Abstract
B cell lymphoma consists of multiple individual diseases arising throughout the lifespan of B cell development. From pro-B cells in the bone marrow, through circulating mature memory B cells, each stage of B cell development is prone to oncogenic mutation and transformation, which can lead to a corresponding lymphoma. Therapies designed against individual types of lymphoma often target features that differ between malignant cells and the corresponding normal cells from which they arise. These genetic changes between tumor and normal cells can include oncogene activation, tumor suppressor gene repression and modified cell surface receptor expression. G protein-coupled receptors (GPCRs) are an important class of cell surface receptors that represent an ideal target for lymphoma therapeutics. GPCRs bind a wide range of ligands to relay extracellular signals through G protein-mediated signaling cascades. Each lymphoma subgroup expresses a unique pattern of GPCRs and efforts are underway to fully characterize these patterns at the genetic level. Aberrations such as overexpression, deletion and mutation of GPCRs have been characterized as having causative roles in lymphoma and such studies describing GPCRs in B cell lymphomas are summarized here.
Keywords: GPCR, lymphoma, cancer, mutation, chemokine receptors, lipid receptors
1. Introduction
B cell lymphomas encompass a variety of neoplasms affecting lymphocytes in the immune system. In all cases, a normal B cell acquires a set of mutations or genetic aberrations that result in clonal proliferation of malignant cells. The stage of development a B cell is in when it acquires these genetic alterations dictates the subsequent lymphoma that develops. Through advances such as next generation sequencing and high throughput screening technologies, the underlying genetics and mechanisms of lymphomagenesis are becoming more clearly defined.
GPCRs are seven-transmembrane domain cell surface-spanning receptors that regulate and transmit extracellular signals to induce numerous intracellular signaling pathways. There are hundreds of known GPCRs, each of which binds a specific ligand or set of ligands, which then induce conformational changes that lead to downstream signaling events. The cell surface ligand-binding capabilities of GPCRs make them highly desirable as drug targets for oncogenic cells. A complete and thorough understanding of GPCR expression in normal and malignant cells, coupled with a detailed knowledge of the molecules that bind to each GPCR and the downstream signaling pathways they activate, will reveal numerous opportunities for targeted therapeutics to improve disease outcome. A summary of what is known regarding GPCR expression and mutation in B cell lymphomas is reviewed here and outlined in Figure 1.
Figure 1. GPCRs in B cell lymphoma.
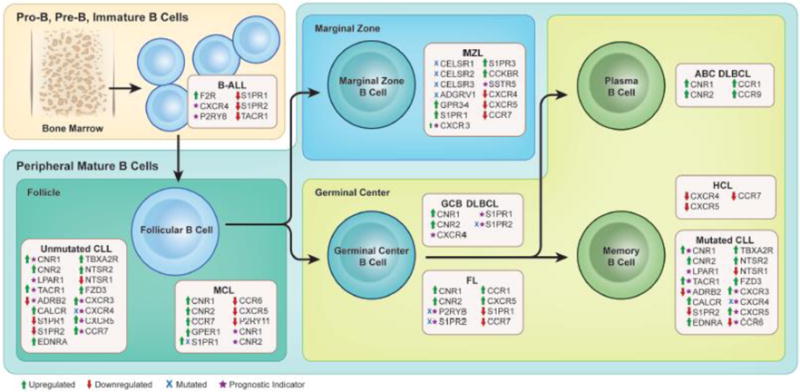
GPCRs that are upregulated (green arrow), downregulated (red arrow), mutated (blue X) or are known to significantly inform prognosis (purple star) are indicated for individual lymphomas. The cell of origin that gives rise to each type of lymphoma is shown in the context of normal B cell development.
2. B-cell Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL) is a neoplasm of the lymphoid precursor cells and is characterized as either B cell ALL (B-ALL) or T cell ALL (T-ALL) based on the cell of origin. B-ALL is the most common childhood malignancy and occurs less frequently in adults [1]. B-ALL cases are often subgrouped based on cell of origin, patient age, or the presence of a chromosomal aberration.
Multiple chemokine receptors are expressed in B-ALL and these findings are summarized in Table 1. Chemokine receptor expression frequently varies between lineage-derived subgroups of B-ALL and this can provide insight into the underlying function of GPCRs in B-cell development and lymphomagenesis. For example, CXCR1 surface protein was not found in pro-B or pre-B ALL but was present in 5/17 (29%) cases of early pre-B and 3/5 (60%) cases of B-ALL [2]. Meanwhile, flow cytometry of CXCR2 and CXCR3 found these receptors to be expressed in all early pre-B, pre-B and B-ALL samples tested along with more than half of pro-B samples [2, 3]. However, another study that used immunohistochemistry (IHC) instead of flow cytometry to detect surface protein only found CXCR3 in 3/9 (33%) lymphoblastic leukemia/lymphoma patients [4].
Table 1. Chemokine receptor expression in B cell lymphomas.
The proportion of tumor samples that were reported as positive for individual chemokine receptors are shown across thirteen NHL subtypes. Data were grouped according to the method used to identify the receptor (IHC = immunohistochemistry; FC = flow cytometry; mRNA = quantitative PCR). Blank cells indicate that measurements have not been reported. Cell color reflects the total percentage of positive tumor cases (grey = 0%; lightest blue = 1–24.9%; medium blue = 25–49.9%; darker blue = 50–74.9%; darkest blue = 75–100%).
| GPCR | Method | Pro B-ALL | Early Pre B-ALL | Pre B-ALL | B-ALL | CLL | SLL | MCL | BL | FL | DLBCL | MZL | MALT | HCL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| CCR1 | IHC | 1/17 | 0/4 | 122/349 | 85/217 | 3/11* | 12/32 | 0/4 | ||||||
| FC | 0/13 | 0/17 | 0/12 | 0/9 | 9/13 | 0/6 | 0/5 | 0/4 | 0/5 | 0/5 | 4/9 | |||
| mRNA | 18/18 | 24/26 | ||||||||||||
|
| ||||||||||||||
| CCR2 | IHC | |||||||||||||
| FC | 0/13 | 0/17 | 0/12 | 0/9 | 6/58 | 1/6 | 3/5 | 0/4 | 8/11 | 0/5 | 6/9 | |||
| mRNA | 6/6 | |||||||||||||
|
| ||||||||||||||
| CCR3 | IHC | |||||||||||||
| FC | 5/8 | 6/17 | 6/12 | 3/5 | 13/13 | 1/6 | 2/5 | 0/4 | 0/5 | 0/5 | 7/9 | |||
| mRNA | ||||||||||||||
|
| ||||||||||||||
| CCR4 | IHC | 11/91 | 7/19 | |||||||||||
| FC | 7/11 | 8/17 | 6/12 | 3/5 | 0/45 | 0/4 | ||||||||
| mRNA | ||||||||||||||
|
| ||||||||||||||
| CCR5 | IHC | 8/8 | 16/17 | |||||||||||
| FC | 0/13 | 0/17 | 0/32 | 0/9 | 1/81 | 3/15 | 5/9 | 0/4 | 4/24 | 3/5 | 16/21 | |||
| mRNA | 18/18 | 24/26 | ||||||||||||
|
| ||||||||||||||
| CCR6 | IHC | 7/11 | 42/76* | 19/19 | ||||||||||
| FC | 0/13 | 0/17 | 0/31 | 0/9 | 29/52 | 10/10 | 12/12 | 0/4 | 4/24 | 5/5 | 21/21 | |||
| mRNA | 8/8 | 16/16 | ||||||||||||
|
| ||||||||||||||
| CCR7 | IHC | 3/15 | ||||||||||||
| FC | 4/11 | 6/17 | 5/12 | 4/5 | 21/21 | 4/8 | 0/4 | 1/13 | 0/4 | |||||
| mRNA | 16/18 | 23/26 | ||||||||||||
|
| ||||||||||||||
| CXCR1 | IHC | |||||||||||||
| FC | 0/8 | 5/17 | 0/12 | 3/5 | 3/58 | 3/6 | 0/5 | 0/4 | 2/5 | 2/5 | 4/9 | |||
| mRNA | ||||||||||||||
|
| ||||||||||||||
| CXCR2 | IHC | 7/51 | ||||||||||||
| FC | 4/7 | 15/15 | 12/12 | 5/5 | 0/58 | 4/6 | 0/5 | 4/4 | 2/5 | 2/5 | 2/9 | |||
| mRNA | ||||||||||||||
|
| ||||||||||||||
| CXCR3 | IHC | 3/9 | 1/30 | 1/27 | 9/11 | 36/67* | 77/127* | 5/7 | ||||||
| FC | 8/10 | 15/15 | 19/19 | 5/5 | 62/65 | 13/15 | 4/14 | 4/4 | 15/22 | 5/5 | 15/29 | |||
| mRNA | 16/18 | 20/26 | ||||||||||||
|
| ||||||||||||||
| CXCR4 | IHC | 255/505 | 2/5 | |||||||||||
| FC | 11/13 | 8/17 | 8/12 | 3/5 | 57/57 | 15/15 | 9/9 | 2/4 | 24/24 | 5/5 | 21/21 | |||
| mRNA | 7/20 | 15/26 | ||||||||||||
|
| ||||||||||||||
| CXCR5 | IHC | 13/15 | ||||||||||||
| FC | 3/9 | 5/17 | 4/12 | 13/13 | 113/115 | 12/15 | 10/10 | 4/4 | 26/26 | 6/7 | 18/22 | |||
| mRNA | 17/18 | 23/26 | ||||||||||||
| Color scale: | 0% | 1–24.9% | 25–49.9% | 50–74.9% | 75–100% |
CXCR4 is the most frequently studied chemokine receptor in lymphoma and its role in signaling in B-ALL has been extensively reviewed [5]. CXCR4 is normally expressed on pre-B and mature B cells and is essential for migration in lymphoma [4]. Elevated mRNA or protein expression of CXCR4 in B-ALL has been correlated with unfavorable clinical outcome [6], extramedullary organ invasiveness [7, 8], and time point and site of relapse [9]. CXCR4 has been identified in the majority of pro-B, pre-B and B-ALL cases and in some early pre-B cases [2, 3, 8, 10]. The estrogen receptor GPER1 (also known as GPR30) is also believed to regulate CXCR4 signaling in pre-B cell ALL [11].
CXCR5 is known to play a role in signaling and cell migration in lymphoma, however there are conflicting reports regarding its cell surface expression in B-ALL. Two flow cytometry studies did not identify any precursor-B, pro-B or pre-B patients with CXCR5 expressed [3, 10] while a third study found CXCR5+ cases in 3/9 (33%) pro-B, 5/17 (29%) early pre-B, 4/12 (33%) pre-B and 5/5 (100%) B-ALL cases [2]. A fourth report identified CXCR5 expression only on CD23+CD5+ B-ALL cells [12], while a final study found the receptor to be expressed in all B-ALL cases [4].
Other chemokine receptors that have been detected by flow cytometry in B-ALL include CCR3 and CCR4 [2, 12] as well as CXCR7 (also known as ACKR3), which is strongly upregulated in the bone marrow in B-ALL compared to normal tissue and plays a role in controlling CXCR4-mediated migration [13]. CCR7 cell surface expression has been found to vary between studies [2, 3, 12] while CCR1, CCR2, CCR5 and CCR6 were extremely rarely or never expressed [2, 3, 10, 14].
In addition to grouping cases of B-ALL by cell of origin, these lymphomas can also be subcharacterized by genetic rearrangements. Fusion of the purinergic receptor P2RY8 promoter to the CRLF2 (cytokine receptor like factor 2) gene frequently occurs in B-ALL but reports of the functional consequences of this rearrangement have been conflicting. Although P2RY8-CRLF2 fusions were significantly correlated with poor outcome in adolescent and adult patients [15, 16], the consequences of the fusion in pediatric B-ALL is less clear as some studies have found it to be associated with poor outcome [17–19] whereas others have not found any significant correlation [20, 21]. A meta-analysis of these studies concluded that presence of the P2RY8-CRLF2 fusion was in fact significantly associated with poor prognosis of relapse-free survival and event-free survival [22]. Another finding among these studies was that P2RY8-CRLF2 was more common in B-ALL patients with trisomy 21 [19, 23–26] or intrachromosomal amplification of chromosome 21 [27] than those with normal chromosome 21 [1, 18–21, 25, 26, 28–30].
P2RY8-CRLF2 rearrangements were significantly enriched in patients harboring deletion of the lymphoid lineage-directing transcription factor IKZF1 in one study [31] but not correlated in another study, and this discrepancy is suggested to be correlated to ethnicity [1]. Interestingly, reduced protein expression of IKZF1 has also been associated with upregulation of the orphan GPCR GPR132 (also known as G2A) in B-ALL patients that do not harbor the BCR-ABL fusion, which encodes a tyrosine kinase oncoprotein [32]. In fact, mice transplanted with BCR-ABL transduced bone marrow deficient of GPR132 developed tumors more quickly and had accelerated tumor progression and death compared to those with wild type GPR132 suggesting that GPR132 acts as a negative regulator of BCR-ABL induced leukemogenesis [33].
Other GPCRs that have been described in B-ALL include oncogenic roles for the prostaglandin receptor PTGER2 [34–37] and orphan receptor GPR34 [38], increased protein expression of the thrombin receptor F2R (also known as PAR1) at significantly higher levels than lymphocytes from healthy donors [39] and decreased expression of S1PR1 and S1PR2 transcripts [40] and the tachykinin receptor TACR1 in tumor compared to normal B lymphocytes [41]. A summary of all GPCRs reported in B-ALL is shown in Table 2.
Table 2. GPCRs in B cell lymphoma studies.
GPCRs that have been investigated in B cell lymphoma are shown corresponding to the specific diseases in which each receptor has been studied.
| GPCR | B-ALL | CLL/SLL | MCL | BL | FL | DLBCL | MZL/MALT | LPL/WM | HCL |
|---|---|---|---|---|---|---|---|---|---|
| ACKR3 | [13] | [80] | [173] | [173] | |||||
| ADGRV1 | [183] | ||||||||
| ADORA2A | [55] | ||||||||
| ADORA2B | [55] | ||||||||
| ADRB2 | [52][53] | [116] | [161] | ||||||
| CALCR | [56] | ||||||||
| CCKBR | [186] | ||||||||
| CCR1 | [2][14] | [14][72] | [14][72][116] | [2][14][116] | [14][72][116][148] | [14][172][173] | [14][72][172][173] | [14][193] | [14][72] |
| CCR2 | [2] | [72] | [72] | [2] | [72][151] | [72] | [193] | [72] | |
| CCR3 | [2] | [72] | [72][116] | [2][116] | [72][116][150] | [72] | [193] | [72] | |
| CCR4 | [2][12] | [12] | [2] | [175][180–182] | [172][175] | [193] | |||
| CCR5 | [2][3][10] | [3][10][72] | [10][72] | [2] | [3][10][72] | [172][173] | [72][172][173] | [193] | [3][10][72] |
| CCR6 | [2][3][10] | [3][10][61][72][93][102] | [10][72][102][115][116][118] | [2][116] | [3][10][72][102][116] | [172][178] | [72][102][135][172] | [193] | [3][10][72] |
| CCR7 | [2][3][12][94] | [3][12][43][46][61][71][76][94][96–101] | [71][116][118] | [2][116] | [3][71][116][118][142] | [118][161][166][172][173][178] | [71][118][172][173] | [193] | [3][71][206] |
| CCR8 | [136][172][173] | [172][173] | [193] | ||||||
| CCR9 | [12] | [12] | [149] | [149][173][178] | [173] | ||||
| CCR10 | [172] | [172] | |||||||
| CCRL2 | [99] | ||||||||
| CELSR1 | [183] | ||||||||
| CELSR2 | [183] | ||||||||
| CELSR3 | [183] | ||||||||
| CNR1 | [51] | [50][51] | [51][107–116] | [51] | [51][103] | [51] | [51] | ||
| CNR2 | [51] | [50][51] | [51][107–116] | [51] | [51][135] | [51][161–162] | [51] | ||
| CX3CR1 | [103–105] | [103] | [103][150] | [103] | [103] | ||||
| CXCR1 | [2] | [72] | [72] | [72] | [172] | [72][172] | [193] | [72] | |
| CXCR2 | [2] | [72][106] | [72] | [72] | [72] | [193] | [72] | ||
| CXCR3 | [2][3][4][12] | [3][4][12][66][68–70][72][76] | [4][68][72] | [4] | [3][4][72] | [4][172][173][175] | [4][72][172][173][175–177][189–191] | [193] | [3][4][68][72] |
| CXCR4 | [2][3][5–11] | [3][10][46][71–89][91][92][100][125][192][202] | [10][71][72][102][118] | [2][123–126][132] | [3][10][71][72][118][123][131–133][139][144–147][151][202] | [118][132][139][163–173][178][202] | [71][72][118][139][172][173][183][192] | [192–204] | [3][10][71][72] |
| CXCR5 | [2][3][4][10][12] | [3][4][10][12][71][72][90–95][137] | [4][10][71][72][90][115][116][118][137] | [2][4][116][122] | [3][4][10][71][72][90][116][118][136–142] | [118][136][139][141][166][172][173][178] | [4][71][72][118][137][172][173] | [4][193] | [3][4][10][71][72] |
| CXCR6 | [12] | [12] | [172][173] | [173] | |||||
| CYSLT1 | [57][179] | [179] | [179] | [179] | [179] | [179] | |||
| EDNRA | [58] | ||||||||
| F2R | [39] | [39] | |||||||
| FZD3 | [65][66] | ||||||||
| FZD6 | [63][64] | ||||||||
| GPER1 | [11] | [121] | [121] | [121] | |||||
| GPR34 | [38] | [184] | [184][185] | ||||||
| GPR65 | [59] | ||||||||
| GPR82 | [185] | ||||||||
| GPR132 | [32][33] | ||||||||
| GPR183 | [103] | [161] | |||||||
| LPAR1 | [47–49] | ||||||||
| LPAR3 | [47] | ||||||||
| LPAR5 | [47] | ||||||||
| LTB4R | [60] | ||||||||
| MRGPRF | [120] | ||||||||
| NTSR1 | [62] | ||||||||
| NTSR2 | [62] | ||||||||
| P2RY8 | [1][15–31] | [130–132][134] | |||||||
| P2RY11 | [55] | [115][116] | |||||||
| PTGER1 | [37] | [127] | |||||||
| PTGER2 | [34–37] | [127] | |||||||
| PTGER3 | [37] | [127] | |||||||
| PTGER4 | [37] | [127][128] | [103] | ||||||
| S1PR1 | [40][117] | [40][43–46][117] | [117–119] | [117] | [117][118][142] | [117][118][156–161] | [117][118] | [117] | |
| S1PR2 | [40] | [40][43] | [118] | [118][131][132] | [118][136][152–155] | [118] | |||
| S1PR3 | [118] | [118] | [118] | [118] | |||||
| S1PR4 | [40] | [40] | |||||||
| SSTR1–SSTR5 | [187][188] | ||||||||
| TAAR1 | [129] | [129] | [129] | [129] | |||||
| TACR1 | [41] | [41] | |||||||
| TBXA2R | [61] | ||||||||
| XCR1 | [172–174] | [172][173] |
3. Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease that arises from CD5+ B cells. CLL is the most frequently occurring leukemia in Western countries and has an extremely variable prognosis that can range from stable, indolent disease not requiring intervention to aggressive malignancy [42]. CLL is often characterized by immunoglobulin heavy-chain variable region gene (IGHV) mutation status. Unmutated IGHV CLL originates from pre-germinal center cells and is defined as having less than a 2% difference between leukemic clone and germline DNA sequence in the IGHV region. Mutated IGHV arises from post-germinal center cells and contains a 2% or greater difference between leukemic clone and germline IGHV sequence and has a significantly better prognosis than unmutated IGHV [43]. CLL that localizes to lymph nodes is also known as small lymphocytic lymphoma (SLL) and represents a subset of CLL.
A variety of receptors spanning multiple GPCR families have been studied in CLL and recent attempts to target members of the lipid receptors in vitro and in vivo have shown a range of success. The sphingosine-1-phosphate (S1P) receptors S1PR1 and S1PR2 transcripts were found to be downregulated in CLL compared to control B cells [40], with S1PR1 expression particularly reduced in unmutated IGHV CLL patients and S1PR2 impaired in both mutated and unmutated CLL [43]. This downregulation is thought to be due to cell interaction with the tumor microenvironment to regulate egress of malignant cells from the lymphoid tissues to peripheral blood [44]. Treatment with Syk, Btk, and B cell receptor (BCR) inhibitors has been effective at increasing S1PR1 protein expression to induce CLL cell mobilization into the blood so that cells are more sensitive to cytotoxic drugs [44–46].
Contrary to the downregulation of S1PR family GPCRs, CLL cells have increased mRNA expression of the lysophosphatidic acid (LPA) family receptors LPAR1, LPAR3 and LPAR4 compared to normal B cells [47]. Increased LPAR1 mRNA has been shown to be associated with more aggressive disease [47] and LPA signaling was found to act as a survival factor by protecting primary CLL cells from spontaneous and chemotherapy-induced apoptosis [48]. Further study revealed that treatment of B cell lines with LPA induced vascular endothelial growth factor (VEGF) expression via activation of c-Jun N-terminal kinases (JNK) and nuclear factor-kappa B (NF-κB) and protected cells against apoptosis [47, 49].
Cannabinoid signaling pathways have been investigated for potentially containing novel therapeutic targets in CLL/SLL. The cannabinoid receptor transcripts CNR1 and CNR2 were found to be overexpressed in CLL and SLL compared to normal B cells and high CNR1 expression was significantly associated with shorter overall survival [50, 51]. Although treatment with cannabinoids reduced viability of CLL cells in culture, the simultaneous death of healthy cells suggested that targeting cannabinoid receptors could have poor therapeutic value [50].
Numerous GPCRs have significantly altered expression in CLL as compared to healthy lymphocytes and these expression patterns can serve as biomarkers of disease subtype or progression. For example, tachykinin receptor TACR1 mRNA is overexpressed in CLL patient cells compared to normal B lymphocytes and expression is higher in aggressive IGHV-unmutated CLL compared to indolent IGHV-mutated CLL [41]. Conversely, CLL mononuclear leukocytes contain fewer beta-2 adrenergic receptors (ADRB2) than healthy cells and increased dysfunction of the receptor complex is correlated with disease progression [52]. ADRB2 agonists have been shown to induce apoptotic cell death in CLL cells alone and synergistically with other agents [53] and expression of alpha-2 adrenergic receptors has also been described in CLL [54].
Multiple GPCRs are believed to affect cyclic adenosine monophosphate (cAMP) and calcium signaling in CLL. RNA transcripts from the adenosine receptors ADORA2A and ADORA2B and purinergic receptor P2RY11 were found to be expressed in CLL lymphocytes it is believed that adenosine induces cAMP accumulation via ADORA2A while adenosine triphosphate (ATP) induces cAMP through P2RY11 [55]. The calcitonin receptor CALCR mRNA and protein were shown to be overexpressed in CLL cells compared to healthy B cells and it is suspected that an increase in CALCR expression increases the concentration of intracellular calcium to promote lymphocyte activation and proliferation [56]. In addition, mRNA from the cysteinyl leukotriene receptor CYSLTR1 was found to be well-expressed in CD19+ CLL cells, albeit at lower levels than normal CD19+ cells, and was found to mediate intracellular calcium and cell migration in response to leukotrienes [57].
Notable oncogenic hallmarks such as increased DNA synthesis, cell cycle progression, and adaptation to the tumor microenvironment are all influenced by GPCRs in CLL. The endothelin receptor EDNRA was found to be overexpressed at both the mRNA and protein level in CLL cells compared to normal cells and activation of EDNRA via endothelin-1 resulted in increased proliferation, cell cycle progression and mitogen-activated protein kinase (MAPK) signaling [58]. The acid sensing GPCR GPR65 transcript levels in CLL were significantly correlated with expression of the apoptosis-regulating proteins Bcl-2, Mcl-1 and Bcl-x1, suggesting that GPR65 may aid CLL cells to survive in the acidic tumor microenvironment [59]. Finally, CLL cells express the leukotriene receptor LTB4R (BLT1) protein and treatment of these cells with leukotriene biosynthesis inhibitors inhibited DNA synthesis and antigen expression and thus represent a novel CLL therapeutic [60].
Other GPCRs notable for changes in expression on CLL cells include upregulation of the thromboxane A2 receptor TBXA2R mRNA [61] and up and downregulation of mRNA and protein from the neurotensin receptors NTSR2 and NTSR1, respectively [62].
The Eμ-TCL1 mouse model of CLL has been used to study multiple aspects of the disease including changes in gene expression during disease development. In this model, the gene expression of the Wnt/Fzd pathway GPCRs FZD6 and FZD1 both increased during leukemogenesis and knockout of FZD6 in the model resulted in a delay and decreased incidence of disease [63]. However, differences between mouse and human FZD6 have made it challenging to understand the relation of this finding to humans [64]. In a study of human mRNA, FZD3 was the only Fzd receptor that had significantly higher expression in mutated or unmutated CLL when compared to normal B cells [65]. FZD3 mRNA was also found to be overexpressed in a separate cohort of early-stage CLL patients compared to normal volunteers [66].
The expression levels of chemokine receptors in CLL and SLL are summarized in Table 1 and their role in disease has been reviewed elsewhere [67]. Of note, CXCR3 is highly expressed in CLL and plays a role in mediating cell chemotaxis. In one study, 31/31 (100%) CLL patients had CD5+ B cells positive for CXCR3 via flow cytometry compared to 0/6 (0%) healthy subjects [68] while in another study CXCR3 was positively identified via IHC in 34/36 (94%) CLL/SLL patients and by flow cytometry in 12/13 (92%) cases [4]. CXCR3 was also found to be overexpressed in low stage CLL compared to healthy B cells via gene expression profiling [66]. With regards to the consequences of CXCR3 expression, one study with a small cohort of 21 patients found no correlation between CXCR3 flow cytometry mean fluorescence intensity (MFI) and the number of lymphocytes or clinical stage of disease [69] whereas a larger study of 149 CLL patients found that patients with high CXCR3 and low CXCR4 MFI had a significantly lower risk of disease progression in both IGHV mutated and unmutated CLL [70].
CLL cells express an abundance of CXCR4 protein [3, 10, 46, 71–73] to enable migration and infiltration of bone marrow [74]. Increased protein levels of CXCR4 were found to be associated with survival in familial CLL [75] and lymphadenopathy [46] but it is unclear whether they do [76] or do not correlate with disease stage [77]. Multiple genetic aberrations of CXCR4 in CLL have been discovered including 3/186 (2%) familial cases of CLL containing a mutation in the coding region of the gene [78] and a case study of a translocation resulting in a CXCR4/MAML2 (mastermind like transcriptional coactivator 2) fusion protein [79].
As CLL cells do not express CXCR7 protein, all CXCL12-mediated responses are accomplished via CXCR4 and numerous downstream signaling molecules including activation of ZAP-70, MAPK and Akt signaling pathways [80–82]. CXCR4 was observed to be hyperphosphorylated in CLL compared to normal lymphocytes and inhibition of PIM kinase caused dephosphorylation and internalization of CXCR4 [83]. Numerous other therapeutics targeting CXCR4 downregulation have been investigated including suberoylanilide hydroxamic acid [84], AMD3100 (plerixafor) [85], the combination of CXCR4 antagonists and passive immunotherapy [86], PI3K inhibitors [87], small peptide inhibitors [88] and ibrutinib-mediated BTK inhibition [46, 89].
The chemokine receptor CXCR5 plays a significant role in CLL/SLL pathogenesis. The receptor protein has been identified on greater than 90% of patient samples [3, 4, 10, 72] and shown not to be affected by IGHV mutation status [90]. Depending on the study, CXCR5 protein was either similarly expressed or significantly overexpressed relative to normal CD5+ B cells [12, 71, 90] and more highly expressed in CLL compared to other B cell neoplasms [3, 10, 71, 90]. Downregulation of CXCR4 and CXCR5 via BCR signaling was found to correlate with unfavorable disease outcome [91, 92] and reduced CXCR5 MFI was observed in del 17p/11q CLL, which has a worse prognosis than normal CLL [93]. Experiments in cell lines revealed that overexpression of CXCR5 along with CCR7 in CLL and B-ALL enabled resistance to apoptosis via upregulation of PEG10 and stabilization of caspase-3 and caspase-8 [12, 94]. In concordance with these findings and its proposed role as on oncogene, knockout of CXCR5 in the Eμ-TCL1 CLL mouse model resulted in significantly delayed disease onset and disrupted leukemia cell migration [95].
CCR7 is another chemokine receptor that is upregulated in CLL [3, 61, 71, 96] and is believed to play a role in enabling cell entry into lymph nodes [97]. Higher mRNA and protein expression of CCR7 has been observed in unmutated compared to mutated IGHV CLL [46] and increased expression has been correlated with disease stage [76] and lymphadenopathy [46]. ZAP-70 has been shown to upregulate CCR7 via an ERK1/2-dependent mechanism that is also believed to regulate CXCR4 and CXCR5 [98]. The atypical chemoattractant GPCR CCRL2 (also known as CRAM) surface protein is expressed on CLL cells and is believed to regulate CCR7- and CCL19-mediated cell responses [99]. Finally, CCR7 and CXCR4 function together to upregulate matrix metallopeptidase 9 (MMP-9) to enable CLL cell migration [100]. Treatment of CLL patients with anti-CCR7 monoclonal antibody has shown promise in mice and humans [101].
Many chemokine receptors have varied expression in CLL based on the subtype of CLL being studied and the method used to identify the receptor. Similar to CXCR5, CCR6 protein expression was significantly reduced in poor-prognosis del 17p/11q CLL compared to normal CLL [93]. In addition, gene expression profiling showed that CCR6 was downregulated compared to memory B cells [61] while studies have reported a range from 23–100% of CLL/SLL cases that were positive for CCR6 [3, 10, 72, 102].
CX3CR1 protein was found to be present in 27–100% of CLL/SLL cases [103, 104] and CX3CR1 gene expression was higher in IGHV mutated than unmutated patients [105]. Interestingly, binding of CX3CL1 to CX3CR1 resulted increased CXCR4 MFI on CLL cells in vitro [104].
CCR1 and CXCR2 have both been detected in CLL/SLL. 4/35 (11%) cases of CLL/SLL were positive for CCR1 via an IHC analysis [14] and 9/13 CLL patient samples were positive for CCR1 as measured by flow cytometry [72]. Conversely, CXCR2 was detected in 7/51 (14%) CLL cases via IHC [106] but 0/13 (0%) cases via flow cytometry [72].
4. Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) arises from B cells located in the mantle cell zone surrounding the germinal center in secondary lymph nodes. MCL is traditionally characterized by overexpression of the oncogene cyclin D1 (CCND1), which frequently results due to a t(11;14) reciprocal translocation.
Among Non-Hodgkin’s lymphoma (NHL) subtypes, the endocannabinoid system has been most extensively described in MCL due to the consistent overexpression of the cannabinoid receptors CNR1 and CNR2 in malignant tissue compared to normal B lymphocytes [51, 107–112]. In a study of 107 MCL tissue samples, CNR1 and CNR2 mRNA was overexpressed in 98% and 100% of samples, respectively, and the degree of overexpression informed clinical factors [113]. CNR1 was significantly more strongly expressed in conventional MCL compared to indolent MCL [114] and significant associations were found between low CNR1 expression and lymphocytosis and leukocytosis as well as between high CNR2 expression and anemia [113]. Gene expression profiling of MCL primary tissue and cell lines compared to normal B cells found upregulation of CNR1 and the adrenergic receptor ADRB2 along with downregulation of the chemokine receptors CXCR5 and CCR6 and purinergic receptor P2RY11 in MCL although further flow cytometry found variable expression of the chemokine receptors [115, 116]. Therapeutic efforts to treat MCL primary cells and cell lines with cannabinoids resulted in decreased cell viability along with upregulated ceramide synthesis, apoptosis and cell death in MCL but not normal cells [107, 111, 112].
Another receptor that is highly expressed in MCL compared to other NHLs is the S1P receptor S1PR1. S1PR1 was detected via immunohistochemistry in 19/19 lymph node, 10/10 gastrointestinal tract and 9/9 bone marrow MCL cases but less frequently in other NHLs [117]. S1PR1 protein was also more highly expressed than S1PR2 and S1PR3 in MCL [118]. Finally, exome sequencing of patient samples identified mutations in the S1PR1 gene in 2/11 (18%) cases [119] as well as in the Mas-related GPCR MRGPRF in 3/56 (5%) cases [120].
The estrogen receptor GPER1 is enriched in MCL as it was detected via IHC in 94/157 (60%) MCL patient samples compared to 1/20 (5%) FL cases and 6/20 (30%) diffuse large B cell lymphoma (DLBCL) cases. Knockdown of GPER1 in MCL cell lines resulted in stabilization of microtubules, inhibition of proliferation, and activation of apoptotic pathways and combining GPER1 inhibitors with additional chemotherapeutics had a synergistic effect [121].
Chemokine receptor expression in MCL is shown in Table 1. CXCR4, CXCR5 and CCR6 protein was detected in the majority of patients [10, 72, 102, 118] and CX3CR1 was found to be present in up to 75% of cases depending on the method used to identify the receptor [103]. CCR7 was more highly expressed in MCL than normal B cells [71] and its protein expression varied based on anatomical site [118]. CCR1, CCR3 and CXCR3 were also detected in a small percentage of patients [4, 14, 68, 72].
5. Burkitt Lymphoma
Burkitt lymphoma, follicular lymphoma and the germinal center subtype of DLBCL (GCB DLBCL) are all believed to arise from germinal center B cells. The germinal center forms in response to foreign antigen presentation in order to produce specific antibodies against the antigen. B cells rapidly proliferate within the germinal center and pass through multiple rounds of selection to identify cells producing highly specific antibodies. B cells that successfully produce high-quality antibodies are retained while the remaining cells succumb to apoptosis.
Germinal center B cells rely on expression of chemokine receptors CXCR4 and CXCR5 to move between the dark and light zone of the germinal center where proliferation and selection occur, respectively. CXCR5 was discovered in BL and was originally named Burkitt Lymphoma Receptor 1 (BLR1) [122]. Flow cytometry on a small number of samples found that CXCR5 was well expressed in BL patient samples while CXCR4 was expressed in some cases [2]. Multiple CXCR4 inhibitors have shown success inducing cell death in BL cell lines including BKT140 [123], plerixafor and GEZ-644494 [124] and palmitoylated peptides called pepducins [125]. Interestingly, treatment with the CD20 antibody rituximab resulted in increased surface expression of CXCR4 whereas treatment with Shiga-like toxin reduced the amount of CXCR4 available [126].
The prostaglandin E receptors PTGER1, PTGER2, PTGER3 and PTGER4 are believed to play a proinflammatory role in BL [127]. In particular, PTGER4 has been identified on a variety of B cell lymphoblast cell lines, including BL, and is thought to inactivate NF-κB to sensitize cells to chemotherapeutics and induce apoptosis [128]. Other GPCRs which have been identified in BL include the cannabinoid receptor CNR1, which demonstrated strong and uniform staining via IHC in a BL patient sample [51], and the trace amine associated receptor TAAR1 protein, which was detected by Western blot in normal B cells along with a variety of B cell lymphoma cell lines including BL. Treatment of the BL cell line L3055 with a TAAR1 agonist was toxic to the cells via an apoptotic mechanism [129].
6. Follicular Lymphoma
Follicular lymphoma encompasses a range of lymphomas emerging from the germinal center B cell follicle that can vary in presentation from indolent to aggressive disease. FL can transform from a small cell to a large cell disease and this transformation is associated with poor clinical outcome.
Next generation sequencing technologies have revolutionized the identification of genetic aberrations in NHL and have revealed multiple mutations in GPCRs in FL. Whole exome sequencing identified loss of function mutations in CXCR4 and P2RY8 [130] as well as copy number loss of CXCR4, P2RY8 and S1PR2 in FL [131]. Mutation or copy number loss of these receptors has also been noted as significantly more common in transformed FL compared to non-transformed FL [131, 132]. These studies suggest a tumor suppressive role for these receptors. CXCR4 protein is well-expressed in FL [133] but S1PR2 surface expression is not as highly expressed compared to other NHLs [118]. P2RY8 has also been noted for a fusion to the SOX5 transcription factor in primary splenic FL [134].
Gene expression profiling of FL cases revealed an increase in the GPCRs PTGER4, CX3CR1, GPR183 (also known as Epstein-Barr virus-induced GPCR 2; EBI2) and CNR1 compared to normal tonsillar B cells [103]. Further investigation into the role of cannabinoid receptors in FL found that CNR1 and CNR2 mRNA were both upregulated compared to reactive lymph node tissue but that their expression did not correlate to tumor grade [51]. In addition, CNR2 transcript expression was found to be anatomically controlled as it was significantly upregulated in duodenal as compared to nodal FL [135].
The expression of chemokine receptors in FL is summarized in Table 1. IHC and flow cytometry of patient samples found that CXCR5 is well-expressed in FL [3, 10, 71, 72, 118] and SNPs in CXCR5 were associated with increased risk of FL and subsequent survival [136, 137]. FL cells are known to produce the ligand for CXCR5, CXCL13, and it is believed they express CXCR5 to respond to CXCL13 and form the architecture of lymphoid follicles [138]. Monoclonal antibodies targeting CXCR5 were found to be reactive against primary cutaneous FL but not closely related lymphomas [139] and have been shown to inhibit tumor growth in mouse models [140]. A trifunctional bispecific antibody targeting CXCR5::CD3 suppressed tumor growth and increased survival in a xenograft model of B cell lymphoma by recruiting T cells to CXCR5-expressing B cells and co-stimulating the activation of CD4+ and CD8+ T cells to lyse B cells [140].
CXCR5 is not only critical in the B cell lineage in FL. A high proportion of CXCR5-expressing follicular helper T cells (TFH) were observed in FL but not in closely related DLBCL [141] and further investigation revealed that FL regulatory T cells (Tregs) used a CXCL13-CXCR5 autocrine loop for positioning and upregulation of additional chemokine receptors [142]. There was also an increase in the number of CXCR5-expressing memory T cells along with changes in specific CXCR5+ memory T cell subsets in particular [143]. Finally, gene expression profiling showed that as a cell transitioned from normal lymph node to reactive lymph node to FL, CXCR5 expression increased in regulatory T cells whereas S1PR1 and CCR7 decreased [142].
While CXCR5 promotes migration towards CXCL13, CXCR4 mediates migration towards CXCL12-producing stromal cells [144] and is expressed on the surface of normal, relapse and transformed FL cells [3, 10, 71, 72, 118, 131, 133, 145]. Multiple therapeutic possibilities involving CXCR4 have been attempted including monoclonal antibodies [139], the pan-PI3K inhibitor BKM120 [146] and crosslinking of LLT1 [147].
CCR1 expression is highly enriched in FL compared to other lymphoid malignancies [14]. Although increased CCR1 mRNA expression was found to be associated with worse outcome in a one study [148], CCR1 protein expression was not correlated with overall survival or disease stage in a larger study [14].
Presentation of FL can vary in anatomical location and multiple chemokine receptors have expression patterns specific to anatomic subtype. CCR9 was found to be significantly more immunopositive in gastrointestinal FL compared to nodal FL and its presence in nodal FL was found to be an indicator for future gastrointestinal involvement [149]. Similarly, CCR6 was expressed in 28/32 (88%) duodenal FL patients as compared to only 14/27 (52%) nodal follicular lymphoma patients via immunohistochemistry [135] although a separate study did not observe CCR6 in any of 17 patient samples [102].
CX3CR1 was identified in 25–80% of cases depending on the method used to identify the receptor [103] and both CX3CR1 and CCR3 mRNA expression were significantly decreased in FL compared to reactive follicular hyperplasia [150]. Individual studies have also concluded that CCR2 protein is expressed in FL [151] whereas CCR7 [118] and CXCR3 are not as highly expressed [4].
7. Diffuse Large B Cell Lymphoma
DLBCL is a clinically and molecularly heterogeneous disease comprised of two major subgroups that can be identified through gene expression profiling: activated B-cell like (ABC) and germinal center B-cell like (GCB) DLBCL. DLBCL can originate at multiple anatomical sites and GPCR expression can vary between DLBCL subtype and disease location.
The S1P receptor family plays a prominent role in DLBCL. S1PR2 acts as a tumor suppressor in DLBCL and high gene expression of S1PR2 has been identified as a prognostic factor indicating increased likelihood of survival [152]. Sequencing of 106 DLBCL patient samples and cell lines found 28/106 (26%) had a mutation in the 5′ region of the S1PR2 gene within the intron between exons 1 and 2 [153]. Mutations have also been identified in the coding region of the gene and are suspected to be a result of aberrant somatic hypermutation [153–155]. In an effort to confirm the role of S1PR2 as a driver mutation in DLBCL, knockout of S1PR2 in mice were found to develop spontaneous disease resembling DLBCL [153]. In addition to mutations in S1PR2, polymorphisms in CCR8 were found to be associated with DLBCL and SNPs in CXCR5 were associated with increased DLBCL risk in the GCB subgroup in particular [136].
IHC of tumor samples found that S1PR1 was expressed on 54–58% of primary testicular DLBCL and 13–40% DLBCL not otherwise specified (DLBCL-NOS) cases [156, 157]. S1PR1 expression was an independent indicator of poor outcome in patients with stage I and II DLBCL [156] and for rituximab-treated patients [157]. S1PR1 was shown to colocalize with phospho-STAT3 in ABC patient samples and treatment of mice with lymphoma with the S1P antagonist FTY720 inhibited Stat3 activity and reduced tumor growth [158]. Similarly, knockout of S1PR1 in cell lines reduced tumor growth and invasion after transplant into mice [158].
Gene expression profiling with corresponding clinical data supported the notion that increased expression of S1PR1 in DLBCL was associated with poor outcome [159–161]. These studies also identified increased expression of the GPCRs GPR183, CCR7, ADRB2 and CNR2 as risk factors for poor outcome [161]. However, a conflicting study found that CNR2 protein expression was not associated with outcome or related to ABC/GCB subtype [162]. While the significance of cannabinoid receptor expression in DLBCL remains to be elucidated, it has been shown that CNR1 and CNR2 mRNA were both upregulated in DLBCL compared to reactive lymph node tissue [51] and that 45/79 (57%) patients were CNR2 immunopositive [162].
Chemokine receptor expression in DLBCL is shown in Table 1. Consistent with its role in NHL, CXCR4 plays an important role in enabling cell migration in DLBCL. Increased CXCR4 expression was associated with worse survival in both ABC and GCB subtypes for 468 patients treated with the standard therapy R-CHOP [163] and a separate study of 94 patients found that those positive for CXCR4 has reduced survival and increased recurrence of disease [164]. However, a smaller cohort of 70 Korean patients did not identify an association between CXCR4 expression and survival [165]. At the cellular level, strong nuclear CXCR4 staining was correlated with systemic DLBCL whereas strong cytoplasmic CXCR5 staining was correlated with CNS involvement [166]. Furthermore, hypoxia was associated with upregulation of CXCR4 protein [167] and such an increase in CXCR4 expression is believed to increase cell dissemination [164]. IHC revealed that 80% of patients had CXCR4 coexpressed with NF-κB [165], which is often mutated in DLBCL, while CXCR4 itself contains an AIDCA somatic hypermutation hotspot that is suspect to mutation [168]. Various therapeutics targeting CXCR4 in DLBCL have been studied including the CXCR4 antagonist plerixafor which enhanced rituximab treatment [169], BTK140 which inhibited growth in cell lines [163], PIM inhibitors which impaired proliferation and CXCR4-mediated migration [170], and in vivo blocking of CXCR4 which was critical for regulatory T cell attraction to lymphoma [171].
CCR1 expression was IHC positive in 77/209 (37%) of DLBCL cases and associated with the ABC subtype of DLBCL but did not correlate with survival [14]. At least 75% of gastric extranodal DLBCL cases had positive mRNA expression for CCR1 along with the chemokine receptors CCR5, CCR7, CCR8, CCR9, CXCR3, CXCR5, CXCR6, CXCR7 and XCR1 [172, 173]. IHC was also positive in the majority of extranodal cases for CCR8, CCR9, CXCR4, CXCR6 and CXCR7 [173].
Multiple reports have highlighted the difference in chemokine receptor expression based on anatomical location of DLBCL. Similar to FL, IHC staining of CCR9 was stronger in gastrointestinal DLCBL compared to nodal DLBCL [149]. XCR1 surface protein was found to be significantly elevated in DLBCL cases manifesting in the bone marrow [174] and also present in the majority of extranodal DLBCL cases [173]. CXCR3 protein expression was higher in thyroid DLBCL than stomach DLBCL [175] and has also been observed in case reports of epidermotropic B cell lymphoma [176] and intravascular large B cell lymphoma [177]. Closely related primary mediastinal large B-cell lymphomas (PMBCLs or MLBCLs) have been characterized by increased CCR9 and decreased CCR6, CCR7 and CXCR5 immunoreactivity compared to nonmediastinal DLBCL [14, 178]. Finally, although not a chemokine receptor, analysis of 57 NHL biopsies found that 9/10 (90%) PMBCL and 1/9 (11%) DLBCL cases were positive for the leukotriene receptor CYSLT1 but no positive samples were found in other NHL subtypes [179].
Other chemokine receptors that have been observed in DLBCL include CX3CR1, which was found to be present in up to 43% of cases and enriched in the GCB subtype [103]. IHC of 80 DLBCL cases found that 10 (12.5%) were positive for CCR4 expression but this had no correlation with patient outcome [180]. CCR4 expression may be subtype specific as DLBCL originating from the thyroid and stomach had very few cells positive for CCR4 protein expression [175] but multiple case reports of CCR4 in other DLBCL subgroups have been reported [180–182].
8. Marginal Zone Lymphoma
Marginal zone lymphoma (MZL) comprises three lymphoma entities that arise from the marginal zone surrounding the germinal center: extranodal MZL or mucosa-associated lymphoid tissue (MALT), splenic MZL, and nodal MZL. Deep sequencing of nodal MZL patients found that 5/35 (14%) cases had a mutation in the adhesion receptor ADGRV1 (also known as GPR98) and the cadherin receptors CELSR1, CELSR2 and CELSR3 were each mutated in 2/35 (6%) cases [183]. In addition to mutation, t(X;14) translocations causing upregulation of purinergic receptor GPR34 were observed in 2/61 (3%) cases of MALT, 1/43 (2%) cases of nodal MZL and 1/19 (5%) cases of extranodal DLBCL [184]. When compared to healthy B and T cells, GPR34 mRNA expression was significantly upregulated in MALT, nodal and splenic MZL and increased gene expression of GPR34 in was correlated with high expression of the orphan receptor GPR82. Overexpression of GPR34 in cell culture experiments resulted in increased cell proliferation and increased phosphorylation of the kinases ERK and PKC and cAMP response element-binding protein (CREB) [185].
Other GPCRs that had elevated expression in MZL include the S1P family receptors S1PR1 and S1PR3 [118] and the gastrin/cholecystokinin receptor CCKBR [186]. A fraction of MALT patients were immunopositive for the somatostatin receptors SSTR1 (1/55, 2%), SSTR2 (15/55, 27%), SSTR3 (20/55, 36%), SSTR4 (10/55, 18%) and SSTR5 (28/55, 50%) and there was a significant association between SSTR5 negativity and poor patient outcome [187]. SSTR-receptor scintigraphy was demonstrated to be useful for staging and follow-up in MALT [188] although there is disagreement concerning whether gastric tumors have higher SSTR3, SSTR4 and SSTR5 than extragastric tumors [187, 188].
Chemokine receptor expression in MZL subtypes is shown in Table 1. CXCR3 is strongly expressed on the surface of the majority of MZLs and was identified via IHC in 14/14 (100%) patients with splenic MZL, 15/16 (94%) patients with extranodal MZL [4] and 5/5 (100%) cases of epidermotropic MZL [176]. One study found that only 13% of cutaneous MZLs were CXCR3-positive compared to 85% of other extranodal MZLs implying that CXCR3-negative patients comprise a unique subtype of MZL [189]. CXCR3 is a predictor of non-responsiveness for H. pylori eradication therapy implying that CXCR3 helps protect tumors from this treatment [190]. MALT lymphoma cells are known to express CXCR3 and migrate to the CXCR3 ligand CXCL9 (also known as MIG) [191] and both high- and low-grade MALT originating from the thyroid and stomach had high CXCR3 and low CCR4 cell surface expression [175]. However, CCR4 protein expression itself was significantly more highly expressed in trisomy 3-positive compared to trisomy 3-negative MALT and correlated with advanced disease stage and poor prognosis [172].
Although CXCR4 and CXCR5 are generally expressed in MZL [72, 118, 172, 173], flow cytometry of splenic MZL tissues with minimal lymphadenopathy had significantly reduced expression of CXCR4, CXCR5 and CCR7 compared to normal B cells and B cell neoplasm with nodular dissemination such as CLL, MCL and FL [71]. Exome sequencing found 2/35 (6%) cases of MZL had a mutation in CXCR4 [183] and the CXCR4WHIM mutation was found in 1/20 (5%) MZL patients [192]. A significant correlation has been observed between CXCR4 expression and bone marrow involvement and loss of CXCR4 expression combined with upregulation of CXCR7 has been suggested to correlate with MALT progression to DLBCL [173].
Other chemokine receptors that are expressed in MZL include CCR8, CCR9, CXCR6 and CXCR7, which were immunopositive in the majority of gastric MALT lymphomas [173]. Two independent studies found that 84–88% of MALT cases stained positive for CCR6, which is known to play a role in migration and B cell maturation [102, 135]. In addition, CCR1 protein expression was found to be subtype-specific as 3/6 (50%) nodal MZL cases expressed CCR1 compared to only 1/21 (5%) extranodal MZL and 0/5 splenic MZL cases [14].
9. Lymphoplasmacytic Lymphoma/Waldenstrom Macroglobulinemia
Lymphoplasmacytic lymphoma (LPL) and its subgroup Waldenstrom macroglobulinemia (WM) are rare and indolent lymphomas that arise from fully differentiated B cells. The only family of GPCRs that has been well studied in LPL/WM is the chemokine receptor family. Flow cytometry of WM cell lines and patient samples found 13 CXC and CC chemokine receptors were expressed to some degree [4, 14, 193] and CXCR4, in particular, was highly expressed and found to be necessary for WM migration to bone marrow [193]. CXCR4 was shown to interact with integrin α4β1 (also known as Very Late Antigen-4; VLA-4) to regulate homing and adhesion, and treatment of WM cell lines with a CXCR4 antagonist inhibited migration and signaling [193, 194].
Driver mutations in CXCR4 are an important determinant of clinical phenotype and survival in WMLPL [195] and the role of CXCR4 in homing, migration and dissemination of WMLPL has been well reviewed [196]. Mutations in CXCR4 were detected in 17/47 (36%) LPL cases and mutation correlated with increased bone marrow infiltration and lower leucocyte, hemoglobin and platelet counts [197]. CXCR4 mutations were also identified in 24–27% of WM patients and the mutations were heterozygous and located in the carboxy-terminal tail [198–200]. The CXCR4 C1013G mutation was detected in 37/131 (28%) cases of LPL and a novel anti-CXCR4 antibody was shown to affect survival and apoptosis signaling in WM cells [201]. Separating LPL/WM into subgroups revealed CXCR4WHIM mutations in 2/12 (17%) Immunoglobulin M monoclonal gammopathy of undetermined significance (IgM MGUS), 0/7 (0%) Non-IgM MGUS, 44/102 (43%) untreated WM and 21/62 (34%) treated WM [192].
CXCR4 cell surface expression was increased in CXCR4-mutated cases of WM compared to CXCR4-wild type [198] but overall CXCR4 was found to be lower in WM than other NHLs [202]. Nearly all cases that have CXCR4 mutations also have MYD88 mutations [197, 200], however a small study found that only about 29% of patients with MYD88 mutations in WM also have a CXCR4 mutation [203]. Frameshift mutations in CXCR4 have also been identified and shown to result in receptor internalization in response to CXCL12 [204].
10. Hairy Cell Leukemia
Hairy cell leukemia (HCL) is a rare disease that accounts for approximately 2% of leukemias and is typically defined by cells that contain the BRAF kinase p.V600E mutation [205]. Gene expression profiling of HCL has suggested that these tumors arise from a memory B cell of origin and that transformation to lymphoma involves changes in expression of chemokine and adhesion receptors [206]. Similar to LPL/WM, studies of GPCRs in HCL mainly concern chemokine receptors and these findings are summarized in Table 1. At least two independent reports have concluded that CX3CR1, CXCR3. CXCR4, CXCR5, CCR5 and CCR6 have been identified in patient cases [3, 4, 10, 72, 103]. Meanwhile, cell surface expression of CCR7, CXCR4 and CXCR5 was found to be significantly lower in HCL compared to normal B cells or B cell neoplasms with nodular dissemination [3, 71, 206].
11. Conclusions
GPCRs represent an important component of B cell signaling and play critical roles in cell migration, proliferation, apoptosis, development, and function. Genetic events that change expression or function of GPCRs, such as mutations, fusions or copy number alterations, have significant consequences and can serve as driver events in B cell malignancies. Although many of these events have been described, significant research remains to elucidate the full expression panel of GPCRs during all stages of B cell development and their function in the normal immune system and oncogenesis. Deciphering these elements will lead to an increase in therapeutics targeting these receptors and ultimately improve patient outcomes.
Highlights.
GPCRs regulate B cell migration, proliferation, apoptosis, development and function
Mutation, fusion and copy number alterations of GPCRs occur in B cell malignancies
B cell lymphoma subtypes contain unique GPCR expression and aberration patterns
GPCR mutations and changes in expression can serve as biomarkers of lymphoma
Better understanding of GPCRs in lymphoma will improve clinical outcomes
Acknowledgments
Funding Sources: This research was supported by the Intramural Research Programs of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health.
Abbreviations
- ABC
Activated B cell-like
- ALL
Acute lymphoblastic leukemia
- ATP
Adenosine triphosphate
- B-ALL
B cell acute lymphoblastic leukemia
- BCR
B cell receptor
- BL
Burkitt lymphoma
- BLR1
Burkitt lymphoma receptor 1
- cAMP
Cyclic adenosine monophosphate
- CCND1
Cyclin D1
- CLL
Chronic lymphocytic leukemia
- CREB
Cyclic adenosine monophosphate response element-binding protein
- CRLF2
Cytokine receptor like factor 2
- DLBCL
Diffuse large B cell lymphoma
- DLBCL-NOS
Diffuse large B cell lymphoma-not otherwise specified
- EBI2
Epstein-Barr virus-induced G protein-coupled receptor 2
- FL
Follicular lymphoma
- GCB
Germinal center B cell-like
- GPCR
G protein-coupled receptor
- HCL
Hairy cell leukemia
- IGHV
Immunoglobulin heavy-chain variable region gene
- IgM
Immunoglobulin M
- IHC
Immunohistochemistry
- JNK
c-Jun N-terminal kinase
- LPA
Lysophosphatidic acid
- LPL
Lymphoplasmacytic lymphoma
- MALT
Mucosa-associated lymphoid tissue
- MAML2
Mastermind like transcriptional coactivator 2
- MAPK
Mitogen-activated protein kinase
- MCL
Mantle cell lymphoma
- MFI
Mean or median fluorescence intensity
- MGUS
Monoclonal gammopathy of undetermined significance
- MMP-9
Matrix metallopeptidase 9
- MZL
Marginal zone lymphoma
- NF-κB
Nuclear factor-kappa B
- NHL
Non-Hodgkin’s lymphoma
- PMBCL
Primary mediastinal large B-cell lymphoma
- S1P
Sphingosine-1-phosphate
- SLL
Small lymphocytic lymphoma
- T-ALL
T cell acute lymphoblastic leukemia
- TFH
Follicular helper T cell
- Treg
Regulatory T cell
- VEGF
Vascular endothelial growth factor
- VLA-4
Very late antigen-4
- WM
Waldenstrom macroglobulinemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts of interest: None
Author contributions: AN conceptualized, wrote and edited the review; RLP conceptualized and edited the review. All authors have approved the final article.
References
- 1.Asai D, et al. IKZF1 deletion is associated with a poor outcome in pediatric B – cell precursor acute lymphoblastic leukemia in Japan. Cancer medicine. 2013;2(3):412–419. doi: 10.1002/cam4.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corcione A, et al. Chemokine receptor expression and function in childhood acute lymphoblastic leukemia of B-lineage. Leukemia research. 2006;30(4):365–372. doi: 10.1016/j.leukres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Wong S, Fulcher D. Chemokine receptor expression in B-cell lymphoproliferative disorders. Leukemia & lymphoma. 2004;45(12):2491–2496. doi: 10.1080/10428190410001723449. [DOI] [PubMed] [Google Scholar]
- 4.Jones D, et al. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95(2):627–632. [PubMed] [Google Scholar]
- 5.de Lourdes Perim A, et al. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target. Cellular and Molecular Life Sciences. 2015;72(9):1715–1723. doi: 10.1007/s00018-014-1830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berk LC, et al. Disturbed CXCR4/CXCL12 axis in paediatric precursor B – cell acute lymphoblastic leukaemia. British journal of haematology. 2014;166(2):240–249. doi: 10.1111/bjh.12883. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel A, et al. Unique SDF-1—induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103(8):2900–2907. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 8.Crazzolara R, et al. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol. 2001;115(3):545–53. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu S, et al. Chemokine IL-8 and chemokine receptor CXCR3 and CXCR4 gene expression in childhood acute lymphoblastic leukemia at first relapse. Journal of pediatric hematology/oncology. 2006;28(4):216–220. doi: 10.1097/01.mph.0000212908.14642.a5. [DOI] [PubMed] [Google Scholar]
- 10.Dürig J, Schmücker U, Dührsen U. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001;15(5):752. doi: 10.1038/sj.leu.2402107. [DOI] [PubMed] [Google Scholar]
- 11.Catusse J, et al. Attenuation of CXCR4 responses by CCL18 in acute lymphocytic leukemia B cells. Journal of cellular physiology. 2010;225(3):792–800. doi: 10.1002/jcp.22284. [DOI] [PubMed] [Google Scholar]
- 12.Chunsong H, et al. CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+ CD5+ B cells. The Journal of Immunology. 2006;177(10):6713–6722. doi: 10.4049/jimmunol.177.10.6713. [DOI] [PubMed] [Google Scholar]
- 13.Melo RdCC, et al. CXCR7 is highly expressed in acute lymphoblastic leukemia and potentiates CXCR4 response to CXCL12. PloS one. 2014;9(1):e85926. doi: 10.1371/journal.pone.0085926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MW, et al. CC chemokine receptor 1 expression in human hematolymphoid neoplasia. American journal of clinical pathology. 2010;133(3):473–483. doi: 10.1309/AJCP1TA3FLOQTMHF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiaretti S, et al. CRLF2 overexpression identifies an unfavourable subgroup of adult B-cell precursor acute lymphoblastic leukemia lacking recurrent genetic abnormalities. Leukemia research. 2016;41:36–42. doi: 10.1016/j.leukres.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Moorman AV, et al. IGH@ translocations, CRLF2 deregulation, and microdeletions in adolescents and adults with acute lymphoblastic leukemia. J Clin Oncol. 2012;30(25):3100–8. doi: 10.1200/JCO.2011.40.3907. [DOI] [PubMed] [Google Scholar]
- 17.Cario G, et al. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non—high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 2010;115(26):5393–5397. doi: 10.1182/blood-2009-11-256131. [DOI] [PubMed] [Google Scholar]
- 18.Palmi C, et al. Poor prognosis for P2RY8-CRLF2 fusion but not for CRLF2 over-expression in children with intermediate risk B-cell precursor acute lymphoblastic leukemia. Leukemia. 2012;26(10):2245–2253. doi: 10.1038/leu.2012.101. [DOI] [PubMed] [Google Scholar]
- 19.Morak M, et al. Small sizes and indolent evolutionary dynamics challenge the potential role of P2RY8-CRLF2—harboring clones as main relapse-driving force in childhood ALL. Blood. 2012;120(26):5134–5142. doi: 10.1182/blood-2012-07-443218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krawczyk J, et al. No prognostic impact of P2RY8 – CRLF2 fusion in intermediate cytogenetic risk childhood B – cell acute lymphoblastic leukaemia. British journal of haematology. 2013;160(4):555–556. doi: 10.1111/bjh.12130. [DOI] [PubMed] [Google Scholar]
- 21.Yano M, et al. An overall characterization of pediatric acute lymphoblastic leukemia with CRLF2 overexpression. Genes, Chromosomes and Cancer. 2014;53(10):815–823. doi: 10.1002/gcc.22190. [DOI] [PubMed] [Google Scholar]
- 22.Jia M, et al. Prognostic significance of cytokine receptor-like factor 2 alterations in acute lymphoblastic leukemia: a meta-analysis. Springer; 2015. [DOI] [PubMed] [Google Scholar]
- 23.Hanada I, et al. Gene alterations involving the CRLF2 – JAK pathway and recurrent gene deletions in Down syndrome – associated acute lymphoblastic leukemia in Japan. Genes, Chromosomes and Cancer. 2014;53(11):902–910. doi: 10.1002/gcc.22201. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaev SI, et al. Frequent cases of RAS-mutated Down syndrome acute lymphoblastic leukaemia lack JAK2 mutations. Nature communications. 2014;5 doi: 10.1038/ncomms5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullighan CG, et al. Rearrangement of CRLF2 in B-progenitor—and Down syndrome—associated acute lymphoblastic leukemia. Nature genetics. 2009;41(11):1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ensor HM, et al. Demographic, clinical, and outcome features of children with acute lymphoblastic leukemia and CRLF2 deregulation: results from the MRC ALL97 clinical trial. Blood. 2011;117(7):2129–2136. doi: 10.1182/blood-2010-07-297135. [DOI] [PubMed] [Google Scholar]
- 27.Rand V, et al. Genomic characterization implicates iAMP21 as a likely primary genetic event in childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2011;117(25):6848–6855. doi: 10.1182/blood-2011-01-329961. [DOI] [PubMed] [Google Scholar]
- 28.Chen IM, et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119(15):3512–3522. doi: 10.1182/blood-2011-11-394221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dou H, et al. Prognostic significance of P2RY8-CRLF2 and CRLF2 overexpression may vary across risk subgroups of childhood B-cell acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2017;56(2):135–146. doi: 10.1002/gcc.22421. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, et al. Genome-Wide Single-Nucleotide Polymorphism Array Analysis Improves Prognostication of Acute Lymphoblastic Leukemia/Lymphoma. The Journal of Molecular Diagnostics. 18(4):595–603. doi: 10.1016/j.jmoldx.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Dörge P, et al. IKZF1 deletion is an independent predictor of outcome in pediatric acute lymphoblastic leukemia treated according to the ALL-BFM 2000 protocol. Haematologica. 2013;98(3):428–432. doi: 10.3324/haematol.2011.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bond J, et al. Direct interaction of Ikaros and Foxp1 modulates expression of the G protein-coupled receptor G2A in B-lymphocytes and acute lymphoblastic leukemia. Oncotarget. 2016;7(40):65923–65936. doi: 10.18632/oncotarget.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le LQ, et al. Positron emission tomography imaging analysis of G2A as a negative modifier of lymphoid leukemogenesis initiated by the BCR-ABL oncogene. Cancer Cell. 2002;1(4):381–391. doi: 10.1016/s1535-6108(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 34.Naderi EH, et al. Bone marrow stroma-derived PGE 2 protects BCP-ALL cells from DNA damage-induced p53 accumulation and cell death. Molecular cancer. 2015;14(1):14. doi: 10.1186/s12943-014-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naderi EH, et al. Selective inhibition of cell death in malignant vs normal B-cell precursors: implications for cAMP in development and treatment of BCP-ALL. Blood. 2013;121(10):1805–1813. doi: 10.1182/blood-2012-08-452698. [DOI] [PubMed] [Google Scholar]
- 36.Denizot Y, et al. Functional EP2 receptors on blast cells of patients with acute leukemia. International journal of cancer. 2005;115(3):499–501. doi: 10.1002/ijc.20877. [DOI] [PubMed] [Google Scholar]
- 37.Malissein E, et al. PGE 2 receptor subtype functionality on immature forms of human leukemic blasts. Leukemia research. 2006;30(10):1309–1313. doi: 10.1016/j.leukres.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Zuo B, et al. G-protein coupled receptor 34 activates Erk and phosphatidylinositol 3-kinase/Akt pathways and functions as alternative pathway to mediate p185Bcr—Abl-induced transformation and leukemogenesis. Leukemia & lymphoma. 2015;56(7):2170–2181. doi: 10.3109/10428194.2014.981177. [DOI] [PubMed] [Google Scholar]
- 39.de SB Veiga C, et al. Increased expression of protease-activated receptor 1 (PAR-1) in human leukemias. Blood Cells, Molecules, and Diseases. 2011;46(3):230–234. doi: 10.1016/j.bcmd.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Sic H, et al. Sphingosine-1-phosphate receptors control B-cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. Journal of Allergy and Clinical Immunology. 2014;134(2):420–428.e15. doi: 10.1016/j.jaci.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 41.Grassin-Delyle S, et al. Expression and proliferative effect of hemokinin-1 in human B-cells. Peptides. 2011;32(5):1027–1034. doi: 10.1016/j.peptides.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Cha Z, et al. Association of peripheral CD4+ CXCR5+ T cells with chronic lymphocytic leukemia. Tumor Biology. 2013;34(6):3579–3585. doi: 10.1007/s13277-013-0937-2. [DOI] [PubMed] [Google Scholar]
- 43.Capitani N, et al. S1P1 expression is controlled by the pro-oxidant activity of p66Shc and is impaired in B-CLL patients with unfavorable prognosis. Blood. 2012;120(22):4391–4399. doi: 10.1182/blood-2012-04-425959. [DOI] [PubMed] [Google Scholar]
- 44.Borge M, et al. The expression of sphingosine-1 phosphate receptor-1 in chronic lymphocytic leukemia cells is impaired by tumor microenvironmental signals and enhanced by piceatannol and R406. The Journal of Immunology. 2014;193(6):3165–3174. doi: 10.4049/jimmunol.1400547. [DOI] [PubMed] [Google Scholar]
- 45.Till KJ, Pettitt AR, Slupsky JR. Expression of Functional Sphingosine-1 Phosphate Receptor-1 Is Reduced by B Cell Receptor Signaling and Increased by Inhibition of PI3 Kinase δ but Not SYK or BTK in Chronic Lymphocytic Leukemia Cells. The Journal of Immunology. 2015;194(5):2439–2446. doi: 10.4049/jimmunol.1402304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patrussi L, et al. Enhanced chemokine receptor recycling and impaired S1P1 expression promote leukemic cell infiltration of lymph nodes in chronic lymphocytic leukemia. Cancer research. 2015;75(19):4153–4163. doi: 10.1158/0008-5472.CAN-15-0986. [DOI] [PubMed] [Google Scholar]
- 47.Kumar SA, et al. Lysophosphatidic acid receptor expression in chronic lymphocytic leukemia leads to cell survival mediated though vascular endothelial growth factor expression. Leukemia & lymphoma. 2009;50(12):2038–2048. doi: 10.3109/10428190903275586. [DOI] [PubMed] [Google Scholar]
- 48.Hu X, et al. Lysophosphatidic acid (LPA) protects primary chronic lymphocytic leukemia cells from apoptosis through LPA receptor activation of the anti-apoptotic protein AKT/PKB. Journal of Biological Chemistry. 2005;280(10):9498–9508. doi: 10.1074/jbc.M410455200. [DOI] [PubMed] [Google Scholar]
- 49.Hu X, et al. Lysophosphatidic acid (LPA) induces the expression of VEGF leading to protection against apoptosis in B-cell derived malignancies. Cellular signalling. 2008;20(6):1198–1208. doi: 10.1016/j.cellsig.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Freund P, et al. Cannabinoid receptors are overexpressed in CLL but of limited potential for therapeutic exploitation. PloS one. 2016;11(6):e0156693. doi: 10.1371/journal.pone.0156693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gustafsson K, et al. Expression of cannabinoid receptors type 1 and type 2 in non – Hodgkin lymphoma: Growth inhibition by receptor activation. International journal of cancer. 2008;123(5):1025–1033. doi: 10.1002/ijc.23584. [DOI] [PubMed] [Google Scholar]
- 52.Kamp T, et al. Defects of ß2 – adrenergic signal transduction in chronic lymphocytic leukaemia: relationship to disease progression. European journal of clinical investigation. 1997;27(2):121–127. doi: 10.1046/j.1365-2362.1997.700623.x. [DOI] [PubMed] [Google Scholar]
- 53.Mamani-Matsuda M, et al. Long – acting ß2 – adrenergic formoterol and salmeterol induce the apoptosis of B – chronic lymphocytic Leukaemia cells. British journal of haematology. 2004;124(2):141–150. doi: 10.1046/j.1365-2141.2003.04746.x. [DOI] [PubMed] [Google Scholar]
- 54.Goin JC, et al. Active alpha 2 and beta adrenoceptors in lymphocytes from patients with chronic lymphocytic leukemia. Int J Cancer. 1991;49(2):178–81. doi: 10.1002/ijc.2910490205. [DOI] [PubMed] [Google Scholar]
- 55.Conigrave AD, et al. P2Y 11 receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. European journal of pharmacology. 2001;426(3):157–163. doi: 10.1016/s0014-2999(01)01222-5. [DOI] [PubMed] [Google Scholar]
- 56.Cafforio P, et al. Functional expression of the calcitonin receptor by human T and B cells. Human immunology. 2009;70(9):678–685. doi: 10.1016/j.humimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Drost AC, et al. The G protein-coupled receptor CysLT1 mediates chemokine-like effects and prolongs survival in chronic lymphocytic leukemia. Leukemia & lymphoma. 2012;53(4):665–673. doi: 10.3109/10428194.2011.625578. [DOI] [PubMed] [Google Scholar]
- 58.Maffei R, et al. Endothelin-1 Promotes Survival and Chemoresistance in Chronic Lymphocytic Leukemia B Cells through ET A Receptor. PLoS One. 2014;9(6):e98818. doi: 10.1371/journal.pone.0098818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosko AE, et al. Acidosis Sensing Receptor GPR65 Correlates with Anti-Apoptotic Bcl-2 Family Member Expression in CLL Cells: Potential Implications for the CLL Micro environment. Journal of leukemia (Los Angeles, Calif) 2014;2(5) doi: 10.4172/2329-6917.1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Runarsson G, et al. Leukotriene B4 plays a pivotal role in CD40-dependent activation of chronic B lymphocytic leukemia cells. Blood. 2005;105(3):1274–1279. doi: 10.1182/blood-2004-07-2546. [DOI] [PubMed] [Google Scholar]
- 61.Klein U, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. Journal of Experimental Medicine. 2001;194(11):1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saada S, et al. Differential expression of neurotensin and specific receptors, NTSR1 and NTSR2, in normal and malignant human B lymphocytes. The Journal of Immunology. 2012;189(11):5293–5303. doi: 10.4049/jimmunol.1102937. [DOI] [PubMed] [Google Scholar]
- 63.Wu QL, Zierold C, Ranheim EA. Dysregulation of Frizzled 6 is a critical component of B-cell leukemogenesis in a mouse model of chronic lymphocytic leukemia. Blood. 2009;113(13):3031–3039. doi: 10.1182/blood-2008-06-163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gandhirajan RK, et al. Wnt/β-catenin/LEF-1 signaling in chronic lymphocytic leukemia (CLL): a target for current and potential therapeutic options. Current cancer drug targets. 2010;10(7):716–727. doi: 10.2174/156800910793605794. [DOI] [PubMed] [Google Scholar]
- 65.Lu D, et al. Activation of the Wn t signaling pathway in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahadevan D, et al. Gene expression and serum cytokine profiling of low stage CLL identify WNT/PCP, Flt-3L/Flt-3, and CXCL9/CXCR3 as regulators of cell proliferation, survival, and migration. Human Genomics and Proteomics. 2009;1(1) doi: 10.4061/2009/453634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burger JA. Seminars in cancer biology. Elsevier; 2010. Chemokines and chemokine receptors in chronic lymphocytic leukemia (CLL): from understanding the basics towards therapeutic targeting. [DOI] [PubMed] [Google Scholar]
- 68.Trentin L, et al. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. The Journal of clinical investigation. 1999;104(1):115–121. doi: 10.1172/JCI7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dao-Ung LP, et al. CXCR4 but not CXCR3 expression correlates with lymphocyte counts in B-cell chronic lymphocytic leukemia. Annals of hematology. 2004;83(5):326–327. doi: 10.1007/s00277-004-0846-y. [DOI] [PubMed] [Google Scholar]
- 70.Ganghammer S, et al. Combined CXCR3/CXCR4 measuremen ts are of high prognostic value in chronic lymphocytic leukemia due to negative cooperativity of the receptors. haematologica. 2016;101(3):e99–e102. doi: 10.3324/haematol.2015.133470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.López-Giral S, et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. Journal of leukocyte biology. 2004;76(2):462–471. doi: 10.1189/jlb.1203652. [DOI] [PubMed] [Google Scholar]
- 72.Trentin L, et al. Homeostatic chemokines drive migration of malignant B cells in patients with non-Hodgkin lymphomas. Blood. 2004;104(2):502–508. doi: 10.1182/blood-2003-09-3103. [DOI] [PubMed] [Google Scholar]
- 73.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–3667. [PubMed] [Google Scholar]
- 74.Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leukemia & lymphoma. 2002;43(3):461–466. doi: 10.1080/10428190290011921. [DOI] [PubMed] [Google Scholar]
- 75.Ishibe N, et al. CXCR4 expression is associated with survival in familial chronic lymphocytic leukemia, but CD38 expression is not. Blood. 2002;100(3):1100–1101. doi: 10.1182/blood-2002-03-0938. [DOI] [PubMed] [Google Scholar]
- 76.Ghobrial IM, et al. Mayo Clinic Proceedings. Elsevier; 2004. Expression of the chemokine receptors CXCR4 and CCR7 and disease progression in B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma. [DOI] [PubMed] [Google Scholar]
- 77.Barretina J, et al. CXCR4 and SDF-1 expression in B-cell chronic lymphocytic leukemia and stage of the disease. Annals of hematology. 2003;82(8):500–505. doi: 10.1007/s00277-003-0679-0. [DOI] [PubMed] [Google Scholar]
- 78.Crowther-Swanepoel D, et al. Genetic variation in CXCR4 and risk of chronic lymphocytic leukemia. Blood. 2009;114(23):4843–4846. doi: 10.1182/blood-2009-07-235184. [DOI] [PubMed] [Google Scholar]
- 79.Acunzo M, et al. Translocation t (2; 11) in CLL cells results in CXCR4/MAML2 fusion oncogene. Blood. 2014;124(2):259–262. doi: 10.1182/blood-2014-02-554675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calpe E, et al. ZAP-70 promotes the infiltration of malignant B-lymphocytes into the bone marrow by enhancing signaling and migration after CXCR4 stimulation. PloS one. 2013;8(12):e81221. doi: 10.1371/journal.pone.0081221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stamatopoulos B, et al. Gene expression profiling reveals differences in microenvironment interaction between patients with chronic lymphocytic leukemia expressing high versus low ZAP70 mRNA. haematologica. 2009;94(6):790–799. doi: 10.3324/haematol.2008.002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Hayre M, et al. Elucidating the CXCL12/CXCR4 signaling network in chronic lymphocytic leukemia through phosphoproteomics analysis. PloS one. 2010;5(7):e11716. doi: 10.1371/journal.pone.0011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Decker S, et al. PIM kinases are essential for chronic lymphocytic leukemia cell survival (PIM2/3) and CXCR4-mediated microenvironmental interactions (PIM1) Molecular cancer therapeutics. 2014;13(5):1231–1245. doi: 10.1158/1535-7163.MCT-13-0575-T. [DOI] [PubMed] [Google Scholar]
- 84.Stamatopoulos B, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) induces apoptosis, downregulates the CXCR4 chemokine receptor and impairs migration of chronic lymphocytic leukemia cells. Haematologica. 2010 doi: 10.3324/haematol.2009.013847. p. haematol. 2009.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stamatopoulos B, et al. AMD3100 disrupts the cross-talk between chronic lymphocytic leukemia cells and a mesenchymal stromal or nurse-like cell-based microenvironment: pre-clinical evidence for its association with chronic lymphocytic leukemia treatments. haematologica. 2012;97(4):608–615. doi: 10.3324/haematol.2011.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchner M, et al. The microenvironmen t differen tially impairs passive and active immunotherapy in chronic lymphocytic leukaemia – CXCR4 antagonists as potential adjuvants for monoclonal antibodies. Br J Haematol. 2010;151(2):167–78. doi: 10.1111/j.1365-2141.2010.08316.x. [DOI] [PubMed] [Google Scholar]
- 87.Niedermeier M, et al. Isoform-selective phosphoinositide 3 ‘ -kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009;113(22):5549–5557. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burger M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 89.Chen SS, et al. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2015 doi: 10.1038/leu.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bürkle A, et al. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110(9):3316–3325. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- 91.Vlad A, et al. Down-regulation of CXCR4 and CD62L in chronic lymphocytic leukemia cells is triggered by B-cell receptor ligation and associated with progressive disease. Cancer research. 2009;69(16):6387–6395. doi: 10.1158/0008-5472.CAN-08-4750. [DOI] [PubMed] [Google Scholar]
- 92.Saint Georges S, et al. Protein kinase D-dependent CXCR4 down-regulation upon BCR triggering is linked to lymphadenopathy in chronic lymphocytic leukaemia. Oncotarget doi. 2016;10 doi: 10.18632/oncotarget.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rawstron AC, et al. Chronic lymphocytic leukaemia (CLL) and CLL – type monoclonal B-cell lymphocytosis (MBL) show differential expression of molecules involved in lymphoid tissue homing. Cytometry Part B: Clinical Cytometry. 2010;78(S1):S42–S46. doi: 10.1002/cyto.b.20534. [DOI] [PubMed] [Google Scholar]
- 94.Hu C, et al. PEG10 Activation by Co-Stimulation of CXCR5 and CCR7 Essentially Contributes toResistance to Apoptosis in CD19+CD34+ B Cells from Patients with B Cell Lineage Acute and Chronic Lymphocytic Leukemia. 2004 [PubMed] [Google Scholar]
- 95.Heinig K, et al. Access to follicular dendritic cells is a pivotal step in murine chronic lymphocytic leukemia B-cell activation and proliferation. Cancer discovery. 2014;4(12):1448–1465. doi: 10.1158/2159-8290.CD-14-0096. [DOI] [PubMed] [Google Scholar]
- 96.Zent CS, et al. The distinct gene expression profiles of chronic lymphocytic leukemia and multiple myeloma suggest different anti-apoptotic mechanisms but predict only some differences in phenotype. Leukemia research. 2003;27(9):765–774. doi: 10.1016/s0145-2126(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 97.Till KJ, et al. The chemokine receptor CCR7 and α4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99(8):2977–2984. doi: 10.1182/blood.v99.8.2977. [DOI] [PubMed] [Google Scholar]
- 98.Calpe E, et al. ZAP-70 enhances migration of malignant B lymphocytes toward CCL21 by inducing CCR7 expression via IgM-ERK1/2 activation. Blood. 2011;118(16):4401–4410. doi: 10.1182/blood-2011-01-333682. [DOI] [PubMed] [Google Scholar]
- 99.Catusse J, et al. Role of the atypical chemoattractant receptor CRAM in regulating CCL19 induced CCR7 responses in B-cell chronic lymphocytic leukemia. Molecular cancer. 2010;9(1):297. doi: 10.1186/1476-4598-9-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redondo-Muñoz J, et al. Matrix metalloproteinase-9 is up-regulated by CCL21/CCR7 interaction via extracellular signal-regulated kinase-1/2 signaling and is involved in CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and migration. Blood. 2008;111(1):383–386. doi: 10.1182/blood-2007-08-107300. [DOI] [PubMed] [Google Scholar]
- 101.Cuesta-Mateos C, et al. Preclinical activity of anti-CCR7 immunotherapy in patients with high-risk chronic lymphocytic leukemia. Cancer Immunology, Immunotherapy. 2015;64(6):665–676. doi: 10.1007/s00262-015-1670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodig SJ, et al. CCR6 is a functional chemokine receptor that serves to identify select B-cell non-Hodgkin’s lymphomas. Human pathology. 2002;33(12):1227–1233. doi: 10.1053/hupa.2002.129417. [DOI] [PubMed] [Google Scholar]
- 103.Andréasson U, et al. B cell lymphomas express CX 3 CR1 a non-B cell lineage adhesion molecule. Cancer letters. 2008;259(2):138–145. doi: 10.1016/j.canlet.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 104.Ferretti E, et al. A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment. Leukemia. 2011;25(8):1268–1277. doi: 10.1038/leu.2011.88. [DOI] [PubMed] [Google Scholar]
- 105.Ferrer A, et al. Different gene expression in immunoglobulin-mutated and immunoglobulin-unmutated forms of chronic lymphocytic leukemia. Cancer genetics and cytogenetics. 2004;153(1):69–72. doi: 10.1016/j.cancergencyto.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 106.Levidou G, et al. Immunohistochemical Analysis of IL-6, IL-8/CXCR2 Axis,-STAT-3, and SOCS-3 in Lymph Nodes from Patients with Chronic Lymphocytic Leukemia: Correlation between Microvascular Characteristics and Prognostic Significance. BioMed research international. 2014;2014 doi: 10.1155/2014/251479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wasik A, et al. WIN55, 212–2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells. Cell death & disease. 2011;2(11):e225. doi: 10.1038/cddis.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wasik AM, Christensson B, Sander B. Seminars in cancer biology. Elsevier; 2011. The role of cannabinoid receptors and the endocannabinoid system in mantle cell lymphoma and other non-Hodgkin lymphomas. [DOI] [PubMed] [Google Scholar]
- 109.Islam T, et al. High level of cannabinoid receptor 1, absence of regulator of G protein signalling 13 and differential expression of Cyclin D1 in mantle cell lymphoma. Leukemia. 2003;17(9):1880–1890. doi: 10.1038/sj.leu.2403057. [DOI] [PubMed] [Google Scholar]
- 110.Gustafsson K, et al. Potentiation of cannabinoid-induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Molecular Cancer Research. 2009;7(7):1086–1098. doi: 10.1158/1541-7786.MCR-08-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gustafsson K, et al. Cannabinoid receptor-mediated apoptosis induced by R (+)-methanandamide and Win55, 212–2 is associated with ceramide accumulation and p38 activation in mantle cell lymphoma. Molecular pharmacology. 2006;70(5):1612–1620. doi: 10.1124/mol.106.025981. [DOI] [PubMed] [Google Scholar]
- 112.Flygare J, et al. Cannabinoid receptor ligands mediate growth inhibition and cell death in mantle cell lymphoma. FEBS letters. 2005;579(30):6885–6889. doi: 10.1016/j.febslet.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 113.Wasik AM, et al. Perturbations of the endocannabinoid system in mantle cell lymphoma: correlations to clinical and pathological features. Oncoscience. 2014;1(8):550–7. doi: 10.18632/oncoscience.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fernàndez V, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer research. 2010;70(4):1408–1418. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 115.Ek S, Ortega E, Borrebaeck CA. Transcriptional profiling and assessment of cell lines as in vitro models for mantle cell lymphoma. Leukemia research. 2005;29(2):205–213. doi: 10.1016/j.leukres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 116.Ek S, et al. Mantle cell lymphomas express a distinct genetic signature affecting lymphocyte trafficking and growth regulation as compared with subpopulations of normal human B cells. Cancer research. 2002;62(15):4398–4405. [PubMed] [Google Scholar]
- 117.Nishimura H, et al. Expression of sphingosine-1-phosphate receptor 1 in mantle cell lymphoma. Modern Pathology. 2010;23(3):439–449. doi: 10.1038/modpathol.2009.194. [DOI] [PubMed] [Google Scholar]
- 118.Middle S, et al. Immunohistochemical analysis indicates that the anatomical location of B-cell non-Hodgkin’s lymphoma is determined by differentially expressed chemokine receptors, sphingosine-1-phosphate receptors and integrins. Experimental hematology & oncology. 2015;4(1):10. doi: 10.1186/s40164-015-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu C, et al. Genetic heterogeneity in primary and relapsed mantle cell lymphomas: Impact of recurrent CARD11 mutations. Oncotarget. 2016;7(25):38180–38190. doi: 10.18632/oncotarget.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang J, et al. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood. 2014;123(19):2988–96. doi: 10.1182/blood-2013-07-517177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rudelius M, et al. The G protein-coupled estrogen receptor 1 (GPER-1) contributes to the proliferation and survival of mantle cell lymphoma cells. haematologica. 2015;100(11):e458–e461. doi: 10.3324/haematol.2015.127399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dobner T, et al. Differentiation – specific expression of a novel G protein – coupled receptor from Burkitt’s lymphoma. European journal of immunology. 1992;22(11):2795–2799. doi: 10.1002/eji.1830221107. [DOI] [PubMed] [Google Scholar]
- 123.Beider K, et al. Targeting the CD20 and CXCR4 pathways in non-hodgkin lymphoma with rituximab and high-affinity CXCR4 antagonist BKT140. Clinical Cancer Research. 2013;19(13):3495–3507. doi: 10.1158/1078-0432.CCR-12-3015. [DOI] [PubMed] [Google Scholar]
- 124.Hu Y, et al. Enhancement of the anti-tumor activity of therapeutic monoclonal antibodies by CXCR4 antagonists. Leukemia & lymphoma. 2012;53(1):130–138. doi: 10.3109/10428194.2011.601698. [DOI] [PubMed] [Google Scholar]
- 125.O’Callaghan K, et al. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood. 2012;119(7):1717–1725. doi: 10.1182/blood-2011-04-347518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jarvis RM, et al. Dynamic interplay between the neutral glycosphingolipid CD77/Gb 3 and the therapeutic antibody target CD20 within the lipid bilayer of model B lymphoma cells. Biochemical and biophysical research communications. 2007;355(4):944–949. doi: 10.1016/j.bbrc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 127.Paul AG, Chandran B, Sharma-Walia N. Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi’s sarcoma-associated herpes virus associated malignancies. Transl Res. 2013;162(2):77–92. doi: 10.1016/j.trsl.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gobec M, et al. Chemo-sensitizing effects of EP 4 receptor-induced inactivation of nuclear factor-κB. European journal of pharmacology. 2014;742:81–88. doi: 10.1016/j.ejphar.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 129.Wasik AM, et al. Evidence for functional trace amine associated receptor-1 in normal and malignant B cells. Leukemia research. 2012;36(2):245–249. doi: 10.1016/j.leukres.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pastore A, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. The Lancet Oncology. 2015;16(9):1111–1122. doi: 10.1016/S1470-2045(15)00169-2. [DOI] [PubMed] [Google Scholar]
- 131.Bouska A, et al. Combined copy number and mutation analysis identifies oncogenic pathways associated with transformation of follicular lymphoma. Leukemia. 2016 doi: 10.1038/leu.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kridel R, et al. Histological Transformation and Progression in Follicular Lymphoma: A Clonal Evolution Study. PLoS Med. 2016;13(12):e1002197. doi: 10.1371/journal.pmed.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arai J, et al. Stromal cells in lymph nodes attractB – lymphoma cells via production ofstromal cell – derived factor -1. European journal of haematology. 2000;64(5):323–332. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]
- 134.Storlazzi C, et al. Upregulation of the SOX5 by promoter swapping with the P2RY8 gene in primary splenic follicular lymphoma. Leukemia. 2007;21(10):2221–2221. doi: 10.1038/sj.leu.2404784. [DOI] [PubMed] [Google Scholar]
- 135.Takata K, et al. Duodenal follicular lymphoma: comprehensive gene expression analysis with insights into pathogenesis. Cancer science. 2014;105(5):608–615. doi: 10.1111/cas.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Charbonneau B, et al. CXCR5 polymorphisms in non-Hodgkin lymphoma risk and prognosis. Cancer Immunology, Immunotherapy. 2013;62(9):1475–1484. doi: 10.1007/s00262-013-1452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skibola CF, et al. Genome-wide association study identifies five susceptibility loci for follicular lymphoma outside the HLA region. Am J Hum Genet. 2014;95(4):462–71. doi: 10.1016/j.ajhg.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Husson H, et al. CXCL13 (BCA -1) is produced by follicular lymphoma cells: role in the accumulation of malignant B cells. British journal of haematology. 2002;119(2):492–495. doi: 10.1046/j.1365-2141.2002.03832.x. [DOI] [PubMed] [Google Scholar]
- 139.Fanoni D, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunology letters. 2011;134(2):157–160. doi: 10.1016/j.imlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 140.Panjideh H, et al. Immunotherapy of B – cell non – Hodgkin lymphoma by targeting the chemokine receptor CXCR5 in a preclinical mouse model. International journal of cancer. 2014;135(11):2623–2632. doi: 10.1002/ijc.28893. [DOI] [PubMed] [Google Scholar]
- 141.Amé-Thomas P, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignan t B cells. Leukemia. 2012;26(5):1053–1063. doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nedelkovska H, et al. Follicular lymphoma tregs have a distinct transcription profile impacting their migration and retention in the malignant lymph node. PloS one. 2016;11(5):e0155347. doi: 10.1371/journal.pone.0155347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hilchey SP, et al. Follicular lymphoma tumor—infiltrating T-helper (TH) cells have the same polyfunctional potential as normal nodal TH cells despite skewed differentiation. Blood. 2011;118(13):3591–3602. doi: 10.1182/blood-2011-03-340646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Corcione A, et al. Stromal cell-derived factor-1 as a chemoattractant for follicular center lymphoma B cells. Journal of the National Cancer Institute. 2000;92(8):628–635. doi: 10.1093/jnci/92.8.628. [DOI] [PubMed] [Google Scholar]
- 145.Husson H, et al. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood. 2002;99(1):282–289. doi: 10.1182/blood.v99.1.282. [DOI] [PubMed] [Google Scholar]
- 146.Matas-Céspedes A, et al. Disruption of Follicular Dendritic Cells–Follicular Lymphoma Cross-talk by the Pan-PI3K Inhibitor BKM120 (Buparlisib) Clinical Cancer Research. 2014;20(13):3458–3471. doi: 10.1158/1078-0432.CCR-14-0154. [DOI] [PubMed] [Google Scholar]
- 147.Llibre A, et al. LLT1 and CD161 expression in human germinal centers promotes B cell activation and CXCR4 downregulation. The Journal of Immunology. 2016;196(5):2085–2094. doi: 10.4049/jimmunol.1502462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Byers RJ, et al. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR—based gene expression profiling. Blood. 2008;111(9):4764–4770. doi: 10.1182/blood-2007-10-115915. [DOI] [PubMed] [Google Scholar]
- 149.Wu W, et al. Strong expression of chemokine receptor CCR9 in diffuse large B-cell lymphoma and follicular lymphoma strongly correlates with gastrointestinal involvement. Human pathology. 2014;45(7):1451–1458. doi: 10.1016/j.humpath.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 150.AKIRAFUJII K, et al. Differential expression of cytokines, chemokines and their receptors in follicular lymphoma and reactive follicular hyperplasia: assessment by complementary DNA microarray. Oncology reports. 2005;13:819–824. [PubMed] [Google Scholar]
- 151.Husson H, et al. MCP – 1 modulates chemotaxis by follicular lymphoma cells. British journal of haematology. 2001;115(3):554–562. doi: 10.1046/j.1365-2141.2001.03145.x. [DOI] [PubMed] [Google Scholar]
- 152.Flori M, et al. The hematopoietic oncoprotein FOXP1 promotes tumor cell survival in diffuse large B-cell lymphoma by repressing S1PR2 signaling. Blood. 2016;127(11):1438–1448. doi: 10.1182/blood-2015-08-662635. [DOI] [PubMed] [Google Scholar]
- 153.Cattoretti G, et al. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer research. 2009;69(22):8686–8692. doi: 10.1158/0008-5472.CAN-09-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morin RD, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood. 2013;122(7):1256–1265. doi: 10.1182/blood-2013-02-483727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Muppidi JR, et al. Loss of signalling via G [agr] 13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516(7530):254–258. doi: 10.1038/nature13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koresawa R, et al. Sphingosine – 1 – phosphate receptor 1 as a prognostic biomarker and therapeutic target for patients with primary testicular diffuse large B – cell lymphoma. British journal of haematology. 2016;174(2):264–274. doi: 10.1111/bjh.14054. [DOI] [PubMed] [Google Scholar]
- 157.Paik JH, et al. Overexpression of sphingosine-1-phosphate receptor 1 and phospho-signal transducer and activator of transcription 3 is associated with poor prognosis in rituximab-treated diffuse large B-cell lymphomas. BMC cancer. 2014;14(1):911. doi: 10.1186/1471-2407-14-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu Y, et al. S1PR1 is an effective target to block STAT3 signaling in activated B cell–like diffuse large B-cell lymphoma. Blood. 2012;120(7):1458–1465. doi: 10.1182/blood-2011-12-399030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shuyuli SH, Peng S. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. International journal of oncology. 2005;27:1329–1339. [PubMed] [Google Scholar]
- 160.Rosenwald A, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. New England Journal of Medicine. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 161.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27(5):1329–39. [PubMed] [Google Scholar]
- 162.Rayman N, et al. The expression of the peripheral cannabinoid receptor CB2 has no effect on clinical outcome in diffuse large B – cell lymphomas. European journal of haematology. 2011;86(6):466–476. doi: 10.1111/j.1600-0609.2011.01596.x. [DOI] [PubMed] [Google Scholar]
- 163.Chen J, et al. Dysregulated CXCR4 expression promotes lymphoma cell survival and independently predicts disease progression in germinal center B-cell-like diffuse large B-cell lymphoma. Oncotarget. 2015;6(8):5597. doi: 10.18632/oncotarget.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moreno MJ, et al. CXCR4 expression enhances diffuse large B cell lymphoma dissemination and decreases patient survival. The Journal of pathology. 2015;235(3):445–455. doi: 10.1002/path.4446. [DOI] [PubMed] [Google Scholar]
- 165.Shin HC, et al. Clinical significance of nuclear factor κB and chemokine receptor CXCR 4 expression in patients with diffuse large B-cell lymphoma who received rituximab-based therapy. The Korean journal of internal medicine. 2014;29(6):785–792. doi: 10.3904/kjim.2014.29.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lemma SA, et al. Similar chemokine receptor profiles in lymphomas with central nervous system involvement–possible biomarkers for patient selection for central nervous system prophylaxis, a retrospective study. European journal of haematology. 2015 doi: 10.1111/ejh.12626. [DOI] [PubMed] [Google Scholar]
- 167.Piovan E, et al. Differential regulation of hypoxia-induced CXCR4 triggering during B-cell development and lymphomagenesis. Cancer research. 2007;67(18):8605–8614. doi: 10.1158/0008-5472.CAN-06-4722. [DOI] [PubMed] [Google Scholar]
- 168.Jiang Y, et al. Genome-wide detection of genes targeted by non-Ig somatic hypermutation in lymphoma. PloS one. 2012;7(7):e40332. doi: 10.1371/journal.pone.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reinholdt L, et al. The CXCR4 antagonist plerixafor enhances the effect of rituximab in diffuse large B-cell lymphoma cell lines. Biomarker Research. 2016;4(1):12. doi: 10.1186/s40364-016-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brault L, et al. PIM kinases are progression markers and emerging therapeutic targets in diffuse large B-cell lymphoma. British journal of cancer. 2012;107(3):491–500. doi: 10.1038/bjc.2012.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dürr C, et al. CXCL12 mediates immunosuppression in the lymphoma microenvironment after allogeneic transplantation of hematopoietic cells. Cancer research. 2010;70(24):10170–10181. doi: 10.1158/0008-5472.CAN-10-1943. [DOI] [PubMed] [Google Scholar]
- 172.Deutsch A, et al. Distinct signatures of B – cell homeostatic and activation – dependent chemokine receptors in the development and progression of extragastric MALT lymphomas. The Journal of pathology. 2008;215(4):431–444. doi: 10.1002/path.2372. [DOI] [PubMed] [Google Scholar]
- 173.Deutsch AJ, et al. Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Modern Pathology. 2013;26(2):182–194. doi: 10.1038/modpathol.2012.134. [DOI] [PubMed] [Google Scholar]
- 174.Yamashita Y, et al. XCR1 expression and biased VH gene usage are distinct features of diffuse large B-cell lymphoma initially manifesting in the bone marrow. American journal of clinical pathology. 2011;135(4):556–564. doi: 10.1309/AJCPCTDC5PY3LXBP. [DOI] [PubMed] [Google Scholar]
- 175.Ohshima K, et al. Expression of chemokine receptor CXCR3 and its ligand, mig, in gastric and thyroid marginal zone lymphomas. Possible migration and autocrine mechanism. Leukemia & lymphoma. 2003;44(2):329–336. doi: 10.1080/1042819031000060546. [DOI] [PubMed] [Google Scholar]
- 176.Magro CM, et al. Epidermotropic B-cell lymphoma: a unique subset of CXCR3-positive marginal zone lymphoma. The American Journal of Dermatopathology. 2016;38(2):105–112. doi: 10.1097/DAD.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 177.Kato M, et al. Analysis of CXCL9 and CXCR3 expression in a case of intravascular large B-cell lymphoma. Journal of the American Academy of Dermatology. 2009;61(5):888–891. doi: 10.1016/j.jaad.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 178.Rehm A, et al. Identification of a chemokine receptor profile characteristic for mediastinal large B – cell lymphoma. International journal of cancer. 2009;125(10):2367–2374. doi: 10.1002/ijc.24652. [DOI] [PubMed] [Google Scholar]
- 179.Schain F, et al. Differential expression of cysteinyl leukotriene receptor 1 and 15-lipoxygenase-1 in non-Hodgkin lymphomas. Clinical Lymphoma and Myeloma. 2008;8(6):340–347. doi: 10.3816/CLM.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 180.Nakayama S, et al. Aberrant expression of CCR4 in diffuse large B-cell lymphoma, not otherwise specified. Leukemia. 2013;27(12):2382. doi: 10.1038/leu.2013.128. [DOI] [PubMed] [Google Scholar]
- 181.Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer science. 2011;102(1):44–50. doi: 10.1111/j.1349-7006.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 182.Nannini PR, et al. CCR4 expression in a case of cutaneous Richter’s transformation of chronic lymphocytic leukemia (CLL) to diffuse large B-cell lymphoma (DLBCL) and in CLL patients with no skin manifestations. Eur J Haematol. 2011;87(1):80–6. doi: 10.1111/j.1600-0609.2011.01613.x. [DOI] [PubMed] [Google Scholar]
- 183.Spina V, et al. The genetics of nodal marginal zone lymphoma. Blood. 2016 doi: 10.1182/blood-2016-02-696757. p. blood-2016-02-696757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baens M, et al. t (X; 14)(p11. 4; q32. 33) is recurrent in marginal zone lymphoma and upregulates GPR34. haematologica. 2011 doi: 10.3324/haematol.2011.052639. p. haematol. 2011.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ansell SM, et al. t (X; 14)(p11; q32) in MALT lymphoma involving GPR34 reveals a role for GPR34 in tumor cell growth. Blood. 2012;120(19):3949–3957. doi: 10.1182/blood-2011-11-389908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Konturek PC, et al. Helicobacter pylori-gastrin link in MALT lymphoma. Aliment Pharmacol Ther. 2000;14(10):1311–8. doi: 10.1046/j.1365-2036.2000.00832.x. [DOI] [PubMed] [Google Scholar]
- 187.Stollberg S, et al. Differential somatostatin and CXCR4 chemokine receptor expression in MALT-type lymphoma of gastric and extragastric origin. Journal of cancer research and clinical oncology. 2016;142(11):2239–2247. doi: 10.1007/s00432-016-2220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Raderer M, et al. Somatostatin-receptor scintigraphy for staging and follow-up of patients with extraintestinal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT)-type. British journal of cancer. 2001;85(10):1462. doi: 10.1054/bjoc.2001.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van Maldegem F, et al. The majority of cutaneous marginal zone B-cell lymphomas expresses class-switched immunoglobulins and develops in a T-helper type 2 inflammatory environment. Blood. 2008;112(8):3355–3361. doi: 10.1182/blood-2008-01-132415. [DOI] [PubMed] [Google Scholar]
- 190.Yamamoto H, et al. Significance of CXCR3 expression in gastric low – grade B – cell lymphoma of mucosa – associated lymphoid tissue type for predicting responsiveness to Helicobacter pylori eradication. Cancer science. 2008;99(9):1769–1773. doi: 10.1111/j.1349-7006.2008.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Suefuji H, et al. CXCR3 – positive B cells found at elevated frequency in the peripheral blood of patients with MALT lymphoma are attracted by MIG and belong to the lymphoma clone. International journal of cancer. 2005;114(6):896–901. doi: 10.1002/ijc.20823. [DOI] [PubMed] [Google Scholar]
- 192.Xu L, et al. Clonal architecture of CXCR4 WHIM – like mutations in Waldenström Macroglobulinaemia. British journal of haematology. 2016;172(5):735–744. doi: 10.1111/bjh.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ngo HT, et al. SDF-1/CXCR4 and VLA-4 interaction regulates homing in Waldenstrom macroglobulinemia. Blood. 2008;112(1):150–158. doi: 10.1182/blood-2007-12-129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dar A, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25(8):1286–1296. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Treon SP, et al. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123(18):2791–6. doi: 10.1182/blood-2014-01-550905. [DOI] [PubMed] [Google Scholar]
- 196.Kapoor P, Paludo J, Ansell SM. Waldenstrom macroglobulinemia: familial predisposition and the role of genomics in prognosis and treatment selection. Current treatment options in oncology. 2016;17(3):1–15. doi: 10.1007/s11864-016-0391-7. [DOI] [PubMed] [Google Scholar]
- 197.Schmidt J, et al. MYD88 L265P and CXCR4 mutations in lymphoplasmacytic lymphoma identify cases with high disease activity. Br J Haematol. 2015;169(6):795–803. doi: 10.1111/bjh.13361. [DOI] [PubMed] [Google Scholar]
- 198.Poulain S, et al. Genomic landscape of CXCR4 mutations in Waldenström macroglobulinemia. Clinical Cancer Research. 2016;22(6):1480–1488. doi: 10.1158/1078-0432.CCR-15-0646. [DOI] [PubMed] [Google Scholar]
- 199.Hunter ZR, et al. Transcriptome sequencing reveals a profile that corresponds to genomic variants in Waldenström macroglobulinemia. Blood. 2016;128(6):827–838. doi: 10.1182/blood-2016-03-708263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hunter ZR, et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014;123(11):1637–46. doi: 10.1182/blood-2013-09-525808. [DOI] [PubMed] [Google Scholar]
- 201.Roccaro AM, et al. C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma. Blood. 2014;123(26):4120–4131. doi: 10.1182/blood-2014-03-564583. [DOI] [PubMed] [Google Scholar]
- 202.Mancuso P, et al. If it is in the marrow, is it also in the blood? An analysis of 1,000 paired samples from patients with B-cell non-Hodgkin lymphoma. BMC cancer. 2010;10(1):644. doi: 10.1186/1471-2407-10-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ballester LY, et al. Clinical Validation of a CXCR4 Mutation Screening Assay for Waldenstrom Macroglobulinemia. Clinical Lymphoma Myeloma and Leukemia. 2016;16(7):395–403.e1. doi: 10.1016/j.clml.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 204.Cao Y, et al. CXCR4 WHIM – like frameshift and nonsense mutations promote ibrutinib resistance but do not supplant MYD88L265P – directed survival signalling in Waldenström macroglobulinaemia cells. British journal of haematology. 2015;168(5):701–707. doi: 10.1111/bjh.13200. [DOI] [PubMed] [Google Scholar]
- 205.Grever MR, Blachly JS, Andritsos LA. Hairy cell leukemia: Update on molecular profiling and therapeutic advances. Blood Rev. 2014;28(5):197–203. doi: 10.1016/j.blre.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 206.Basso K, et al. Gene expression profiling of hairy cell leukemia reveals a phenotype related to memory B cells with altered expression of chemokine and adhesion receptors. Journal of Experimental Medicine. 2004;199(1):59–68. doi: 10.1084/jem.20031175. [DOI] [PMC free article] [PubMed] [Google Scholar]


