Abstract
People with schizophrenia typically show visual processing deficits on masking tasks and other performance-based measures, while people with bipolar disorder may have related deficits. The etiology of these deficits is not well understood. Most neuroscientific studies of perception in schizophrenia and bipolar disorder have focused on visual processing areas in the cerebral cortex, but perception also depends on earlier components of the visual system that few studies have examined in these disorders. Using diffusion weighted imaging (DWI), we investigated the structure of the primary sensory input pathway to the cortical visual system: the optic radiations. We used probabilistic tractography to identify the optic radiations in 32 patients with schizophrenia, 31 patients with bipolar disorder, and 30 healthy controls. The same participants also performed a visual masking task outside the scanner. We characterized the optic radiations with three structural measures: fractional anisotropy, mean diffusivity, and tract volume. We did not find significant differences in those structural measures across groups. However, we did find a significant correlation between the volume of the optic radiations and visual masking thresholds that was unique to the schizophrenia group and explained variance in masking performance above and beyond that previously accounted for by differences in visual cortex. Thus, individual differences in the volume of the optic radiations explained more variance in visual masking performance in the schizophrenia group than the bipolar or control groups. This suggests that individual differences in the structure of the subcortical visual system have an important influence on visual processing in schizophrenia.
Keywords: Schizophrenia, Bipolar Disorder, Diffusion Weighted Imaging, Tractography, Optic Radiations, Visual Perception
1. Introduction
Abnormalities in visual perception have been well-characterized in schizophrenia using various methods (Butler et al., 2008; Green et al., 2009a; 2012; Javitt, 2009; Javitt and Freedman, 2015). Similar types of perceptual dysfunction might also exist in other mental illnesses that share genetic risk factors and clinical characteristics with schizophrenia, including bipolar disorder (Chen et al., 2005; Chkonia et al., 2012; Jahshan et al., 2014). The neural bases of these abnormalities remain largely unknown. Most studies of the visual system in schizophrenia have focused on the cerebral cortex, but some effects observed there may be downstream reflections of abnormal inputs to the cortical visual system.
The main sensory input pathway to the cortical visual system is from the lateral geniculate nucleus of the thalamus to primary visual cortex (V1). This white matter tract is known as the optic radiations, and its structure can be assessed in vivo using diffusion-weighted magnetic resonance imaging (DWI). Some whole-brain DWI analyses have reported differences in the properties of the optic radiations between schizophrenia or bipolar groups and controls (Douaud et al., 2007; Lee et al., 2014; Mitelman et al., 2007; Versace et al., 2008; Wu et al., 2014). However, those whole-brain studies focused on only one or two measures of optic radiation structure and did not address the possible significance of group differences in the structure of that tract. To our knowledge, only two previous papers have specifically and more comprehensively investigated the structure of the optic radiations in schizophrenia (Butler et al., 2006; Henze et al., 2014). Both found patient-control differences in the structure of the optic radiations, but both had small patient samples (N<20) and used methodologies that are no longer current (e.g., lower field strength and angular resolution). There appear to be no published studies specifically examining the structure of the optic radiations in bipolar disorder. Furthermore, no study has linked any structural property of the optic radiations to a performance-based measure of perception in schizophrenia or bipolar disorder.
In this study, we used probabilistic tractography to investigate the optic radiations in schizophrenia, bipolar disorder, and healthy controls. We assessed three DWI-based measures: fractional anisotropy (FA), mean diffusivity (MD), and tract volume. Traditionally, these measures have typically been reported as indirect indices of “white matter integrity,” but the relationships between these measures and the microstructural properties of white matter are now acknowledged to be more nuanced (Jones et al., 2013). Intact, well-organized, well-myelinated axons within a voxel tend to limit diffusion perpendicular to the axons, allowing relatively little diffusion overall and mostlu constraining diffusion that does occur to the axis parallel to the axons, making the directionality of diffusion high (Beaulieu, 2002). FA is a measure of the directionality of diffusion; higher FA values indicate that diffusion is more directional. MD is an index of the total amount of diffusion in all directions. Tract volume is a simple measure of the size of a white matter pathway.
When differences in DWI measures between these patient and control groups have been found, FA typically has been lower in schizophrenia and bipolar disorder (e.g., Skudlarski et al., 2013). While MD is less often reported, it is typically higher in those populations (e.g., Clark et al., 2011). Reductions in white matter volume also tend to be found in schizophrenia and bipolar disorder (e.g., Oertel-Knöchel et al., 2015). Therefore, we expected that FA and tract volume would be reduced in schizophrenia and bipolar disorder, while MD would be higher in the patient groups, compared to controls. We also examined correlations between each DWI measure and visual masking performance within each group.
2. Methods
2.1 Participants
Participants in this study came from a larger, ongoing, NIMH-sponsored study of visual processing in major mental illness. The sample included 32 patients with schizophrenia, 31 bipolar disorder patients, and 30 healthy controls. All patient participants were clinically stable outpatients with a DSM-IV diagnosis of either schizophrenia or bipolar disorder who were not in a current mood episode. Healthy participants were a matched community sample. Full details about participant selection criteria and recruitment, are included in the Supplementary Methods.
Patients' clinical symptoms were characterized using the Brief Psychiatric Rating Scale (BPRS), Young Mania Rating Scale (YMRS), and Hamilton Depression rating scale (HAM-D) (Hamilton, 1960; Overall and Gorham, 1962; Thompson et al., 1994; Ventura et al., 1993; Young et al., 1978). The study used the 24-item version of the BPRS developed at the UCLA Clinical Research Center for Schizophrenia and Psychiatric Rehabilitation, in which each item is rated on a scale of 1 to 7, and the 21-item version of the HAM-D scale (Hamilton, 1960; Overall and Gorham, 1962; Ventura et al., 1993).
2.2 MRI data collection
All MRI data was collected at the UCLA Staglin Center for Cognitive Neuroscience on a 3-Tesla Siemens Tim Trio scanner with a 12-channel head coil (Siemens Medical Solutions; Erlangen, Germany). T1-weighted structural scans were collected using a Magnetization-Prepared Rapid Gradient Echo (MPRAGE) sequence (1.9 sec TR, 3.4ms TE, 9 flip-angle, 1mm isotropic voxels, 256 x 256 x 160 voxel field of view). DWI scans were collected with 64 diffusion directions at a B-value of 1000 s/mm2 (8sec TR, 93ms TE, 2.3 mm isotropic voxels, 96x96x58 voxel FOV).
2.3 MRI processing
DWI data were preprocessed, and FA and MD measures calculated for each voxel, using a well-documented standard FSL pipeline (Jenkinson et al., 2012; Smith et al., 2004). Thalamus and V1 region masks were created from automated FreeSurfer reconstructions of each participant’s T1 anatomical scan (Dale et al., 1999; Fischl et al., 1999). We used a standard approach and parameters recommended by the FSL developers to perform bidirectional tractography between these two region masks in each hemisphere with FSL (Behrens et al., 2007; 2003). The results of this tractography analysis were thresholded according to the number of streamlines passing through each voxel and other criteria (see Supplementary Methods) to create a unique volumetric mask of the optic radiations for each participant. A detailed description of all steps and parameters used to process the MRI data is in the Supplementary Methods. Mean FA and MD within the optic radiations were computed by averaging FA and MD scores within the mask of all tract voxels in each hemisphere, then averaging across hemispheres. The volume of the optic radiations, in mm3, was calculated based on the final, bilateral, optic radiation mask of each participant (see Supplementary Methods).
2.4 Masking Task
In a separate testing session without MRI, participants’ visual perception was assessed with a task in which they attempted to identify backward-masked objects from one of six categories of household items. For each participant, we estimated the length of the delay at which that person would be able to identify the type of object correctly 50% of the time, using standard psychometric curve-fitting methods (Prins and Kingdom, 2009; Wichmann and Hill, 2001). These analyses are described in detail in the Supplementary Methods. Threshold ISIs were used for correlations with DWI measures.
3. Results
Demographic and clinical characteristics of the sample, as well as group comparisons of those variables, are in Table 1. There were no significant differences in age, handedness, or parental education across the three groups, and the two patient groups did not differ in the number of years since their diagnosis. The groups did differ significantly in gender and years of personal education. The two patient groups also differed significantly in the number of individuals taking antipsychotic and mood stabilizing medication, and in antipsychotic medication dosage. Schizophrenia patients had higher scores on the BPRS than bipolar patients, but the two patient groups did not have significantly different scores on the YMRS or HAM-D. A one-way ANOVA comparing backward masking thresholds did not show a significant difference across groups (F(2,82) = 2.16, p = 0.12). Means (standard deviations) were similar across groups for the masking thresholds: schizophrenia = 58.30 (20.90) ms, bipolar = 48.68 (20.17) ms, controls = 60.18 (24.09) ms.
Table 1.
Characterization of participants.
| SZ Patients (N=32) | BD Patients (N=31) | Controls (N=30) | Group Comparison | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Statistic | p | |
| Age | 44.66 (12.44) | 43.74 (13.15) | 48.00 (6.54) | F(2,90)=1.23 | p=0.30 |
| Illness Duration | 23.91 (13.51) | 22.50 (12.61) | t(58)=0.42 | p=0.68 | |
| Education | 12.81 (1.94) | 13.94 (2.34) | 14.33 (1.83) | F(2,90)=4.64 | p=0.01 |
| Parental Education | 12.88 (2.56) | 13.83 (2.74) | 13.43 (3.01) | F(2,82)=0.86 | p=0.43 |
| Gender (M/F) | 23 / 9 | 13 / 18 | 15 / 15 | Χ2(2)=6.12 | p=0.05 |
| Handedness (R/L) | 27 / 5 | 27 / 4 | 26 / 4 | Χ2(2)=0.11 | p=0.95 |
| BD Type: I / II | 20 / 11 | ||||
|
| |||||
| BPRS (Total) | 40.47 (12.23) | 32.77 (9.09) | t(61)=2.83 | p=0.01 | |
| HAM-D (Total) | 7.38 (6.26) | 7.26 (4.61) | t(61)=0.08 | p=0.93 | |
| YMRS (Total) | 5.06 (3.93) | 4.06 (5.02) | t(61)=0.88 | p=0.38 | |
| Antipsychotic medication (Y/N) | 28 / 4 | 20 / 11 | Χ2(1)=4.59 | p=0.03 | |
| CPZ-equivalent dosage (mg/day) | 516.15 (407.08) | 257.60 (160.03) | t(33)=2.32 | p=0.03 | |
| Mood stabilizer frequency (Y/N) | 10 / 23 | 23 / 8 | Χ2(1)=12.33 | p=0.01 | |
Figure 1 shows an example unilateral optic radiation tract mask, and the FA values of voxels within it. Across subjects, FA, MD, and tract volume were all normally distributed, so we compared those measures across groups using ANOVAs. Descriptive and inferential statistics for each ANOVA are in Table 2. Because there was a significant gender difference across the three groups, we included gender as a factor in the ANOVAs. There were no significant main effects of group, nor group-by-gender interactions, for any of the three DWI measures.
Figure 1.
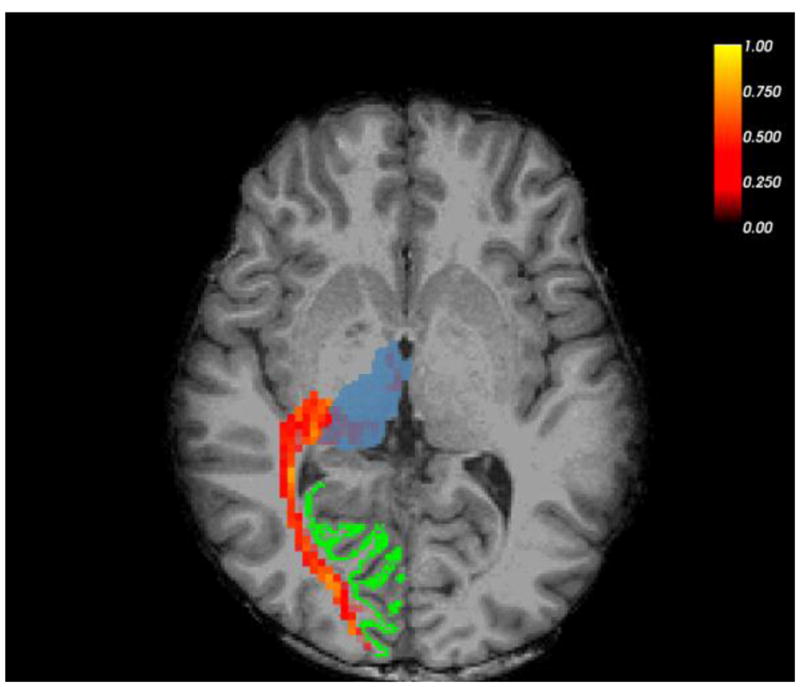
Example right-hemisphere optic radiation tract and seed masks in a healthy control participant, overlaid on that participant’s high-resolution anatomical scan (T1). Thalamus mask is in blue, V1 mask in green. Thresholded FA map is in red/yellow; brighter colors indicate higher FA.
Table 2.
Above: Means (standard errors) of DWI measures by group. Below: ANOVA statistics for each DWI measure.
| Schizophrenia | Bipolar Disorder | Healthy Controls | |
|---|---|---|---|
| FA (ratio) | 0.432 (0.004) | 0.428 (0.004) | 0.427 (0.004) |
| MD (μm2/sec) | 79.182 (0.703) | 81.169 (0.651) | 81.213 (0.653) |
| Tract Volume (cm3) | 18.488 (1.098) | 17.692 (1.016) | 19.976 (1.020) |
| Main effect: Group | Main Effect: Gender | Interaction: Group-by-Gender | |
|---|---|---|---|
|
dfwithin = 2 dferror = 87 |
dfwithin = 1 dferror = 87 |
dfwithin = 2 dferror = 87 |
|
| FA |
F = 0.392 p = 0.677 η2p = 0.009 |
F = 0.278 p = 0.599 η2p = 0.003 |
F = 0.855 p = 0.429 η2p = 0.019 |
| MD |
F = 2.858 p = 0.063 η2p = 0.062 |
F = 0.734 p = 0.394 η2p = 0.008 |
F = 2.761 p = 0.069 η2p = 0.060 |
| Tract Volume |
F = 1.292 p = 0.280 η2p = 0.029 |
F = 0.606 p = 0.438 η2p = 0.007 |
F = 0.414 p = 0.662 η2p = 0.009 |
We examined the associations between visual masking thresholds and the three DWI measures in each of the three participant groups. A Bonferroni-corrected α-level of 0.005 was used for the correlations. As shown in Figure 2, tract volume was significantly correlated with masking thresholds, but only in the schizophrenia group (schizophrenia r(29) = −0.584, p = 0.001; bipolar r(26) = 0.037, p = 0.857; controls r(29) = −0.052, p = 0.784). Indeed, follow-up Fisher’s r-to-z comparisons of correlation magnitudes showed that the correlation between tract volume and masking thresholds was significantly larger in the schizophrenia group than the other groups (schizophrenia vs. bipolar Z = 2.46, p =0.01; schizophrenia vs. controls Z = 2.24, p =0.03).
Figure 2.
Correlations between masking thresholds tract volume for each group.
Neither FA nor MD was correlated with masking performance in any of the three groups. FA correlations were as follows: schizophrenia r(29) = −0.297, p = 0.118; bipolar r(26) = 0.107, p = 0.602; controls r(29) = −0.193, p = 0.307. MD correlations were: schizophrenia r(29) = 0.227, p = 0.236; bipolar r(26) = 0.051, p = 0.804; controls r(29) = 0.038, p = 0.841.
4. Discussion
Contrary to our expectations, we found no evidence of group differences in FA, MD, or volume of the optic radiations across patients with schizophrenia, patients with bipolar disorder, and healthy controls, even though differences in FA have been reported in previous studies. Our sample was larger than those in many previous reports, and our scanning and analysis techniques took advantage of numerous recent methodological advances. These methodological differences could be related to the difference between our findings and those of previous studies.
Although we found no group differences in the properties of the optic radiations between patients and controls, we did find a significant correlation between tract volume and perceptual performance in schizophrenia. To our knowledge, this is the first time that the structure of a subcortical visual pathway has been linked to performance on a visual masking task in schizophrenia. This result suggests that individual differences in brain structure as early as the optic radiations may have cascading effects on perception in the disorder. The specificity of the link between neuroanatomical structure and perceptual performance to the schizophrenia group suggests that there is a different relationship between the structure of the optic radiations and perception in schizophrenia than in bipolar disorder or healthy controls. Specifically, individual differences in the volume of this tract appear to matter more for the perception of masked stimuli in the schizophrenia group than in the other groups.
We recently identified another neuroanatomical correlate of performance on the same masking task in an overlapping sample of participants. In that study, we found that the thickness of visual cortex was significantly correlated with masking thresholds in schizophrenia patients (Reavis et al., 2016). To determine whether optic radiation volume accounts for additional variance in schizophrenia patients’ masking performance, above and beyond that explained by cortical thickness, we performed a multiple regression. Specifically, we added optic radiation tract volume to thickness of early visual cortex in a stepwise regression model. We found that adding the tract volume measure accounted for significantly more variance in masking performance than cortical thickness alone (ΔF (1,26)=14.34, Δr2 = 0.27, p=0.001). Thus, these two neuroanatomical features appear to have non-overlapping influences on visual perception in schizophrenia. Together, optic radiation volume and the thickness of visual cortex accounted for about half of the variance in schizophrenia patients’ performance on the masking task (F(2,26)=13.24, r2=0.51, p < 0.001). The surprising ability of just two neuroanatomical features of the early visual system to account for such a large amount of perceptual variance raises the possibility that individual differences in the structure of the early visual system have profound effects on the way people with schizophrenia experience the visual world.
The present study had several limitations. First, there was an imbalance in the gender distribution of the participant groups. Although we found no effects of gender in our analyses, a more balanced distribution of gender across groups would be desirable in future studies. Second, there were no significant differences in visual masking performance between the groups. Many previous studies have found visual masking deficits in schizophrenia, and it is unclear why such differences were not apparent in this sample. On the positive side, the lack of group differences in masking makes the interpretation of correlational differences between groups clearer. Third, patients were on clinically-determined doses of medication. To rule out any possible medication effects, it would be informative to test participants who have never received psychotropic medication (e.g., first episode patients). Fourth, current tractography methods cannot differentiate the optic radiations proper from adjacent pathways that connect other thalamic nuclei (e.g., pulvinar) to V1.
Despite these limitations, the results of this study suggest that perception is more strongly influenced by the structure an early visual pathway in schizophrenia than in bipolar disorder or healthy controls. This is consistent with the possibility that some of the differences in the function of the cortical visual system which have been reported between schizophrenia patients and controls (e.g., Green et al., 2009b; Silverstein et al., 2015; Yoon et al., 2008) might be downstream effects related to individual differences in inputs from the subcortical visual system. It will be important for future work to investigate how such individual differences in the structure of early visual areas might relate to individual differences in the function of those areas in schizophrenia. It also remains an important question to what extent these individual differences in the structure (and perhaps function) of early visual areas are correlated with performance on other measures of perception and cognition, as well as disease characteristics (e.g., symptoms). Processing in many cognitive domains depends on accurate perceptual input (Javitt and Freedman, 2015), and cognition is a major determinant of outcomes in schizophrenia (Green, 1996; Green et al., 2000). Thus, it is conceivable that individual differences in the structure and function of early visual areas could have wide-ranging effects in the disorder. Fortunately, the structure and physiology of the early visual system is well-understood in healthy populations, making investigation of the early visual system in schizophrenia an especially promising topic for future research.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health: R01 MH095878, to MFG, “Visual Tuning in Psychosis.” EAR was also supported by the NIH under RuthL. Kirschstein National Research Service Award F32MH108317.
We thank Ana Ceci Myers and Julio Iglesias for their help with data collection.
Footnotes
Contributors: MFG is PI of the larger project from which the present data came. EAR initiated the current project and developed the strategy for the analyses in consultation with JL, JKW, KLN, and MFG. SNN preprocessed the data and EAR performed the rest of the data analysis. Together, EAR, JL, JKN, KLN, SAE, and MFG interpreted the results. EAR wrote the manuscript with input from the other authors. All authors contributed to and approved the final manuscript.
Conflicts of interest: Dr. Green has been a consultant to AbbVie, ACADIA, DSP, FORUM, Lundbeck, and Takeda. He is on the Scientific Board of Luc and has received research support from Amgen and Forum. All other authors report no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benson NC, Butt OH, Brainard DH, Aguirre GK. Correction of Distortion in Flattened Representations of the Cortical Surface Allows Prediction of V1–V3 Functional Organization from Anatomy. PLoS Comput Biol. 2014;10:e1003538–9. doi: 10.1371/journal.pcbi.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson NC, Butt OH, Datta R, Radoeva PD, Brainard DH, Aguirre GK. The Retinotopic Organization of Striate Cortex Is Well Predicted by Surface Topology. Current Biology. 2012;22:2081–2085. doi: 10.1016/j.cub.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Hoptman MJ, Nierenberg J, Foxe JJ, Javitt DC, Lim KO. Visual white matter integrity in schizophrenia. AJP. 2006;163:2011–2013. doi: 10.1176/ajp.2006.163.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual Perception and Its Impairment in Schizophrenia. Biological Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophrenia Research. 2005;74:271–281. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Chkonia E, Roinishvili M, Reichard L, Wurch W, Puhlmann H, Grimsen C, Herzog MH, Brand A. Patients with functional psychoses show similar visual backward masking deficits. Psychiatry Res. 2012;198:235–240. doi: 10.1016/j.psychres.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Clark KA, Nuechterlein KH, Asarnow RF, Hamilton LS, Phillips OR, Hageman NS, Woods RP, Alger JR, Toga AW, Narr KL. Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia. Journal of Psychiatric Research. 2011;45:980–988. doi: 10.1016/j.jpsychires.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J, Benjamin L. Structured clinical interview for DSM-IV axis II personality disorders. State Psychiatric Institute; New York: 1996. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis I disorders Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? AJP. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophrenia Bulletin. 2009a;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From Perception to Functional Outcome in Schizophrenia. Arch Gen Psychiatry. 2012;69:1216–9. doi: 10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Lee J, Cohen MS, Engel SA, Korb AS, Nuechterlein KH, Wynn JK, Glahn DC. Functional Neuroanatomy of Visual Masking Deficits in Schizophrenia. Arch Gen Psychiatry. 2009b;66:1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze R, Brunner R, Thiemann U, Parzer P, Klein J, Resch F, Stieltjes B. The optic radiation and the cerebellar peduncles in adolescents with first-admission schizophrenia--a diffusion tensor imaging study. J Neuroimaging. 2014;24:111–116. doi: 10.1111/j.1552-6569.2011.00668.x. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, McCleery A, Glahn DC, Altshuler LL, Green MF. Cross-diagnostic comparison of visual processing in bipolar disorder and schizophrenia. Journal of Psychiatric Research. 2014;51:42–48. doi: 10.1016/j.jpsychires.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Freedman R. Sensory Processing Dysfunction in the Personal Experience and Neuronal Machinery of Schizophrenia. AJP. 2015;172:17–31. doi: 10.1176/appi.ajp.2014.13121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Lee JS, Han K, Lee SK, Seok JH, Kim JJ. Altered structural connectivity and trait anhedonia in patients with schizophrenia. Neuroscience Letters. 2014;579:7–11. doi: 10.1016/j.neulet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: A diffusion tensor imaging survey. Schizophrenia Research. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Oertel-Knöchel V, Lancaster TM, Knöchel C, Stäblein M, Storchak H, Reinke B, Jurcoane A, Kniep J, Prvulovic D, Mantripragada K, Tansey KE, O'Donovan MC, Owen MJ, Linden DEJ. Schizophrenia risk variants modulate white matter volume across the psychosis spectrum: evidence from two independent cohorts. NeuroImage: Clinical. 2015;7:764–770. doi: 10.1016/j.nicl.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Prins N, Kingdom FAA. Palamedes: Matlab routines for analyzing psychophysical data. 2009 www.palamedestoolbox.org.
- Reavis EA, Lee J, Wynn JK, Engel SA, Jimenez AM, Green MF. Cortical Thickness of Functionally Defined Visual Areas in Schizophrenia and Bipolar Disorder. Cereb Cortex. 2016:bhw151. doi: 10.1093/cercor/bhw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Harms MP, Carter CS, Gold JM, Keane BP, MacDonald A, III, Daniel Ragland J, Barch DM. Cortical contributions to impaired contour integration in schizophrenia. Neuropsychologia. 2015;75:469–480. doi: 10.1016/j.neuropsychologia.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O'Neil K, Pearlson GD. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170:886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thompson PA, Buckley PF, Meltzer HY. The brief psychiatric rating scale: effect of scaling system on clinical response assessment. J Clin Psychopharmacol. 1994;14:344–346. [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters. Int J Methods Psychiatr Res. 1993;3:221–224. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JRC, Hassel S, Walsh ND, Novelli M, Klein CR, Kupfer DJ, Phillips ML. Elevated Left and Reduced Right Orbitomedial Prefrontal Fractional Anisotropy in Adults With Bipolar Disorder Revealed by Tract-Based Spatial Statistics. Arch Gen Psychiatry. 2008;65:1041. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wu C-H, Hwang T-J, Chen Y-J, Hsu Y-C, Lo Y-C, Liu C-M, Hwu H-G, Liu C-C, Hsieh MH, Chien YL, Chen C-M, Isaac Tseng W-Y. Altered integrity of the right arcuate fasciculus as a trait marker of schizophrenia: A sibling study using tractography-based analysis of the whole brain. Hum Brain Mapp. 2014;36:1065–1076. doi: 10.1002/hbm.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Tamir D, Minzenberg MJ, Ragland JD, Ursu S, Carter CS. Multivariate pattern analysis of functional magnetic resonance imaging data reveals deficits in distributed representations in schizophrenia. Biological Psychiatry. 2008;64:1035–1041. doi: 10.1016/j.biopsych.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



