Abstract
The objectives of this study were to evaluate the effects of cryoprotectant, osmotic pressure, cooling rate, equilibration time, and sperm-to-extender ratio, as well as somatic relationships of body length, body weight, and testis weight to sperm density in the platyfish Xiphophorus couchianus. Sperm motility and survival duration after thawing were significantly different between cryopreservation with dimethyl sulfoxide (DMSO) and glycerol, with the highest motility at 10 min after thawing obtained with 14% glycerol. With subsequent use of 14% glycerol as cryoprotectant, the highest motility after thawing was observed with Hanks’ balanced salt solution (HBSS) across a range of 240–300 mOsm/kg. Samples cooled from 5 to −80 °C at 25 °C/min yielded the highest post-thaw motility, although no significant difference was found for cooling rates across the range of 20–30 °C/min. In addition, the highest motility after thawing was found in samples equilibrated from 10 to 30 min with 14% glycerol and cooled at 25 °C/min. The post-thaw motility declined rapidly with use of 10% glycerol and cooling at 5 °C/min across the equilibration range of 10 min to 2 h. Sperm motility with a dilution ratio of sperm to extender of 1:10 was not different at 10 min after thawing with those samples at greater dilutions, but declined significantly from Day 1 after thawing and showed lower survival duration when stored at 4 °C. However, the additional dilution of sperm solutions with HBSS (300 mOsm/kg) immediately after thawing significantly slowed the decline of motility and prolonged the duration of survival. Based on the above findings, the highest average sperm motility (78 ± 3%) at 10 min after thawing was obtained when sperm were suspended in HBSS at 300 mOsm/kg with 14% glycerol as cryoprotectant, diluted at a ratio of sperm to HBSS–glycerol of 1:20, equilibrated for 10 min, cooled at 25 °C/min from 5 to −80 °C before plunging into liquid nitrogen, and thawed at 40 °C in a water bath for 7 s. If diluted within 5 h after thawing, sperm frozen by the above protocol retained continuous motility for 15 days when stored at 4 °C.
Keywords: Cryopreservation, Xiphophorus couchianus, Sperm, Germplasm repositories
1. Introduction
The first paper on fish sperm cryopreservation was published 50 years ago [1]; since then, more than 200 fish species have been studied [2]. Small teleost fishes have been studied little except for the research models zebrafish (Danio rerio) and Japanese medaka (Oryzias latipes) [3]. Published studies of sperm cryopreservation of small live-bearing fish are completely lacking except for our initial research on the green swordtail Xiphophorus helleri [4]. Live-bearing fishes as a group are distinct from other fishes for many reasons, most notably for internal fertilization in which males transfer sperm in packages (spermatophores) to females which store the sperm in the receptaculum seminis or “Delle” of the genital tract for as long as several months after insemination [5–8].
Worldwide, there are more than 1000 species of live-bearing fishes [8] and many of them have important research and commercial value. For example, swordtails and platyfish of the genus Xiphophorus can be hybridized and are widely used in diverse areas of contemporary scientific research, including evolution [9], sex determination [10], endocrinology [11], ethology and behavioral ecology [12,13], toxicology [14], parasitology [15], immunology [16,17], and cancer genetics [18–20]. The increasing research value and the continuous decline of diversity in the wild of these fishes has expanded the need to preserve their genetic material. However, cryopreservation techniques that can be applied to small fishes are limited [21]. In addition, the internal fertilization mode of live-bearing fishes presents significant challenges in sperm quality assessment after cryopreservation.
Our studies of sperm cryopreservation with the green swordtail developed basic techniques necessary for cryopreservation of sperm from small live-bearing fishes [4]. The present study was designed to develop practical techniques of sperm cryopreservation for the platyfish Xiphophorus couchianus. Our areas of investigation included relationships of body length, body weight and testis weight to sperm density, osmolality of blood plasma and sperm motility, the effect of cryoprotectants (CPAs) and concentrations, cooling rate, and equilibration time on sperm viability, and finally, the effect of dilution ratio of sperm to extender before freezing and after thawing.
2. Materials and methods
2.1. Sperm collection and dilution
A total of 70 male X. couchianus ranging from 6 to 12 months of age were shipped weekly by overnight delivery from the Xiphophorus Genetic Stock Center (XGSC) of Texas State University, San Marcos, to the Aquaculture Research Station (ARS) of the Louisiana State University Agricultural Center in January and February of 2003. Within 2–7 days of arrival at the ARS, fish were anesthetized in 0.01% tricaine–methane sulfonate (Western Chemical Inc., Ferndale, WA) for 2 min, and standard lengths (tip of snout to the crease of the caudal peduncle) and wet weights were measured after blotting excess water with a paper tower. Blood samples were collected in micro-hematocrit tubes with an internal diameter of 0.5–0.6 mm (VWR Scientific, Niles, IL) by severing the tail. After centrifugation at 10,000 rpm for 10 min, six 10-μl replicates were obtained from pooled blood samples from 45 fish. Osmolality of the blood plasma was measured by vapor pressure osmometry (Model 5500; Wescor Inc., Logan, UT).
Sperm were collected by surgical removal of the testis. Adherent tissue was dissected away and testes were pooled and placed in tared resealable plastic bags (NASCO whirl-pak, MBCOCT, New Haven, CT) and weighed. Hanks’ balanced salt solution (NaCl 8.0 g, KCl 0.4 g, CaCl2·2H2O 0.16 g, MgSO4·7H2O 0.2 g, Na2HPO4 0.06 g, KH2PO4 0.06 g, NaHCO3 0.35 g, and C6H12O6 1.0 g in 1000 ml distilled water) was added before crushing testes to release sperm. Dilutions with HBSS were based on testes weight. Except for the experiment used to evaluate different ratios of testis to HBSS, the ratio of testis to HBSS (mass:volume) was always 1:100. Based on our preliminary research on X. helleri [4], HBSS at 300 mOsm/kg was used for sperm suspension after collection. Within this manuscript, HBSS at specific osmolalities such as 300 mOsm/kg are abbreviated as HBSS 300. Sperm numbers per testis were obtained from the average of duplicate counts using a hemocytometer (using a dilution factor of 101).
2.2. Motility estimation
The motility of Xiphophorus sperm is distinct from that of other teleosts. The sperm were motile upon collection and they remained continuously motile after suspension in HBSS, and therefore activation solutions were not necessary for motility estimates [4]. For estimation, a 5-μl aliquot was removed from each sample and placed on a glass microscope slide without a cover slip. The final ratio of sperm to HBSS-CPA (extender with cryoprotectant) was about 1:100 before estimation. Sperm motility was estimated visually at 200× magnification using darkfield microscopy (Optiphot 2, Nikon Inc., Garden City, NY) and was expressed as the percentage of cells (in increments of 2% when motility was less than 10%, otherwise in increments of 5%) actively moving in a forward direction (e.g. [22]). Sperm vibrating in place were not considered to be motile. After thawing, samples were stored at 4 °C and motility was monitored at various intervals based on experimental design until motility ceased or the entire sample volume was depleted.
2.3. Freezing procedures
Aliquots (80 μl) of sperm suspensions with cryoprotectant (detailed below) were drawn into 0.25-ml French straws (IMV International, Minneapolis, MI) and held (equilibrated) for 8 min at room temperature (23–25 °C) and 2 min at 4 °C before cooling in a controlled-rate freezer (Kryo 10 Series II; Planer Products, Sunbury-on-Thames, UK) at 45 °C/min from 5 to −80 °C. The straws were transferred to a liquid nitrogen storage dewar after the temperature reached −80 °C. After a minimum of 12 h, the straws were thawed for 7 s in a 40 °C water bath (Model 1141, VWR Scientific).
2.4. Effect of cryoprotectants and concentrations
There were two trials in this experiment. In the first trial, sperm samples from six males were used. Sperm–extender suspension was prepared from the pooled sperm suspension at a ratio of sperm to HBSS 300 of 1:50, and was divided into eighteen 40-μl sub-samples. Each sub-sample was mixed with equal volumes of freshly prepared HBSS-CPA solution. The studied cryoprotectants were dimethyl sulfoxide (DMSO) and glycerol, and each was evaluated with three final concentrations of 6, 10, and 14% [23]. To minimize differences in final osmolality (that can affect sperm motility [23]), suspensions were prepared with HBSS 240 for DMSO and HBSS 300 for glycerol (according to our previous studies with X. helleri). Three replicate samples were used for each CPA concentration combination. Motility was estimated at 10 min and at 12, 24, 36, 60, 84, 108, 132, and 156 h after thawing.
In the second trial, involving sperm of six males, the final concentrations of glycerol were increased to 5, 8, 11, 14, 17, and 20%. Motility was estimated at 10 min and at 12, 24, 36, 60, 84, and 108 h after thawing.
2.5. Effect of osmolality of HBSS
Based on the results of the previous experiments, 14% glycerol was used for subsequent studies. Nine males were used for osmolality evaluation in this experiment. The final osmolality of the sperm–HBSS–glycerol mixtures was prepared by adjusting the ratio of HBSS 800 to distilled water. Each of the four sub-samples from the pooled sperm suspensions was used to evaluate the nine osmolalities: 150, 180, 210, 240, 270, 300, 330, 360, and 390 mOsm/kg. Motility was estimated at 10 min and at 4, 16, 40, 64, 88, 112, 136, and 160 h after thawing.
2.6. Effect of cooling rate
There were two trials in this experiment. In the first trial, sperm from three males were used to evaluate three cooling rates: 5, 25, and 45 °C/min from 5 to −80 °C before plunging into liquid nitrogen. Four sub-samples from the pooled sperm suspension were used for each cooling rate. Motility was estimated at 10 min and at 12, 36, 60, 84, 108, and 156 h after thawing.
To more accurately identify the appropriate cooling rate, the second trial used sperm from five males and evaluated rates at a narrower interval: 15, 20, 25, 30, and 35 °C/min from 5 to −80 °C. Motility was estimated at 10 min, 9 h, and at 1, 2, 3, 5, and 7 day(s) after thawing.
2.7. Effect of equilibration time combined with cooling rate
Five equilibration periods and two cooling rates were tested with four or five sub-samples from the pooled sperm suspension for each period and cooling rate of combination. Samples were equilibrated for 10, 20, 30, 60, and 120 min before cooling at a rate of 25 or 5 °C/min from 5 to −80 °C. Before the addition of HBSS–glycerol, samples were stored at 4 °C. Two trials with eight fish used in each trial were performed. The first trial used 14% glycerol, and motility was estimated at 10 min, 12 h, and at 1, 2, 3, 5, 7, and 9 day(s) after thawing. The second trial used 10% glycerol, and motility was estimated only at 10 min after thawing.
2.8. Effect of dilution ratio: before freezing and after thawing
There were three trials in this experiment. Based on the results of the previous experiments, a cooling rate of 25 °C/min from 5 to −80 °C was used in this experiment, and 60 μl aliquots of sperm were drawn into 0.25-ml French straws in the second and third trials. In the first trial, eight sperm suspensions were prepared with the pooled sperm of five fish at a ratio of sperm to HBSS–glycerol of 1:20 before freezing. Immediately after thawing, four samples remained undiluted, but each of another four samples was divided into two sub-samples: one of which was diluted with HBSS 300 to yield a final ratio of 1:60, another was diluted with HBSS–glycerol (14%) to the final ratio of 1:60 (Table 1).
Table 1.
Final sperm-to-extender ratios with single (before freezing) and second dilution (after thawing)
| Trial | Single dilution factor | Second dilution factor | Final ratio | Dilution media (second dilution) | Storage temperature (°C) |
|---|---|---|---|---|---|
| First | 1:20 | 0 | 1:20 | – | 4 |
| 1:3 | 1:60 | HBSS | 4 | ||
| 1:3 | 1:60 | HBSS–glycerol | 4 | ||
| Second | 1:10 | 1:2 | 1:20 | HBSS | 4 |
| 1:3 | 1:30 | HBSS | 4 | ||
| 1:20 | 1:2 | 1:40 | HBSS | 4 | |
| 1:3 | 1:60 | HBSS | 4 | ||
| Third | 1:10 | 1:2 | 1:20 | HBSS | 4 |
| 1:20 | 1:2 | 1:40 | HBSS | 4 | |
| 1:2 | 1:40 | HBSS | RTa |
RT: room temperature (~25–26 °C).
In the second trial, sperm pooled from four males were used in this experiment. To obtain the final ratios of sperm to HBSS–glycerol of 1:10 and 1:20, initial ratios of sperm to HBSS of 1:5 and 1:10 were prepared. Three samples were used for each dilution ratio. Each sample was divided into two sub-samples immediately after thawing: one of which was diluted with HBSS 300 to yield final ratios of 1:20 or 1:40, the other was diluted to yield final ratios of 1:30 or 1:60 (Table 1). Motility was estimated at 10 min and at 1, 3, and 6 day(s) after thawing.
In the third trial, 15 sperm samples pooled from eight males were frozen with a sperm-to-HBSS–glycerol ratio of 1:10, and another 30 sperm samples pooled from eight males were frozen with a sperm-to-HBSS–glycerol ratio of 1:20. Three samples with a ratio of 1:10 or six samples with 1:20 were diluted with HBSS 300 to yield 1:20 or 1:40 at 5 min and at 1, 3, 5, and 8 h after thawing. The samples with a ratio of 1:10 were stored at 4 °C before and after dilution, but for each of the six samples with a ratio of 1:20, three were stored at 4 °C and the other three at room temperature before second dilution (Table 1). Motility was estimated at 10 min and at 1, 3, 6, 9, 12, and 15 day(s) after second dilution (after thawing). Samples with dilutions before freezing only are referred to as “single dilution,” and samples with a second dilution (after thawing) are referred to as “double dilution” in this paper.
2.9. Data analysis
Simple linear regression was used to evaluate the relationships of testis weight to sperm per testis, body weight to testes weight, and standard length to body weight. Repeated measures designs were employed for most experiments, using the between-subjects design and the within-subjects design. The former refers to the treatment design and the experiment design used for the experimental units (sperm samples); the latter refers to the repeated measures on each experimental unit. General linear model (GLM) repeated measures was used to test for differences among results for the different cryoprotectants, concentrations of cryoprotectant, osmolalities of HBSS with glycerol as cryoprotectant, cooling rates, equilibration times, and among the different volume ratios of milt to extender–cryoprotectant before freezing and for dilution with HBSS 300 after thawing. Results were presented as mean ± S.D., and probability values of P < 0.05 were considered significant. Data for sperm motility were arcsine transformed prior to analysis when heterogeneity of variance occurred. The software used was SPSS 10.0 for Windows (1999).
3. Results
3.1. Basic parameters
The sizes of the male X. couchianus used in this study were 2.32 ± 0.20 cm (n = 70) for standard length and 295 ± 97 mg (n = 70) for body wet weight. The standard length was positively (P < 0.0001) related to the body wet weight (Fig. 1). The average testis wet weight was 4.7 ± 2.0 mg (n = 70), and was positively (P < 0.0001) related to the body wet weight (Fig. 1). There were 4.42 ± 2.42 × 107 sperm cells per testis (n = 64), and sperm density was 8.66 ± 2.45 × 109 cell/g of testis. There was a significant (P < 0.0001) polynomial relationship between testis weight and sperm per testis (Fig. 1), but no significant relationship was found between testis weight and sperm density. Osmolality of the blood plasma was 324 ± 4 mOsm/kg (n = 6).
Fig. 1.
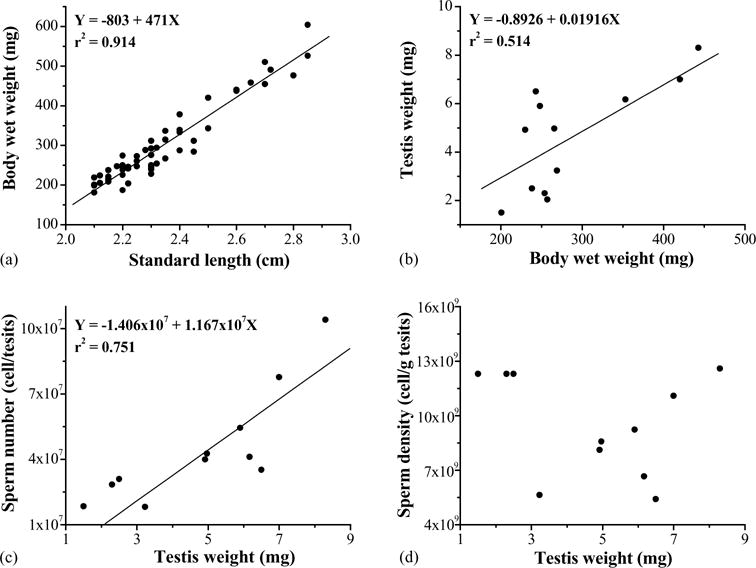
Relationships between standard length and body wet weight (a), body wet weight and testes weight (b), testes weight and sperm number (c), and testis weight and sperm density (d) of Xiphophorus couchianus.
3.2. Effect of cryoprotectants and concentrations
In the first trial, motility of pooled sperm before the addition of cryoprotectants was 80%. At 10 min after thawing, motility was highest with 14% glycerol (52 ± 3%), followed by 10% glycerol (33 ± 3%), 10% DMSO (23 ± 3%), and 6% glycerol (12 ± 3%) (Fig. 2). Motility in the samples with 14% DMSO was lowest (4 ± 3%) at 10 min after thawing, and ceased at 24 h after thawing. Samples with 10% glycerol retained motility the longest, 156 h after thawing. In all of the cryoprotectant and concentration combinations, the only non-significant differences found were between 6% DMSO and 14% DMSO (P = 0.126), and between 10% DMSO and 6% glycerol (P = 0.174).
Fig. 2.
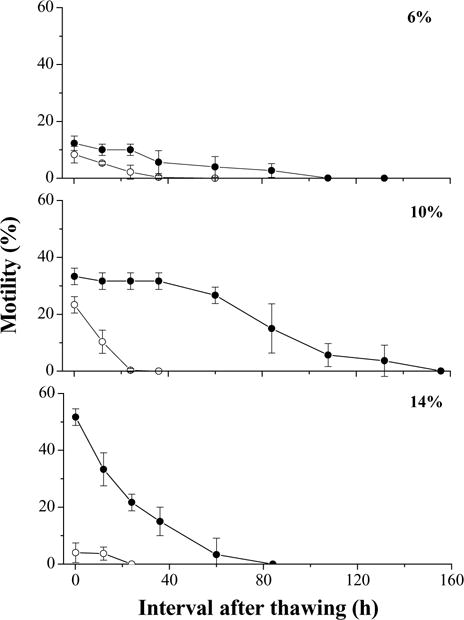
Motility during storage at 4 °C for 156 h (mean ± S.D.) after thawing of Xiphophorus couchianus sperm cryopreserved with dimethyl sulfoxide (DMSO; open circles) or glycerol (filled circles) at concentrations of 6, 10, or 14%. Suspensions were prepared with 240 mOsm/kg HBSS for DMSO and 300 mOsm/kg for glycerol.
In the second trial with glycerol as cryoprotectant, sperm motility before addition of glycerol was 80%. At 10 min after thawing, sperm in the samples with 14 and 17% glycerol had the highest motility (47 ± 6%), followed by 20% (38 ± 8%), 11% (35 ± 5%), and 8% (18 ± 3%) (Fig. 3). Although higher motilities were found at 10 min after thawing for concentrations above 14% than for those below 14%, with prolonged storage the motility of sperm in higher concentration declined faster than did that of sperm in lower concentrations. In this experiment when stored at 4 °C, sperm retained motility for 108 h with 8 and 11% glycerol, for 84 h with 14 and 17%, for 60 h with 6%, and for 36 h with 20%. Sperm motilities in the samples with 11, 14, and 17% glycerol were higher (P = 0.050) than those at 5, 8, and 20%, but there was no difference among 11, 14, and 17% glycerol (P = 0.061).
Fig. 3.
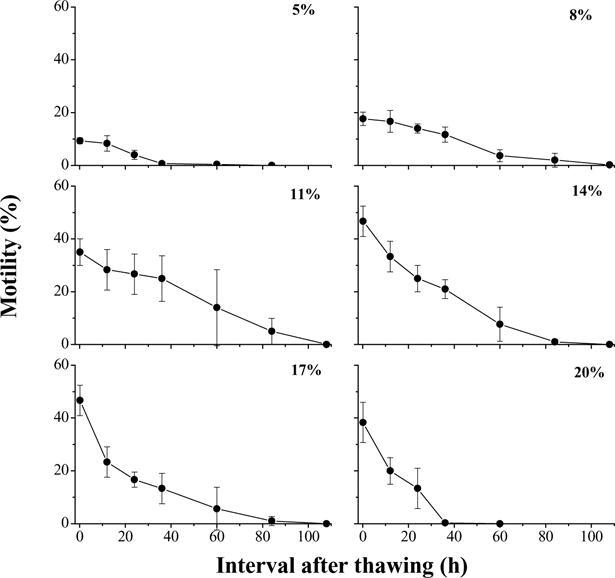
Motility during storage at 4 °C for 108 h (mean ± S.D.) after thawing of Xiphophorus couchianus sperm cryopreserved with glycerol at six concentrations ranging from 5 to 20%. Suspensions were prepared with 300 mOsm/kg HBSS.
3.3. Effect of osmolality of HBSS
The motility of fresh semen in this experiment was 90%. At 10 min after thawing, the lowest motility (14 ± 8%) was found with HBSS at the lowest osmolality (150 mOsm/kg) (Fig. 4). Sperm motility increased with the osmolality of HBSS to the highest level (71 ± 3%) with 270 mOsm/kg and declined thereafter with the increase to HBSS 390. The motility also declined over time, but the same pattern persisted throughout the entire experiment as observed at 160 h after thawing, with the highest motility found at HBSS 270, except for the case at 136 h after thawing when the highest motility was found at HBSS 240. When stored at 4 °C, only sperm suspended in HBSS 240 to HBSS 300 retained motility for 160 h after thawing. Sperm suspended in HBSS 270 had higher motility (P = 0.047) than did those in other osmolalities, except for samples in HBSS 240.
Fig. 4.
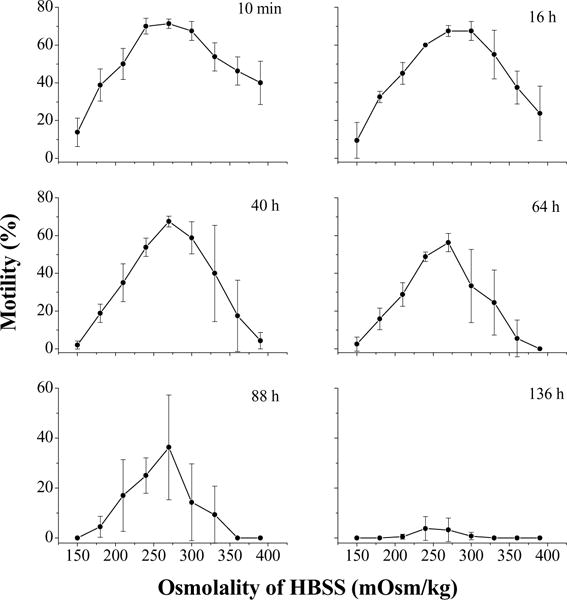
Motility during storage at 4 °C for 160 h (mean ± S.D.) after thawing of Xiphophorus couchianus sperm suspended in Hanks’ balanced salt solution (HBSS) at nine osmolalities ranging from 150 to 390 mOsm/kg.
3.4. Effect of cooling rate
In the first trial, motility of fresh semen was 85%. At 10 min after thawing, the highest motility (68 ± 9%) was found in samples cooled at 25 °C/min, compared with 59 ± 7% at 45 °C/min and 43 ± 9% at 5 °C/min (Fig. 5). The motility declined over time, but the same pattern persisted throughout the entire experiment as observed at Day 7 after thawing, with the highest motility found in samples cooled at 25 °C/min. The motility of samples cooled at 25 °C/min was higher (P = 0.014) than that of those cooled at 45 °C/min, which was higher (P = 0.008) than that cooled at 5 °C/min.
Fig. 5.
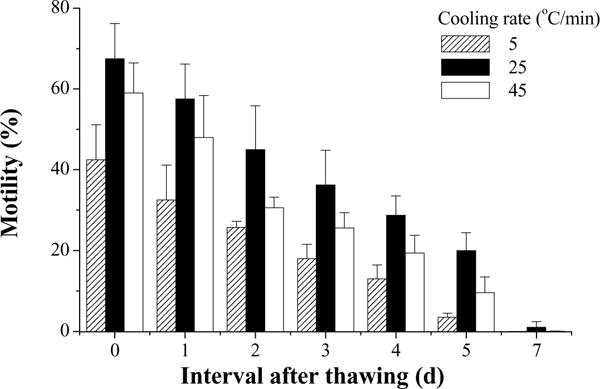
Motility during storage at 4 °C for 7 days (mean ± S.D.) after thawing of Xiphophorus couchianus sperm frozen with three cooling rates.
In the second trial, motility of fresh semen was also 85%. At 10 min after thawing, the highest motility (70 ± 7%) was found in samples cooled at 25 °C/min, followed by those at 30 °C/min (Fig. 6). In this experiment, sperm retained motility over 7 days when stored at 4 °C. The sperm motilities in samples cooled at 20, 25, and 30 °C/min did not have any significant differences among each other, but were higher (P = 0.050) than the motility of samples cooled at 15 and 35 °C/min except for the comparison between 20 and 35 °C/min (P = 0.237).
Fig. 6.
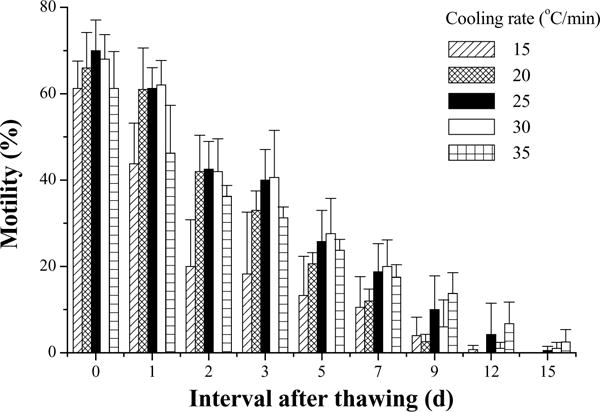
Motility during storage at 4 °C for 15 days (mean ± S.D.) after thawing of Xiphophorus couchianus sperm frozen with five cooling rates.
3.5. Effect of equilibration time combined with cooling rate
In this experiment, motility of fresh semen was 85%. In the first trial with 14% glycerol, the highest motility (66 ± 5%) at 10 min after thawing was found with samples equilibrated for 10 min and cooled at 25 °C/min, and the lowest motility (25 ± 4%) was with 60 min equilibration and a cooling rate of 5 °C/min (Fig. 7). Regardless of the equilibration time, the motilities in samples cooled at 25 °C/min were always higher (P = 0.001) throughout the entire experiment (216 h after thawing) than the motility of samples cooled at 5 °C/min. No interaction was observed between the equilibration time and cooling rate. For the samples cooled at 25 °C/min, the sperm motility with a 60-min equilibration time was lower (P = 0.032) than those with an equilibration time of 10, 20, and 30 min, which were not significantly different among each other (P = 0.379).
Fig. 7.
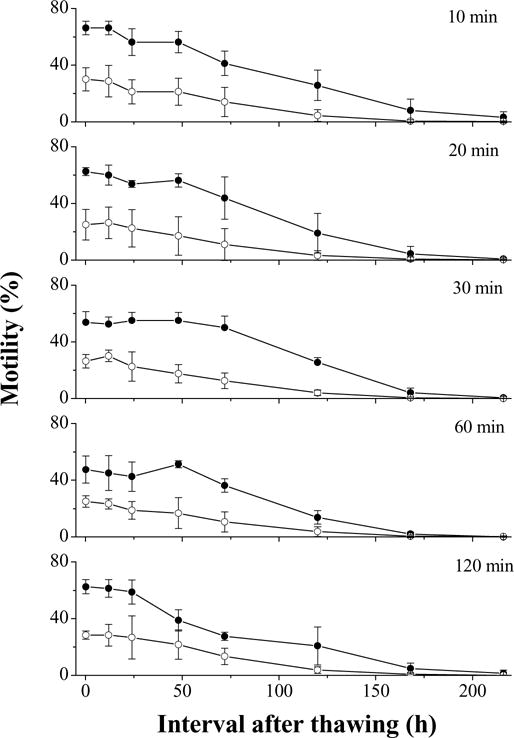
Motility during storage at 4 °C for 9 days (mean ± S.D.) after thawing of Xiphophorus couchianus sperm cryopreserved with 14% glycerol, equilibration times of 10, 20, 30, 60, and 120 min, and cooling from 5 to −80 °C at 5 °C/min (open circles) or 25 °C/min (filled circles). Suspensions were prepared with 300 mOsm/kg HBSS.
In the second trial with 10% glycerol, the highest motility (16 ± 11%) at 10 min after thawing was found with samples equilibrated for 120 min and cooled at 25 °C/min (Fig. 8). For the samples cooled at 5 °C/min, average motility at 10 min after thawing was ≤ 2%. The sperm motility in the samples with 120-min equilibration was not different (P = 0.376) from those with shorter equilibration time. In general, the motility in this trial was much lower than that of the first trial.
Fig. 8.
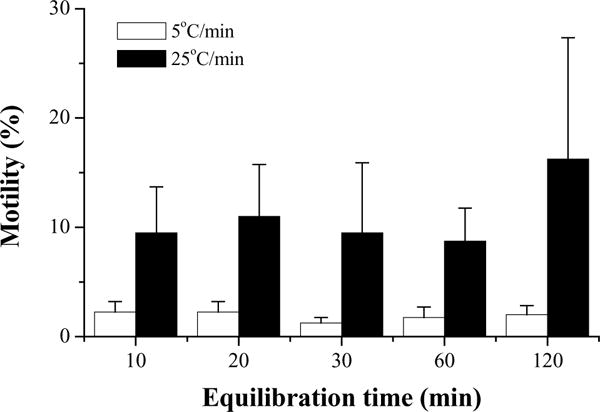
Motility at 10 min (mean ± S.D.) after thawing of Xiphophorus couchianus sperm cryopreserved with 10% glycerol, equilibration times of 10, 20, 30, 60, or 120 min, and cooling from 5 to −80 °C at 5 °C/min (open bars) or 25 °C/min (filled bars). Suspensions were prepared with 300 mOsm/kg HBSS.
3.6. Effect of dilution ratio: before freezing and after thawing
In these experiments, single dilutions (before freezing) were compared to double dilutions (before freezing and after thawing). Initial motility of fresh semen was 85% for the first trial. At 10 min after thawing, the highest motility (65 ± 4%) was found for a single dilution with a volume ratio of sperm to HBSS–glycerol of 1:20, and the lowest motility (58 ± 8%) was found for a double dilution of 1:20 (before cooling) and 1:60 (after thawing) (Fig. 9). Over time, sperm motility in the samples with double dilutions using HBSS declined more slowly, while motility with double dilutions using HBSS–glycerol declined most rapidly. The sperm retained motility for 36 h with a double dilution using HBSS–glycerol, for 108 h with a single dilution, and for 368 h with a double dilution using HBSS. Sperm motility with a double dilution using HBSS was higher (P = 0.011) than those with single dilution or double dilution using HBSS–glycerol (Table 1). The sperm motility with double dilution using HBSS–glycerol was lower (P = 0.002) than that with single dilution (Table 1).
Fig. 9.
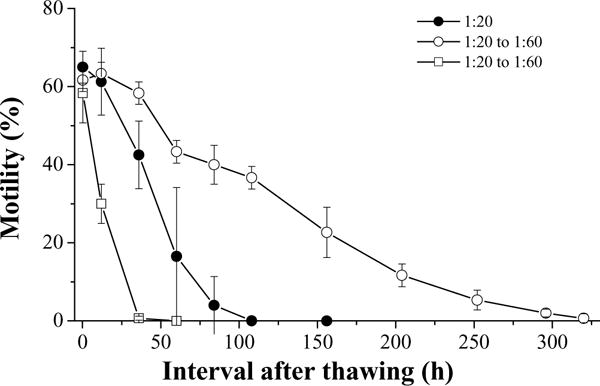
Motility during storage at 4 °C for 15 days (mean ± S.D.) after thawing of Xiphophorus couchianus sperm diluted before freezing at volume ratios of semen to extender–cryoprotectant (HBSS–glycerol) of 1:20, and diluted again immediately after thawing at a ratio of sperm suspension to HBSS of 1:3 (open circles) or to HBSS–glycerol of 1:3 (open squares), or without dilution after thawing (filled circles). Suspensions were prepared with 300 mOsm/kg HBSS.
In the second trial, initial motility of fresh semen was 90%. At 10 min after thawing, the sperm motilities were not different (78 ± 3%) in samples with the four different dilution ratios (Fig. 10). Although the sperm motilities in all samples declined over time after thawing, samples with double dilution from a volume ratio of 1:10 (before cooling) and 1:20 (after thawing) declined most rapidly, followed by those diluted twice from 1:10 to 1:30. However, sperm in the samples with a double dilution of from 1:10 to 1:20 retained 48 ± 8% motility at Day 3 after thawing. Motility after thawing ceased at Day 9 for samples double diluted from 1:10 to 1:20, at Day 12 for double dilution from 1:10 to 1:30, and at Day 15 for the samples with single dilution of 1:20 before freezing. There were differences in sperm motility between samples from 1:10 to 1:20 and from 1:10 to 1:30 (P = 0.028), and between samples from 1:10 to 1:20 and from 1:20 to 1:40 (P = 0.012) (Table 1).
Fig. 10.
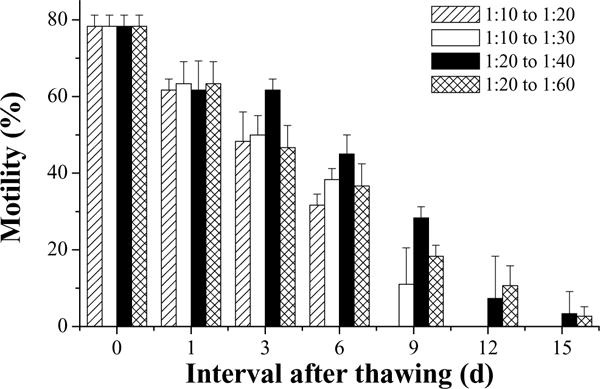
Motility during storage at 4 °C for 15 days (mean ± S.D.) after thawing of Xiphophorus couchianus sperm diluted at ratios of semen to extender–cryoprotectant (HBSS–glycerol) of 1:10 and 1:20 before freezing, and diluted again immediately after thawing with HBSS at ratios of sperm suspension to HBSS of 1:2 or 1:3.
In the third trial, initial motility of fresh semen was 90%. Sperm motilities were highest (78 ± 3%) in all samples at 10 min after thawing before dilution (Fig. 11). Although double dilution prolonged storage after thawing, sperm motilities declined with time. Samples with a final ratio of 1:40 and stored at room temperature before the second dilution declined most sharply, followed by the final ratio of 1:20 and stored at 4 °C before the second dilution (Fig. 11). When stored at 4 °C before a second dilution at 3 h after thawing, sperm retained 68 ± 10% motility in the samples with a final ratio of 1:40, compared to motility of 68 ± 3% with a final ratio of 1:20 and stored at 4 °C and 30 ± 9% with a final ratio of 1:40 and stored at room temperature. When the second dilution was made at 8 h after thawing, sperm retained 67 ± 8% motility in the samples with a final ratio of 1:40 and stored at 4 °C before dilution, compared to 50 ± 10% in the samples with a final ratio of 1:20 and stored at 4 °C, and 6 ± 11% with a final ratio of 1:40 and stored at room temperature. When stored at 4 °C before the second dilution, only samples with a double dilution at 8 h after thawing showed lower (P = 0.024) motilities than those diluted earlier than 5 h after thawing, but when stored at room temperature, differences (P = 0.004) were found between the samples diluted at 3 h and those diluted earlier. When stored at 4 °C, the samples with a final ratio of 1:40 always had higher motilities (P = 0.050) than those with a final ratio of 1:20, regardless of when the second dilution occurred.
Fig. 11.
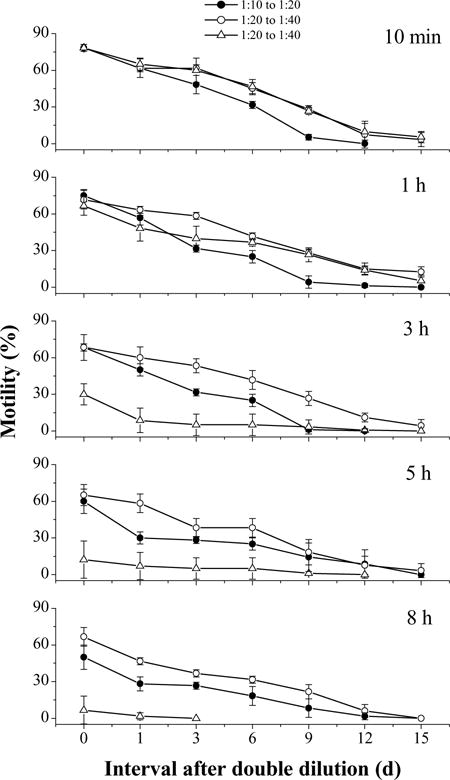
Motility during storage at 4 °C for 15 days (mean ± S.D.) after thawing of Xiphophorus couchianus sperm diluted before freezing at ratios of semen to extender–cryoprotectant (HBSS–glycerol) of 1:10 (open circles) or 1:20 (open squares and open triangles), and diluted again at 10 min and at 1, 3, 5, and 8 h after thawing with HBSS at a ratio of sperm suspension to HBSS of 1:2. For the samples with a final ratio of sperm suspension to HBSS of 1:40, the samples were stored prior to the second dilution at 4 °C (open squares) or at room temperature (open triangles).
4. Discussion
Fishes of the genus Xiphophorus present several challenges for cryopreservation, not the least of which is their small body size [21]. The mature X. couchianus males used in the present study were <2.5 cm long and weighed <300 mg. The average testis weight of these fish was <5 mg, about half of the weight (9.2 mg) found for mature X. helleri males [4]. If we assume a specific gravity of 1.0 for the testis and assume that the entire testis is filled with sperm, these male X. couchianus would produce a maximum volume of <5 μl of sperm. Therefore, it is almost impossible to use single male X. couchianus to perform research trials of sperm cryopreservation with multiple variables and motility estimates after thawing. Even if the procedures developed in our previous study for X. helleri [4] including the use of 0.25-ml straws and dilution ratios as high as 1:100 were used for X. couchianus, it was necessary to pool testes from several males in the present study.
In the present study, glycerol yielded significantly higher post-thaw motility than did DMSO, although DMSO is the most commonly used cryoprotectant for aquatic species and is generally useful in most species [24–27]. In addition, higher concentrations (14–17%) of glycerol yielded the highest motility immediately after thawing, but lower concentrations (8–11%) retained motility longer when stored at 4 °C, as found in our previous study with X. helleri [23].
In the present study, the optimal osmolality of HBSS for X. couchianus was 240–300 mOsm/kg when using 14% glycerol as cryoprotectant, with the highest motilities found at 270 mOsm/kg overall. Therefore, the optimal osmolality of HBSS used to suspend sperm seems to vary for species of Xiphophorus when using the same cryoprotectant. For X. helleri, the appropriate osmolality of HBSS was similar to the osmolality of blood plasma (~320 mOsm/kg) [4], but an osmolality of HBSS lower than that of its blood plasma (324 mOsm/kg) should be used to suspend sperm of X. couchianus when using glycerol as cryoprotectant.
Cooling rate is another important factor affecting motility of semen after thawing. In the present study, sperm of X. couchianus had a wide adaptability to cooling rate, and yielded the highest motility after thawing when frozen at cooling rates of 20–30 °C/min. For fish sperm, optimal reported rates vary from 5 to 45 °C/min for cooling from 5 to −80 °C, but some species show highest post-thaw motility with a combination of different cooling rates [28–31]. For example, the optimal cooling procedure for semen of common carp, Cyprinus carpio, was 5 °C/min from 2 to −7 °C and then 25 °C/min from −7 to −70 °C [32]. Although 70% motility after thawing would be acceptable for most fishes, the demands of internal fertilization in fishes of the family Poeciliidae may necessitate higher motility levels, and development of two-step freezing regimes may be necessary.
Equilibration times of 10–20 min are most commonly used for fish semen [33], although semen of sea bass (Decentrarchus labrax) equilibrated for 6 h with 10% ethylene glycol at 0–2 °C had post-thaw motility comparable to that of fresh semen [31]. Equilibration time seemed to be a minor factor for sperm cryopreservation of X. couchianus with glycerol as the cryoprotectant; no consistent differences were found in this experiment across a range of equilibration times of from 10 to 30 min for cooling rates of 5 and 25 °C/min. Changes of equilibration time or cooling rate that yield improved post-thaw motility at lowered concentrations of cryoprotectant would be especially desirable for sperm preservation of Xiphophorus because cryoprotectant toxicity is an important constraint for sperm storage and poses unknown risks for females after artificial insemination. Overall, for this study, optimization of cryoprotectant concentration or cooling rate would seem more effective than optimization of equilibration time, because a longer equilibration (>30 min) had no effect on post-thaw motility at a lower concentration of glycerol (10%) and at a slow cooling rate (5 °C/min).
In this study, a second dilution of 1:2 or 1:3 with HBSS 300 after thawing decreased the decline of sperm motility of X. couchianus and significantly prolonged the storage of sperm at 4 °C to more than 15 days, compared to storage of 9 days without dilution after thawing. However, if the diluent contained glycerol at the same concentration (14%) as used for cryoprotectant, sperm motility of X. couchianus decreased more rapidly than those without dilution, indicating that a rapid elimination of cryoprotectant from sperm suspensions after thawing is important to minimize toxicity. Elimination of cryoprotectant before insemination may also be a key point for fertilization to minimize toxicity to females, which can store sperm for months and release broods about every 30th day [34].
Comparing the present results with our previous studies [23], we found that X. couchianus shared physiological characteristics and responses to different sperm cryopreservation protocols with X. helleri, although that species is about double in body size. Based on the experiments in the present study, however, we improved post-thaw motility of sperm of X. couchianus to as high as 78% (average) at 10 min after thawing when sperm were suspended in HBSS at 300 mOsm/kg with 14% glycerol as cryoprotectant at a dilution ratio of sperm to HBSS–glycerol of 1:10 or 1:20, equilibrated for 10–20 min, cooled at 25 °C/min from 5 to −80 °C before plunging into liquid nitrogen, and thawed at 40 °C in a water bath for 7 s. Moreover, if diluted with HBSS within 5 h after thawing, the sperm frozen by the above protocol would retain motility for as long as 15 days when stored at 4 °C; this was double the storage time we observed for X. helleri even with comparable motility immediately after thawing [23].
This improvement of post-thaw motility and storage time after thawing is extremely important for live-bearing fishes because of internal fertilization, which requires sustainable long-term storage within the female reproductive tract of viable sperm before fertilization occurs. Although an average of 78% post-thaw motility and storage at 4 °C for over 15 days would generally be considered quite acceptable for cryopreservation research with typical teleost fishes, a key point is whether these freezing protocols would yield fertilization and development of viable offspring in X. couchianus. At present, basic techniques exist for fertilization of Xiphophorus females with undiluted fresh sperm ([35]; http://www.xiphophorus.org). Future research is needed to develop insemination protocols suitable for use with diluted cryopreserved sperm and assays that can yield fertilization estimates as an alternative to comparing brood sizes after 30 days of gestation.
Acknowledgments
This work was supported by USPHS grants, RR-17072 from the National Center for Research Resources and CA-75137 from the National Cancer Institute, with additional support provided by the Roy F. and Joanne Cole Mitte Foundation and the U.S. Department of Agriculture. We thank L. Hazlewood and R. Bowers of the XGSC of Texas State University—San Marcos for providing fish, R. Walter and S. Kazianus for advice and discussion, and R. Smeal for administrative help. This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 03-11-1550.
References
- 1.Blaxter JHS. Sperm storage and cross-fertilization of spring and autumn spawning herring. Nature. 1953;172:1189–90. [Google Scholar]
- 2.Figiel CR, Jr, Tiersch TR. Cryopreservation methods: a review. Aquaculture 1998 (book of abstracts) 1998:178. [abstract] [Google Scholar]
- 3.Aoki K, Okamoto M, Tatsumi K, Ishikawa Y. Cryopreservation of medaka spermatozoa. Zool Sci. 1997;14:641–4. [Google Scholar]
- 4.Huang C, Dong Q, Walter RB, Tiersch TR. Initial studies on sperm cryopreservation of a live-bearing fish, the green swordtail Xiphophorus helleri. Theriogenology. doi: 10.1016/j.theriogenology.2003.09.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallman KD. The platyfish, Xiphophorus maculates. In: King RC, editor. Handbook of genetics. New York: Plenum Publishing Corporation; 1975. pp. 81–132. [Google Scholar]
- 6.Paaben U, Paris F, Schlierenkamp H, Blum V. Sperm storage and fertilization in Xiphophorus helleri. In: Braucker R, editor. Proceedings of the CEBAS workshops annual issue 1996. RUHR-University of Bochum: Proc. C.E.B.A.S. Workshops; 1996. pp. 17–24. [Google Scholar]
- 7.Paris F, Paassen U, Bluem V. Sperm storage of the female swordtail (Xiphophorus helleri) Verh Ges Ichthyol. 1998;1:157–65. [Google Scholar]
- 8.Tamaru CS, Cole B, Bailey R, Brown C, Ako H. A manual for commercial production of the swordtail, Xiphophorus helleri. Honolulu: 2001. (CTSA Publication Number 128). Available at: http://www.soest.hawaii.edu/SEAGRANT. [Google Scholar]
- 9.Meyer A, Morrissey JM, Schartl M. Recurrent origin of a sexually selected trait in Xiphophorus fishes inferred from a molecular phylogeny. Nature. 1994;368:539–42. doi: 10.1038/368539a0. [DOI] [PubMed] [Google Scholar]
- 10.Kallman KD. The sex determining mechanism of the poeciliid fish, Xiphophorus montezumae, and the genetic control of the sexual maturation process and adult size. Copeia. 1983;3:755–69. [Google Scholar]
- 11.Breuckmann A, Paris F, Schreibman Martin P, Bluem V. Immunoreactive gonadotropin-releasing hormone (GnRH) in the brain and pituitary of adult and juvenile swordtails (Xiphophorus helleri, Teleostei, Poeciliidae) J Morphol. 1996;230:55–67. doi: 10.1002/(SICI)1097-4687(199610)230:1<55::AID-JMOR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Hoefler CD, Morris MR. A technique for the temporary application and augmentation of pigment patterns in fish. Ethology. 1999;105:431–8. [Google Scholar]
- 13.Beaugrand JP, Goulet C. Distinguishing kinds of prior dominance and subordination experiences in males of green swordtail fish (Xiphophorus helleri) Behav Process. 2000;50:131–42. doi: 10.1016/s0376-6357(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 14.Trainor BC, Basolo AL. An evaluation of video playback using Xiphophorus helleri. Anim Behav. 2000;59:83. doi: 10.1006/anbe.1999.1289. [DOI] [PubMed] [Google Scholar]
- 15.De Wolf W, Seinen W, Hermens JLM. N-acetyltransferase activity in rainbow trout liver and in vitro biotransformation of chlorinated anilines and benzenes in fish. Xenobiotica. 1993;29:1045–56. doi: 10.3109/00498259309057042. [DOI] [PubMed] [Google Scholar]
- 16.Dove AD. Richness patterns in the parasite communities of exotic poeciliid fishes. Parasitology. 2000;120:609–23. doi: 10.1017/s0031182099005958. [DOI] [PubMed] [Google Scholar]
- 17.Hogarth PJ. Protection of the developing foetus from immunological attack in Xiphophorus helleri. J Reprod Fertil. 1970;23:532. doi: 10.1530/jrf.0.0230532. [DOI] [PubMed] [Google Scholar]
- 18.McConnell TJ, Godwin UB, Norton SF, Nairn RS, Kazianis S, Morizot DC. Identification and mapping of two divergent, unlinked major histocompatibility complex class II B genes in Xiphophorus fishes. Genetics. 1998;149:1921–34. doi: 10.1093/genetics/149.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schartl M. Platyfish and swordtails: a genetic system for the analysis of molecular mechanisms in tumor formation. Trends Genet. 1995;11:185–9. doi: 10.1016/S0168-9525(00)89041-1. [DOI] [PubMed] [Google Scholar]
- 20.Nairn RS, Kazianis S, McEntire BB, Della CL, Walter RB, Morizot DC. A CDKN2-like polymorphism in Xiphophorus LG V is associated with UV-B-induced melanoma formation in platyfish-swordtail hybrids. Proc Natl Acad Sci USA. 1996;93:13042–7. doi: 10.1073/pnas.93.23.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiersch TR. Cryopreservation in aquarium fishes. Marine Biotechnol. 2001;3:212–23. doi: 10.1007/s10126001-0044-z. [DOI] [PubMed] [Google Scholar]
- 22.Paniagua-Chavez CG, Tiersch TR. Laboratory studies of cryopreservation of sperm and trochophore larvae of the eastern oyster. Cryobiology. 2001;43:211–23. doi: 10.1006/cryo.2001.2346. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Dong Q, Walter RB, Tiersch TR. Sperm cryopreservation of green swordtail Xiphophorus helleri, a fish with internal fertilization. Cryobiology. doi: 10.1016/j.cryobiol.2004.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorogood J, Blackshaw A. Factors affecting the activation, motility, and cryopreservation of the spermatozoa of the yellowfin bream Acanthopagrus australis (Guűther) Aquacult Fish Manage. 1992;23:337–44. [Google Scholar]
- 25.Billard R, Cosson J, Crim LW. Motility of fish and aged halibut sperm. Aquat Living Resour. 1993;6:67–75. [Google Scholar]
- 26.Tiersch TR, Mazik PM, editors. Cryopreservation in aquatic species. Baton Rouge: World Aquaculture Society; 2000. [Google Scholar]
- 27.Tanaka S, Zhang H, Horie N, Yamada Y, Okamura A, Utoh T, et al. Long-term cryopreservation of sperm of Japanese eel. J Fish Biol. 2002;60:139–46. [Google Scholar]
- 28.Rana KJ, Gilmour A. Cryopreservation of fish spermatozoa: effect of cooling methods on the reproducibility of cooling rates and viability; Refrigeration and Aquaculture Conference; Bordeaux. 20–22 March; 1996. pp. 3–12. [Google Scholar]
- 29.Linhart O, Rodina M, Cosson J. Cryopreservation of sperm in common carp Cyprinus carpio: sperm motility and hatching success of embryos. Cryobiology. 2000;41:241–50. doi: 10.1006/cryo.2000.2284. [DOI] [PubMed] [Google Scholar]
- 30.Lang RP, Riley KL, Chandler JE, Tiersch TR. The use of dairy protocols for sperm cryopreservation of blue catfish Ictalurus furcatus. J World Aquaclut Soc. 2003;34:66–75. [Google Scholar]
- 31.Sansone G, Fabbrocini A, Ieropoli S, Langellotti A, Occidente M, Matassino D. Effects of extender composition, cooling rate, and freezing on the motility of sea bas (Dicentrarchus labrax, L) spermatozoa after thawing. Cryobiology. 2002;44:229–39. doi: 10.1016/s0011-2240(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 32.Cognie F, Billard R, Chao NH. Cryopreservation of carp, Cyprinus carpio, sperm. J Appl Ichthyol. 1989;5:165–76. [Google Scholar]
- 33.Billard R, Zhang T. Techniques of genetic resource banking in fish. In: Walson PF, Holt WV, editors. Cryobanking the genetic resource: wildlife conservation for the future. New York: Yaylor & Francis; 2001. pp. 143–70. [Google Scholar]
- 34.Gordon M. Hereditary basis of melanosis in hybrid fishes. Am J Cancer. 1931;15:1495–523. [Google Scholar]
- 35.McGovern-Hopkins K, Tamaru CS, Takeshita G, Yamamoto M. Procedural guide for the artificial insemination of the lyretail swordtail, Xiphophorus helleri. Honolulu: 2003. (CTSA Publication Number 149). Available at: http://www.soest.hawaii.edu/SEAGRANT. [Google Scholar]


