Developmental delays in mid-gastrula zebrafish from UVB and UVA exposure
| UVB dose (× 0.31 J/cm2) | Hatch (%) | UVA dose (×42.3 J/cm2) | Hatch (%) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Day 3 | Day 4 | Day 3 | Day 4 | Day 5 | ||
| 0 | 100 ± 0 | – | 0 | 100 ± 0 | – | – |
| 1 | 99 ± 2 | – | 8 | 97 ± 6 | 100±0 | – |
| 3 | 99 ± 2 | – | 10 | 89 ± 17 | 99 ± 3 | 100 ± 0 |
| 5 | 73 ± 7 | 74 ± 9 | 12 | 74 ± 36 | 93 ± 13 | 100 ± 0 |
| 10 | 25 ± 18 | † | 15 | 63 ± 54 | 88 ± 22 | 92 ± 14 |
| 18 | 38 ± 33 | 60 ± 33 | † | |||
| 20 | 32 ± 15 | 55 ± 13 | † | |||
Percent hatch (mean ± SD) of gastrulated zebrafish embryos at days 3, 4, and 5 after exposure to UVB for 0, 1, 3, 5, and 10 min at a dosage of 0.31 J/cm2 per min, and UVA for 0, 8, 10, 12, 15, 18, and 20 min at a dosage of 42.3 J/cm2 per min.
“–” indicates termination of hatch rate monitoring due to complete hatching in prior observation, whereas “†” indicates no surviving embryos present.
4.2. UVB and UVA sensitivities of zebrafish
The dose–responses for zebrafish embryo mortality, hatch rates, and malformation rates as a result of UVA and UVB light followed typical sigmoidal profiles (Fig. 2). As we expected, zebrafish toxicities were greater for UVB than UVA exposure, with more than a 350-fold difference between the LD50 values (UVB, 2.32 J/cm2 vs. UVA, 855 J/cm2). The high resistance of zebrafish embryos to UVA exposure seen here confirms the suitability of this animal model for photoactivation studies. In comparison, it is understandable that the tolerable doses of UV in zebrafish embryos are greater than those observed in cell culture. Cells without protective structures or pigment can tolerate only lower doses of UVA (1–100 J/cm2), and these responses have been shown to vary depending on cell type and biochemical assay for photodamage (reviewed in [18]).
Significant malformations in zebrafish resulting from UVB exposure was seen at 0.93 J/cm2, indicating that malformation was a more subtle indicator of UVB damage than hatch rates or mortality. However, there are likely more sensitive indicators of UVB damage, as 0.005 J/cm2 was sufficient to stimulate some aspects of DNA repair in zebrafish, although the specific mechanisms have not been identified [33]. While to our knowledge, a systematic characterization of UVA dosing regimes in zebrafish embryos has not been presented, data have been gathered for responses of zebrafish to UVB as part of environmental studies.
Thorough assessments of zebrafish embryo survival and antioxidant status resulting from UVB exposures have been carried out in newly fertilized and recently hatched eggs [16]. The results indicate that survival rates for newly hatched larvae exposed to 1.95 W/m2 for 6 h each day over 2 d (8.4 J/cm2 total dose), were zero. While the exact ages of the hatched larvae were not specified, they were presumably greater than the oldest larvae (24 hpf) we analyzed. However, similar trends can be observed, with earlier developmental stages being more tolerant to UVB exposure. However, important differences can be noted between these studies such as in the duration and intensity of exposures, spectra of the UVB lamps, and the strains and sources of the zebrafish studied. These factors prompted our study of the potential differences that stage of development as well as source of fish can have on UVB tolerances in zebrafish.
4.3. Influences of embryo source and stage on UV sensitivity
Direct DNA damage from UV exposure is more serious for embryos, larvae, and cells in culture in which gene expression is more active [34]. Additionally, there are differences in UV sensitivity among the developmental stages of zebrafish embryos (e.g., Fig. 4). Embryos at the 1000-cell stage (3 hpf) were significantly more tolerant to UVB exposure than were embryos at later stages. Embryos at the segmentation stage (12 hpf) were most vulnerable, suggesting significant changes in photoprotective or photorepair mechanisms as a function of developmental stage. Medaka embryos (Oryzias latipes) also showed differing susceptibility to UVA exposure at different stages of development, suggesting that morphological events at these stages were vulnerable to oxidative stress [35]. In zebrafish, the mid-blastula transition in embryos occurring at cell cycle 10 is associated with the onset of transcription [36], potentially explaining the increased sensitivity to UV seen after the 3 hpf age.
In addition to stage sensitivity of zebrafish embryos, we evaluated differences in UV responses between fish derived from two different environments (indoors and outdoors). Significant differences in hatch and mortality rates of embryos derived from laboratory-raised versus pond-raised fish were observed at various UVB doses (Fig. 5). The pond-raised zebrafish were reared in shallow ponds and thus presumably received more UV exposure than did laboratory-raised fish that had not been exposed to natural sunlight for generations. In addition, there are other potential differences between these environments that could affect results, such as fish strain, degree of inbreeding, diet, fish behavior or social cues. Lastly, differences in the genetic backgrounds of the fish used in this study may contribute to the varying abilities to tolerate UV exposure. It may be the case that the laboratory-raised fish have a slightly modified genome based on the fact that these fish, especially those from the AB strain, have been intercrossed for generations. If this intercrossing has indeed modified the genome, it is possible that some genes related to the ability to tolerate UV exposure may have been modified or lost in these fish. At this time we cannot rule against this possibility. Regardless, these differences highlight the need for caution in making direct comparisons across zebrafish from different strains or sources for photobiological studies. There were no observable differences in embryo pigmentation between these sources at any of the stages we evaluated, as the chromophores are generally not seen until later stages of development. Zebrafish embryos used in microscopy studies can be treated with phenylthiourea to inhibit pigment formation altogether [37], but the consequences of such treatments were not considered in this study. These changes in UV tolerance could also be due to differential levels of maternal protective compounds and pathways, or increased susceptibility due to onset of UV-sensitive metabolic processes such as gene expression.
4.4. A competent photorepair system in zebrafish
In general, the two most frequent types of DNA damage induced by UVB radiation are 5,6-dipyrimidines, referred to as cyclobutane pyrimidine dimers (CPDs,) and pyrimidine (6-4) pyrimidones, often referred to as (6-4) photoproducts [38]. Repair of DNA damage usually takes place through two main mechanisms: (1) photoenzymatic repair (PER), which involves the direct monomerization of CPDs by DNA photolyase in the presence of light, particularly UVA and short wavelength visible light [20,38,39]; and (2) nucleotide excision repair (NER,) a generalized DNA repair mechanism acting on both CPDs and the (6-4) photoproducts. Because there is no requirement for light, NER is also known as dark repair and is present in all organisms. In contrast, PER is not observed in all organisms, and has been documented primarily in marine phytoplankton [13]. Here we suggest that zebrafish have a competent PER system that can be driven by UVA exposure. Zebrafish embryos exposed to 0.93 or 1.56 J/cm2 of UVB during mid-gastrulation underwent significant mortality if maintained in the dark or kept on a normal ambient light cycle. However, exposure to UVA immediately after the UVB exposure can induce morphological recovery in these embryos in a dose-dependent manner (Fig. 3). Many of the protection-repair mechanisms operating in cells are inducible, and cells exposed gradually to bright light or UV are less prone to damage or are tolerant to UV exposure (e.g., [40]). However, in this study, exposure of embryos to UVA before UVB exposure did not reduce percent hatch, mortality or malformation (data not shown), suggesting the PER system could not be “primed” to act on subsequent injury. Malformation in embryos obtained form pond-raised and laboratory-raised zebrafish was reduced to background levels by exposure to UVA after UVB radiation during the mid-gastrula stage of development (Fig. 6), indicating that zebrafish from either source have an efficient PER system not affected by rearing conditions. Direct DNA damage in the form of CPDs may be the causative factors in malformation from UVB. However, PER also targets non-CPD lesions in fish DNA such as (6-4) pyrimidones [41,42], suggesting that this type of damage is also involved in malformation. Investigation of light-sensitivity in the context of circadian development confirmed the presence of 6-4 photolyase in zebrafish embryos [21]. That study showed that 6-4 photolyase mRNA was present at 6 hpf, prior to the start of zygotic transcription, yet decreased thereafter unless stimulated with UV light. While the spectra of the UV light used was not specified, the stage-associated vulnerabilities noted are similar to those that we observed with UVB. Together these studies suggest that in addition to widespread use for genomic and photoactivation studies, zebrafish represent an appropriate model for studying photorepair. Numerous future studies are necessary to fully characterize photorepair in zebrafish, such as determining the exact onset of photorepair in development, the time window (or delay of UVA exposure) allowed for recovery from UVB, specific molecular markers confirming photorepair in zebrafish, and different clues that could be more sensitive indicators of photodamage than gross morphology.
Supplementary Material
Acknowledgments
This work was supported in part by the Louisiana State University Agricultural Center Biotechnology Education for Students and Teachers (BEST) Postdoctoral Fellowship to QD. We thank R. Tulley for administrative assistance, J. Forman, R. Blidner, M. Huguet, J. Casey, and M. Lapré for laboratory help, and R. Pollet for fish care. This manuscript was approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 07-22-0.
Abbreviations
- UVA
Ultraviolet A
- UVB
Ultraviolet B
- DAPI
4′,6-diamidino-2-phenylindole
- CPDs
cyclopyrimidine dimers
- hpf
hours post-fertilization
- PR
photorepair
- PER
photoenzymatic repair
- NER
nucleotide excision repair
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jphotobiol.2007.07.002.
References
- 1.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dynam. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 2.Udvadia AJ, Linney E. Windows into development: historic, current, and future perspectives on transgenic zebrafish. Dev Biol. 2003;256:1–17. doi: 10.1016/s0012-1606(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 3.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 4.Langheinrich U, Hennen E, Stott G, Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol. 2002;12:2023–2028. doi: 10.1016/s0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- 5.Berghmans S, Jette C, Langenau D, Hsu K, Stewart R, Look T, Kanki JP. Making waves in cancer research: new models in the zebrafish. Biotechniques. 2005;39:227–237. doi: 10.2144/05392RV02. [DOI] [PubMed] [Google Scholar]
- 6.Beis D, Stainier DYR. In vivo cell biology: following the zebrafish trend. Trends Cell Biol. 2006;16:105–112. doi: 10.1016/j.tcb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem Cell Biol. 1997;75:551–562. [PubMed] [Google Scholar]
- 8.Ando H, Furuta T, Tsien RY, Okamoto H. Photo-mediated gene activation using caged RNA/DNA in zebrafish embryos. Nat Genet. 2001;28:317–325. doi: 10.1038/ng583. [DOI] [PubMed] [Google Scholar]
- 9.Ando H, Okamoto H. Practical procedures for ectopic induction of gene expression in zebrafish embryos using Bhc-diazo-caged mRNA. Method Cell Sci. 2003;25:25–31. doi: 10.1023/B:MICS.0000006846.13226.38. [DOI] [PubMed] [Google Scholar]
- 10.Stark DA, Kulesa PM. Photoactivatable green fluorescent protein as a single-cell marker in living embryos. Dev Dynam. 2005;233:983–992. doi: 10.1002/dvdy.20385. [DOI] [PubMed] [Google Scholar]
- 11.Häder DP, Kumar HD, Smith RC, Worrest RC. Aquatic ecosystems: effects of solar ultraviolet radiation and interactions with other climatic change factors. Photochem Photobiol Sci. 2003;2:39–50. doi: 10.1039/b211160h. [DOI] [PubMed] [Google Scholar]
- 12.Farman JC, Gardiner BG, Shanklin JD. Large losses of total ozone in Antarctica reveal seasonal clox/nox interaction. Nature. 1985;315:207–210. [Google Scholar]
- 13.Jeffrey WH, Kase JP, Wilhelm SW. UV radiation effects on heterotrophic bacterioplankton and viruses in marine ecosystems. In: de Mora S, Demers S, Vernet M, editors. The Effects of UV radiation in the Marine Environment, Cambridge Environmental Chemistry Series. Vol. 10. Cambridge University Press; New York: 2000. pp. 206–236. [Google Scholar]
- 14.Vernet M. Effects of UV radiation on the physiology and ecology of marine phytoplankton. In: de Mora S, Demers S, Vernet M, editors. The Effects of UV radiation in the Marine Environment, Cambridge Environmental Chemistry Series. Vol. 10. Cambridge University Press; New York: 2000. pp. 237–278. [Google Scholar]
- 15.Zagarese HE, Williamson CE. Impact of solar UV radiation on zooplankton and fish. In: de Mora S, Demers S, Vernet M, editors. The Effects of UV radiation in the Marine Environment, Cambridge Environmental Chemistry Series. Vol. 10. Cambridge University Press; New York: 2000. pp. 279–309. [Google Scholar]
- 16.Charron RA, Fenwick JC, Lean DR, Moon TW. Ultraviolet-B radiation effects on antioxidant status and survival in the zebrafish, Brachydanio rerio. Photochem Photobiol. 2000;72:327–333. doi: 10.1562/0031-8655(2000)072<0327:ubreoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Meunier JR, Sarasin A, Marrot L. Photogenotoxicity of mammalian cells: a review of the different assays for in vitro testing. Photochem Photobiol. 2002;75:437–447. doi: 10.1562/0031-8655(2002)075<0437:POMCAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Forman J, Dietrich M, Monroe WT. Photobiological and thermal effects of photoactivating UVA light doses on cell cultures. Photochem Photobiol Sci. 2007;6:649–658. doi: 10.1039/b616979a. [DOI] [PubMed] [Google Scholar]
- 19.Tyrrell RM, Keyse SM. New trends in photobiology – the interaction of UVA radiation with cultured cells. J Photochem Photobiol B. 1990;4:349–361. doi: 10.1016/1011-1344(90)85014-n. [DOI] [PubMed] [Google Scholar]
- 20.Sinha RP, Häder DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 21.Tamai TK, Vardhanabhuti V, Foulkes NS, Whitmore D. Early embryonic light detection improves survival. Curr Biol. 2004;14:R104–R105. [PubMed] [Google Scholar]
- 22.Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon Press; 1995. [Google Scholar]
- 23.Buma AGJ, Boelen P, Jeffrey WH. UVR-induced DNA damage in aquatic organisms. In: Helbling EW, Zagarese H, editors. UV Effects in Aquatic Organisms and Ecosystems, Comprehensive Series in Photochemistry and Photobiology. Vol. 1. Royal Society of Chemistry; Cambridge: 2003. pp. 291–329. [Google Scholar]
- 24.Sancar A, Sancar GB. DNA-repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- 25.Pelliccioli AP, Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 26.Daiyasu H, Ishikawa T, Kuma K, Iwai S, Todo T, Toh H. Identification of cryptochrome DASH from vertebrates. Genes Cells. 2004;9:479–495. doi: 10.1111/j.1356-9597.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- 27.Thompson CL, Sancar A. Photolyase/cryptochrome blue-light photoreceptors use photon energy to repair DNA and reset the circadian clock. Oncogene. 2002;21:9043–9056. doi: 10.1038/sj.onc.1205958. [DOI] [PubMed] [Google Scholar]
- 28.Wiegand MD, Young DLW, Gajda BM, Thuen DJM, Rittberg DAH, Huebner JD, Loadman NL. Ultraviolet light-induced impairment of goldfish embryo development and evidence for photorepair mechanisms. J Fish Biol. 2004;64:1242–1256. [Google Scholar]
- 29.Schmitz B, Camposortega JA. Dorsoventral polarity of the zebrafish embryo is distinguishable prior to the onset of gastrulation. Roux Arch Dev Biol. 1994;203:374–380. doi: 10.1007/BF00188685. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol Pharmacol. 2002;62:234–249. doi: 10.1124/mol.62.2.234. [DOI] [PubMed] [Google Scholar]
- 31.Reimers MJ, Flockton AR, Tanguay RL. Ethanol- and acetaldehyde-mediated developmental toxicity in zebrafish. Neurotoxicol Teratol. 2004;26:769–781. doi: 10.1016/j.ntt.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Winckler K, Fidhiany L. Significant influence of UVA on the general metabolism in the growing cichlid fish, Cichlasoma nigrofasciatum. J Photochem Photobiol B. 1996;33:131–135. doi: 10.1111/j.1751-1097.1996.tb03074.x. [DOI] [PubMed] [Google Scholar]
- 33.Cui ZB, Yang Y, Kaufman CD, Agalliu D, Hackett PB. RecA-mediated, targeted mutagenesis in zebrafish. Mar Biotechnol. 2003;5:174–184. doi: 10.1007/s10126-002-0059-0. [DOI] [PubMed] [Google Scholar]
- 34.Naganuma T, Inoue T, Uye S. Photoreactivation of UV-induced damage to embryos of a planktonic copepod. J Plankton Res. 1997;19:783–787. [Google Scholar]
- 35.Bass EL, Sistrun SN. Effect of UVA radiation on development and hatching success in Oryzias latipes, the Japanese medaka. Bull Environ Contam Toxicol. 1997;59:537–542. doi: 10.1007/s001289900512. [DOI] [PubMed] [Google Scholar]
- 36.Kane DA, Kimmel CB. The zebrafish mid-blastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson J, Hofsten Jv, Olsson P. Generating transparent zebrafish: a refined method to improve detection of gene expression during embryonic development. Mar Biotechnol. 2001;V3:522–527. doi: 10.1007/s1012601-0053-4. [DOI] [PubMed] [Google Scholar]
- 38.Vincent WF, Neale PJ. Mechanisms of UV damage to aquatic organisms. In: de Mora S, Demers S, Vernet M, editors. The Effects of UV radiation in the Marine Environment, Cambridge Environmental Chemistry Series. Vol. 10. Cambridge University Press; New York: 2000. pp. 149–176. [Google Scholar]
- 39.Mitchell DL, Nairn RS, Johnston DA, Byrom M, Kazianis S, Walter RB. Decreased levels of (6-4) photoproduct excision repair in hybrid fish of the genus Xiphophorus. Photochem Photobiol. 2004;79:447–452. doi: 10.1562/ca-03-14.1. [DOI] [PubMed] [Google Scholar]
- 40.Roos JC, Vincent WF. Temperature dependence of UV radiation effects on Antarctic cyanobacteria. J Phycol. 1998;34:118–125. [Google Scholar]
- 41.Meador JA, Walter RB, Mitchell DL. Induction, distribution and repair of UV photodamage in the platyfish, Xiphophorus signum. Photochem Photobiol. 2000;72:260–266. doi: 10.1562/0031-8655(2000)072<0260:idarou>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Uchida N, Mitani H, Todo T, Ikenaga M, Shima A. Photoreactivating enzyme for (6-4) photoproducts in cultured goldfish cells. Photochem Photobiol. 1997;65:964–968. doi: 10.1111/j.1751-1097.1997.tb07955.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.