Abstract
Cytochrome P450 1B1 (CYP1B1), a member of CYP450s, is expressed in liver and extrahepatic tissues carries out the metabolism of numerous xenobiotics, including the metabolic activation of polycyclic aromatic hydrocarbons. Surprisingly, CYP1B1 was also shown to be important in regulating endogenous metabolic pathways, including the metabolism of steroid hormones, fatty acids, melatonin, and vitamins. CYP1B1 and nuclear receptors including peroxisome proliferator-activated receptors (PPARs), estrogen receptor (ER), and retinoic acid receptors (RAR) contribute to the maintenance of the homeostasis of these endogenous compounds. Many natural flavonoids and synthetic stilbenes show the inhibitory activity toward CYP1B1 expression and function, notably isorhamnetin and 2,4,3′,5′-tetramethoxystilbene. Accumulating data indicate that modulation of CYP1B1 can decrease adipogenesis and tumorigenesis, and prevent obesity, hypertension, atherosclerosis, and cancer. Therefore, it may be feasible to consider CYP1B1 as a therapeutic target for the treatment of metabolic diseases.
Keywords: CYP1B1, Metabolic diseases, Metabolic pathways, Metabolomics
1. Introduction
Cytochrome P450 1B1 (CYP1B1) is a heme-thiolate monooxygenase involved in NADPH-dependent phase I metabolism of a variety of xenobiotics. In 1991, a novel cytochrome P-450 (P450EF) that can be induced by benzo[α]anthracene and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was purified from C3H10T1/2 mouse embryo fibroblasts (Pottenger, et al., 1991). P450EF was subsequently identified as a new member of CYP1B subfamily in 1994 (Savas, et al., 1994). In the same year, the cDNA sequence of human CYP1B1 was characterized from primary cultures of normal human epidermal keratinocytes (Sutter, et al., 1994). In contrast to two other CYP1 family enzymes, CYP1A1 and CYP1A2, CYP1B1 is expressed in a variety of tumor tissues. Oral benzo[a]pyrene (B[a]P)-treated Cyp1a1/1a2/1b1(−/−) mice showed the same the “rescued” response as that seen in the Cyp1a1/1b1(−/−) mouse, whereas Cyp1a1(−/−) mice ingesting B[a]P died due to severe immunosuppression (Dragin, et al., 2008), suggesting that the CYP1B1 is necessary in immune tissues. In this study, it was also observed that the phenotype of oral B[a]P-treated Cyp1a1/1a2/1b1(−/−) mice was similar to that of the BaP-treated wild-type and the corn oil-treated control mice, whereas there was substantial bone marrow hypocellularity in oral B[a]P-treated Cyp1a1(−/−) and Cyp1a1/1a2(−/−) mice. These studies suggest that inhibition of CYP1B1 expression may contribute to the protection against bone marrow hypocellularity. An early study revealed that the expression of CYP1B1 was found in 122 of 127 tumor tissues, including brain, breast, and colon tumor (Murray, et al., 1997). The higher expression of CYP1B1 in tumor cells than the surrounding normal tissue has led to much interest on the role of CYP1B1 in tumorigenesis and its treatment.
The human CYP1B1 gene spanning 12 kb in length and located on chromosome region 2p21–22, consists of 3 exons and 2 introns (Tang, et al., 1996). Human CYP1B1 shows approximately 40% homology with CYP1A1 and CYP1A2, but its gene structure is simpler. The mRNA is 4.2 kb and its open reading frame begins at the second intron of 5′ end, coding a 543 amino acids residues protein (Murray, et al., 2001). Several positive and negative regulators exist in the promoter region of the human CYP1B1 gene, but it is structurally different from CYP1A1 and CYP1A2 genes (Murray, et al., 2001; Wo, et al., 1997). CYP1B1 is involved in the metabolism of a wide variety of xenobiotics, such as ethoxyresorufin, theophylline and caffeine, and shows some overlapping metabolic activities with CYP1A1 and CYP1A2. Unlike most CYPs, CYP1B1 expression is not detected in human liver, but CYP1B1 is expressed in many extra-liver tissues, including lung, colon, eye and kidney. CYP1B1 shows activity toward activation of environmental carcinogens via the hydroxylation of procacinogens, including 27 polycyclic aromatic hydrocarbons and their derivatives, 17 heterocyclic and aryl amine and aminoazo dyes, 3 mycotoxins, 2 nitroaromatic hydrocarbons (Tsutomu Shimada, et al., 1996). Consistent with its role in carcinogen activation, carcinogenesis is reduced in Cyp1b1-null mice (J. T. Buters, et al., 1999), suggesting that CYP1B1 plays an important role in metabolic activation of environmental carcinogens.
The expression of CYP1B1 can be induced by xenobiotics such as TCDD, through the aryl hydrocarbon receptor (AHR) (Hankinson, 2016). In addition to the oxidation of xenobiotics, CYP1B1 is involved in the metabolism of many important physiological compounds, including estrogen, arachidonic acid, melatonin and retinoids. A recent study revealed that Cyp1b1 disruption altered the expression of 560 liver genes, including suppression of peroxisome proliferator-activated receptor γ (PPARγ) and many genes regulated by PPARα (Larsen, et al., 2015). PPARs are a group of nuclear receptor that regulates the expression of many down-stream genes, and play a key role in the homeostasis of lipids and glucose, closely related to metabolic diseases. Metabolic diseases are associated with the disorder of endogenous metabolism, ranging from obesity and atherosclerosis to hypertension and cancer. In terms of obesity, its incidence has been dramatically increased worldwide in recent years. It is estimated that more than 1/3 of adults and nearly 17% of children in the United States are obese. In 2008, the cost for obesity-related medical diseases was an estimated $147 billion. Some studies have shown that Cyp1b1 disruption can protect against obesity induced by high-fat diet (HFD) (Larsen, et al., 2015; Fei Li, et al., 2014). Previous reviews have discussed the role of CYP1B1 in glaucoma (Vasiliou & Gonzalez, 2008). In this review, recent findings are summarized on the impact of CYP1B1 in the regulation of metabolic pathways and the development of metabolic diseases, and the potential therapy for the treatment of metabolic diseases using CYP1B1 modulators are discussed.
2. Discovery of CYP1B1 inhibitors
CYP1B1 is known to show high frequency expression in a wide of variety of cancers, such as prostate, uterus, and colon cancer. CYP1B1 is involved in the metabolic activation of many environmental procarcinogens. Mutant CYP1B1 alleles have been detected in cancer and glaucoma patients. These findings suggest that the regulation of CYP1B1 expression can act as a therapeutic strategy, especially for cancer treatment. To date, more than 50 natural products and synthetic compounds have been developed or identified as CYP1B1 inhibitors (Table 1). Stilbene, flavonoid, coumarin, and anthraquinone are the four major types of compounds that inhibit CYP1B1 activity (Figure 1). 2,4,3′,5′-Tetramethoxystilbene (TMS), a methoxy derivative resveratrol, is a highly potent and selective inhibitor of CYP1B1. Its inhibitory ability for CYP1B1 (IC50 = 6 nM) is over 50-fold greater than against CYP1A1 (IC50 = 300 nM) and 500-fold higher than for CYP1A2 (IC50 = 3000 nM) (Chun, Kim, et al., 2001). It was reported that TMS can protect against hypertensions from chemical induction and gene mutation. Natural flavonoids are an important source of CYP1B1 inhibitors. Methoxy types of flavones and flavonols were shown to selectively inhibit CYP1B1 activity, such as chrysoeriol and isorhamnetin. The synthetic α-naphthoflavone is a strong inhibitor of CYP1B1 (IC50 = 5 nM) and CYP1A2 (IC50 = 6 nM), compared to CYP1A1 (IC50 = 60 nM) (T. Shimada, et al., 1998). More recently, a potent inhibitor of CYP1B1 (IC50 = 0.043 nM) was synthesized from α-naphthoflavone, and its water-soluble derivative can eliminate the resistance of docetaxel in MCF-7/1B1 cells (Cui, et al., 2015). Several flavonoids from St. John’s wort also show inhibitory activity on CYP1B1, including quercetin, rutin, apigenin, and amentoflavone (Chaudhary & Willett, 2006). Some CYP1B1 inhibitors, such as kaempferol and isorhamnetin, can also antagonize the expression of AHR (Rajaraman, et al., 2009), which may show synergetic inhibition on the expression of CYP1B1. Thus, the inhibitory activity of CYP1B1 in mouse studies is difficult to interpret for both CYP1B1 inhibitors and AHR antagonists. Interestingly, some anticancer agents widely used in clinical are competitive inhibitors of CYP1B1, such as flutamide (IC50 = 1.0 μM), paclitaxel (IC50 = 31.6 μM), mitoxantrone (IC50 = 11.6 μM), and docetaxel (IC50 = 28.0 μM) (Rochat, et al., 2001). CYP1B1 inhibitors can be used to dissect CYP1B1 function and might be considered as therapeutic agents for the treatment of certain diseases as noted below.
Table 1.
Inhibitors of CYP1B1
| Year | Compounds | References |
|---|---|---|
| 1997 | 1,2-,1,3- and 1,4-phenylenebis(methylene)selenocyanate | (T. Shimada, et al., 1997) |
| 1998 | α-naphthoflavone, acetylenes,2-ethynylpyrene, | (T. Shimada, et al., 1998) |
| 2000 | hesperetin, homoeriodictyol, acacetin, diosmetin | (Doostdar, et al., 2000) |
| 2000 | resveratrol | (T. K. Chang, et al., 2000) |
| 2000 | oltipraz | (Langouet, et al., 2000) |
| 2001 | 2,4,3′,5′-tetramethoxystilbene | (Chun, Kim, et al., 2001) |
| 2001 | hydroxystilbenes | (Chun, Ryu, et al., 2001) |
| 2001 | flutamide, pacilitaxel, mitoxantrone, docetaxel, tamoxifen, doxorubicin, daunomycin | (Rochat, et al., 2001) |
| 2002 | 2,3′,4,5′-Tetramethoxystilbene, trans-stilbene analogues | (Kim, et al., 2002) |
| 2002 | imperatorin, isopimpinellin | (Kleiner, et al., 2002) |
| 2002 | purpurin, alizarin | |
| 2006 | polycyclic aromatic hydrocarbons | (T. Shimada & Guengerich, 2006) |
| 2006 | pyricetin, apigenin, kaempferol, quercetin, amentoflavone, quercitrin, rutin | (Chaudhary & Willett, 2006) |
| 2007 | trans-resveratrol methyl ethers | (Mikstacka, et al., 2007) |
| 2007 | 3′,4′-dimethoxyflavone, 5,7,4′-trimethoxyflavone, curcumin7,4′-dimethoxyflavone, 7,3′-dimethoxyflavone, quercetin | (Walle & Walle, 2007) |
| 2008 | thiomethylstilbenes | (Mikstacka, et al., 2008) |
| 2009 | 2,2′,4,6′-Tetramethoxystilbene | (Chun, et al., 2009) |
| 2010 | methoxyflavonoids | (Takemura, et al., 2010) |
| 2010 | melatonin | (T. K. H. Chang, et al., 2010) |
| 2012 | 2,3,4-trimethoxy-4′-methylthio-trans-stilbene | (Mikstacka, et al., 2012) |
| 2013 | propargyloxyflavones 2-(4-(3′-fluoro-6,7,10-trimethoxy-α-naphthoflavonol)octyloxy) | (J. Liu, et al., 2013) |
| 2015 | -2-oxoethanaminium chloride | (Cui, et al., 2015) |
Figure 1.
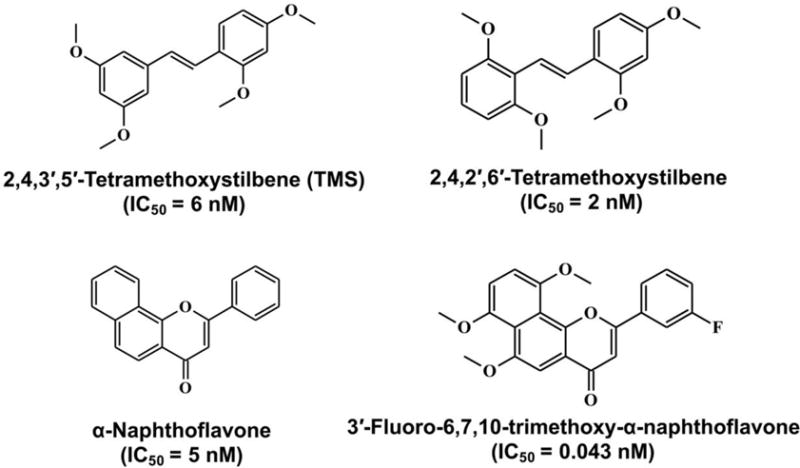
Highly potent and selective CYP1B1 inhibitors.
3. Transgenic mouse models to determine the biological functions of CYP1B1
In 1999, a Cyp1b1 knockout mouse line on the 129/sv background was generated to determine the role of CYP1B1 in metabolic activation of 7,12-dimethylbenz[a] anthracene (DMBA) (J. T. Buters, et al., 1999). It was noted that 70% of wild-type mice developed highly malignant lymphomas after administration with DMBA, whereas only 7.5% of Cyp1b1-null mice had lymphomas. Cyp1b1 disruption also reduced the tumorigenesis-induced by other procarcinogens, including benzo[a]pyrene (B[a]P) (Uno, et al., 2006) and dibenzo[a,l]pyrene (DB[a,l]P) (J. T. Buters, et al., 2002). These studies demonstrated that CYP1B1 plays a key role in the metabolic activation of environmental procarcinogens. The role of CYP1B1 in primary congenital glaucoma (PCG) was also determined using Cyp1b1-null mice on a mixed 129×1/SvJ × C57BL/6J background. The results showed that CYP1B1 deficiency damaged the ocular drainage structure and increased intraocular pressure (IOP), similar to the injury in human PCG patients (Libby, et al., 2003). Recently, Cyp1b1-null mice on a pure C57BL/6J background were used to determine the effects of CYP1B1 on hypertension and obesity. To better understand the biological functions of CYP1B1, CYP1B1-humanized mice were created through the insertion of the human CYP1B1 gene into the Cyp1b1-null mice genome. When fed with a high-fat diet (HFD), the weight gain in CYP1B1-humanized mice was similar to wild-type mice, which were higher than Cyp1b1-null mice (Fei Li, et al., 2014). CYP1B1-humanized showed a similar response to the HFD as the wild-type mouse. Therefore, transgenic CYP1B1 mice models are effective to define the biological effects of CYP1B1 on the development of metabolic diseases, endogenous metabolism and xenobiotic metabolism.
4. Metabolic pathways regulated by CYP1B1
4.1 Steroid hormone metabolism
Mouse models are effective to define the role of CYP1B1 in the development of metabolic diseases, endogenous metabolism and xenobiotic metabolism. Steroid hormones are the organic compound with four rings arranged in a specific configuration that can act as hormone. They can easily across cell membrane and bind to specific receptors forming hormone-receptor composite in cytoplasm. Steroid hormones are composed of five group based: glucocorticoid, mineralocorticoid, androgen, estrogen, and progestogen. Estrogen is the primary sex hormone in females, and is responsible for development and regulation of the female reproductive system. It is known that estrogen metabolism is associated with cancers and hypertension. Estrogen is converted to 17β-estradiol by 17β-hydroxylase. CYP1B1 carries out 4-hydroxylation of estradiol and exhibits the minor metabolic activity towards 2-hydroxylation (Figure 2) (Hayes, et al., 1996). The hydroxylated estradiol can be eliminated after further metabolism, such as methylation, glucuronidation or sulphonation; however, methylated metabolites of 4-OH-estradiol and 2-OH-estradiol also undergo demethylation by CYP1B1 (Dawling, et al., 2004). 4-OH-Estradiol and 2-OH-estradiol are further transformed to semiquinones and quinones, which can form DNA adducts with resulting in oncogenic mutations (Embrechts, et al., 2003; Markushin, et al., 2003). The quinones/semiquinones also undergo redox cycling and generate the reactive oxygen species (ROS), resulting in the oxidative damage (Parl, et al., 2009). Further examination revealed a role for CYP1B1 in estradiol carcinogenicity depended on the type of amino acids in the residue of Val395 in the human CYP1B1 gene (Nishida, et al., 2013). Therefore, the carcinogenesis induced by estrogen metabolites attributes to CYP1B1, the monitor of the level of 4-OH-estradiol and its DNA adduct could predict cancer risk.
Figure 2.
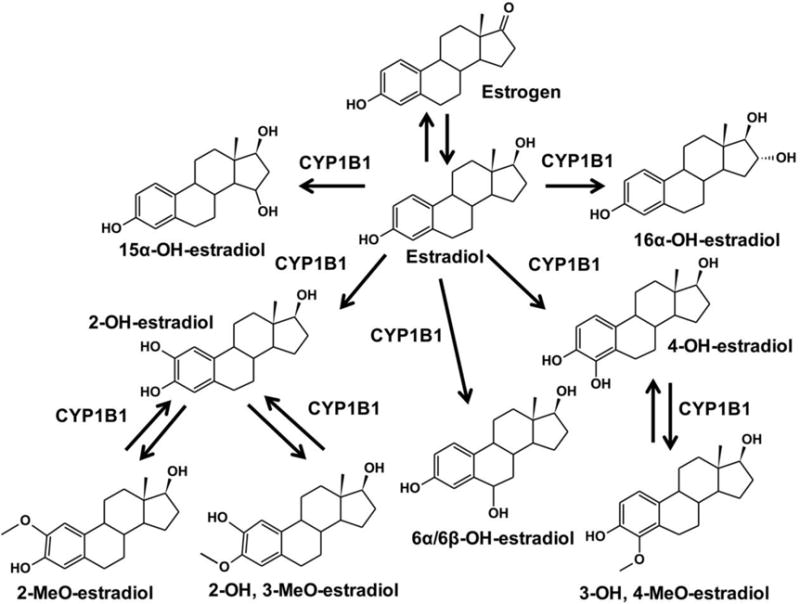
Metabolism of estrogen and estradiol. The Km values for 2- and 4-hydroxylation of estradiol are determined as 0.78 and 0.71 μM (Hayes, et al., 1996). The Vmax values for 2-, 4-, 6α, 6β-, 15α-, and 16α-hydroxylation of estradiol are 0.63, 1.14, 0.15, 0.08, 0.28 and 0.10 nmol/min/nmol P450 (Jansson, et al., 2001).
In addition, CYP1B1 is involved in the metabolism of testosterone and progesterone. Testosterone is a steroid hormone from the androgen group, which regulates hypothalamic-pituitary-adrenal (HPA) axis response under activation of the thromboxane A2 receptor (Ajayi, et al., 1995; Mehta, et al., 2008). Metabolomics revealed a link between HPA axis and PPARα in controlling energy homeostasis and immune system (Wang, et al., 2010). Progesterone is a progestogen hormone involved in the production of other endogenous steroids, including sex hormones and corticosteroids, and plays an important role in the central nervous system. By comparison of the metabolism of testosterone, progesterone, and estradiol, the B- and D-ring metabolites are produced from testosterone and progesterone by CYP1B1 (Figures 3 and 4), whereas the A-, B- and D-ring metabolites are transformed from estradiol (Jansson, et al., 2001). Compared to estrogen, testosterone and progesterone are the poor substrate for CYP1B1. Approximately 70–80% metabolites from CYP1B1 metabolism are A-ring hydroxylation metabolites. Other minor metabolites are 6α/β-, 15α- and 16α-hydroxy products of estrogen, testosterone or progesterone. Androgen signaling can be reduced via the increased CYP1B1 expression in a mouse model (Hwang, et al., 2003). Thus, CYP1B1 might be relevant to androgen-mediated cancers.
Figure 3.
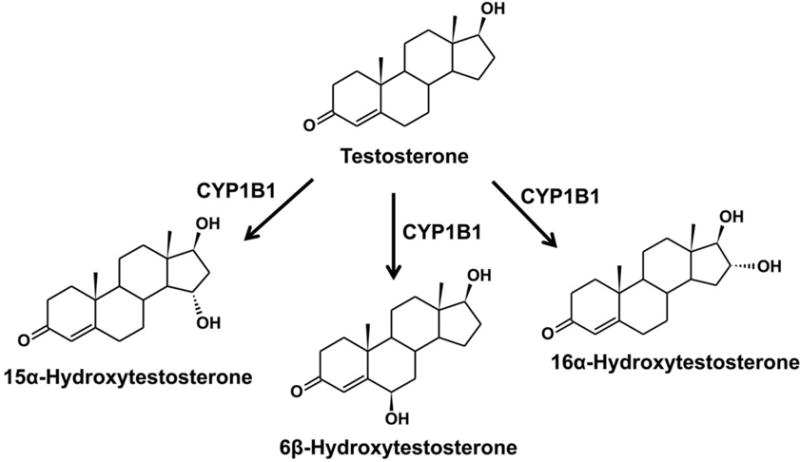
Metabolism of testosterone. The Vmax values for 6β-, 15α- and 16α-hydroxylation of testosterone are 0.15, 0.02 and 0.09 nmol/min/nmol P450 (Jansson, et al., 2001).
Figure 4.
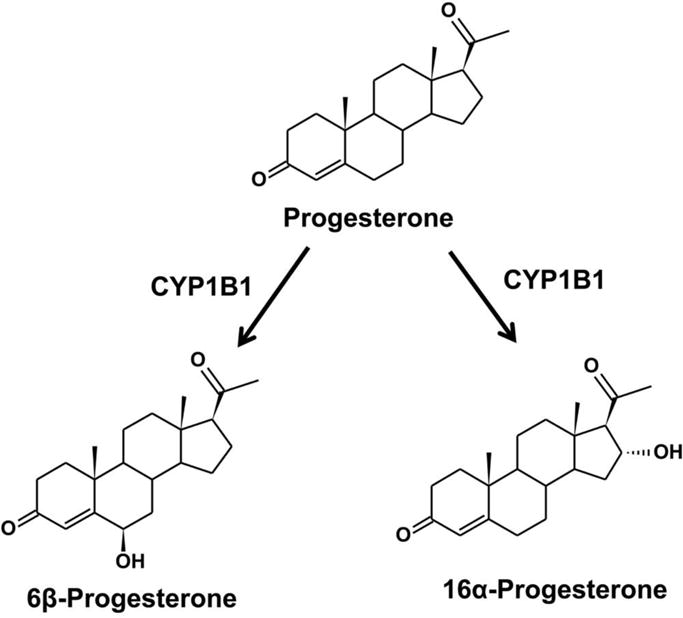
Metabolism of progesterone. The Vmax values for 6β- and 16α-hydroxylation of progesterone are 0.74 and 0.91 nmol/min/nmol P450 (Jansson, et al., 2001).
4.2 Fatty acid metabolism
Fatty acids are a family of non-polar molecules classified as the lipid class. The role of fatty acids is energy production in the form of adenosine triphosphate (ATP) synthesis and energy storage in the free and conjugated form, including triacylglycerol and phospholipid. It is known that many important diseases such as cancer and obesity are closely related to the disruption of fatty acid metabolism. Fatty acid metabolism is regulated by a set of nuclear receptors, including PPARα and PPARγ that play key roles in maintaining energy homeostasis. A recent study revealed that Cyp1b1 disruption suppressed the expression of PPARγ and PPARα target genes, including differentiation 36 (CD36), CYP4A14 and acyl-CoA thioesterase 1 (ACOT1) (Larsen, et al., 2015). The body weight gain of Cyp1b1-null mice is significantly lower than wild-type mice when fed a HFD (Fei Li, et al., 2014). The lipid synthesis including phospholipids and triglyceride were also decreased in Cyp1b1-null mice. These results indicate that CYP1B1 is an important modulator of fatty acids homeostasis.
Arachidonic acid (AA) is oxidized to prostaglandins, leukotrienes, and epoxyeicosatrienoic acids (EETs) by cyclooxygenase (COXs), lipoxygenase (LOs), and CYP (Capdevila & Falck, 2001; Roman, 2002). Expoxygenases and hydroxylases are two major pathways of AA regulated by CYPs, which transform AA to EETs and hydroxyeicosatetraenoic acids (HETEs) (Figure 5), respectively (Roman, 2002). The major metabolites of AA by human CYP1B1 are HETEs, including 20-HETE and 12-HETE (Choudhary, et al., 2004). The function of HETEs in cardiovascular has been summarized (Elbekai & El-Kadi, 2006; Roman, 2002). Specifically, 20-HETEs takes part in anti-platelet activity (Hill, et al., 1992), inhibition of renal Na+-K+-ATPase activity and sodium transport in proximal tubules (M. Schwartzman, et al., 1985), and regulation of the growth response in vascular smooth muscle cells (Muthalif, et al., 1998). 12-HETEs affects corneal susceptibility which has an influence on corneal clouding associated with glaucoma by regulating Na+-K+-ATPase activity (Jaime L Masferrer, et al., 1990; Stiemke, et al., 1991). One study showed that CYP1B1 preferentially produced midchain HETEs, whereas CYP1A1 showed a high specificity for ω-terminal region HETEs and CYP1A2 produced EETs (Choudhary, et al., 2004). In contrast to human CYP1B1, the major metabolites of arachidonic acid by mouse CYP1B1 were EETs. EETs were shown to have anti-inflammation (Node, et al., 1999), anti-apoptotic (Chen, et al., 2001), vasodilating (Pomposiello, et al., 2001), fibrinolytic (Node, et al., 2001) and anti-fibrotic (Levick, et al., 2007) functions. The differences in AA metabolism between human CYP1B1 and mouse CYP2B1 might be due to differences in catalytic efficiency of mouse CYP1B1 that is significantly lower than the human ortholog.
Figure 5.
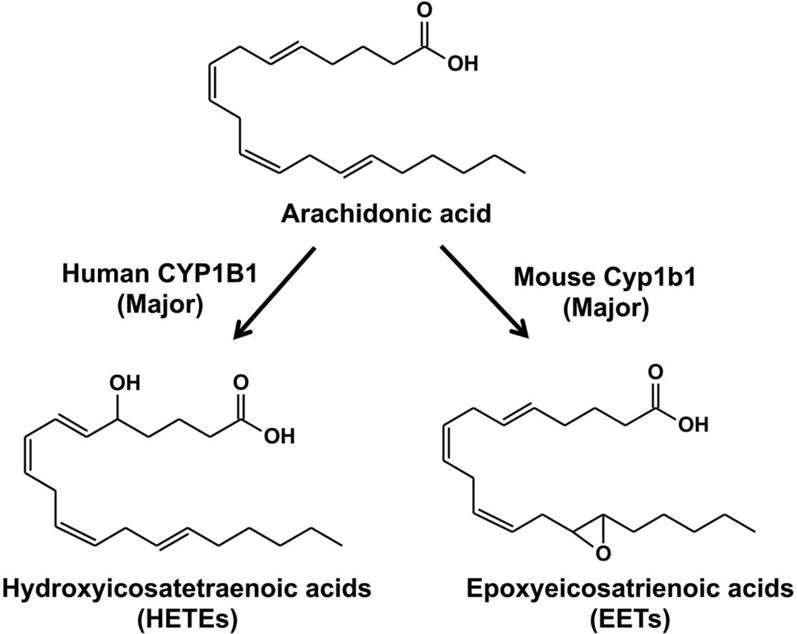
Metabolism of arachidonic acid. The CYP1B1 and Cyb1b1 Km values for the generation of HETEs and EETS are 29.8 and 500.0 μM, respectively (Choudhary, et al., 2004).
4.3 Vitamin Metabolism
Vitamin is a family of organic compounds with diverse biochemical functions essential normal physiological functions. CYP1B1 is involved in the metabolism of vitamin A and shows a synergistic effect with other vitamins. Vitamin A is important for the growth and development, and maintains the immune system and good vision. The major forms of vitamin A include retinol, retinal and retinoic acid. Studies revealed that CYP1B1 was able to catalyze the oxidative metabolism of retinol to retinal and transform retinal into retinoic acid (Figure 6) (Choudhary, et al., 2004; Q. Y. Zhang, et al., 2000). Further studies indicated that oxidation of retinol to retinoic acid was regulated by both human and mouse CYP1B1, but neither human or mouse CYP1B1 was found to oxidize retinoic acid. Compared to mouse CYP1B1, human CYP1B1 shows higher catalytic efficiency for the two step oxidation from retinol to retinoic acid (Choudhary, et al., 2004). Retinoic acid can trigger retinoid-mediated signaling pathways via binding to retinoic acid receptors (RAR) that has been considered as a therapeutic target in the treatment of dyslipidemia, atherosclerosis and cancer (Connolly, et al., 2013; Jakel, et al., 2006). More recently, metabolomics analysis reveals that α-tocopherol, a biologically active form of vitamin E, can be oxidized to several metabolites via β-oxidation and ω-oxidation by CYPs, however, the specific CYPs carrying out these reactions were not identified in this study (Johnson, et al., 2012). Additionally, a clinical study indicated that genetic variations in CYP1B1 and serum 25-hydroxyvitamin D levels showed synergistic effect on blood pressure (H. Y. Park, et al., 2015). These data suggest that CYP1B1 might be an important regulator for the diseases related to vitamin deficiency.
Figure 6.
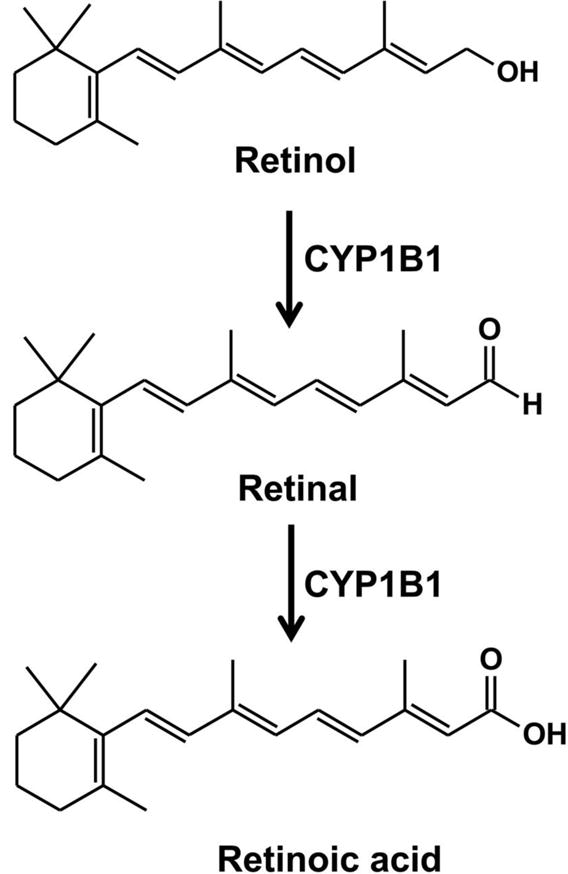
Metabolism of vitamin A. The Km values for retinol and retinal are 18.5 and 8.5 μM (Choudhary, et al., 2004).
4.4 Melatonin Metabolism
Melatonin is a functionally diverse and ubiquitously distributed methoxyindole molecular with activity from unicellular organisms to animals. Melatonin is produced from tryptophan and serotonin in the pineal gland and then released to the blood by the control of sympathetic nerve, and the circulating melatonin can then be absorbed into liver. There are three main metabolic pathways for melatonin, hydroxylation, O-demethylation and deacetylation (X. C. Ma, et al., 2005). 6-Hydroxylation of melatonin is catalyzed by hepatic CYPs, and its products are further sulfated and excreted in urine (Skene, et al., 2001). It was demonstrated using recombinant human CYPs that CYP1A1, CYP1A2, and CYP1B1 6-hydroxylate of melatonin (Figure 7) (X. C. Ma, et al., 2005; Tijmes, et al., 1996). 6-Hydroxylation of melatonin can be decreased in wild-type mouse brain homogenate by the CYP1B1 inhibitor TMS, and shows lower activity in Cyp1b1-null mouse brain homogenates compared to wild-type mice (X. C. Ma, et al., 2005). However, TMS treatment did not further reduced 6-hydroxylation of melatonin in Cyp1b1-null mouse brain homogenates, suggesting a role for CYP1B1 in the generation of 6-hydroxymelatonin. Metabolomics analysis indicated that 6-hydroxymelatonin, 6-hydroxymelatonin glucuronide, and 6-hydroxymelatonin sulfate can be detected in urine after intraperitoneal injection of melatonin (X. Ma, et al., 2008; X. Ma, et al., 2006). A recent study revealed that melatonin is an inhibitor of CYP1A1, CYP1A2 and CYP1B1, and can decrease the incidence of tumorigenesis caused by benzo[a]anthracene (T. K. H. Chang, et al., 2010). Clinical studies revealed that melatonin can lower the intraocular pressure of glaucoma patients (Samples, et al., 1988) and improve sleep disorders (Gringras, et al., 2012). Numerous studies revealed that melatonin metabolism affects the nervous system and vision (Hardeland, et al., 2015; Lundmark, et al., 2006). Thus, CYP1B1 may affect the nervous system and vision via its regulation on melatonin metabolism.
Figure 7.
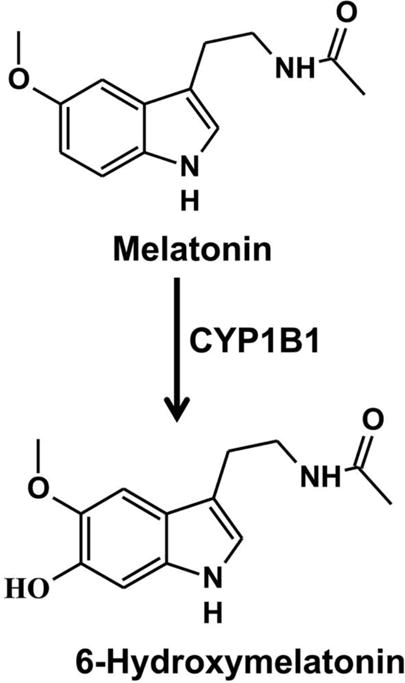
Metabolism of melatonin. The Km and Vmax values for 6-hydroxylation of melatonin are determined as 30.9 μM and 5.31 pmol/min/pmol P450 (X. C. Ma, et al., 2005).
5. Role of CYP1B1 in metabolic diseases
5.1 Inhibition of CYP1B1 prevents against obesity
Obesity, contributes to other metabolic diseases, particularly cardiovascular diseases, type-2 diabetes mellitus, non-alcoholic fatty liver and cancer. In 1998, it was first reported that the basal CYP1B1 protein expression was elevated by the differentiation of C3H10T1/2 cells (L. Zhang, et al., 1998). Subsequent studies indicated that CYP1B1 expression can be substantially induced following the stimulation of C3H10T1/2 cells by an adipogenic hormonal mixture consisting of insulin, dexamethasone, and methylisobutyl-xanthine (Cho, et al., 2005; Zheng, et al., 2013). The induction of PPARγ expression involves in adipogenesis was increased during the stimulation of C3H10T1/2 cells. Consistent with these observations, a review of 49 obesity-related genome-wide sequencing experiments covering 16186 genes indicated that CYP1B1, interleukin 1 receptor, type I (IL1R1), and adiponectin, C1Q and collagen domain containing (ADIPOQ) were three highest scoring genes associated with obesity (English & Butte, 2007), suggesting that CYP1B1 may have an important role in adipogenesis and obesity. In the mouse obesity model induced by HFD for 11-week, the body weight, epididymis fat pad weight, and fat content in liver were significantly lower in CYP1B1-null mice than wild-type mice (X. Liu, et al., 2012). Microarray analysis indicated that CYP1B1 disruption altered the expression of 560 genes in the liver, including suppression of PPARγ, stearoyl CoA desaturase 1 (Scd1) and many genes stimulated by PPARα which are associated with energy homeostasis (Larsen, et al., 2015). Among these genes, 17 genes changes in Cyp1b1-null mice contributed to the attenuation of diet-induced diabetes. A recent study indicated that CYP1B1 deficiency can attenuate HFD-induced obesity and improved glucose tolerance (X. Liu, et al., 2015). The expressions of PPARγ signaling, CD36, fatty acid synthase (FAS), and SCD1 involved in fatty acid transport and synthesis were decreased in the liver, and the genes uncoupling protein 2 (Ucp2) and carnitine palmitoyltransferase 1a (Cpt1a), responsible for fatty acid oxidation, were increased. However, knock down of CYP1B1 in C3H10T1/2 cells did not abolish adipogenesis induced by adipogenic agents (X. Liu, et al., 2015), suggesting that the mechanism of CYP1B1 regulating obesity needs further examination.
Metabolomics profiling revealed changes in endogenous metabolites associated with obesity. Examples include plasma lysophosphatidylcholines and phosphatidylcholines in adult obese men and children with obesity (Pietilainen, et al., 2007). A metabolomics study found lysophosphatidylcholine 18:0 (LPC 18:0) is a biomarker positively related to HFD-induced obesity in mice (Fei Li, et al., 2014). Consistent with LPC 18:0 changes, the hepatic expression of phosphatecytidylyltransferase 1β (PCYT1β) involved in lysophosphatidylcholine synthesis showed a similar trend between lean and obese mice. The increased serum LPC 18:0 in wild-type mice could be significantly reduced in Cyp1b1-null mice with the lean phenotype. Compared to wild-type mice, CYP1B1-humanized mice showed a similar level of LPC 18:0 as wild-type mice, suggesting human CYP1B1 and mouse Cyp1b1 showed the same response to the HFD challenge. Further studies indicated that hepatic SCD1 expression also contributed to the changes of lysophosphatidycholines. SCD1 catalyzes saturated fatty acids from the diet to monounsaturated fatty acids, which is a critical control point in the development of obesity and insulin resistance (Flowers & Ntambi, 2008). Taken together, these data demonstrate that CYP1B1 influences adipogenesis (Figure 8).
Figure 8.
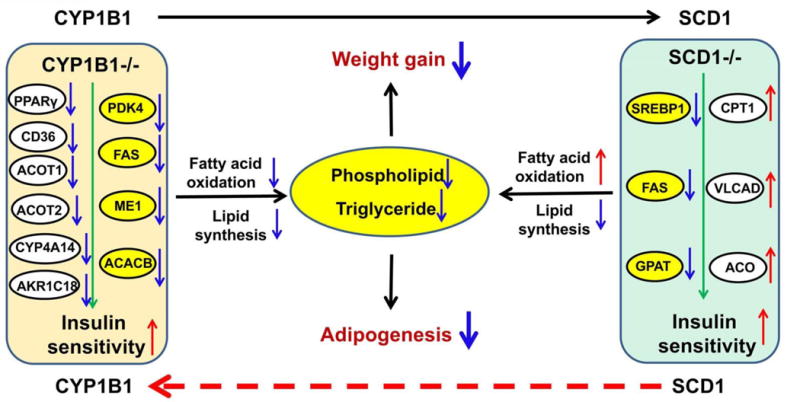
Inhibition of CYP1B1 prevents obesity. CYP1B1 deletion diminishes the level of SCD1 expression in the liver, but SCD1 expression level can be normalized in CYP1B1-humanized mice. Studies showed that the deficiency of both CYP1B1 and SCD1 can enhance insulin sensitivity and prevent obesity from HFD challenge. In Cyp1b1-null mice, the expression of lipid synthesis genes are down-regulated, including pyruvate dehydrogenase kinase, isozyme 4 (PDK4), fatty acid synthase (FAS), malic enzyme 1 (ME1), and acetyl-coenzyme A carboxylase beta (ACACB), and the target genes of PPARα involved in the fatty acid oxidation also are decreased, including CD36, ACOT1, ACOT2, CYP4A14, and aldo-keto reductase family 1, member C18 (AKR1C18) (Larsen, et al., 2015). These metabolic changes will cause the decreased generation of phospholipid and tryglyceride. Study in Scd1-null mice have shown the similar changes that the expression of the genes related to lipid synthesis can be decreased such as sterol regulatory element-binding protein 1 (SREBP1), FAS, and, glycerol phosphate acyl-CoA transferase (GPAT). Conversely, the expressions of gene involved in fatty acid oxidation are increased, including CPT1, very long chain acyl-CoA dehydrogenase (VLCAD), and acyl-CoA oxidase (ACO)(Ntambi, et al., 2002). The reduced adipogenesis finally leads to the decrease of weight gain.
5.2 Inhibition of CYP1B1 minimizes hypertension
Hypertension is the leading cause of cardiovascular diseases. CYP1B1 is mainly expressed in extra-hepatic tissues, including cardiovascular system (Korashy & El-Kadi, 2006). In blood vessels, CYP1B1 is mostly expressed in vessel smooth muscle cells (VSMCs) and slightly expressed in endothelial cells (Conway, et al., 2009). CYP1B1 mediates some important physiological processes in the blood vessel, notably the development and maintenance of hypertension. In angiotensin II (Ang II)-dependent hypertension, CYP1B1 is responsible for Ang II induced-vascular smooth muscle cell migration, proliferation and protein synthesis via the regulation of AA metabolism (Yaghini, et al., 2010). AA is metabolized by CYP1B1 to 12- and 20-hydroxyeicosatetraenoic acids, which generate reactive oxygen species (ROS) (Trevisi, et al., 2002). Inhibition of CYP1B1 by TMS can reduce ROS production and extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase activity, resulting in the decrease of hypertension (Yaghini, et al., 2010). A subsequent study found that the blood pressure increased by Ang II can be reduced in Cyp1b1-null mice compared to wild-type mice (Brett L. Jennings, et al., 2010). Consistent with this study, the modulation of CYP1B1 by 2-methoxyestradiol can protect against Ang II-induced hypertension and cardiovascular changes in female mice (B. L. Jennings, George, et al., 2014). 6β-Hydroxytestoterone produced by CYP1B1 can restore the increased systolic blood pressure and cardiac hypertrophy, and fibrosis induced by Ang II, whereas these pathological characteristics were minimized in Cyp1b1-null or castrated Cyp1b1-null mice (Pingili, et al., 2015). Similarly, CYP1B1 also mediates Ang II-independent hypertension by reducing ROS production and activity of ERK1/2 and p38 MAPK. TMS can normalize the blood pressure deoxycorticosterone acetate (DOCA)-salt-induced hypertension which elevates salt and water retention, and increases the levels of vasopressin, endothelin-1 and catecholamine (Sahan-Firat, et al., 2010). In a model of pulmonary arterial hypertension induced by dexfenfluramine, the expression of CYP1B1 was elevated in human pulmonary artery smooth muscle cells. Another study found that CYP1B1 was critical in the development of dexfenfluramine-induced pulmonary arterial hypertension, and this effect was related to the metabolism of estrogen (Dempsie, et al., 2013). Additionally, the aortic lesions, hypertension, and associated pathogenesis in Apoe−/−/Cyp1b1+/+ mice on an atherogenic diet were dependent on CYP1B1-generated oxidative stress (Song, et al., 2015). Therefore, CYP1B1 could serve as a novel target for the developing drugs to treat different types of hypertension.
5.3 Inhibition of CYP1B1 contributes to the decrease of tumorigenesis
Cancer is a group of deadly diseases involving abnormal cell growth that has the potential to spread to other parts of the body. Numerous metabolomics studies have shown that the changes in endogenous metabolites are found in the fluids and tissues of cancer patients. By comparison of patients with liver cirrhosis, acute myeloid leukemia and healthy individuals, it was noted that the levels of glycodeoxycholate, deoxycholate 3-sulfate, and bilirubin were increased in hepatocellular carcinoma, whereas the levels of lysophosphocholines and free fatty acids such as lignoceric acid and nervonic acid were reduced in hepatocellular carcinoma (Patterson, et al., 2011). Another metabolomics analysis showed that the changes in asymmetric dimethylarginine, hexanoylglycine, and nicotinamide 1-oxide in urine were associated with the tumor size and number after squamous cell carcinoma (SCCVII) tumor cells grown as xenographs in mice (F. Li, et al., 2013). It was shown that CYP1B1 expression is enhanced in tumors, including prostate cancer (Tokizane, et al., 2005), breast cancer (Larsen, et al., 1998) and colon cancer (Gibson, et al., 2003). Meta-analysis of clinical studies indicated that a CYP1B1 polymorphism was associated with a wide of variety of cancers, including lung cancer, breast cancer, and colon cancer (C. Li, et al., 2015). The detection of CYP1B1 expression might offer a novel method to diagnose renal cancer (M. C. E. McFadyen, et al., 2004). Some CYP1B1 inhibitors and monoclonal antibodies have been developed for the treatment of certain cancers which show highly expressed CYP1B1 (M. C. E. McFadyen, et al., 1999). Thus, CYP1B1 may be a potential therapeutic target of cancer.
Metabolism of both endobiotics and xenobiotics by CYP1B1 might be assocated with increased carcinogenesis. As noted above, hydroxylation of estradiol catalyzed by CYP1B1 can generate DNA adducts. Many environmental procarcinogens can be activated by CYP1B1, including polycyclic aromatic hydrocarbons (PAH), aromatic amines, and nitropolycyclic hydrocarbons (Tsutomu Shimada, et al., 1996). In mouse models, ovarian cancer and skin cancer are the main cancers induced by DB[a,l]P, at approximately 71% and 47%, respectively. DB[a,l]P can be oxidized to (−)-trans-11,12-dihydrodiol by CYP1B1, and this metabolite is subsequently converted to (−)-anti-DB[a,l]PDE by CYP1B1 (Luch, et al., 2000). Reaction of (−)-anti-DB[a,l]PDE with 2′-deixyadenosine (Ado) generated DNA adducts, which was detected in mouse skin cancer after treated with DB[a,l]P (Figure 9) (J. T. Buters, et al., 2002). The tumor rate of DB[a,l]P was 100% in wild-type mice, however, the tumor rate was decreased to 62% in Cyp1b1-null mice(J. T. Buters, et al., 2002). Also, the tumorigenesis of other procarcinogens such as 7,12-dimethylbenz[a]anthracene (DMBA) and benzo[a]pyrene (B[a]P) can be decreased in Cyp1b1-null mice (J. Buters, et al., 2003; Uno, et al., 2006), demonstrating a role for CYP1B1 in procarcinogen-induced tumor formation. Thus, inhibition of CYP1B1 activity contributes to the decrease in the cancer risk induced by environmental procarcinogens.
Figure 9.
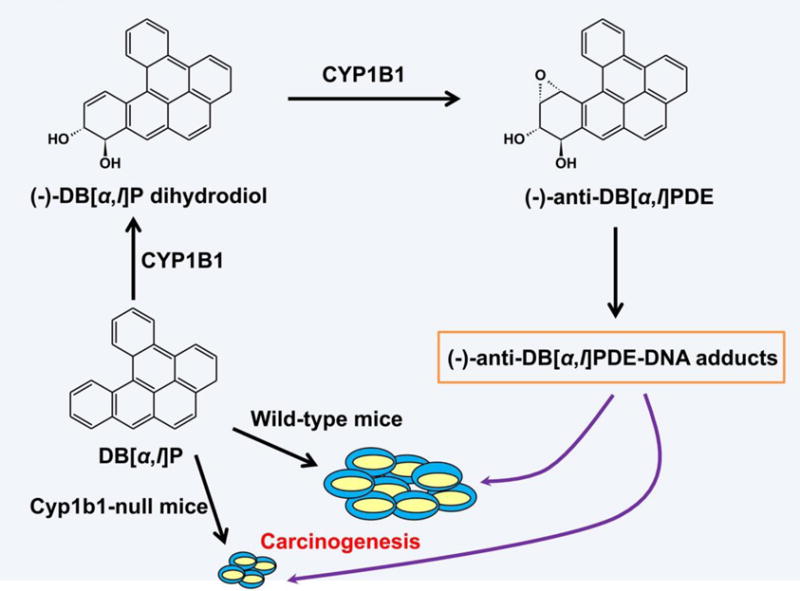
Metabolic activation of procarcinogens by CYP1B1 (Adapted from Reference 18). DB[a,l]P is activated by CYP1B1 to the formation of (−)-trans-11,12- dihydrodiol, which is subsequently converted by CYP1B1 to (−)-anti-DB[a,l]PDE. The reaction of (−)-anti-DB[a,l]PDE with 2′-deixyadenosine (Ado) generates (−)-anti-DB[a,l]PDE-DNA adduct that shows the activity of carcinogenesis. Cyp1b1 deletion can reduce the carcinogenesis of DB[a,l]P.
Overexpression of human CYP1B1 protein has been detected in a variety of tumors, but its level is lower or undetectable in normal tissues, indicating that CYP1B1 may be a target for cancer therapy. The anticancer agents, flutamide, pacilitaxel, mitoxantrone and docetaxel are competitive inhibitor of CYP1B1, and tamoxifen is a noncompetitive inhibitor (Rochat, et al., 2001). In the clinic, cancer patients frequently acquire the resistance after long-term use of the anticancer agents. One study showed that flutamide can be transformed into 2-hydroxylation flutamide by CYP1B1. However, 2-hydroxylation of flutamide did not show the anticancer effect (Rochat, et al., 2001). Interestingly, the anti-cancer drug flutamide was identified as an AHR agonist (Gao, et al., 2016), which may induce CYP1B1 expression, and the increased expression of CYP1B1 in certain tumors could decrease the tumor killing efficacy of flutamide. In another study, after long-term exposure to docetaxel, the cytotoxic effects of docetaxel was decreased in a hamster ovary cell line, however, the sensitivity of its cytotoxicity can be recovered by co-incubation with the CYP1B1 inhibitor α-naphthoflavone (M. C. McFadyen, et al., 2001). Therefore, co-administration of the anticancer agent and CYP1B1 inhibitors might decrease cancer resistance and enhance the outcome of anti-cancer therapy.
5.4 Glaucoma is associated with CYP1B1 mutation
Glaucoma is a leading cause of irreversible blindness. It is estimated that glaucoma will affect 80 million people by 2020 worldwide (Quigley & Broman, 2006). Glaucoma is caused by continuous or intermittent elevation of IOP and anterior segment dysgenesis (ASD). Generally, glaucoma can be classified into three categories, primary open-angle glaucoma (POAG), primary congenital glaucoma (PCG), and primary angle closure glaucoma (PACG). Genetic analysis of glaucoma patients has identified CYP1B1 as a causative gene for PCG, and as a modifier gene in POAG. Study reported that 147 distinct mutations of CYP1B1 were verified in glaucoma patients, including missense mutation, nonsense mutation, duplication mutation, insertion mutation and deletion mutation (N. Li, et al., 2011). The percentage of CYP1B1 mutations in patients is associated with their races. Generally, the incidence of African Americans is higher than Caucasians, and the incidence of Caucasians is higher than Asian (Bejjani, et al., 1998; Colomb, et al., 2003; Lim, et al., 2013; Mashima, et al., 2001; Melki, et al., 2004). In 2003, Cyp1b1-null mice were first used to determine the role of CYP1B1 in PCG. It was noted that Cyp1b1-null mice exhibit ocular drainage structure abnormalities, which resemble the eye injury in human PCG patients (Libby, et al., 2003). Most recently, morphometric and semiquantitative analysis revealed that Cyp1b1-null mice present a decreased amount of trabecular meshwork (TM) collagen, higher TM endothelial cell, and collagen lesion scores (P < 0.005) compared to the age-matched controls; the collagen loss and lesion scores were progressively increased in older animals (Teixeira, et al., 2015). Using the Cyp1b1-null mouse model, it was found that the tyrosinase gene (Tyr) was a modifier of the drainage structure phenotype, and the severe dysgenesis in eyes lacking both CYP1B1 and TYR can be alleviated by dihydroxyphenylalanine (L-dopa) (Libby, et al., 2003). Thus, selective upregulation of CYP1B1 expression in the eye could open new therapeutic avenues for the treatment of glaucoma.
It was proposed that several metabolic pathways regulated by CYP1B1 are relevant to the glaucoma. CYP1B1 is involved in the metabolism of vitamin A in which retinol is first oxidized to retinaldehyde, and then retinaldehyde is oxidized to retinoic acid. It is known that retinoid signaling regulates embryonic pattern formation during the development of eye, and vitamin A deficiency causes severe malformations of the developing eyes. Melatonin is metabolized primarily to 6-hydromelatonn by CYP1B1. Melatonin can be synthesized in the retina, lacrimal gland, lens, and ciliary body in the eye (Abe, et al., 1999; Mhatre, et al., 1988; Tosini & Menaker, 1998), and melatonin was detected in aqueous humor and the ciradia rhythm of the plasma during the night cycle (Yu, et al., 1990). It was reported that the acute suppression of melatonin output to light was markedly reduced when chick pineal cells were depleted of vitamin A. Addition of retinaldehyde specifically restored the acute response, suggesting that there is a link between CYP1B1-mediated retinal and melatonin metabolism (Zatz, 1994). However, the exact mechanism remains unclear. Melatonin modulates retinomotor movements, photoreceptors, dopamine synthesis and release, and IOP. 12-(R)-HETE formed from arachidonic acid by CYP1B1 is a potent inhibitor of Na+-K+-ATPase activity, which regulates corneal transparency and affects corneal susceptibility to pressure (Jaime L Masferrer, et al., 1990). Modulation of this ATPase activity further promotes the corneal clouding associated with glaucoma. Another study indicated that 12-(R)-HETE can lower IOP in rabbits (J. L. Masferrer, et al., 1990). A clinical study found that the levels of fasting serum glucose and uric acid were increased in glaucoma patients compared to the control population (Elisaf, et al., 2001). The incidences of diabetes mellitus, hyperlipidemia, diabetes, and hypertension in glaucoma patients were significantly higher than the control population (Chopra, et al., 2012). Lipidomics analysis revealed that a number of phospholipids were uniquely present in controls but absent in glaucomatous TM and vice versa (Aribindi, et al., 2013). These data indicated that a disorder in endogenous metabolism in the eye might be related to the development of glaucoma.
5.5 Inhibition of CYP1B1 improves renal dysfunction
CYP1B1 is expressed in both normal and neoplastic kidney, and oxidizes AA to generate EETs and HETEs (Choudhary, et al., 2004). It was reported that 19- and 20-HETE regulate the vasoconstrictor response of endothelin-1(Oyekan, et al., 1997) and Ang II (Carroll, et al., 1996) in kidney, causing a decrease in glomerular filtration rate. 20-HETE inhibits Na+ transport in the proximal tubule by blunting Na+-K+-ATPase, conversely, 19-HETE accelerates Na+ transport (M. L. Schwartzman, et al., 1985). EETs also regulate the glomerular filtration by stimulating Na+/H+ exchanger as well as natriuretic actions of Ang II in the proximal tubule (Harris, et al., 1990). Thus, the metabolites of AA produced by CYP1B1 may be harmful to the renal function. Consistent with these observations, the enhanced levels of 12- and 20-HETEs in the mice kidney by Ang II infusion cause renal dysfunction, including the increase of water consumption and urine output, decreased urine osmolality, increased urinary Na+ and K+ excretion, and proteinuria and albuminuria (B. L. Jennings, Anderson, et al., 2012). The renal dysfunction and reactive oxygen species induced by Ang II can be diminished by TMS treatment and in Cyp1b1-null mice (B. L. Jennings, Anderson, et al., 2012). The CYP1B1 deficiency also can minimize renal fibrosis, tubular damage, and inflammation after infusion with Ang II for 28 days. However, 50% of the 12-month old female Cyp1b1-null mice developed an unusual progressive glomerulonephritis while similar renal lesions were found in old male Cyp1b1-null mice later in life (Ward, et al., 2004). In another renal dysfunction model induced by DOCA, CYP1B1 deficiency resulted in decreased renal vascular resistance, renal infiltration of macrophages and T lymphocytes, and the production of reactive oxygen species (B. L. Jennings, Estes, et al., 2012). The increased activities of extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase, and cellular-Src were reduced in the kidney of Cyp1b1-null mice. Additionally, TMS improved the renal dysfunction and renal fibrosis in spontaneously hypertensive rats (SHR) (B. L. Jennings, Montanez, et al., 2014). Taken together, inhibition of CYP1B1 may be of value for the protection against renal damage induced by chemical or genetic mutation.
6. The potency of CYP1B1 inhibitors as clinical therapeutic agents
The fact that CYP1B1 modulates the metabolism of hormones, fatty acids, vitamin A and melatonin, suggest that CYP1B1 influences the development of metabolic diseases (Figure 10). Thus, CYP1B1 modulators could be considered as therapeutic agents to protect against metabolic diseases. TMS is a selective and potent CYP1B1 inhibitor and its effects on several metabolic diseases have been determined, including tumorigenesis, hypertension, atherosclerosis, and adipogenesis. TMS could decrease blood pressure in several hypertension models induced by chemicals and gene mutation. The blood pressure from Ang II- and DOCA-induced hypertension was normalized by TMS via the decrease of ROS production and extracellular signal-regulated kinase activity (Sahan-Firat, et al., 2010; Yaghini, et al., 2010). In ApoE−/−/Cyp1b1+/+ mice on an atherogenic diet, TMS minimized the blood pressure, plasma lipid levels, and ROS (Song, et al., 2015). In spontaneously hypertensive rats, TMS reversed the increased blood pressure, reduced the plasma levels of nitrite/nitrate, pro-inflammatory cytokines, and diminished ROS production (B. L. Jennings, Montanez, et al., 2014). More recently, one study indicated that TMS can suppress adipogenic differentiation of C3H10T1/2 cells by inhibiting PPARγ (Fan, et al., 2015), suggesting that TMS treatment might protect against the obesity. Since CYP1B1 is overexpressed in tumor cells, it may be a potential therapeutic target of cancer. However, its expression in many normal tissues, albeit at low levels suggest caution. However, the fact that Cyp1b1-null mice are normal, other than an increase in IOP suggest that inhibition of or CYP1B1 would not result in toxicities (Jeroen TM Buters, et al., 1999). An increasing number of CYP1B1 inhibitors have developed and patented (Cui, et al., 2015; Kumar & Gupta, 2016). In addition, ZYC300 is a CYP1B1-based vaccine which stimulates the immune system to elicit a cytotoxic T lymphocyte response against tumor cells expressing CYP1B1 (Maecker, et al., 2003). A phase I trial of ZYC300 was conducted in late stage cancer patients at Dana-Farber Cancer Institute at Boston, and the results indicated that the amount of vaccine dosed to patients was correlated with a decrease of cancer progression, and increased survival when an immune response was produced to CYP1B1 (M. C. McFadyen & Murray, 2005). Another phase I open-label study of the safety and feasibility of ZYC300 administration with cyclophosphamide pre-dosing (NCT00381173) was completed at Dana-Farber Cancer Institute and M. D. Anderson Cancer Center (M. C. McFadyen & Murray, 2005). A phase II clinical trial of ZYC300 is underway in recurrent glioblastoma multiforme patients (M. C. McFadyen & Murray, 2005). These results suggested that a CYP1B1 inhibitor could be a promising therapeutic agent for metabolic diseases.
Figure 10.
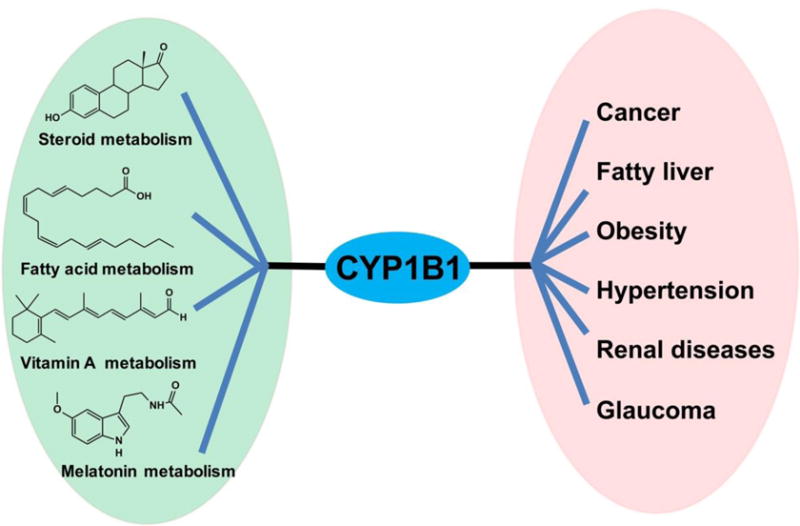
Biochemical links of CYP1B1 between metabolic pathways and metabolic diseases.
One potential application of CYP1B1 inhibitors is the treatment of anticancer drug resistance. An in vitro study showed that TMS inhibited the growth of tamoxifen-treated MCF-7 cells by 80% and fulvestrant-treated MCF-7 cells by 70% (H. Park, et al., 2007). A subsequent in vivo study found that 8 weeks of treatment with TMS reduced tumor volume of breast cancer from tamoxifen-resistant MCF-7 cells xenograft by 53%. Additionally, TMS also can inhibit tubulin polymerization, microtubule formation, the activated focal adhesion kinase, and mammalian target of rapamycin, and stimulate c-jun-NH2-kinase and p38 mitogen-activated protein kinase activity, suggesting that TMS is a promising therapeutic agent for hormone-resistance breast cancer. A recent study revealed that the CYP1B1 was overexpressed in primary and metastatic loci of epithelial ovarian cancers. Exposure to α-naphthoflavone, a specific CYP1B1 inhibitor, can reduce paclitaxel resistance and enhance the sensitivity of ovary cells to paclitaxel in vitro and in xenograft model of nude mice (Zhu, et al., 2015). In renal cell cancer (RCC), the loss of miR-200c upregulated the expression of CYP1B1, which might be the underlying mechanism for the resistance of RCC cells to docetaxel (I. Chang, et al., 2015). Consistent with this study, a new water-soluble α-naphthoflavone derivative which shows highly selective inhibitory activity toward CYP1B1 (IC50 = 0.043 nM) can eliminate the docetaxel-resistance caused by the enhanced CYP1B1expression in MCF-7/1B1 cells (Cui, et al., 2015). Taken together, these studies suggest that CYP1B1 inhibitors could overcome anticancer drug resistance.
7. Conclusion
CYP1B1 is one unique and important CYP. CYP1B1 catalyzes the metabolism of many xenobiotics and is involves in the metabolic activation of procarcinogens, the utilization of both CYP1B1 inhibitors and Cyp1b1-null mice has demonstrated that CYP1B1 modulates many metabolic pathways. The studies discussed in the current review suggest that CYP1B1 inhibitors are effective in the treatment of hypertension, obesity, and atherosclerosis. However, to our knowledge, the clinical use of CYP1B1 inhibitors has not been reported to date. In contrast to other CYPs, CYP1B1 is expressed in many extra-hepatic tissues and a variety of tumor tissues. Studies indicate that CYP1B1 inhibitors can decrease the carcinogenesis of procarcinogens, and minimize the resistance of some anticancer drugs. Thus, specific CYP1B1 inhibitors may be considered as a potential therapeutic strategy for the treatment of metabolic diseases.
Acknowledgments
This work was supported by the National Cancer Institute Intramural Research Program to F.J.G., and the National Natural Science Foundation of China (81360509) as well as the Thousand Young Talents Program of China to F.L.
Abbreviations
- AA
Arachidonic acid
- AHR
aryl hydrocarbon receptor
- Ang II
angiotensin II
- B[a]P
benzo[a]pyrene
- CYP
Cytochrome P450
- DB[a,l]P
dibenzo[a,l]pyrene
- EETs
epoxyeicosatrienoic acids
- ER
estrogen receptor
- HETE
hydroxyeicosatetraenoic acids
- HFD
high-fat diet
- IOP
intraocular pressure
- LPC
lysophosphatidylcholine
- PCG
primary congenital glaucoma
- POAG
primary open-angle glaucoma
- PPAR
peroxisome proliferator-activated receptor
- RAR
retinoic acid receptor
- RCC
renal cell cancer
- ROS
reactive oxygen species
- Scd1
stearoyl CoA desaturase 1
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TMS
2,4,3′,5′-Tetramethoxystilbene
- Tyr
tyrosinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abe M, Itoh MT, Miyata M, Ishikawa S, Sumi Y. Detection of melatonin, its precursors and related enzyme activities in rabbit lens. Exp Eye Res. 1999;68:255–262. doi: 10.1006/exer.1998.0601. [DOI] [PubMed] [Google Scholar]
- Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–2747. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- Aribindi K, Guerra Y, Lee RK, Bhattacharya SK. Comparative phospholipid profiles of control and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2013;54:3037–3044. doi: 10.1167/iovs.12-10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani BA, Lewis RA, Tomey KF, Anderson KL, Dueker DK, Jabak M, Astle WF, Otterud B, Leppert M, Lupski JR. Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. American Journal of Human Genetics. 1998;62:325–333. doi: 10.1086/301725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buters J, Quintanilla-Martinez L, Schober W, Soballa VJ, Hintermair J, Wolff T, Gonzalez FJ, Greim H. CYP1B1 determines susceptibility to low doses of 7,12-dimethylbenz[a]anthracene-induced ovarian cancers in mice: correlation of CYP1B1-mediated DNA adducts with carcinogenicity. Carcinogenesis. 2003;24:327–334. doi: 10.1093/carcin/24.2.327. [DOI] [PubMed] [Google Scholar]
- Buters JT, Mahadevan B, Quintanilla-Martinez L, Gonzalez FJ, Greim H, Baird WM, Luch A. Cytochrome P450 1B1 determines susceptibility to dibenzo[a,l]pyrene-induced tumor formation. Chem Res Toxicol. 2002;15:1127–1135. doi: 10.1021/tx020017q. [DOI] [PubMed] [Google Scholar]
- Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz [a] anthracene-induced lymphomas. Proceedings of the National Academy of Sciences. 1999;96:1977–1982. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci U S A. 1999;96:1977–1982. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR. The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochemical and Biophysical Research Communications. 2001;285:571–576. doi: 10.1006/bbrc.2001.5167. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Balazy M, Margiotta P, Huang DD, Falck JR, McGiff JC. Cytochrome P-450-dependent HETEs: Profile of biological activity and stimulation by vasoactive peptides. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1996;271:R863–R869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- Chang I, Mitsui Y, Fukuhara S, Gill A, Wong DK, Yamamura S, Shahryari V, Tabatabai ZL, Dahiya R, Shin DM, Tanaka Y. Loss of miR-200c up-regulates CYP1B1 and confers docetaxel resistance in renal cell carcinoma. Oncotarget. 2015;6:7774–7787. doi: 10.18632/oncotarget.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TK, Lee WB, Ko HH. Trans-resveratrol modulates the catalytic activity and mRNA expression of the procarcinogen-activating human cytochrome P450 1B1. Can J Physiol Pharmacol. 2000;78:874–881. [PubMed] [Google Scholar]
- Chang TKH, Chen J, Yang G, Yeung EYH. Inhibition of procarcinogen-bioactivating human CYP1A1, CYP1A2 and CYP1B1 enzymes by melatonin. Journal of Pineal Research. 2010;48:55–64. doi: 10.1111/j.1600-079X.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary A, Willett KL. Inhibition of human cytochrome CYP1 enzymes by flavonoids of St. John’s wort. Toxicology. 2006;217:194–205. doi: 10.1016/j.tox.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Chen JK, Capdevila J, Harris RC. Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Molecular and Cellular Biology. 2001;21:6322–6331. doi: 10.1128/MCB.21.18.6322-6331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YC, Zheng WC, Yamamoto M, Liu XQ, Hanlon PR, Jefcoate CR. Differentiation of pluripotent C3H10T1/2 cells rapidly elevates CYP1B1 through a novel process that overcomes a loss of ah receptor. Archives of Biochemistry and Biophysics. 2005;439:139–153. doi: 10.1016/j.abb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Chopra R, Chander A, Jacob JJ. Ocular associations of metabolic syndrome. Indian J Endocrinol Metab. 2012;16(Suppl 1):S6–S11. doi: 10.4103/2230-8210.94244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug metabolism and disposition. 2004;32:840–847. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- Chun YJ, Kim S, Kim D, Lee SK, Guengerich FP. A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res. 2001;61:8164–8170. [PubMed] [Google Scholar]
- Chun YJ, Oh YK, Kim BJ, Kim D, Kim SS, Choi HK, Kim MY. Potent inhibition of human cytochrome P450 1B1 by tetramethoxystilbene. Toxicol Lett. 2009;189:84–89. doi: 10.1016/j.toxlet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Chun YJ, Ryu SY, Jeong TC, Kim MY. Mechanism-based inhibition of human cytochrome P450 1A1 by rhapontigenin. Drug Metab Dispos. 2001;29:389–393. [PubMed] [Google Scholar]
- Colomb E, Kaplan J, Garchon HJ. Novel Cytochrome P450 1B1 (CYP1B1) Mutations in Patients with Primary Congenital Glaucoma in France. Human Mutation. 2003;22 doi: 10.1002/humu.9197. [DOI] [PubMed] [Google Scholar]
- Connolly RM, Nguyen NK, Sukumar S. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin Cancer Res. 2013;19:1651–1659. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovascular Research. 2009;81:669–677. doi: 10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Meng Q, Zhang X, Cui Q, Zhou W, Li S. Design and Synthesis of New alpha-Naphthoflavones as Cytochrome P450 (CYP) 1B1 Inhibitors To Overcome Docetaxel-Resistance Associated with CYP1B1 Overexpression. Journal of Medicinal Chemistry. 2015;58:3534–3547. doi: 10.1021/acs.jmedchem.5b00265. [DOI] [PubMed] [Google Scholar]
- Dawling S, Hachey DL, Roodi N, Parl FF. In vitro model of mammary estrogen metabolism: structural and kinetic differences between catechol estrogens 2- and 4-hydroxyestradiol. Chem Res Toxicol. 2004;17:1258–1264. doi: 10.1021/tx0498657. [DOI] [PubMed] [Google Scholar]
- Dempsie Y, MacRitchie NA, White K, Morecroft I, Wright AF, Nilsen M, Loughlin L, Mair KM, MacLean MR. Dexfenfluramine and the oestrogen-metabolizing enzyme CYP1B1 in the development of pulmonary arterial hypertension. Cardiovascular Research. 2013;99:24–34. doi: 10.1093/cvr/cvt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doostdar H, Burke MD, Mayer RT. Bioflavonoids: selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology. 2000;144:31–38. doi: 10.1016/s0300-483x(99)00215-2. [DOI] [PubMed] [Google Scholar]
- Dragin N, Shi Z, Madan R, Karp CL, Sartor MA, Chen C, Gonzalez FJ, Nebert DW. Phenotype of the Cyp1a1/1a2/1b1−/− triple-knockout mouse. Mol Pharmacol. 2008;73:1844–1856. doi: 10.1124/mol.108.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbekai RH, El-Kadi AO. Cytochrome P450 enzymes: central players in cardiovascular health and disease. Pharmacology and Therapeutics. 2006;112:564–587. doi: 10.1016/j.pharmthera.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Elisaf M, Kitsos G, Bairaktari E, Kalaitzidis R, Kalogeropoulos C, Psilas K. Metabolic abnormalities in patients with primary open-angle glaucoma. Acta Ophthalmol Scand. 2001;79:129–132. doi: 10.1034/j.1600-0420.2001.079002129.x. [DOI] [PubMed] [Google Scholar]
- Embrechts J, Lemiere F, Van Dongen W, Esmans EL, Buytaert P, Van Marck E, Kockx M, Makar A. Detection of estrogen DNA-adducts in human breast tumor tissue and healthy tissue by combined nano LC-nano ES tandem mass spectrometry. J Am Soc Mass Spectrom. 2003;14:482–491. doi: 10.1016/S1044-0305(03)00130-2. [DOI] [PubMed] [Google Scholar]
- English SB, Butte AJ. Evaluation and integration of 49 genome-wide experiments and the prediction of previously unknown obesity-related genes. Bioinformatics. 2007;23:2910–2917. doi: 10.1093/bioinformatics/btm483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CF, Zhu AN, Huang TT, Li L, Wang SQ. Tetramethoxystilbene, a selective CYP1B1 inhibitor, suppresses adipogenesis of C3H10T1/2 pluripotent stem cells. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:72–76. [PubMed] [Google Scholar]
- Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XX, Xie C, Wang YY, Luo YH, Yagai T, Sun DX, Qin XM, Krausz KW, Gonzalez FJ. The antiandrogen flutamide is a novel aryl hydrocarbon receptor ligand that disrupts bile acid homeostasis in mice through induction of Abcc4. Biochemical Pharmacology. 2016;119:93–104. doi: 10.1016/j.bcp.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P, Gill JH, Khan PA, Seargent JM, Martin SW, Batman PA, Griffith J, Bradley C, Double JA, Bibby MC, Loadman PM. Cytochrome P4501B1 (CYP1B1) is overexpressed in human colon adenocarcinomas relative to normal colon: Implications for drug development. Molecular Cancer Therapeutics. 2003;2:527–534. [PubMed] [Google Scholar]
- Gringras P, Gamble C, Jones AP, Wiggs L, Williamson PR, Sutcliffe A, Montgomery P, Whitehouse WP, Choonara I, Allport T, Edmond A, Appleton R, M.S. Group Melatonin for sleep problems in children with neurodevelopmental disorders: randomised double masked placebo controlled trial. BMJ. 2012;345:e6664. doi: 10.1136/bmj.e6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O. The role of AHR-inducible cytochrome P450s in metabolism of polyunsaturated fatty acids. Drug Metabolism Reviews. 2016;48:342–350. doi: 10.1080/03602532.2016.1197240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR. Melatonin and brain inflammaging. Progress in Neurobiology. 2015;127–128:46–63. doi: 10.1016/j.pneurobio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Harris RC, Homma T, Jacobson HR, Capdevila J. EPOXYEICOSATRIENOIC ACIDS ACTIVATE NA+/H+ EXCHANGE AND ARE MITOGENIC IN CULTURED RAT GLOMERULAR MESANGIAL CELLS. Journal of Cellular Physiology. 1990;144:429–437. doi: 10.1002/jcp.1041440310. [DOI] [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Fitzpatrick F, Murphy RC. Biological activity and metabolism of 20- hydroxyeicosatetraenoic acid in the human platelet. British Journal of Pharmacology. 1992;106:267–274. doi: 10.1111/j.1476-5381.1992.tb14327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Cho JS, Chae KR, Kang TS, Hwang JH, Lim CH, Lee SH, Lim HJ, Min SH, Sheen YY, Jang IS, Kim YK. Differential expression of the tetracycline-controlled transactivator-driven human CYP1B1 gene in double-transgenic mice is due to androgens: application for detecting androgens and antiandrogens. Arch Biochem Biophys. 2003;415:137–145. doi: 10.1016/s0003-9861(03)00218-2. [DOI] [PubMed] [Google Scholar]
- Jakel H, Fruchart-Najib J, Fruchart JC. Retinoic acid receptor-related orphan receptor alpha as a therapeutic target in the treatment of dyslipidemia and atherosclerosis. Drug News Perspect. 2006;19:91–97. doi: 10.1358/dnp.2006.19.2.977445. [DOI] [PubMed] [Google Scholar]
- Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Effect of two mutations of human CYP1B1, G61E and R469W, on stability and endogenous steroid substrate metabolism. Pharmacogenetics and Genomics. 2001;11:793–801. doi: 10.1097/00008571-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Jennings BL, Anderson LJ, Estes AM, Yaghini FA, Fang XR, Porter J, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 Contributes to Renal Dysfunction and Damage Caused by Angiotensin II in Mice. Hypertension. 2012;59:348–354. doi: 10.1161/HYPERTENSIONAHA.111.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BL, Estes AM, Anderson LJ, Fang XR, Yaghini FA, Fan Z, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 gene disruption minimizes deoxycorticosterone acetate-salt-induced hypertension and associated cardiac dysfunction and renal damage in mice. Hypertension. 2012;60:1510–1516. doi: 10.1161/HYPERTENSIONAHA.112.202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BL, George LW, Pingili AK, Khan NS, Estes AM, Fang XR, Gonzalez FJ, Malik KU. Estrogen metabolism by cytochrome P450 1B1 modulates the hypertensive effect of angiotensin II in female mice. Hypertension. 2014;64:134–140. doi: 10.1161/HYPERTENSIONAHA.114.03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BL, Montanez DE, May ME, Jr, Estes AM, Fang XR, Yaghini FA, Kanu A, Malik KU. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc Drugs Ther. 2014;28:145–161. doi: 10.1007/s10557-014-6510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, Fang XR, Gonzalez FJ, Malik KU. Cytochrome P450 1B1 Contributes to Angiotensin II-Induced Hypertension and Associated Pathophysiology. Hypertension. 2010;56:667–U204. doi: 10.1161/HYPERTENSIONAHA.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Slanar O, Krausz KW, Kang DW, Patterson AD, Kim JH, Luecke H, Gonzalez FJ, Idle JR. Novel metabolites and roles for alpha-tocopherol in humans and mice discovered by mass spectrometry-based metabolomics. Am J Clin Nutr. 2012;96:818–830. doi: 10.3945/ajcn.112.042929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ko H, Park JE, Jung S, Lee SK, Chun YJ. Design, synthesis, and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem. 2002;45:160–164. doi: 10.1021/jm010298j. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Vulimiri SV, Reed MJ, Uberecken A, DiGiovanni J. Role of cytochrome P450 1a1 and 1b1 in the metabolic activation of 7,12-dimethylbenz[a]anthracene and the effects of naturally occurring furanocoumarins on skin tumor initiation. Chem Res Toxicol. 2002;15:226–235. doi: 10.1021/tx010151v. [DOI] [PubMed] [Google Scholar]
- Korashy HM, El-Kadi AOS. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metabolism Reviews. 2006;38:411–450. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gupta D. Identification of CYP1B1-specific candidate inhibitors using combination of in silico screening, integrated knowledge-based filtering, and molecular dynamics simulations. Chemical Biology & Drug Design. 2016;88:730–739. doi: 10.1111/cbdd.12803. [DOI] [PubMed] [Google Scholar]
- Langouet S, Furge LL, Kerriguy N, Nakamura K, Guillouzo A, Guengerich FP. Inhibition of human cytochrome P450 enzymes by 1,2-dithiole-3-thione, oltipraz and its derivatives, and sulforaphane. Chem Res Toxicol. 2000;13:245–252. doi: 10.1021/tx990189w. [DOI] [PubMed] [Google Scholar]
- Larsen MC, Angus WGR, Brake PB, Eltom SE, Sukow KA, Jefcoate CR. Characterization of CYP1B1 and CYP1A1 expression in human mammary epithelial cells: Role of the aryl hydrocarbon receptor in polycyclic aromatic hydrocarbon metabolism. Cancer Research. 1998;58:2366–2374. [PubMed] [Google Scholar]
- Larsen MC, Bushkofsky JR, Gorman T, Adhami V, Mukhtar H, Wang S, Reeder SB, Sheibani N, Jefcoate CR. Cytochrome P450 1B1: An unexpected modulator of liver fatty acid homeostasis. Arch Biochem Biophys. 2015;571:21–39. doi: 10.1016/j.abb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick SP, Loch DC, Taylor SM, Janicki JS. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. The Journal of Immunology. 2007;178:641–646. doi: 10.4049/jimmunol.178.2.641. [DOI] [PubMed] [Google Scholar]
- Li C, Long B, Qin X, Li W, Zhou Y. Cytochrome P1B1 (CYP1B1) polymorphisms and cancer risk: a meta-analysis of 52 studies. Toxicology. 2015;327:77–86. doi: 10.1016/j.tox.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Li F, Jiang C, Larsen MC, Bushkofsky J, Krausz KW, Wang T, Jefcoate CR, Gonzalez FJ. Lipidomics reveals a link between cyp1b1 and scd1 in promoting obesity. Journal of Proteome Research. 2014;13:2679–2687. doi: 10.1021/pr500145n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Patterson AD, Krausz KW, Jiang C, Bi H, Sowers AL, Cook JA, Mitchell JB, Gonzalez FJ. Metabolomics reveals that tumor xenografts induce liver dysfunction. Mol Cell Proteomics. 2013;12:2126–2135. doi: 10.1074/mcp.M113.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhou Y, Du L, Wei M, Chen X. Overview of Cytochrome P450 1B1 gene mutations in patients with primary congenital glaucoma. Experimental Eye Research. 2011;93:572–579. doi: 10.1016/j.exer.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Libby RT, Smith RS, Savinova OV, Zabaleta A, Martin JE, Gonzalez FJ, John SWM. Modification of ocular defects in mouse developmental glaucoma models by tyrosinase. Science. 2003;299:1578–1581. doi: 10.1126/science.1080095. [DOI] [PubMed] [Google Scholar]
- Lim S-H, Khanh-Nhat T-V, Yanovitch TL, Freedman SF, Klemm T, Call W, Powell C, Ravichandran A, Metlapally R, Nading EB, Rozen S, Young TL. CYP1B1, MYOC, and LTBP2 Mutations in Primary Congenital Glaucoma Patients in the United States. American Journal of Ophthalmology. 2013;155:508–517. doi: 10.1016/j.ajo.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Taylor SF, Dupart PS, Arnold CL, Sridhar J, Jiang Q, Wang Y, Skripnikova EV, Zhao M, Foroozesh M. Pyranoflavones: a group of small-molecule probes for exploring the active site cavities of cytochrome P450 enzymes 1A1, 1A2, and 1B1. J Med Chem. 2013;56:4082–4092. doi: 10.1021/jm4003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang T, Li L, Tang Y, Tian Y, Wang S, Fan C. CYP1B1 deficiency ameliorates obesity and glucose intolerance induced by high fat diet in adult C57BL/6J mice. Am J Transl Res. 2015;7:761–771. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao l, Feng Q, Colin JR, Wang S. Role of CYP1B1 in hepatic lipid metabolism of adult mice and its possible mechanism. Acta Nutrimenta Sinica. 2012;2:143–146. [Google Scholar]
- Luch A, Schober W, Greim H, Doehmer J, Jacob J, Seidel A, Baird WM. Metabolic activation of dibenzo[a,l]-pyrene by cytochrome P450 enzymes to stable DNA adducts occurs exclusively through the formation of the (−)-trans-(11R,-12R)-diol. Polycyclic Aromatic Compounds. 2000;21:87–98. [Google Scholar]
- Lundmark PO, Pandi-Perumal SR, Srinivasan V, Cardinali DP. Role of melatonin in the eye and ocular dysfunctions. Visual Neuroscience. 2006;23:853–862. doi: 10.1017/S0952523806230189. [DOI] [PubMed] [Google Scholar]
- Ma X, Chen C, Krausz KW, Idle JR, Gonzalez FJ. A metabolomic perspective of melatonin metabolism in the mouse. Endocrinology. 2008;149:1869–1879. doi: 10.1210/en.2007-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Idle JR, Krausz KW, Tan DX, Ceraulo L, Gonzalez FJ. Urinary metabolites and antioxidant products of exogenous melatonin in the mouse. J Pineal Res. 2006;40:343–349. doi: 10.1111/j.1600-079X.2006.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XC, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes P450. Drug metabolism and disposition. 2005;33:489–494. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- Maecker B, Sherr DH, Vonderheide RH, von Bergwelt-Baildon MS, Hirano N, Anderson KS, Xia Z, Butler MO, Wucherpfennig KW, O’Hara C, Cole G, Kwak SS, Ramstedt U, Tomlinson AJ, Chicz RM, Nadler LM, Schultze JL. The shared tumor-associated antigen cytochrome P450 1B1 is recognized by specific cytotoxic T cells. Blood. 2003;102:3287–3294. doi: 10.1182/blood-2003-05-1374. [DOI] [PubMed] [Google Scholar]
- Markushin Y, Zhong W, Cavalieri EL, Rogan EG, Small GJ, Yeung ES, Jankowiak R. Spectral characterization of catechol estrogen quinone (CEQ)-derived DNA adducts and their identification in human breast tissue extract. Chem Res Toxicol. 2003;16:1107–1117. doi: 10.1021/tx0340854. [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Dunn MW, Schwartzman ML. 12(R)-hydroxyeicosatetraenoic acid, an endogenous corneal arachidonate metabolite, lowers intraocular pressure in rabbits. Invest Ophthalmol Vis Sci. 1990;31:535–539. [PubMed] [Google Scholar]
- Masferrer JL, Rios AP, Schwartzman ML. Inhibition of renal, cardiac and corneal (Na+− K+ ATPase by 12 (R)-hydroxyeicosatetraenoic acid. Biochemical Pharmacology. 1990;39:1971–1974. doi: 10.1016/0006-2952(90)90617-t. [DOI] [PubMed] [Google Scholar]
- Mashima Y, Suzuki Y, Sergeev Y, Ohtake Y, Tanino T, Kimura I, Miyata H, Aihara M, Tanihara H, Inatani M, Azuma N, Iwata T, Araie A. Novel cytochrome P4501B1 (CYP1B1) gene mutations in Japanese patients with primary congenital glaucoma. Investigative Ophthalmology and Visual Science. 2001;42:2211–2216. [PubMed] [Google Scholar]
- McFadyen MC, McLeod HL, Jackson FC, Melvin WT, Doehmer J, Murray GI. Cytochrome P450 CYP1B1 protein expression: a novel mechanism of anticancer drug resistance. Biochemical Pharmacology. 2001;62:207–212. doi: 10.1016/s0006-2952(01)00643-8. [DOI] [PubMed] [Google Scholar]
- McFadyen MC, Murray GI. Cytochrome P450: a novel anticancer therapeutic agent. Future Oncology. 2005;1:259–263. doi: 10.1517/14796694.1.2.259. [DOI] [PubMed] [Google Scholar]
- McFadyen MCE, Breeman S, Payne S, Stirk C, Miller ID, Melvin WT, Murray GI. Immunohistochemical localization of cytochrome P450CYP1B1 in breast cancer with monoclonal antibodies specific for CYP1B1. Journal of Histochemistry and Cytochemistry. 1999;47:1457–1464. doi: 10.1177/002215549904701111. [DOI] [PubMed] [Google Scholar]
- McFadyen MCE, Melvin WT, Murray GI. Cytochrome P450 CYP1B1 activity in renal cell carcinoma. British journal of cancer. 2004;91:966–971. doi: 10.1038/sj.bjc.6602053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PH, Jones AC, Josephs RA. The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat. J Pers Soc Psychol. 2008;94:1078–1093. doi: 10.1037/0022-3514.94.6.1078. [DOI] [PubMed] [Google Scholar]
- Melki R, Colomb E, Lefort N, Brezin AP, Garchon HJ. CYP1B1 mutations in French patients with early-onset primary open-angle glaucoma. Journal of Medical Genetics. 2004;41:647–651. doi: 10.1136/jmg.2004.020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre MC, van Jaarsveld AS, Reiter RJ. Melatonin in the lacrimal gland: first demonstration and experimental manipulation. Biochem Biophys Res Commun. 1988;153:1186–1192. doi: 10.1016/s0006-291x(88)81353-6. [DOI] [PubMed] [Google Scholar]
- Mikstacka R, Baer-Dubowska W, Wieczorek M, Sobiak S. Thiomethylstilbenes as inhibitors of CYP1A1, CYP1A2 and CYP1B1 activities. Mol Nutr Food Res. 2008;521(Suppl):S77–83. doi: 10.1002/mnfr.200700202. [DOI] [PubMed] [Google Scholar]
- Mikstacka R, Przybylska D, Rimando AM, Baer-Dubowska W. Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers. Mol Nutr Food Res. 2007;51:517–524. doi: 10.1002/mnfr.200600135. [DOI] [PubMed] [Google Scholar]
- Mikstacka R, Rimando AM, Dutkiewicz Z, Stefanski T, Sobiak S. Design, synthesis and evaluation of the inhibitory selectivity of novel trans-resveratrol analogues on human recombinant CYP1A1, CYP1A2 and CYP1B1. Bioorg Med Chem. 2012;20:5117–5126. doi: 10.1016/j.bmc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annual Review of Pharmacology and Toxicology. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- Murray GI, Taylor MC, McFadyen MC, McKay JA, Greenlee WF, Burke MD, Melvin WT. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Research. 1997;57:3026–3031. [PubMed] [Google Scholar]
- Muthalif M, Benter I, Karzoun N, Fatima S, Harper J, Uddin M, Malik K. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proceedings of the National Academy of Sciences. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida CR, Everett S, Ortiz de Montellano PR. Specificity determinants of CYP1B1 estradiol hydroxylation. Mol Pharmacol. 2013;84:451–458. doi: 10.1124/mol.113.087700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Gαs mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. Journal of Biological Chemistry. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyekan A, Balazy M, McGiff JC. Renal oxygenases: Differential contribution to vasoconstriction induced by ET-1 and ANG II. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1997;273:R293–R300. doi: 10.1152/ajpregu.1997.273.1.R293. [DOI] [PubMed] [Google Scholar]
- Park H, Aiyar SE, Fan P, Wang J, Yue W, Okouneva T, Cox C, Jordan MA, Demers L, Cho H, Kim S, Song RX, Santen RJ. Effects of tetramethoxystilbene on hormone-resistant breast cancer cells: biological and biochemical mechanisms of action. Cancer Res. 2007;67:5717–5726. doi: 10.1158/0008-5472.CAN-07-0056. [DOI] [PubMed] [Google Scholar]
- Park HY, Kim JH, Bae S, Choi YY, Park JY, Hong YC. Interaction effect of serum 25-hydroxyvitamin D levels and CYP1A1, CYP1B1 polymorphisms on blood pressure in an elderly population. J Hypertens. 2015;33:69–76. doi: 10.1097/HJH.0000000000000381. [DOI] [PubMed] [Google Scholar]
- Parl FF, Egan KM, Li C, Crooke PS. Estrogen exposure, metabolism, and enzyme variants in a model for breast cancer risk prediction. Cancer Inform. 2009;7:109–121. doi: 10.4137/cin.s2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Maurhofer O, Beyoglu D, Lanz C, Krausz KW, Pabst T, Gonzalez FJ, Dufour JF, Idle JR. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res. 2011;71:6590–6600. doi: 10.1158/0008-5472.CAN-11-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietilainen KH, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, Oresic M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects–a monozygotic twin study. PLoS One. 2007;2:e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingili AK, Kara M, Khan NS, Estes AM, Lin Z, Li W, Gonzalez FJ, Malik KU. 6beta-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension. 2015;65:1279–1287. doi: 10.1161/HYPERTENSIONAHA.115.05396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposiello SI, Carroll MA, Falck JR, McGiff JC. Epoxyeicosatrienoic acid–mediated renal vasodilation to arachidonic acid is enhanced in SHR. Hypertension. 2001;37:887–893. doi: 10.1161/01.hyp.37.3.887. [DOI] [PubMed] [Google Scholar]
- Pottenger LH, Christou M, Jefcoate CR. Purification and immunological characterization of a novel cytochrome P450 from C3H/10T1/2 cells. Arch Biochem Biophys. 1991;286:488–497. doi: 10.1016/0003-9861(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman G, Yang G, Chen J, Chang TK. Modulation of CYP1B1 and CYP1A1 gene expression and activation of aryl hydrocarbon receptor by Ginkgo biloba extract in MCF-10A human mammary epithelial cells. Can J Physiol Pharmacol. 2009;87:674–683. doi: 10.1139/y09-061. [DOI] [PubMed] [Google Scholar]
- Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL. Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther. 2001;296:537–541. [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological Reviews. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Sahan-Firat S, Jennings BL, Yaghini FA, Song CY, Estes AM, Fang XR, Farjana N, Khan AI, Malik KU. 2,3′,4,5′-Tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: contribution of cytochrome P-450 1B1. Am J Physiol Heart Circ Physiol. 2010;299:H1891–1901. doi: 10.1152/ajpheart.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples JR, Krause G, Lewy AJ. Effect of melatonin on intraocular pressure. Curr Eye Res. 1988;7:649–653. doi: 10.3109/02713688809033192. [DOI] [PubMed] [Google Scholar]
- Savas U, Bhattacharyya KK, Christou M, Alexander DL, Jefcoate CR. Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem. 1994;269:14905–14911. [PubMed] [Google Scholar]
- Schwartzman M, Ferreri N, Carroll M, Songu-Mize E, McGiff J. Renal cytochrome P450-related arachidonate metabolite inhibits (Na++ K+) ATPase. Nature. 1985;314:620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- Schwartzman ML, Abraham NG, Masferrer J, Dunn MW, McGiff JC. CYTOCHROME-P450 DEPENDENT METABOLISM OF ARACHIDONIC-ACID IN BOVINE CORNEAL EPITHELIUM. Biochemical and Biophysical Research Communications. 1985;132:343–351. doi: 10.1016/0006-291x(85)91028-9. [DOI] [PubMed] [Google Scholar]
- Shimada T, El-Bayoumy K, Upadhyaya P, Sutter TR, Guengerich FP, Yamazaki H. Inhibition of human cytochrome P450-catalyzed oxidations of xenobiotics and procarcinogens by synthetic organoselenium compounds. Cancer Res. 1997;57:4757–4764. [PubMed] [Google Scholar]
- Shimada T, Guengerich FP. Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem Res Toxicol. 2006;19:288–294. doi: 10.1021/tx050291v. [DOI] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Research. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol. 1998;11:1048–1056. doi: 10.1021/tx980090+. [DOI] [PubMed] [Google Scholar]
- Skene DJ, Papagiannidou E, Hashemi E, Snelling J, Lewis DFV, Fernandez M, Ioannides C. Contribution of CYP1A2 in the hepatic metabolism of melatonin: studies with isolated microsomal preparations and liver slices. Journal of Pineal Research. 2001;31:333–342. doi: 10.1034/j.1600-079x.2001.310408.x. [DOI] [PubMed] [Google Scholar]
- Song CY, Ghafoor K, Ghafoor HU, Khan NS, Thirunavukkarasu S, Jennings BL, Estes AM, Zaidi S, Bridges D, Tso P, Gonzalez FJ, Malik KU. Cytochrome P450 1B1 Contributes to the Development of Atherosclerosis and Hypertension in Apolipoprotein E-Deficient Mice. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.115.06427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiemke MM, Edelhauser HF, Geroski DH. The developing corneal endothelium: correlation of morphology, hydration and Na/K ATPase pump site density. Current Eye Research. 1991;10:145–156. doi: 10.3109/02713689109001742. [DOI] [PubMed] [Google Scholar]
- Sutter TR, Tang YM, Hayes CL, Wo YY, Jabs EW, Li X, Yin H, Cody CW, Greenlee WF. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994;269:13092–13099. [PubMed] [Google Scholar]
- Takemura H, Itoh T, Yamamoto K, Sakakibara H, Shimoi K. Selective inhibition of methoxyflavonoids on human CYP1B1 activity. Bioorg Med Chem. 2010;18:6310–6315. doi: 10.1016/j.bmc.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Tang YM, Wo YYP, Stewart J, Hawkins AL, Griffin CA, Sutter TR, Greenlee WF. Isolation and characterization of the human cytochrome P450 CYP1B1 gene. Journal of Biological Chemistry. 1996;271:28324–28330. doi: 10.1074/jbc.271.45.28324. [DOI] [PubMed] [Google Scholar]
- Teixeira LB, Zhao Y, Dubielzig RR, Sorenson CM, Sheibani N. Ultrastructural abnormalities of the trabecular meshwork extracellular matrix in Cyp1b1-deficient mice. Vet Pathol. 2015;52:397–403. doi: 10.1177/0300985814535613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijmes M, Pedraza R, Valladares L. Melatonin in the rat testis: Evidence for local synthesis. Steroids. 1996;61:65–68. doi: 10.1016/0039-128x(95)00197-x. [DOI] [PubMed] [Google Scholar]
- Tokizane T, Shiina H, Igawa M, Enokida H, Urakami S, Kawakami T, Ogishima T, Okino ST, Li LC, Tanaka Y, Nonomura N, Okuyama A, Dahiya R. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clinical Cancer Research. 2005;11:5793–5801. doi: 10.1158/1078-0432.CCR-04-2545. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Trevisi L, Bova S, Cargnelli G, Ceolotto G, Luciani S. Endothelin-1-induced arachidonic acid release by cytosolic phospholipase A(2) activation in rat vascular smooth muscle via extracellular signal-regulated kinases pathway. Biochemical Pharmacology. 2002;64:425–431. doi: 10.1016/s0006-2952(02)01066-3. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Gonzalez FJ. Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol. 2008;48:333–358. doi: 10.1146/annurev.pharmtox.48.061807.154729. [DOI] [PubMed] [Google Scholar]
- Walle T, Walle UK. Novel methoxylated flavone inhibitors of cytochrome P450 1B1 in SCC-9 human oral cancer cells. J Pharm Pharmacol. 2007;59:857–862. doi: 10.1211/jpp.59.6.0012. [DOI] [PubMed] [Google Scholar]
- Wang T, Shah YM, Matsubara T, Zhen Y, Tanabe T, Nagano T, Fotso S, Krausz KW, Zabriskie TM, Idle JR, Gonzalez FJ. Control of steroid 21-oic acid synthesis by peroxisome proliferator-activated receptor alpha and role of the hypothalamic-pituitary-adrenal axis. J Biol Chem. 2010;285:7670–7685. doi: 10.1074/jbc.M109.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Nikolov NP, Tschetter JR, Kopp JB, Gonzalez FJ, Kimura S, Siegel RM. Progressive glomerulonephritis and histiocytic sarcoma associated with macrophage functional defects in CYP1B1-deficient mice. Toxicologic Pathology. 2004;32:710–718. doi: 10.1080/01926230490885706. [DOI] [PubMed] [Google Scholar]
- Wo YYP, Stewart J, Greenlee WF. Functional Analysis of the Promoter for the HumanCYP1B1 Gene. Journal of Biological Chemistry. 1997;272:26702–26707. doi: 10.1074/jbc.272.42.26702. [DOI] [PubMed] [Google Scholar]
- Yaghini FA, Song CY, Lavrentyev EN, Ghafoor HUB, Fang XR, Estes AM, Campbell WB, Malik KU. Angiotensin II-Induced Vascular Smooth Muscle Cell Migration and Growth Are Mediated by Cytochrome P450 1B1-Dependent Superoxide Generation. Hypertension. 2010;55:1461–U1313. doi: 10.1161/HYPERTENSIONAHA.110.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Yee RW, Howes KA, Reiter RJ. Diurnal rhythms of immunoreactive melatonin in the aqueous humor and serum of male pigmented rabbits. Neurosci Lett. 1990;116:309–314. doi: 10.1016/0304-3940(90)90092-n. [DOI] [PubMed] [Google Scholar]
- Zatz M. Photoendocrine transduction in cultured chick pineal cells: IV. What do vitamin A depletion and retinaldehyde addition do to the effects of light on the melatonin rhythm? J Neurochem. 1994;62:2001–2011. doi: 10.1046/j.1471-4159.1994.62052001.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Savas Ü, Alexander DL, Jefcoate CR. Characterization of the mouse CYP1B1 gene Identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. Journal of Biological Chemistry. 1998;273:5174–5183. doi: 10.1074/jbc.273.9.5174. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Dunbar D, Kaminsky L. Human cytochrome P-450 metabolism of retinals to retinoic acids. Drug Metab Dispos. 2000;28:292–297. [PubMed] [Google Scholar]
- Zheng W, Tong T, Lee J, Liu X, Marcus C, Jefcoate CR. Stimulation of mouse Cyp1b1 during adipogenesis: characterization of promoter activation by the transcription factor Pax6. Arch Biochem Biophys. 2013;532:1–14. doi: 10.1016/j.abb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Mu Y, Qi C, Wang J, Xi G, Guo J, Mi R, Zhao F. CYP1B1 enhances the resistance of epithelial ovarian cancer cells to paclitaxel in vivo and in vitro. Int J Mol Med. 2015;35:340–348. doi: 10.3892/ijmm.2014.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]


