Abstract
Intra-neuronal protein aggregates made of fibrillar alpha-synuclein (α-syn) are the hallmark of Parkinson’s disease (PD). With time, these aggregates spread through the brain following axonal projections. Understanding the mechanism of this spread is central to the study of the progressive nature of PD. Here we review data relevant to the uptake, transport and release of α-syn fibrils. We summarize several cell surface receptors that regulate the uptake of α-syn fibrils by neurons. The aggregates are then transported along axons, both in the anterograde and retrograde direction. The kinetics of transport suggests that they are part of the slow component b of axonal transport. Recent findings indicate that aggregated α-syn is secreted by neurons by non-canonical pathways that may implicate various molecular chaperones including USP19 and the DnaJ/Hsc70 complex. Additionally, α-syn fibrils may also be released and transmitted from neuron-to-neuron via exosomes and tunneling nanotubes. Understanding these different mechanisms and molecular players underlying α-syn spread is crucial for the development of therapies that could halt the progression of α-syn-related degenerative diseases.
Keywords: alpha-synuclein, axonal transport, exosome, fibrils, Parkinson’s disease, prion, spread, tunneling nanotube, unconventional secretion
Introduction
Parkinson’s disease (PD) is characterized by the presence of protein aggregates termed Lewy bodies (LBs) when located in neuron soma, and Lewy neurites (LNs) when located in axons. Both consist mainly of misfolded and fibrillar forms of alpha-synuclein (α-syn) (Spillantini et al., 1997), a protein located predominantly at pre-synaptic terminals where it is soluble and appears to play a role in the homeostasis of the synaptic vesicle pool (Burré et al., 2010; Chandra et al., 2005; Murphy et al., 2000; Withers et al., 1997). When aggregated in LBs and LNs, α-syn displays characteristics of amyloids and is assembled into highly ordered fibrils (Spillantini et al., 1998a). Besides PD, α-syn aggregation is also characteristic of dementia with Lewy bodies (DLB) and multiple system atrophy (MSA). These neurodegenerative diseases are now collectively referred to as synucleinopathies (Baba et al., 1998; Spillantini et al., 1997; 1998b; Wakabayashi et al., 1998).
The Braak model of PD posits that LBs and LNs spread with time between axonally connected structures in the PNS and CNS (Braak et al., 2006; 2003). This model and the prion hypothesis for synucleinopathies are game-changing concepts with implications for both our understanding of the pathogenesis of PD and the development of new therapies. The model has been bolstered by a growing number of studies showing that α-syn fibrils can spread through connected neuronal networks, and seed the misfolding of endogenous α-syn after internalization by neurons (Luk et al., 2012a; Volpicelli-Daley et al., 2011). Here we review the successive steps (internalization, transport and exit) necessary for the spread of α-syn fibrils. The initial misfolding, oligomerization and fibrillization, which triggers the disease, will be touched on only briefly in this introduction. It is assumed that the initial fibrillization results from stochastic α-syn misfolding that is not properly dealt with by quality control mechanisms. It may be favored by high local concentration of the protein in certain vulnerable neuron populations. This may be the case in individuals with duplications and triplications of the α-syn gene (SNCA), or genetic variants located in non-coding genomic regions, which enhance α-syn expression (Ibáñez et al., 2004; Simón-Sánchez et al., 2009; Singleton et al., 2003; Soldner et al., 2016).
Here we review the knowledge on three steps - internalization, transport and exit of α-syn fibrils - central to the spread of α-syn pathology. We will focus on studies that use α-syn fibrils isolated from patient CNS or obtained in vitro from bacterially-expressed recombinant protein (preformed fibrils, PFFs), thereby bypassing the initial seed formation. We will limit the discussion to fibrillar forms of α-syn since only those forms have been shown to trigger the aggregation of endogenous α-syn robustly both in vitro and in vivo (Bousset et al., 2013; Luk et al., 2012a; 2012b; Paumier et al., 2015; Peelaerts et al., 2015; Volpicelli-Daley et al., 2011). Understanding uptake transport and release of α-syn fibrils will not only further our understanding of the progressive nature of synucleinopathies but may also uncover potential targets for therapeutic intervention that could slow or even halt the spread of α-syn pathology.
The uptake of α-syn fibrils by neurons
Primary neurons cultured in vitro rapidly internalize recombinant α-syn fibrils that are added to the medium (Abounit et al., 2016; Brahic et al., 2016; Volpicelli-Daley et al., 2011). In the mouse, α-syn fibrils administered by stereotaxic injection into the brain are taken up within a few hours by neurons and glia surrounding the injection site (N. L. Rey et al., 2013). Uptake can take place both in the soma/dendrites as well as the axon compartment, as shown using microfluidic tissue culture devices that physically separate soma/dendrites from axons (Brahic et al., 2016; Freundt et al., 2012; Tran et al., 2014; Volpicelli-Daley et al., 2011). The extent and the kinetics of uptake reported by different laboratories appear to vary, which could be due to differences in culture conditions and/or the method of sonication used to fragment the fibrils. Furthermore, differences can also be attributed to the use of distinct “α-syn strains” with varying biophysical properties (Bousset et al., 2013; Guo et al., 2013; Sacino et al., 2014).
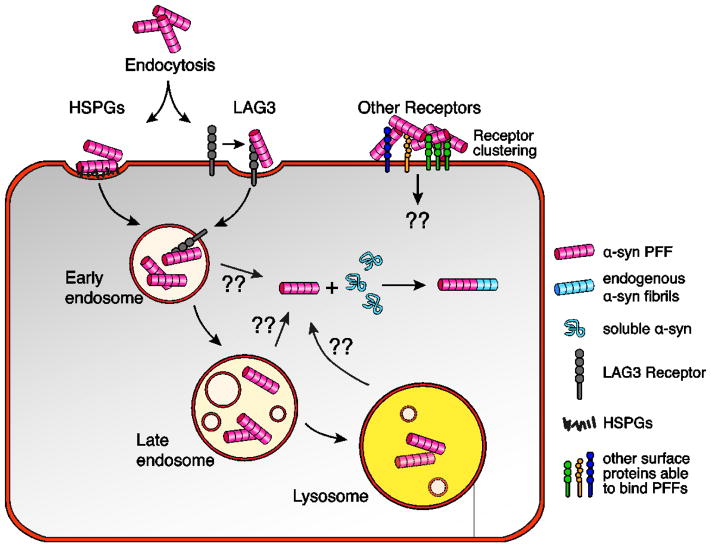
There is strong evidence that α-syn PFFs enter cell lines and primary neurons in culture by endocytosis (Figure 1). Inhibiting endocytosis with low temperature or by expressing a dominant negative version of dynamin 1, markedly reduces the uptake (Desplats et al., 2009; H.-J. Lee et al., 2005a; 2008). Internalized α-syn PFFs co-localize with both early endosomal markers EEA1 and Rab5 and late endosomal/lysosomal marker Rab7 and Lamp1 (Brahic et al., 2016; Desplats et al., 2009; Konno et al., 2012; H.-J. Lee et al., 2008). The majority of endocytosed α-syn PFFs appears to be degraded in lysosomes. However, there are some conflicting reports about the kinetics of degradation, possibly due to variations in cell culture systems, fibril preparations and methods of delivery (H.-J. Lee et al., 2008; Luk et al., 2009)
Figure 1. Internalization of α-synuclein fibrils and aggregation of endogenous α-syn protein.
Recombinant α-syn fibrils are transported into the cell through endocytosis. This process is facilitated by the binding of α-syn PFFs to the cell membrane through interactions with cell surface molecules. In particular, the cell surface receptor LAG3 (lymphocyte activation gene 3) can bind and mediate the endocytosis of fibrillary α-syn. Additionally, α-syn fibrils can bind and cluster a number of other surface receptors at the plasma membrane. It is currently unknown whether any of these cell surface proteins can regulate the uptake of α-syn as well. Heparan sulfate proteoglycans (HSPG), abundant extracellular glycoproteins that are able to interact with a large number of extracellular proteins and ligands, are able to bind α-syn fibrils and promote their uptake. Internalized PFFs travel through the early and late endosomal compartment to the lysosome, where they are destined for degradation. Through some unknown process, α-syn PFFs can escape the lumen of the endosomal compartment and template the misfolding of soluble endogenously expressed α-syn in the cytoplasm. (??) indicate unknown mechanisms and molecular players.
Interestingly, the uptake of α-syn fibrils by neurons may have striking commonalities with the entry of viruses into host cells. Many viruses first bind to heparan sulfates on the cell surface in what is believed to be a non-specific electrostatic interaction (Marsh and Helenius, 2006). Subsequently, the interaction with one, or more, specific protein receptor(s) triggers protein conformation changes that lead to entry through the plasma membrane. In the case of α-syn fibrils, as well as misfolded tau and PrP, it has been shown that the proteins bind heparan sulfate proteoglycans (HSPGs) on the surface of cells (Figure 1) (Holmes et al., 2013; Horonchik et al., 2005; Schonberger et al., 2003; Shrivastava et al., 2015). Holmes et al. demonstrated that internalization by non-neuronal cell lines was reduced by competitive inhibition with heparin. Although blocking the HSPG- α-syn interaction using heparin or heparin mimics is an intriguing potential therapeutic approach, it will be important to examine if the uptake of α-syn PFFs is mediated by HSPGs in CNS neurons.
In addition to HSPGs, a variety of cell surface proteins that bind α-syn fibrils with some specificity have recently been identified (Figure 1). Mao et al. expressed a trans-membrane protein cDNA library in the SH-SY5Y cell line and screened for differential binding to α-syn fibrils over α-syn monomers. Although only a fraction of the transmembrane proteins in the library were known to locate to the plasma membrane, the authors identified several putative receptors for fibrillary α-syn including neurexin 1 and APLP1 but eventually focused on the product of lymphocyte-activation gene 3 (LAG3) (Mao et al., 2016). Using knockout and over-expression systems, they showed that LAG3 is involved in the binding and endocytosis of α-syn PFFs. The absence of LAG3 reduced but did not completely abolish α-syn aggregation and spread in primary neurons as well as in the mouse CNS. LAG3-specific antibodies competitively blocked the interaction and reduced the uptake of α-syn fibrils by cultured neurons and, as a result, prevented the fibrilization of endogenous α-syn. Although the authors indicate that LAG3 is predominantly expressed by neurons, published gene expression datasets show that it is highly expressed by microglia, potentially mediating α-syn fibril uptake by these glial cells (Zhang et al., 2014). These important results point to LAG3 as a potential drug target, although it is currently not known whether α-syn is expressed on relevant human neuronal populations.
In addition to LAG3, other cell surface proteins expressed by neurons have been shown to bind α-syn fibrils. In particular, the Na+/K+ transporting ATPase subunit α3 (α3-NKA) was found in an unbiased proteomics study aimed at identifying neuronal cell-surface binding partners of recombinant α-syn fibrils and oligomers (Shrivastava et al., 2015). Interestingly, ATP1A3, the gene encoding α3-NKA, is linked to rapid-onset dystonia-parkinsonism (de Carvalho Aguiar et al., 2004). Shrivastava et al. demonstrated that α-syn fibrils and oligomers form clusters on the plasma membrane of rodent neurons and that α3-NKA receptors are trapped in these clusters. Functionally, they demonstrated that this redistribution and clustering of α3-NKA leads to changes in Na+ gradient and neuronal excitability in vitro. Although the authors did not investigate α3-NKA or α-syn endocytosis, it seems plausible that changes in membrane dynamics and receptor clustering could affect and mediate the internalization of both the receptors and the bound α-syn fibrils. Interestingly, Shrivastava et al. also identified several HSPGs as binding partners of fibrillary α-syn, again emphasizing the importance of this class of surface molecule. Additionally, both the screens performed by Mao et al. and Shrivastava et al. independently identified neurexins as putative α-syn fibril receptors. However, whether these proteins mediate the uptake of α-syn fibrils and play any role in spread has not been investigated. Interestingly, several genetic risk factors for PD are involved in endocytosis and vesicle trafficking processes in general (Abeliovich and Gitler, 2016). Although they could theoretically be implicated in the uptake of α-syn fibrils, the experimental information on their role in this process is still very limited (Volpicelli-Daley et al., 2016).
As a result of endocytosis, the α-syn PFFs are surrounded by lipid membranes of the endocytic compartment and do not have direct access to soluble cytoplasmic α-syn (Figure 1). How fibrils escape the lumen of the endocytic compartment to trigger the aggregation of endogenous α-syn is still unknown. Can α-syn PFFs somehow interfere with the endo-lysosomal function and permeabilize the lipid membrane? The analogy with virus entry could be relevant here. For instance, if α-syn is still bound to its receptor, the acidification in the endosomal compartment could induce conformation changes of fibrils, receptor or the receptor-fibril complex that would somehow cause transfer (Igonet and F. A. Rey, 2012). Escape from the endosomal lumen could be a relatively rare, rate-limiting event that contributes to the chronicity of PD. Distinct α-syn ‘strains’ may have different affinities for surface proteins causing differences in their ability to escape the endosomal compartment.
The uptake of α-syn fibrils, including binding to surface proteins, endocytosis and escape from endosomal compartment, could be targeted by drugs to slow down or even prevent neuron-to-neuron transmission of pathology. Identifying as many putative targets as possible will be important in the hope that some may be specific enough to the entry of α-syn to avoid general toxicity.
The axonal transport of α-syn fibrils
The evidence for the axonal transport of misfolded α-syn comes from experiments done in vivo, in the mouse, and in vitro using cultured neurons. In the mouse, several groups showed that injecting preformed fibrillar α-syn, or α-syn fibrils extracted form PD CNS material, induces the fibrillization of endogenous α-syn at the site of injection, as well as in distant brain areas (Luk et al., 2012b; 2012a; Masuda-Suzukake et al., 2013; Mougenot et al., 2012; Paumier et al., 2015; Peelaerts et al., 2015; Recasens et al., 2014). Mapping these remote secondary sites showed that they were connected to the site of injection by anterograde and retrograde axonal projections. These results, predicted by the Braak model (Braak et al., 2003), are consistent with axonal transport of fibrils and seeding of misfolding at a distance in secondary neurons, although they do not formally prove it.
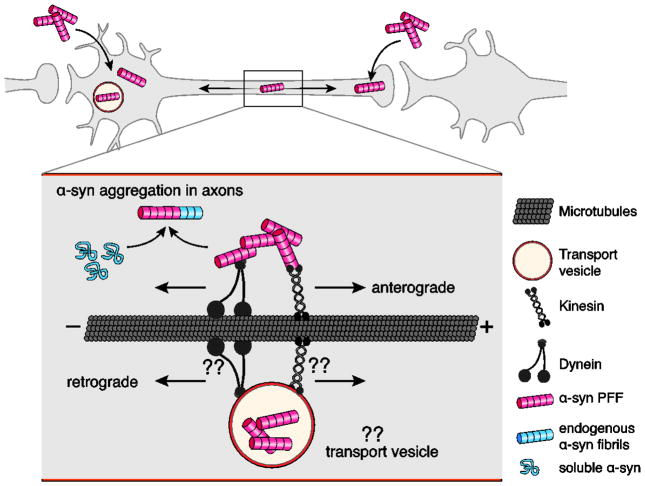
Direct observation of axonal transport of α-syn fibrils was obtained using primary mouse neurons grown in microfluidic devices, which physically separate the soma and axonal compartments in two fluidically isolated channels. In this way, if a product added, for example, to the soma compartment appears in the axon compartment it must have been actively transported by the axons, in this case in the anterograde direction. Using this approach, transport of exogenously added preformed oligomeric misfolded α-syn and α-syn fibrils was observed in both the anterograde and retrograde direction (Figure 2) (Brahic et al., 2016; Danzer et al., 2011; Freundt et al., 2012). Measuring the amount of labeled, preformed fibrils transported in either direction showed that retrograde transport was almost twice as efficient as anterograde transport (Brahic et al., 2016).
Figure 2. Axonal transport of α-syn fibrils.
α-syn fibrils can be internalized both in the dendrite/cell body compartment and in axons. α-syn fibrils are actively transported along microtubules both in the anterograde and retrograde direction. It is currently not well understood if internalized α-syn fibrils are being transported directly in the cytoplasm or in transport vesicles following endocytosis. Kinesins and dynein families of proteins are the main types of molecular motors transporting cargos along microtubules in anterograde and retrograde directions. The motor and adapter proteins mediating α-syn fibril transport, both in the cytosol as well as in transport vesicles, are still unknown. Aggregation is thought to initially occur in axons, where α-syn fibrils can encounter and template the misfolding of soluble endogenous α-syn proteins that are transported along axons for delivery to synapses. (??) indicate unknown mechanisms and molecular players.
Time-lapse microscopy provided a measurement of transport velocity. In the anterograde direction, the fibrils moved at velocities characteristic of fast axonal transport but with pauses of variable lengths, a behavior that defines slow component b of axonal transport. This type of transport moves large assemblies of axonal proteins not enclosed in vesicles (Roy et al., 2007). It was suggested that α-syn fibrils might have properties in common (size, charge, other surface properties) with the physiological cargoes of slow component b transport (Freundt et al., 2012). By adding new neurons to the axon channel of a previously established culture, microfluidic devices provided direct evidence of transfer of fibrils to a still immature second order neuron after anterograde transport. Direct transfer of the originally added PFFs as well as aggregation of endogenous α-syn in the second order neurons have been reported, indicating that the transferred α-syn fibrils maintain the potential to template the misfolding of soluble α-syn (Freundt et al., 2012; Mao et al., 2016; Tran et al., 2014).
Both the in vivo and in vitro approaches described above are limited in their relevance to PD by the use of rodent neurons. Human embryonic stem cells (ES) and induced pluripotent stem cells (iPSC)-derived neurons now makes it possible to study transport of α-syn fibrils in relevant human neurons including midbrain dopaminergic neurons (Chung et al., 2013; Kriks et al., 2011; Mazzulli et al., 2011). The growing number of sporadic and familial PD iPSC lines available will facilitate the study of genetic risk factors and their role in axonal transport and transfer of α-syn fibrils to second order neurons (Devine et al., 2011; Hsieh et al., 2016; Mazzulli et al., 2016).
Many questions about axonal transport of α-syn fibrils remain unanswered. Are the fibrils transported as naked protein assemblies, as suggested by the resemblance with slow component b cargo, or in vesicles (Figure 2)? Live-cell imaging of fluorescently labeled PFFs in combination with florescent-protein tagged Rab GTPases, known to localize to distinct endosomal compartments, might help answer this question (Stenmark, 2009). What are the adaptor proteins which link α-syn fibrils, whether naked or in vesicles, to the kinesin and dynein motors? Interestingly, in the CNS as well as in cultured neurons, fibrillization of endogenous α-syn initially predominates in axons (Braak et al., 1999; Kramer and Schulz-Schaeffer, 2007; Volpicelli-Daley et al., 2011; 2014). Active transport of naked fibrils and high concentration of soluble α-syn may facilitate the fibrillization of endogenous α-syn in axons.
Thus, targeting the axonal transport of α-syn fibrils currently only has limited therapeutic potential owing to the lack of known molecular players as well as the high likelihood to impair a wide range of other cargos sharing the same transport machinery.
The release of α-syn fibrils
It has been known for some time that soluble α-syn can be secreted by neurons in CNS interstitial fluid and cerebrospinal fluid (CSF) (El-Agnaf, 2003; Emmanouilidou et al., 2011). In vitro, secretion appears to be by non-canonical vesicle-mediated exocytosis (Jang et al., 2010; H.-J. Lee et al., 2005a). It is still unclear whether misfolded and fibrillar forms of α-syn are secreted by the same pathway. Two recent studies potentially shed some light on this subject (Figure 3). Lee et al. over-expressed proteins prone to misfold, including wild type and mutant forms of α-syn, in cell lines. They showed that USP19, an ER-bound deubiquitylase, has chaperone activity and mediates the delivery of these misfolded proteins to Rab9-positive late endosomes followed by secretion (J.-G. Lee et al., 2016). Protein secretion was enhanced in the presence of proteasome inhibitors, which is reminiscent of the enhanced α-syn secretion observed with neurons under stress (Jang et al., 2010; H.-J. Lee et al., 2005b). Fontaine et al. showed that the DnaJ/Hsc70 chaperone complex could drive the secretion by neurons of α-syn and of other neurodegenerative-associated proteins such as tau and TDP43. The structure, misfolded or not, of α-syn secreted by these pathways was not determined (Fontaine et al., 2016; J.-G. Lee et al., 2016). At present we do not know if the USP19 and DnaJ/Hsc70 pathways are synergistic or work in parallel. If they operate for the release of misfolded, oligomeric or fibrillar forms of α-syn they may offer new drug targets for PD.
Figure 3. Cellular pathways mediating α-syn release.
Following endocytosis, part of the internalized α-syn PFFs can be secreted again from the endosomal compartment without being targeted to the lysosome for degradation. Neutralizing antibodies can block the secreted α-syn fibrils and prevent the cell to cell transmission. Cytosolic α-syn monomers, oligomers and possibly fibrils can be targeted to secretory vesicles for release. Two molecular players with chaperone activity, including USP19 and DnaJ/Hsc70, can mediated the secretion of cytosolic α-syn. It is currently unknown if these two pathways can also mediate the secretion of α-syn fibrils as well. Small amounts of soluble and oligomeric α-syn are packaged into endosome-derived membrane vesicles called exosomes and can be secreted into the extracellular space. It is currently unclear if α-syn fibrils are present in exosomes. Tunneling nanotubes can also mediate the direct release and transmission of α-syn between cells, possibly in lysosome-derived transport vesicles. Neutralizing antibodies target α-syn fibrils released into the extracellular space and potentially reduce or prevent transmission to a second cell. (??) indicate unknown mechanisms and molecular players.
The release of α-syn fibrils by neurons has been investigated using primary neurons cultured in microfluidic devices (Brahic et al., 2016; Freundt et al., 2012). Fluorescently labeled fibrils were released into the medium after both anterograde and retrograde axonal transport (Figure 3). Since release after anterograde transport was unaffected by the Sarm1−/− and WLDS mutations, which render axons highly resistant to degeneration, the fibrils likely were secreted into the medium independent of cell lysis (Brahic et al., 2016). In CNS, where many axons are myelinated or surrounded by other types of glial cells, secretion may be restricted to areas with high endo- and exocytic activity such as synaptic terminals.
Microfluidic devices were also used to show that α-syn PFFs released in the medium after anterograde transport are internalized by still immature second order neurons (Freundt et al., 2012). Using three chamber microfluidics devices, it was recently shown that PFF added to the first chamber can trigger aggregation of endogenous α-syn in synaptically connected neurons in the second and third chamber, indirectly demonstrating the release and transmission of pathological α-syn species in neuronal networks in vitro (Mao et al., 2016; Tran et al., 2014). Importantly, the aggregation was reduced when certain anti-α-syn monoclonal antibodies were added to the medium. Moreover, the same systemically-administered antibodies were able to reduce the pathology induced by intracerebral injection of α-syn PFFs in mice (Tran et al., 2014). Accessibility to antibodies suggests that α-syn fibrils are not secreted in vesicles and raises the hope of being able to interfere with spread by using soluble extracellular drugs.
The role of exosomes and tunneling nanotubes in neuron-to-neuron transfer of α-syn fibrils
α-syn seeds may also use routes other than direct secretion in the extracellular space to spread from neuron-to-neuron. In particular, soluble and oligomeric forms of α-syn have been found in exosomes (Figure 3). However, there is no data yet demonstrating the presence of α-syn fibrils in exosomes. Overall, the amount of α-syn in exosomes appears to be relatively low, significantly smaller than the amount secreted by exocytosis (Alvarez-Erviti et al., 2011; Emmanouilidou et al., 2010). Additionally, there is no evidence that α-syn-containing exosome can trigger aggregation of endogenous α-syn in recipient neurons. Interestingly, lysosomal impairments have been associated with PD and exosome secretion has been shown to increase in response to lysosomal dysfunction (Budnik et al., 2016; Zappulli et al., 2016). Additionally, PD-associated risk genes are involved in intracellular vesicle traffic and transport to lysosomes (Abeliovich and Gitler, 2016; Mazzulli et al., 2016). It is thus possible that fibrils are more prominent in exosomes in these risk gene associated cases of PD. The PD-linked gene ATP13A2 (PARK9), establishes a more direct link between exosomes and α-syn. Indeed, over-expression of PARK9 in neurons causes increased exosome-mediated release of α-syn (Tsunemi et al., 2014).
Tunneling nanotubes, F-actin containing membranous bridges that can connect cells to each other, have been hypothesized to mediate the transmission of α-syn fibrils between neurons (Abounit and Zurzolo, 2012). Evidence has accumulated that the prion protein PrP can spread from cell to cell by way of tunneling nanotubes (Gousset et al., 2009). More recently, Abounit et al. (2016) showed that fibrillar α-syn could also use tunneling nanotubes to spread from cell to cell and seed fibrillization in the recipient cell in vitro (Abounit et al., 2016). In summary α-syn fibrils may be released form neurons via several pathways (Figure 3). Non-conventional secretion of naked fibrils is now well documented. Exosomes and tunneling nanotubes may participate in the release, most probably to a smaller extent than non-conventional secretion. However, the exact state of α-syn, whether monomeric, oligomeric or fibrillar, as well as the exact molecular players mediating these processes remain to be investigated.
Concluding Remarks
The entry into neurons, axonal transport and release of misfolded α-syn are central steps in the pathogenesis of PD. Now the challenge is to define the mechanisms underlying these steps in detail, at the molecular level. This review described recent advances made towards this goal. The identification of candidate receptors for entry, the characterization of the kinetics and yield of axonal transport and the description of non-canonical exocytosis of α-syn are important preliminary results that should lead to exciting developments. The prion-like behavior of misfolded α-syn forces us to think about analogies between PD and infections, in particular by neurotropic viruses. The cellular barriers and other challenges encountered by α-syn fibrils and by these viruses are remarkably similar. The results obtained for viruses could inspire the work on PD. The future of the field is exciting. Fundamental questions in neurobiology will be solved and it is hoped that the answers will help devise new treatments for this very common neurodegenerative disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L, Olivo-Marin JC, Melki R, Zurzolo C. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 2016;35:e201593411–2138. doi: 10.15252/embj.201593411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abounit S, Zurzolo C. Wiring through tunneling nanotubes--from electrical signals to organelle transfer. Journal of Cell Science. 2012;125:1089–1098. doi: 10.1242/jcs.083279. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJA, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VMY, Lee V, Trojanowski JQ, Iwatsubo T. Aggregation of a-Synuclein in Lewy Bodies of Sporadic Parkinson’s Disease and Dementia with Lewy Bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Böckmann A, Meier BH, Melki R. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, de Vos RAI, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach‘s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Sandmann-Keil D, Gai W, Braak E. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by α-synuclein immunocytochemistry. Neurosci Lett. 1999;265:67–69. doi: 10.1016/S0304-3940(99)00208-6. [DOI] [PubMed] [Google Scholar]
- Brahic M, Bousset L, Bieri G, Melki R, Gitler AD. Axonal transport and secretion of fibrillar forms of α-synuclein, Aβ42 peptide and HTTExon 1. Acta Neuropathol. 2016:1–10. doi: 10.1007/s00401-016-1538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, Mungenast AE, Muffat J, Mitalipova M, Pluth MD, Jui NT, Schüle B, Lippard SJ, Tsai LH, Krainc D, Buchwald SL, Jaenisch R, Lindquist S. Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, Hyman BT, McLean PJ. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MAJ, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. NEURON. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA, Kunath T. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OMA. α-Synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. The FASEB Journal. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, Vekrellis K. Assessment of α-Synuclein Secretion in Mouse and Human Brain Parenchyma. PLoS ONE. 2011;6:e22225. doi: 10.1371/journal.pone.0022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-Produced α-Synuclein Is Secreted in a Calcium-Dependent Manner by Exosomes and Impacts Neuronal Survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine SN, Zheng D, Sabbagh JJ, Martin MD, Chaput D, Darling A, Trotter JH, Stothert AR, Nordhues BA, Lussier A, Baker J, Shelton L, Kahn M, Blair LJ, Stevens SM, Dickey CA. DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J. 2016;35:1537–1549. doi: 10.15252/embj.201593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, Covert M, Melki R, Kirkegaard K, Brahic M. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann Neurol. 2012;72:517–524. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Männel D, Zurzolo C. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VMY. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci USA. 2013;110:E3138–47. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horonchik L, Tzaban S, Ben-Zaken O, Yedidia Y, Rouvinski A, Papy-Garcia D, Barritault D, Vlodavsky I, Taraboulos A. Heparan Sulfate Is a Cellular Receptor for Purified Infectious Prions. Journal of Biological Chemistry. 2005;280:17062–17067. doi: 10.1074/jbc.M500122200. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schüle B, Krainc D, Palmer TD, Wang X. Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez P, Bonnet AM, Débarges B, Lohmann E, Tison F, Pollak P, Agid Y, Dürr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Igonet S, Rey FA. SnapShot: Viral and eukaryotic protein fusogens. Cell. 2012;151:1634–1634.e1. doi: 10.1016/j.cell.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- Konno M, Hasegawa T, Baba T, Miura E, Sugeno N, Kikuchi A, Fiesel FC, Sasaki T, Aoki M, Itoyama Y, Takeda A. Suppression of dynamin GTPase decreases α-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Mol Neurodegener. 2012;7:38. doi: 10.1186/1750-1326-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. Journal of Neuroscience. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. Journal of Neuroscience. 2005a;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. Journal of Neuroscience. 2005b;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alphasynuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lee JG, Takahama S, Zhang G, Tomarev SI, Ye Y. Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat Cell Biol. 2016;18:765–776. doi: 10.1038/ncb3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VMY. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam T-I, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin J-H, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Gonçalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DAA, Dawson VL, Ko HS, Dawson TM. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353:aah3374–aah3374. doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DMA, Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci USA. 2016:201520335–35. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchère J, Lakhdar L, Legastelois S, Baron T. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. Journal of Neuroscience. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, Sandoval IM, Fleming S, Dirr E, Polinski NK, Trojanowski JQ, Lee VM, Sortwell CE. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, Fernagut P-O, Blesa J, Parent A, Perier C, Fariñas I, Obeso JA, Bezard E, Vila M. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Rey NL, Petit GH, Bousset L, Melki R, Brundin P. Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 2013;126:555–573. doi: 10.1007/s00401-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VMY. Rapid and Intermittent Cotransport of Slow Component-b Proteins. J Neurosci. 2007;27:3131–3138. doi: 10.1523/JNEUROSCI.4999-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, Thomas MA, McKinney AB, McGarvey NH, Rutherford NJ, Ceballos-Diaz C, Robertson J, Golde TE, Giasson BI. Amyloidogenic α-synuclein seeds do not invariably induce rapid, widespread pathology in mice. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberger O, Horonchik L, Gabizon R, Papy-Garcia D, Barritault D, Taraboulos A. Novel heparan mimetics potently inhibit the scrapie prion protein and its endocytosis. Biochem Biophys Res Commun. 2003;312:473–479. doi: 10.1016/j.bbrc.2003.10.150. [DOI] [PubMed] [Google Scholar]
- Shrivastava AN, Redeker V, Fritz N, Pieri L, Almeida LG, Spolidoro M, Liebmann T, Bousset L, Renner M, Léna C, Aperia A, Melki R, Triller A. α-synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient. EMBO J. 2015;34:2408–2423. doi: 10.15252/embj.201591397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisán-Ruíz C, Lichtner P, Scholz SW, Hernandez DG, Krüger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nature Genetics. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841–841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, Goldmann J, Myers RH, Young RA, Jaenisch R. Parkinson-associated risk variant in distal enhancer of α-synuclein modulates target gene expression. Nature. 2016;533:95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998a;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998b;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Reviews Molecular Cell Biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Tran HT, Chung CH-Y, Iba M, Zhang B, Trojanowski JQ, Luk KC, Lee VMY. α-Synuclein Immunotherapy Blocks Uptake and Templated Propagation of Misfolded α-Synuclein and Neurodegeneration. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Hamada K, Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. Journal of Neuroscience. 2014;34:15281–15287. doi: 10.1523/JNEUROSCI.1629-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Abdelmotilib H, Liu Z, Stoyka L, Daher JPL, Milnerwood AJ, Unni VK, Hirst WD, Yue Z, Zhao HT, Fraser K, Kennedy RE, West AB. G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons. Journal of Neuroscience. 2016;36:7415–7427. doi: 10.1523/JNEUROSCI.3642-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VMY. Formation of α-Synuclein Lewy Neurite-like aggregates in Axons Impedes the Transport of Distinct Endosomes. Mol Biol Cell. 2014 doi: 10.1091/mbc.E14-02-0741. mbc.E14–02–0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VMY. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. NEURON. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of α-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 1998;96:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Withers GS, George JM, Banker GA, Clayton DF. Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res. 1997;99:87–94. doi: 10.1016/s0165-3806(96)00210-6. [DOI] [PubMed] [Google Scholar]
- Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest. 2016;126:1198–1207. doi: 10.1172/JCI81134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. Journal of Neuroscience. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]