Abstract
Ethnopharmacological relevance
Vernonia guineensis Benth. (Asteraceae) preparations are used in folk medicine in Cameroon to treat a number of ailments, including prostate cancer and malaria, and is used as an anthelmintic, adaptogen and antidote. The aim of this study was to continue the validation of the activity of Vernonia guineensis Benth. extracts and isolated molecules against cancer cell lines following the previous isolation of an anti-prostate cancer sugar ester from the root extract.
Materials and methods
Acetone extracts of Vernonia guineensis Benth. leaves were tested for activity against 10 cancer cell lines (Breast—MDA-MB-231, Breast—MCF-7, Colon—HCT-116, Leukemia—HL-60, Lung—A549, Melanoma—A375, Ovarian—OVCAR3, Pancreas—Mia-paca, Prostate—PC-3 and Prostate—DU-145). The acetone extract was subjected to bioactivity guided fractionation. Anti-proliferation and clonogenic activity of the isolated compounds were tested. The WST-1 assay was used for the anti-proliferation activity, while the standard clonogenic test was used to determine the clonogenic activity.
Results
The acetone extract of Vernonia guineensis Benth. demonstrated in vitro activity ranging from IC50 4–26 mg/mL against the 10 cell lines. Activity guided fractionation of this extract yielded two sesquiterpene lactones, isolated for the first time from the genus Vernonia. The compounds were characterized using spectroscopic experiments, including a combination of 1D and 2D NMR data. Vernopicrin (1) and Vernomelitensin (2) demonstrated in vitro activity against human cancer cell lines with IC50 ranging from 0.35–2.04 μM (P < 0.05) and 0.13–1.5 μM (P < 0.05), respectively, between the most and least sensitive cell lines for each compound. Vernopicrin was most active against the human melanoma (A375) cell line and least active against the lung cancer (A549) cell line, while Vernomelitensin was also most active against the human melanoma (A375) cell line and least active against the breast cancer (MCF-7) cell line. Both compounds also demonstrated anticlonogenic activity.
Conclusion
The cytotoxicity demonstrated by the crude extract and isolated sesquiterpenes against cancer cell lines highlights the medicinal potential of V. guineensis. The selective anti-proliferation and dose dependent anticlonogenic activities suggest that the identified sesquiterpenes could be potential antitumor agents..
Keywords: Vernonia guineensis, Germacranolide, Elemanolide, Vernopicrin, Vernomelitensin, Cytotoxicity, Clonogenic
1. Introduction
Vernonia guineensis Benth. (Asteraceae) is a variable species with three varieties (var. guineensis, var. cameroonica, and var. procera) recognized in the West African region. They are herbaceous with strong erect stems from a perennial woody rootstock, 1.70 m high, distributed across the region from Mali to Western Cameroon, and across central Africa from Cameroon to Sudan (Burkill, 1985). Vernonia guineensis Benth. is a widely used medicinal plant in West Africa and particularly in Cameroon (Tchinda et al., 2002; Focho et al., 2009; Noumi, 2010). Whole carrot-like tubers and powders derived therefrom, packaged in 10–20 g sachets, are sold by herbalists in the open market. Claimed medicinal uses include adaptogenic properties to combat stress and as a stimulant (Toyang et al., 2012). The plant is also used as an anthelmintic, an aphrodisiac, an antidote and to treat malaria and jaundice (Iwu, 1993; Jiofack et al., 2010).
On the phytochemistry and biological activity of V. guineensis, early isolation work on this plant was carried out by Toubiana et al. (1975) who isolated two compounds called Vernodalin and vernolepin. These compounds have also been isolated from other members of the Vernonia genus including V. amygdalina and have been reported to be responsible for the antibacterial activity related to the use of this species by chimpanzees (Jisaka et al., 1993). Tchinda et al. (2002, 2003) reported the isolation of stigmastane derivatives and sucrose ester type compounds from the roots, with one of the stigmastanes showing anti-trypanocidal activity. Recent studies resulted in the isolation of a sucrose ester from the roots with antiprostate cancer activity (Toyang et al., 2012) and a stigmastane steroid with antimicrobial activity (Donfack et al., 2012).
The diagnosis of new cancer cases remains on the rise world-wide, and there is a need for the continuous search for new anticancer agents (Parkin et al., 2008; Ferlay et al., 2010). Based on the preliminary cytotoxicity activity observed with the root extract of V. guineensis (Toyang et al., 2012a), the leaves were evaluated and found to be even more potent than the roots. Bioactivity guided fractionation of the leaf extract afforded a germacranolide and an elemanolide with cytotoxic and clonogenic activity which are reported herein.
2. Materials and methods
2.1. Plant material collection
The leaves of V. guineensis Benth. Var. cameroonica were harvested from young plants at Baicham, Boyo, Division of the North West Region of Cameroon in 2009. A voucher specimen was authenticated at the Limbe Botanic Garden, South West Region Cameroon and deposited at the Limbe Botanic Garden Herbarium with reference no.: SCA 12431.
2.2. Extraction and isolation
The powder of V. guineensis leaves (1.5 kg) was extracted with 10 L of acetone 2 x for 24 h and filtered using a Buchner funnel and Whatman no.1 filter paper. The marc was rinsed with an additional 5 L of acetone. The filtrate was dried in vacuo to obtain a dark green oily residue (81.3 g) which was stored at 4 ° C until use in the bioassay and for fractionation.
2.3. Cell cultures
Ten cancer cell lines (Breast—MDA-MB-231, Breast—MCF-7, Colon—HCT-116, Leukemia—HL-60, Lung—A549, Melanoma—A375, Ovarian—OVCAR3, Pancreatic—Mia-Paca, Prostate—PC-3 and Prostate—DU-145) were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA). The cells were maintained in minimum essential media (MEM) supplemented with 10% fetal calf serum (FCS), 1% L-glutamine, 2% penicillin–streptomycin, and 0.2% gentamicin or in RPMI 1640 medium supplemented with 10% FBS and 1% L-glutamine.
2.4. Anti-proliferation assay
The inhibitory efficacy of the acetone extracts of V. guineensis and compounds 1 and 2 on the growth of cancer cells in vitro was investigated using the WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) (Roche) colorimetric assay (Ngamwongsatit et al., 2008). Briefly, on the day the experiment is initiated, cells are trypsinized and plated into 96 well plates in 50 μL of medium. The compound is added approximately 18 h after plating. Cells are plated at a density so that 72 h post drug addition, the cells are in log phase (500–2000 cells/well). The compounds are solubilized in DMSO at a concentration of 100 mM, aliquoted and stored at − 20 ° C. Following drug addition, the cells are allowed to proliferate for 72 h. The experiment is terminated using WST-1 (Roche) 10 mL per well and absorbance is read at 450 nm/690 nm. The effect of drugs on growth was assessed as percent of cell viability. The IC50 values were determined from the compound dose versus control growth curves using the Graphpad Prism software. All experiments were carried out at least in duplicate and the mean results determined.
2.5. Clonogenic assay
Clonogenic effect of pure cells plated on a 24-well plate in RPMI-10 at 75 cells per well (in 1 mL media). Note: the cell number had been determined by testing various numbers of cells per well ranging from 25 to 10,000. The best results in terms of distinct colony numbers per well was obtained with 75 cells per well. The assay was terminated on day 9 by removing the media; adding 0.5 mL methanol for 15 min (− 20 °C); removing methanol and then adding 0.25% crystal violet for less than 30 s. Plates were rinsed with water and allowed to dry. Colonies were counted under a microscope.
2.6. Statistical analysis
The antiproliferation assay experiments were run at least in duplicate with each experimental concentration duplicated during each run. For the clonogenic assay, each concentration was run in quadruplet during each experiment. The average mean of the different assays were calculated and the data represented as mean ±SEM. All data were analyzed in Graphpad Prism (Graphpad Software, La Jolla, CA) software.
3. Results
3.1. Activity guided isolation
The acetone extract obtained as described above was found to be active (IC50 6.24 μg/mL) against the PC-3 prostate cancer cell line which was used for the bioactivity guided fractionation. The extract (50 g) was absorbed onto Celite and then fractionated over a silica gel column, eluting with a gradient of n-hexane–ethyl acetate. Sixteen fractions were obtained and pooled on the basis of TLC analysis to seven main fractions. Fraction #4 (11 g) obtained with n-hexane/ethyl acetate (1:1) was active with IC50 =2.4 μg/mL. This fraction (10 g) was subjected to dry column chromatography (DCC) using 200 g normal phase silica gel activated for visualization with UV254. The column was run with an isocratic solvent system (n-hexane/ethyl acetate 1:1) until the solvent reached the bottom of the column. The solvent was terminated and the column placed on a flat glass surface. The bands were visualized using a UV254 lamp and a sharp stainless steel knife used to separate the bands. A total of nine bands were cut out and extracted with acetone. The nine bands were eventually pooled to four based on TLC analysis. Bands #2 and #3 were active in the anticancer assay. Compound 1 (570 mg) was isolated from band #3 by recrystallization in methanol. Band #2 (1.6 g) was further subjected to reverse phase column chromatography eluting with Methanol/water 6:4 to yield compound 2 (73 mg).
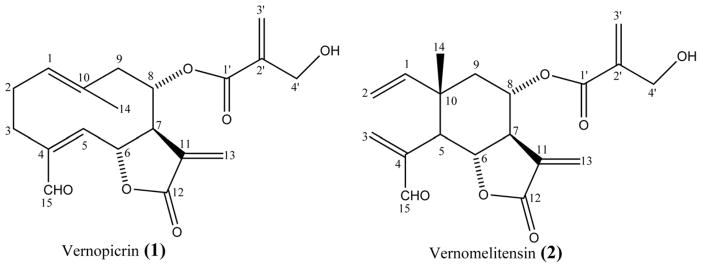
Compound 1 was obtained as white powder. The molecular formula was determined as C19H22O6 from the FABMS, which showed a pseudo-molecular ion peak [M+ H]+ at m/z 347, in conjunction with NMR data. The 1H NMR spectrum showed signals of an aldehydic proton at dH 9.46 (s, H-15), six olefinic protons at dH 5.33 (t, J=6.0 Hz, H-1), 5.82 (brs, H-13b), 5.86 (brs, H-3′ b), 6.17 (brs, H-3′ a), 6.21 (brs, H-13a) and 6.58 (d, J= 10.3 Hz, H-5). This spectrum also showed signals of two oxymethine protons at dH 5.01 (m, H-6) and 5.10 (m, H-8), two hydroxymethylene protons at dH 4.11 (m, H-4′) and three olefinic methyl protons at dH 1.81 (brs, H3-14). The 13C NMR spectrum displayed 19 carbon resonances ascribable to a sesquiterpene skeleton bearing a 4-hydroxymethacryloxy group (Fortuna et al., 2001). The signals at dC 195.2, 168.7, and 164.5 were attributed to an aldehyde carbon (C-15), a lactone carbonyl (C-12) and an ester carbonyl (C-1′) respectively. This spectrum also exhibited signals of ethylenic carbons at dC 124.0 (C-3′), 127.6 (C-13), 127.8 (C-1), 131.9 (C-10), 133.9 (C-11), 140.6 (C-2′), 141.3 (C-4) and 148.1 (C-5). Signals at dC 69.1 (C-8) and 75.7 (C-6) were assignable to oxymethine carbons; that of the oxymethylene carbon was observed at dC 59.3 (C-4′). The 1H–1H COSY spectrum showed the presence of two main spin systems, a CH–CH2–CH2 unit and a CH–CH–CH–CH–CH2 unit. These data were in agreement with a germacranolide sesquiterpene lactone skeleton (Marco et al., 2005). In the HMBC, correlations observed between H-8 (dH 5.21) and C-1′ (dC 168.7) enabled the positioning of the 4-hydroxymethacryloxy group at C-8 of the germacranolide skeleton. Compound 1 was thus identified as (6S, 7R, 8S)-8-(4′-hydroxymethacryloxy)-15-oxogermacra-1(10),4,11(13)-trien-6,12-olide, previously reported from Centaurea tweediei (Fortuna et al., 2001). Rustaiyan et al. (1979) prepared this compound by partial oxidation of onopordopicrin, a sesquiterpene lactone obtained from Onopordon leptolepis. This is the first report on the isolation of this compound from V. guineensis. As no trivial name was previously given to this compound, we trivially named it Vernopicrin (Fig. 1).
Fig. 1.
Chemical structures of Vernopicrin (1) and Vernomelitensin (2) isolated from the leaves of Vernonia guineensis Benth.
Compound 2 was obtained as colorless oil. The molecular formula C19H22O6 was deduced from the FABMS, which showed a pseudo-molecular ion peak [M + H] + at m/z 347, in conjunction with NMR data. 1H NMR spectrum exhibited typical signals of elemane-type sesquiterpenoids (Rustaiyan et al., 1979), including nine olefinic protons at dH 4.85 (2H, brd, J= 16.6 Hz, H-2), 5.55 (1H, d, J= 2.8 Hz, H-13a), 5.65 (1H, dd, J= 11.1 and 16.6 Hz, H-1), 5.89 (1H, d, J= 1.7 Hz, H-3′ a), 5.99 (1H, d, J= 3.2 Hz, H-13b), 6.15 (1H, d, J= 1.2 Hz, H-3′ b), 6.48 (1H, s, H-3a) and 6.49 (1H, s, H-3b). The 13C NMR data of compound 2 were closely related to those of compound 1, except that 2 exhibited two additional ethylenic methylene carbons at dC 112.0 (C-2) and 138.9 (C-3), several aliphatic signals including one tertiary methyl group at dC 17.4 (Me-10), one quaternary carbon at dC 41.5 (C-10) and one methine carbon at dC 44.1 (C-5). In the HMBC spectrum, pertinent correlations were observed between H-2 and C-10, as well as between H-3 and C-15 (dC 194.6), C-4 (dC 144.7) and C-5 (dC 44.1). Compound 2 was thus identified as (5R, 6S, 7R, 8S, 10S)-8-(4′-hydroxymethacryloxy)-15-oxoeleman-1,3,11(13)-trien-6,12-olide, previously reported by Rustaiyan et al. (1979) from Onopordon leptolepis. Several elemane sesquiterpenoids have been reported from Vernonia guineensis (Toubiana et al., 1975). This is the first report on the isolation of this compound from V. guineensis to the best of our knowledge. As no trivial name was previously given to this compound and given the similarities between Melitensin and Compound 2 (Rustaiyan et al., 1979), we trivially named it Vernomelitensin (Fig. 1).
3.2. Anti proliferation assay
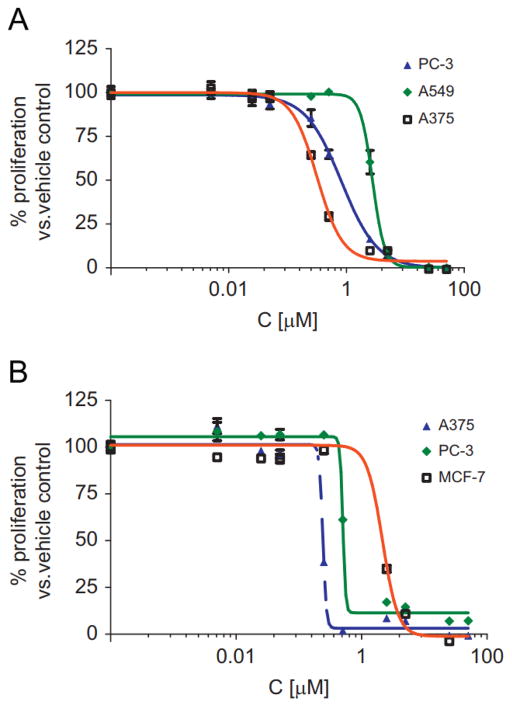
The results of the anti proliferation assay conducted against 10 cancer cell lines utilizing the WST-1 cell proliferation reagent are presented in Table 1. Fig. 2 presents the growth curves of three cell lines for each drug representing low, mid and high anti proliferation activity for Vernopicrin and Vernomelitensin.
Table 1.
Results of anti-proliferation activity of Vernopicrin (1) and Vernomelitensin A (2) against 10 cancer cell lines.
| Cancer cell line | IC50(μM)
|
|
|---|---|---|
| Vernopicrin (1) | Vernomelitensin (2 | |
| Breast—MDA-MD-231 | 1.01 ± 0.200 | 0.367 ±0.030 |
| Breast—MCF-7 | 0.67 ±0.320 | 1.56 ± 0.960 |
| Colon—HCT-116 | 0.45 ± 0.007 | 0.14 ± 0.150 |
| Leukemia—HL-60 | 1.55 ± 0.470 | 1.12 ± 0.750 |
| Lung—A549 | 2.04 ± 1.040 | 1.13 ± 0.380 |
| Human melanoma—A375 | 0.35 ± 0.040 | 0.13 ± 0.150 |
| Ovarian—OVCAR3 | 0.74 ± 0.310 | 0.48 ± 0.008 |
| Pancreas—Mia-Paca | 0.42 ± 0.040 | 0.25 ± 0.110 |
| Prostate—DU-145 | 0.49 ± 0.260 | 0.41 ± 0.120 |
| Prostate—PC-3 | 1.00 ± 0.260 | 0.64 ± 0.14 |
Fig. 2.
Growth curves for in vitro WST-1 assay anti proliferation activity of Vernopicrin (1) (A); and Vernomelitensin (2) (B) on selected cancer cell lines representing low, mid and high activity against the 10 cell lines tested for each compound. IC50 values (Table 1) were determined from the compound dose versus control growth curves using Graphpad Prism software. All experiments were carried out at least in duplicate and the means ±STD determined.
3.3. Results of clonogenic assay
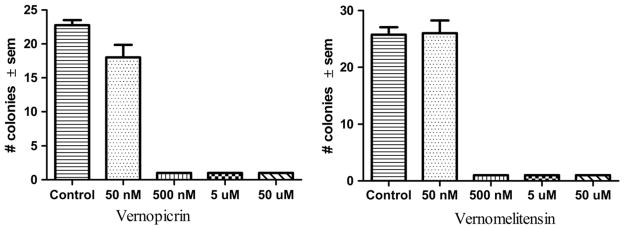
Compounds 1 and 2 inhibited colony formation with an IC50 ≤ 0.5 μM. Fig. 2 presents the results of the clonogenic assay.
4. Discussion
The sesquiterpene lactones, Vernopicrin (1) and Vernomelitensin (2) were isolated for the first time from V. guineensis in this study, to the best of our knowledge, and show cytotoxicity against a range of cancer cell lines. Sesquiterpene lactones are commonly found amongst plants of the Asteraceae family (Merfort, 2011). At least 34 sesquiterpene lactones from the Vernonia genus have been reported to possess anticancer and other biological activities including antimalarial, antibiotic, antileishmania, antioxidant, anti-inflammatory, anti-platelet and insecticidal activities (Ganjian et al., 1983; Laekeman et al., 1985; Koshimizu et al., 1994; Kos et al., 2006; Buskuhl et al., 2010; Farombi and Owoeye, 2011; Pratheeshkumar and Kuttan, 2011a; Toyang and Verpoorte, in press). Notable amongst the sesquiterpene lactones from the Vernonia genus with anticancer activity is Vernolide A (Kuo et al., 2003; Pratheeshkumar and Kuttan, 2011b, 2012).
Vernopicrin and Vernomelitensin exhibited activity against all the 10 cancer cell lines tested with IC50 values ranging from 0.35 to 2.04 μM and 0.13–1.56 μM respectively (Table 1). The human melanoma cell line (A375) was the most sensitive cell line to both compounds, while the lung cancer (A549) and breast (MCF-7) cell lines were least sensitive to Vernopicrin and Vernomelitensin, respectively. The significant difference (P < 0.05) between IC50 values of the most sensitive versus least sensitive cell lines may suggest that the compounds possess selective and not generalized cytotoxicity. Though isomers, the structures are quite different with Vernomelitensin demonstrating more potent activity against the cell lines compared to Vernopicrin. In the clonogenic assay, Vernopicrin however showed slightly higher activity than Vernomelitensin (Fig. 3).
Fig. 3.
Effect of Vernopicrin (1) and Vernomelitensin (2) pretreatment on colony formation of PC-3 cell line in vitro was studied. PC-3 cells (75 cells/well) were treated with 50 nM, 500 nM, 5 mM, 50 mM and 0.25% DMSO control in quadruplet. The assay was terminated on day 9 by removing the media; adding 0.5 mL methanol for 15 min (− 20 °C); removing methanol and then adding 0.25% crystal violet for less than 30 s. Plates were rinsed with water and allowed to dry. Colonies were counted under a microscope and data analyzed to obtain mean ± SEM in Graphpad Prism Software. Both compounds show potent colony growth inhibition activity at 500 nM suggesting an IC50 < 500 nM.
While many sesquiterpenes have been reported to have anticancer activity, to date there are only a few sesquiterpenes in clinical development for cancer treatment including thapsigargin, parthenolide and artemisinin derivatives (Ghantous et al., 2010). Drawbacks to sesquiterpene development for clinical use includes difficulties related to their isolation from plants, poor bioavailability and selectivity (Ghantous et al., 2010; Merfort 2011). However, the success recorded with the development and clinical use of artemisinin derivatives for the treatment of malaria is an indication that some of the drawbacks to developing sesquiterpenes for use in cancer can be overcome by designing analogs with improved bioavailability and selectivity profiles (Kaur et al., 2009). Moreover, some sesquiterpene lactones have been found to be amongst the few known classes of compounds that have the potential of selectively targeting cancer stem cells (Ghantous et al., 2010).
5. Conclusion
The present paper reports the isolation of two cytotoxic sesquiterpene lactones from V. guineensis. The cytotoxicity demonstrated by the crude extract and isolated sesquiterpenes against cancer cell lines supports the medicinal potential of V. guineensis. The anti- proliferation activity and dose dependent anticlonogenic activity suggests that the identified sesquiterpenes could be potential leads for development into antitumor compounds. The in-vivo efficacy studies of Vernopicrin and Vernomelitensin against the most sensitive cell lines is planned as well as other studies to elucidate the mechanism of action and toxicity profile of these compounds.
Acknowledgments
The authors are grateful to Alosyius N. Toyang, Yua Eric and Therese Toyeng for assisting with the collection and processing of the plant material. The technical support of the Translational Core, Greenebaum Cancer Center at the University of Maryland School of Medicine; a NCI Comprehensive Cancer Center are acknowledged.
References
- Burkill HM. The Useful plants of West Tropical Africa. 2. Vol. 1. Royal Botanic Gardens Kew; UK: 1985. p. 510. [Google Scholar]
- Buskuhl H, Oliveira FL, Blind LZ, Freitas RA, Barison A, Campos FR, Corilo YE, Eberlin MN, Caramori GF, Biavatti MW. Sesquiterpene lactones from Vernonia scorpioides and their in vitro cytotoxicity. Phytochemistry. 2010;71:1539–1544. doi: 10.1016/j.phytochem.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Donfack ARN, Toyang NJ, Wabo HK, Tane P, Awoufack MD, Kikuchi H, Tamokou JDD, Kuiate JR, Oshima Y. Stigmatane derivatives from the root extract of Vernonia guineensis and their antimicrobial activity. Phytochemistry Letters. 2012;5:596–599. [Google Scholar]
- Farombi EO, Owoeye O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. International Journal of Environmental Research and Public Health. 2011;8:2533–2555. doi: 10.3390/ijerph8062533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. European Journal of Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Focho DA, Nkeng EAP, Lucha CF, Ndam WT, Afegenui A. Ethnobotanical survey of plants used to treat diseases of the reproductive system and preliminary phytochemical screening of some species of Malvaceae in Ndop central sub-division, Cameroon. Journal of Medicinal Plants Research. 2009;3:301–314. [Google Scholar]
- Fortuna AM, de Riscala EC, Catalan CAN, Gedris TE, Herz W. Sesquiterpene lactones from Centaurea tweediei. Biochemical Systematics and Ecology. 2001;29:967–971. doi: 10.1016/s0305-1978(01)00042-4. [DOI] [PubMed] [Google Scholar]
- Ganjian I, Kubo I, Fludzinski P. Insect antifeedant elemanolide lactones from Vernonia amygdalina. Phytochemistry. 1983;22:2525–2526. [Google Scholar]
- Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials. Drug Discovery Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Iwu MM. Handbook of African Medicinal Plants. CRC Press; London: 1993. p. 415. [Google Scholar]
- Jiofack T, Fokunang C, Guedje N, Kemeuze V, Fongnzossie E, Nkongmeneck BA, Mapongmetsem PM, Tsabang N. Ethnobotanical uses of medicinal plants of two ethnoecological regions of Cameroon. International Journal of Medicine and Medical Sciences. 2010;2:60–79. [Google Scholar]
- Jisaka M, Ohigashi H, Takegawa K, Huffman MA, Koshimizu K. Antitumoral and antimicrobial activities of bitter sesquiterpene lactones of Vernonia amygdalina, a possible medicinal plant used by wild chimpanzees. Bioscience Biotechnolology Biochemistry. 1993;57:833–834. doi: 10.1271/bbb.57.833. [DOI] [PubMed] [Google Scholar]
- Kaur K, Jain M, Kaur T, Jain R. Antimalarials from nature. Bioorganic and Medicinal Chemistry. 2009;17:3229–3256. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Kos O, Castro V, Murillo R, Poveda L, Merfort I. Ent-kaurane glycosides and sesquiterpene lactones of the hirsutinolide type from Vernonia triflosculosa. Phytochemistry. 2006;67:62–69. doi: 10.1016/j.phytochem.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Koshimizu K, Ohigashi H, Huffman MA. Use of Vernonia amygdalina by wild chimpanzee: possible roles of its bitter and related constituents. Physiology and Behavior. 1994;56:1209–1216. doi: 10.1016/0031-9384(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Kuo YH, Kuo YJ, Yu AS, Wu MD, Ong CW, Kuo LMY, Huang JT, Chen CF, Li SY. Two novel sesquiterpene lactones, cytotoxic vernolide-A and -B, from Vernonia cinerea. Chemical and Pharmaceutical Bulletin. 2003;51:425–426. doi: 10.1248/cpb.51.425. [DOI] [PubMed] [Google Scholar]
- Laekeman GM, De Clerck F, Vlietinck AJ, Herman AG. Vernolepin: an antiplatelet compound of natural origin. Naunyn Schmiedebergs Archives of Pharmacology. 1985;331:108–113. doi: 10.1007/BF00498859. [DOI] [PubMed] [Google Scholar]
- Marco JA, Sanz-Cervera JF, Yuste A, Saucenón F, Carda M. Sesquiterpenes from Centaurea aspera. Phytochemistry. 2005;66:1644–1650. doi: 10.1016/j.phytochem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Merfort I. Perspective on sesquiterpene lactones in inflammation and cancer. Current Drug Targets. 2011;12:1560–1573. doi: 10.2174/138945011798109437. [DOI] [PubMed] [Google Scholar]
- Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. Journal of Microbiological Methods. 2008;73:211–215. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Noumi E. Ethno medicines used for treatment of prostatic disease in Foumban, Cameroon. African Journal of Pharmacy and Pharmacology. 2010;4:793–805. [Google Scholar]
- Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Cancer in indigenous Africans: burden, distribution, and trends. The Lancet Oncology. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Kuttan G. Modulation of immune response by Vernonia cinerea L. inhibits the proinflammatory cytokine profile, iNOS, and COX-2 expression in LPS-stimulated macrophages. Immunopharmacology and Immunotoxicology. 2011a;33:73–83. doi: 10.3109/08923971003745977. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Kuttan G. Vernolide-A, a sesquiterpene lactone from Vernonia cinerea, induces apoptosis in B16F-10 melanoma cells by modulating p53 and caspase-3 gene expressions and regulating NF-kB mediated bcl-2 activation. Drug and Chemical Toxicology. 2011b;34:261–270. doi: 10.3109/01480545.2010.520017. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Kuttan G. Antimetastatic potential of vernolide-A, a sesquiterpenoid from Vernonia cinerea L. Human and Experimental Toxicology. 2012;31:66–80. doi: 10.1177/0960327111414279. [DOI] [PubMed] [Google Scholar]
- Rustaiyan A, Nazarians L, Bohlmann F. Two new elemanolides from Onopordon leptolepis. Phytochemistry. 1979;18:879–880. [Google Scholar]
- Tchinda AT, Tsopmo A, Tane P, Ayafor JF, Connolly JD, Sterner O. Vernoguinosterol and vernoguinoside, trypanocidal stigmastane derivatives from Vernonia guineensis (Asteraceae) Phytochemistry. 2002;59:371–374. doi: 10.1016/s0031-9422(01)00448-4. [DOI] [PubMed] [Google Scholar]
- Tchinda AT, Tsopmo A, Tane P, Ayafor JF, Connolly JD. Stigmatane derivatives and isovaleryl sucrose esters from Vernonia guineensis (Asteraceace) Phytochemistry. 2003;63:841–846. doi: 10.1016/s0031-9422(03)00326-1. [DOI] [PubMed] [Google Scholar]
- Toubiana R, Monpon B, Ho CM, Toubiana MJ. Isolement du vernodalin et du vernolepin a partir de Vernonia guineensis: authenticité du squelette elemane. Phytochemistry. 1975;14:115–118. [Google Scholar]
- Toyang NJ, Wabo HK, Ateh EN, Davis H, Tane P, Kimbu SF, Sondengam LB, Bryant J. In vitro anti-prostate cancer and ex vivo antiangiogenic activity of Vernonia guineensis Benth. (Asteraceae) tuber extracts. Journal of Ethnopharmacology. 2012;141:866–871. doi: 10.1016/j.jep.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Toyang NJ, Verpoorte R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae) Journal of Ethnorphamacology. doi: 10.1016/j.jep.2013.01.040. http://dx.doi.org/10.1016/j.jep.2013.01.040i. in press. [DOI] [PubMed]