Abstract
Introduction
Burn trauma damages resting cardiac function; however, it is currently unknown if the cardiovascular response to exercise is likewise impaired. We tested the hypothesis that, in children, burn injury lowers cardiac output (Q) and stroke volume (SV) during submaximal exercise.
Methods
Five children with 49±4% total body surface area (BSA) burned (2 female, 11.7±1 y, 40.4±18 kg, 141.1±9 cm) and eight similar non-burned controls (5 female, 12.5±2 y, 58.0±17 kg, 147.3±12 cm) with comparable exercise capacity (peak oxygen consumption [peak VO2]: 31.9±11 vs. 36.8±8 ml O2·kg·min-1, P=0.39) participated. The exercise protocol entailed a pre-exercise (pre-EX) rest period followed by 3-minute exercise stages at 20 W and 50 W. VO2, heart rate (HR), Q (via non-rebreathing), SV (Q/HR), and arteriovenous O2 difference ([a-v] O2 dif, Q/VO2) were the primary outcome variables.
Results
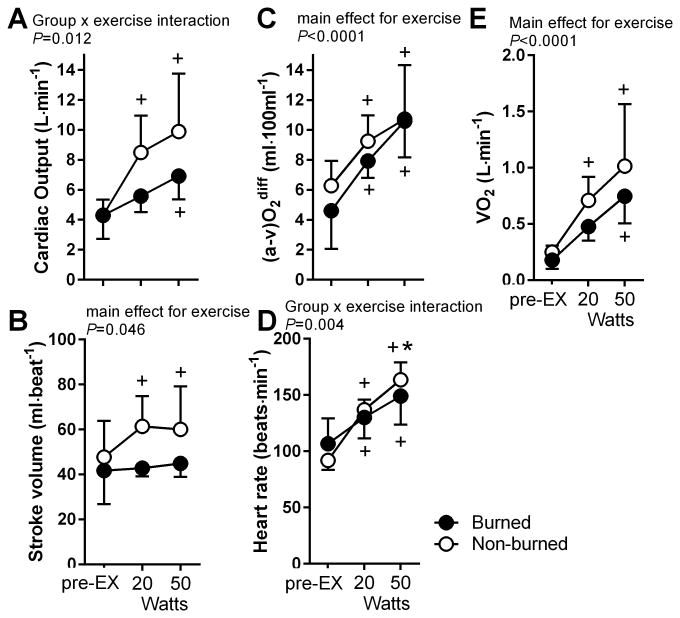
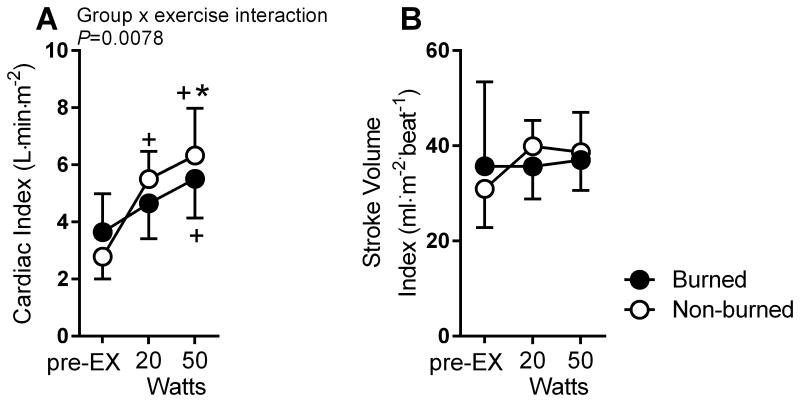
Using a 2-way factorial ANOVA (group [G] × exercise [EX]), we found that Q was ∼27% lower in the burned than the non-burned group at 20 W of exercise (burned 5.7±1.0 vs. nonburned: 7.9±1.8 L·min-1) and 50 W of exercise (burned 6.9±1.6 vs. nonburned 9.2 ±3.2 L·min-1) (G × EX interaction, P=0.012). SV did not change from rest to exercise in burned children but increased by ∼24% in the non-burned group (main effect for EX, P=0.046). Neither [a-v] O2 dif nor VO2 differed between groups at rest or exercise, but HR response to exercise was reduced in the burn group (G × EX interaction, P=0.004). When normalized to BSA, SV (index) was similar between groups; however, Q (index) remained attenuated in the burned group (G × EX interaction, P<0.008).
Conclusions
Burned children have an attenuated cardiovascular response to submaximal exercise. Further investigation of hemodynamic function during exercise will provide insights important for cardiovascular rehabilitation in burned children.
Introduction
Burn trauma causes a profound activation of the sympathetic autonomic nervous system that prompts the classic fight-or-flight response, which increases blood pressure, heart rate (HR), and respiratory rate. While this stressresponse mechanism is beneficial under acute stress, prolonged activation of the adrenergic system is thought to be detrimental to cardiac function and whole-body metabolism (22, 27). During the first 48 hours after burn injury, an increase in cardiac output, oxygen consumption, metabolic rate, and hyper metabolism characterize the “ebb phase.”These responses are thought to be driven by an elevation in catecholamines, which also disrupt insulin, glucose, lipid, and protein metabolism (42). The “flow” phase then follows within 5 days of burn injury to further disrupt metabolism (27).Much of the research on burn injury over the last 20+ years has centered oncharacterizing the hyper metabolic response and has greatly improved burn care (14, 27). In fact, due to these medical advances, patients with 90% total body surface area burns now survive these injuries; however, these improvements in burn care have resulted in children and adults with long-term metabolic and cardiovascular complications that persist for up to 3 years postburn (11, 14, 16, 40)and that are thought to be risk factors for long-term cardiovascular disease (9).
Cardiorespiratory fitness (peak VO2) is a strong predictor of all-cause mortality (18). Beginning from5 years post burn until 10 or more years after the injury, adults have reduced aerobic exercise capacity (10, 41), suggesting thatlong-termcardiorespiratory impairments may be present. However, whether this is due to cardiovascular dysfunction or reduced physical activity post burn is not entirely clear(38).Additionally, burn trauma may affect children differently than adults, and this requires further study and understanding. Adult physiology may differ from that of children due to children's rapid growth and development during puberty coupled with morphologic differences (29).For example, children have a greater body surface area (BSA)-to-mass ratio and are less economical (use greater oxygen at similar exercise workloads) in regard to exercise compared to adults. Moreover, healthy non-burned children have smaller hearts, less blood volume, and lower stroke volume (SV)in response to exercise than adults (37).Reynolds and colleagues found that, in children, burn injury causes cardiac failure, particularly left ventricular myocardial depression, an outcome that was likewise different from that seen in burned adults(28).
Whether cardiovascular response to exercise is impaired in burned childrenis currently unknown. If this is the case, then rehabilitation exercise medicine may need specific guidelines for burned populations, especially if HR- or oxygen uptake-based training (i.e., percent HR/oxygen uptake max) is used as a guide for exercise prescription. Some have reported that burns do not affect children's exercise tolerance during a graded exercise stress test(20), while others have reported limited endurance exercise due to abnormal lung function at 2 to 3 years post burn (7, 21).
The objective of this study was to assess the cardiovascular response to submaximal exercise in childrennear1-yearpost burn (range: 7-15mo). We hypothesized that burn injury would attenuate cardiac output and SV during submaximal exercise in burned children compared to non-burned healthy children matched for age and aerobic capacity.
Methods
Ethical approval
All experiments were approved by the Institutional Review Board of the University of Texas Medical Branch and in agreement with the Declaration of Helsinki. Thirteen children participated in this study. Prior to participation in the study, informed consent was obtained from parents or legal guardians and child assent was obtained as applicable.
Experimental design
Upon admission to our institution, pediatric patients undergo standard of care treatment involving reconstructive surgery and skin grafting. They are then discharged once wounds are 95% healed. Patients return for follow-up at 6, 9, 12, and 24 months after discharge and yearly thereafter for further reconstructive surgery and continued care. This study was conducted in patients returning for the 9- to 12-monthfollow-up visits. Five burned children and 8 non-burned healthy children matched for age, height, weight, and exercise capacity participated in this study. Prior to the main study, an aerobic exercise stress test (peakVO2) and body composition (dual-energy X-ray absorption metry [DXA]) scan were administered. Within 1 week of preliminary assessments, subjects participated in a submaximal exercise protocol that entailed a pre-exercise (pre-EX) standing rest period followed by 3-minute exercise stages at 20W and 50 W. During the rest and submaximal exercise, oxygen consumption, HR, cardiac output, SV, and arteriovenous oxygen difference were measured.
Peak aerobic exercise capacity test
Aerobic exercise capacity (peak VO2) was determined by a modified Bruce protocol maximal treadmill exercise test to volitional exhaustion. Respiratory gasses were analyzed using breath-by-breath data using an automated Med Graphics CardiO2 metabolic cart (St. Paul, MN) after O2 and CO2 gas and air flow were calibrated using known gasses and a 3 L syringe. Initial speed was set at 1.7 miles per hour and angle of elevation at 0%. These were then increased every 3 minutes. Subjects were continually encouraged to complete 3-minute stages, and the test was ended once peak volitional effort was achieved. Notably, no validated, universally accepted criteria exist in children for the determination of peak VO2 [42]. Therefore, we used standards similar to those used in adults [27], and the test was deemed to be maximal once subjects signaled to stop exercise and at least 3 of the following criteria were met: a respiratory exchange ratio (RER) of ≥ 1.05, a leveling off in VO2 with increasing workloads (less than 2 mlkgmin-1), volitional fatigue, exercise final HR of 190 b pm or greater, or a final test time from 8 to 12 min. Similar criteria have been used by others in children [42,47]. All subjects met 3 of the aforementioned criteria.
Cardiac output and calculated stroke volume and arteriovenous difference
During the rest, while standing (pre-exercise), and during the two stages of submaximal exercise (20 W and 50 W), cardiac output and diffusing capacity (DLCO) was assessed using a non-rebreathing, open-circuitcardiac output (CO) and pulmonary diffusing capacity method previously described (17, 34). Briefly, the gas mixture consisted of 0.3% C18O, 21% oxygen, 8% helium, 0.7% acetylene, and balance nitrogen (Scott Medical Products, PA). We used a respiratory mass spectrometer (MGA1100, St. Louis, MO,) to sample gas at a mouthpiece attached to a screen-type pneumotachograph (3813 Hans Rudolph, Kansas City, MO).Athree-way pneumatic valve (8500 Hans Rudolph, Kansas City, MO) was used to switch the inhaled gas between room air and a 20 L bag containing the test gas mixture. During a CO maneuver, subjects inhaled from the bag and exhaled into the atmosphere. After 8 to 12 breaths, the respiratory valve was turned back to make inspiration from room air. Data analysis was performed immediately after each maneuver using a custom computer program that derived cardiac output from acetylene uptake while using gas dilution methods of the helium tracer gas to obtain alveolar volume. The dead space was calculated by gas dilution from helium for every maneuver. The pneumotachograph was calibrated using a 3 L syringe filled with test gas mixtures before each study. Stroke volume (SV, ml) was calculated as cardiac output divided by HR (beats per min), and arteriovenous O2 difference ([a-v] O2 dif, ml/100 ml) was calculated as cardiac output divided by oxygen consumption (VO2). According to Johnson et al. (2000) these methods in comparison to the direct Fick have a reproducibility of results where the coefficient of variation (%) were between 2.1% within sessions and 1.8% and the correlation to Fick is r2=0.9.
Body morphology
Standard, calibrated scales were used to determine weight and height. Body composition (DXA, Hologic QDR 4500 densitometer, Hologic Inc., Bedford, MA)was measured within 7 days of the exercise protocol during the subject's first visit to the laboratory. On the day of each test, the DXA instrument was calibrated using the procedures provided by the manufacturer, and DXA scans were performed and analyzed using pediatric software. Three body mass indices were used: body mass index (BMI), BMI percentile (BMI% ile), and BSA. BMI (kgm-2) was calculated by weight in kilograms divided by the square of height in meters. BMI % ile was determined using the normative values provided by the CDC (3), and BSA (m2) was calculated according to the method of DuBois and DuBois (8). The burn patients in this study were admitted for flame (n=4) and scald (n=1) on the head, chest, back, arms or legs. Total body surface area burned and third-degree burn was calculated by nursing staff. At admission, total body surface area burned was documented in Lund and Browder charts and adjusted accordingly upon demarcation of third-degree burns.
Statisticalanalysis
Baseline subject characteristics were analyzed using an independent t-test. A two-way factorial ANOVA was used to assess the interaction and main effects of group × exercise. If significance was found, the appropriate Holm-Sidak multiple comparison post hoc test was performed. The rate of external work was calculated from the treadmill grade and speed using the following standard formula: W = body mass in kg × 9.81 × (speed in mph × 0.44704) × (%Grade /100). To control for growth and body morphology variations between burned and non-burned children, we normalized oxygen uptake (VO2) and exercise work rate (Watts) to total body mass and lean body mass. Cardiac output and SV were also normalized to BSA. Slopes and intercepts for cardiac output, SV, HR, and arteriovenous difference were compared between burned and non-burned healthy controls. Data were analyzed and figures generated using Graph Pad Prism (Version 6.0, La Jolla, CA, USA), with significance set at P<0.05. All data are reported as mean ± SD.
Results
Subject characteristics
As shown in Table 1, the five burned children (2 female)had 49±4% total body surface area burns with 24.4% third-degree burns. They were comparable to the eight non-burned healthy controls (5 female) with regard to age (burn, 11.7±1 years vs. non-burned, 12.5±2 years), weight (burn, 40.4±18 vs. non-burn, 58.0±17 kg), and height (burn, 141.1±9 vs. non-burn, 147.3±12 cm) (P>0.05 for all). Burn children had a lower growth BMI-for-age percentile than non-burned controls (54.6±37 vs. 92.5±6 %tile; P=0.008). In burn patients, testing occurred at 9.4±3 mo post burn.
Table 1. Subjects' physical and peak exercise characteristics (mean ± SD [range]).
| Characteristic | Burned | Non-Burned | P value |
|---|---|---|---|
| n (male/female) | 5 (3/2) | 8 (3/5) | - |
| Age (y) | 11.7±1.4[10-13] | 12.5±2.3[9-15] | 0.50 |
| Time of testing (months post burn) | 9.4±3.4[7-15] | - | - |
| Length from admit to discharge (days) | 13±3 [8-14] | - | - |
| Body Morphology | |||
| Height (cm) | 141.1±9.0[135-156] | 147.3±12.3[123-162] | 0.19 |
| Weight (kg) | 40.1±17.5[28-71] | 58.0±16.8[31-71] | 0.10 |
| BSA (m-2) | 1.2±0.3[1.0-1.7] | 1.5±0.3[1.0-1.9] | 0.09 |
| BMI (kg·m-2) | 19.7±5.5[15-20] | 25.3±4.9[21-36] | 0.08 |
| BMI (%tile) | *54.6±36.6[13-98] | 92.5±6.2[80-99] | 0.01 |
| Fat mass (kg) | 11.5±10.2[5-41] | 18.1±8.5[10-37] | 0.44 |
| Fat mass (% total body) | 25.3±10.0[17-41] | 30.5±7.2[27-41] | 0.19 |
| Lean mass (kg) | 27.8±7.5[25-41] | 39.1±11.5[21-59] | 0.08 |
| TBSA burn (%) | 49.2±3.6[45-53] | - | - |
| TBSA 3rd-degree burn (%) | 24.4±20.0[0-51] | - | - |
| Peak Aerobic Exercise | |||
| Peak VO2 (L min-1) | *1.2±0.3[0.7-1.4] | 2.1±0.7[1.3-3.6] | 0.02 |
| Peak VO2 (ml O2 kg TBM min-1) | 31.9±11.3[18-43] | 36.8±8.4[31-51] | 0.39 |
| Peak VO2 (ml O2 kg LBM min-1) | 44.4±13.5[29-57] | 53.7±7.3[44-62] | 0.12 |
| Respiratory exchange ratio (AU) | 1.1±0.1[1.0-1.2] | 1.1±0.1[1.0-1.3] | 0.68 |
| Exercise test time (min-1) | 12.2±4.1[9-18] | 13.6±2.4[11-18] | 0.46 |
| Peak HR (beat min-1) | *175±16[152-195] | 197±7[189-209] | 0.01 |
| Peak exercise work rate (W) | *101±79[27-125] | 282±163[94-545] | 0.04 |
BSA, body surface area; BMI, body mass index; BMI %ile, body mass index percentile for age; TBSA, total body surface area;VO2, volume of oxygen; TBM, total body mass; LBM, lean body mass; HR, heart rate.
Statistically different from non-burned healthy controls.
Peak and submaximal aerobic exercise
The burn and non-burn groups had similar peak VO2 capacity relative to total and lean body mass as well as similar final RER and total exercise time (Table 1). However, absolute VO2 was 42% less in the burned group (P<0.05). Additionally, burned children reached a peak exercise work rate (watts) that was 64% lower than that in non-burned healthy children (P<0.05). Burned children also had lower peak HR values (11% or 21 b pm decrease) than non-burned children (P<0.01).
Submaximal exercise was adequately matched between groups (Table 2). Absolute and total body mass- and lean body mass-normalized VO2 and exercise work rate did not differ between groups for either of the two stages of exercise (group × exercise interaction, P>0.05). However, relative intensity (as a percentage of peak) for VO2, HR, and work rate was different between groups. Burned children had greater relative oxygen cost, HR, and work rates than non-burned healthy children (group × exercise interaction, P<0.001).
Table 2. Subjects' absolute and relative submaximal exercise characteristics.
| Exercise Characteristic | Burned | Non-Burned | 2-Way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Interaction | Main Effect | ||||||
| Exercise Stage 1 | Exercise Stage 2 | Exercise Stage 1 | Exercise Stage 2 | Group × Exercise | Group | Exercise | |
| Absolute | |||||||
| VO2 (L min-1) | 0.4±0.1 | 0.7±0.1 | 0.6±0.2 | 0.7±0.3 | 0.68 | 0.13 | 0.008 |
| Exercise work rate (W) | 13.3±11 | 43.3±31 | 23.3±25 | 57.2±26 | 0.83 | 0.35 | 0.002 |
| Normalized (TBM, LBM) | |||||||
| VO2 (ml O2kgTBM min-1) | 12.6±6 | 18.6±7 | 10.5±4 | 13.1±5 | 0.59 | 0.84 | 0.007 |
| VO2 (ml O2kgLBM min-1) | 17.1±7 | 25.6±9 | 18.5±3 | 23.6±3 | 0.30 | 0.92 | <0.0001 |
| Exercise work rate (W kgTBM) | 0.37±0.4 | 1.0±0.5 | 0.40±0.4 | 0.99±0.4 | 0.75 | 0.89 | <0.0001 |
| Exercise work rate (W kgLBM) | 0.28±0.2 | 1.1±1.0 | 0.41±0.4 | 1.1±0.4 | 0.61 | 0.82 | 0.003 |
| Relative Intensity (%peak) | |||||||
| Peak VO2 (%) | 39±9 | 59±13+ | 32±8* | 41±11+ | 0.002 | 0.05 | <0.0001 |
| Peak HR (%) | 74±9 | 85±9+ | 69±4* | 83±9+ | 0.03 | 0.40 | <0.0001 |
| Peak exercise work rate (%) | 17±10 | 44±14+ | 12±16 | 26±19+ | 0.008 | 0.21 | <0.0001 |
VO2, volume of oxygen; TBM, total body mass; LBM, lean body mass; HR, heart rate.
P<0.05 for burned vs. non-burned.
P<0.05 for stage 1 vs. stage 2 exercise.
The cardiovascular response to submaximal exercise is attenuated in burned children
Cardiac output in the burned group was reduced by ∼28% at 20 W and 25% at50 W compared to the non-burned healthy group (group × exercise interaction, P=0.012) (Figure 1). SV did not differ from pre-exercise to 20 W and50 W in burned children. However, in the non-burned healthy group, SV increased from pre-exercise to 20 W by ∼21% and from pre-exercise to50 W by 19%(main effect for exercise, P<0.05).Burned children also had an attenuated HR response compared to non-burned healthy children (group × exercise interaction, P<0.01). The arteriovenous difference increased to a similar degree in each group (main effect for exercise, P<0.01). When cardiac output and SV were normalized to BSA (Figure 2), the SV index was similar between groups; however, the cardiac output index remained lower in the burned group (group × exercise interaction, P<0.01).
Figure 1.
Cardiac output (A), stroke volume (B), arterial-venous difference (C), heart rate (D), and oxygen consumption (E) during pre-exercise standing (pre-EX) and at 20 W and 50 W of exercise for burned (black filled circle; n=5) and non-burned children (open circle, n=8). *P<0.05 for burned vs. non-burned. +P<0.05 vs. pre-EX.
Figure 2.
Cardiac index (A) and stroke volume index (B) (normalized to body surface area) during pre-exercise (pre-EX) and at 20 W and 50 W of exercise for burned (black filled circle; n=5) and non-burned children (open circle, n=8). *P<0.05 for burned vs. non-burned. +P<0.05 vs. pre-EX.
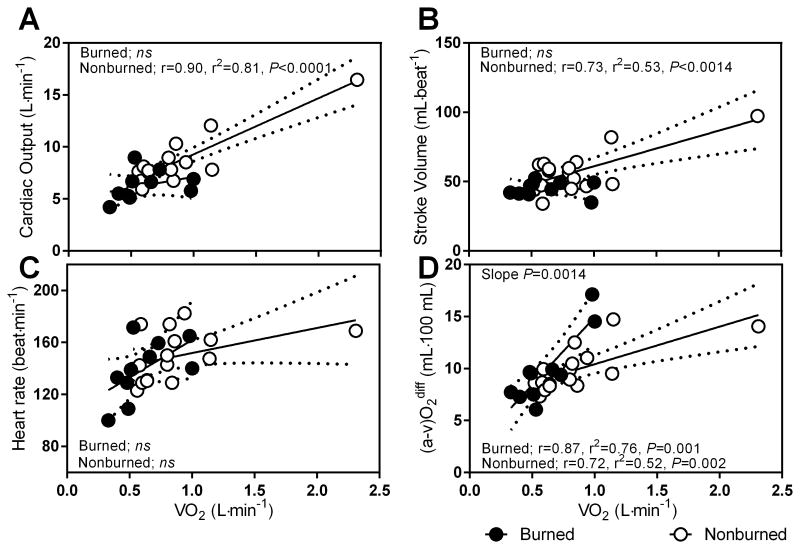
The relationships between oxygen uptake and cardiac output, stroke volume, and arteriovenous oxygen difference are altered in burned children
The cardiac output slope strongly correlated with oxygen uptake in the non-burned group, whereas there was no significant relationship in the burned group (Figure 3A, r=.90, P<0.0001). Likewise, SV slope strongly correlated with VO2 (r=.73, P<0.01), with no significant relationship being detected in the burned group. However, the slope for [a-v] O2 dif, and VO2 were strongly correlated (r=.72-.87) for both groups with significant slope differences (P<0.01).We note that one non-burned individual was larger in body size than the remainder of the non burned group. When we removed this individual, Q (r=.43, P=0.008), and (a-v)O2 (r=.47, P=0.005) remained strongly correlated; whereas SV did was not. In further analysis, we normalized to BSA to control for body size differences between burn and non-burned children and therefore kept this individual in the analysis.
Figure 3.
Slope and intercept for cardiac output (A), stroke volume (B), heart rate (C), and arterial-venous difference ([a-v]O2 diff) (D) during submaximal exercise for burned (black filled circle, n=5) and non-burned children (open circle, n=8).
Discussion
This study tested the hypothesis that burn trauma attenuates the cardiovascular response to submaximal exercise. We show, for the first time, that the cardiovascular response to submaximal exercise is weaker in burned children than innon-burned healthy children. Both cardiac output and SV were attenuated during submaximal exercise, with the former remaining reduced when normalized to BSA. Oxygen uptake and calculated arteriovenous difference were not different between groups. However, burn injury was associated with lower peak HR values during the peak VO2 test along with a reduced HR and cardiac output response. These data suggest that cardiac dysfunction from burn injury may play a role in the impaired cardiovascular response to submaximal exercise in burned children.
It is worth mentioning that no established protocol exists for exercise testing in children. Children do not exhibit clear symptoms of fatigue that are supported by maximal HRs that level off at about 200, a RER>1.0, and a plateau in VO2(1). Notably, this is due to children's development of cellular metabolic capacity (31). Because children generate less lactate and excess CO2, an RER of 1.0 has been commonly used. However, we control for these by including only criteria established in adults and comparing groups with similar peakVO2 characteristics (no difference in time to exhaustion, RER, or peakVO2).Nevertheless, even though groups were matched for peak exercise capacity, peak work rate was significantly attenuated in the burned children. Additionally, we found for the first time that burned children have a significantly lower peak HR than non-burned healthy children.
Others have shown that adults assessed at 5 to 10 years post burn have a lower peak VO2 and time to fatigue when compared to non-burned healthy adults (41) and published norms (10). In fact, Ganio et al. reported that 88% of the burned adult subjects studied had values below American Heart Associationage-adjusted normative values. Innon-burned adults, low cardiorespiratory fitness and aerobic exercise capacity (peak VO2) are strong predictors of all-cause mortality (18),and burned adults with low aerobic fitness may be at greater risk of all-cause mortality relative to the general population. No standardized norms for children younger than 13 years old currently exist. Additionally, because both age and sex reportedly influence peak VO2 in children, use of the ratio scale (i.e., kg total body mass) and absolute values may not be the best way to measure cardio respiratory fitness (2). However, according to normative data for non-burned healthy children aged 13 to 19 years available from The Cooper Institute for Aerobics Research, all of our burned children would fall into the very poor (<25 mlO2·kg-1·min-1)-to-poor (25-31ml O2·kg-1·min-1) category (using standard ratio scaling) (15). In a review by Matecki et al., healthy non-trained boys and girls reportedly had a peak VO2 of 47 ml O2 kg min-1and 40 ml O2·kg·min-1, respectively (19). Cooper et al. reported that healthy childrenaged 6 to 13years had aerobic capacities of 47 ml O2·kg-1·min-1(4). We have reported in several other studies that at hospital discharge our children with severe burn injuries have valuesin the range of 24 to 32 ml O2·kg-1·min-1(12, 26).
Additional work from our institute has shown that metabolic and cardiovascular impairments are sustained for up to 3 years after burn injury (11, 14, 16, 40). How burn trauma affects cardiac function in humans is still not entirely clear. In animal models, burn trauma causes an increase in cardiac myocyte secretion of inflammatory cytokines (i.e., interleukin 1β, interleukin 6, and tumor necrosis factor α) coupled with an alteration in cardiac calcium homeostasis that is associated with impaired cardiac contraction and relaxation (32).There seems to be a parallel between the burn patients and patients with heart failure. A hallmark of heart failure is exercise intolerance, which is reportedly due to inadequate blood flow to active skeletal muscle secondary to impaired cardiac output (33, 43). We have also observed exercise intolerance in our children with severe burn injury (unpublished findings), and we have found that, exercising at 75% of their peak VO2, burned children can complete only 20 min of exercise, while non-burned age-matched controls complete the full 30 min. These children report crying, nausea, and fatigue. Notably, we have previously shown that burned children have profound elevations in skin blood flow perfusion (40-60% of max) compared to non-burned controls (∼10% of max)(30).Because of the hyper metabolic state and inability of damaged skin to regulate body temperature, issues that are similar to those seen in patients with heart failure may contribute to an impaired cardiovascular response to exercise due to the profound redistribution of blood to the peripheral skin; however, this is speculative and requires further understanding.
Notably, Reynolds et al. reported that, in children, burn injury causes cardiac failure, particularly left ventricular myocardial depression (28). In heart failure patients, there is a modest rise in SV (50-65ml) relative to that seen in healthy subjects (>100 ml). We observed a similar effect in our burn and non-burn groups. This attenuated increase in SV in heart failure populations is due to a blunted ability to increase both LV preload and ejection fraction (33, 43). Additionally, the heart failure literature suggests this is also due to a lower maximal heart rate, dilated left ventricle, and reduced resting left ventricular systolic function (25). Our analysis of burned children likewise revealed attenuated peak heart rate values during the peak exercise stress test, as seen by comparison to healthy controls. Most interesting patients with heart failure, the inability to increase left ventricular systolic emptying is due to an impaired intrinsic contractility, reduced(&F062)-adrenergic responsiveness, and peripheral arterial vasodilator response to exercise. Therefore it seems likely that the chronic sympathetic autonomic nervous activation induced by burn trauma may alter(&F062)-adrenergic responsiveness in cardiac muscle; however, this hypothesis requires further investigation.
Burn trauma also increases energy expenditure, and protein catabolism causes the loss of lean body mass and muscle wasting (13). Notably, in this study, we showed that burned children had a reduced growth BMI-for-age percentile compared to non-burned healthy children. In addition, burn patients are subjected to prolonged immobilization due to bed rest, which may cause deconditioning. In non-burned healthy adults, 14 to 60 days of sedentary bed restreduces SV and left ventricular mass (23, 39).Burn patients in the current study were hospitalized for 8-14 days. Therefore, it is not surprising that cardiovascular and strength conditioning may reverse muscle wasting and physical capacity (12, 26). Thus, exercise training immediately after discharge is an important component of the standard of care in hospital settings because of its ability to restore lean body mass and exercise capacity (12, 26). Work from our laboratory has shown that a 12-week exercise program involving strength and aerobic exercise training has been shown to improve peak torque and peakVO2 in conjunction with lean body mass (via improved fractional synthetic rate) (12). However, whether adaptations to exercise training also involve improvements in cardiovascular function in burned populations is currently unknown. Our study controlled for peak aerobic capacity and submaximal oxygen utilization (matched for both) and showed that when oxygen kinetics (VO2 and [a-v]O2diff) were similar, both cardiac output and SV were reduced during submaximal exercise in burned children (Figures1 and 3). This finding suggests that children with burn injury have impaired cardiovascular response and that exercise training may improve cardiovascular physiology, though this requires further study in burn patients.
Only a few others have investigated cardiac output and SV in response to submaximal exercise in healthy children using rebreathing cardiac output methods, and our data in non-burned healthy children are similar (36, 37). Cardiac physiology may differ between burned adults and children. Turley and Wilmore found that in non-burned healthy 7- to 9-year-olds, cardiac output and SV response to exercise differed from that in adults owing to smaller hearts and a smaller absolute amount of muscle doing any given work rate (37). Given that our burned group had significantly less age-sex BMI, this response may have been attributable to blunted growth from burn injury. However, as previously mentioned, because of the confounding effect of body size and growth in children with regard to exercise capacity and the use of standard ratio scaling, others have recommended that SV and cardiac output also be reported (i.e., to BSA)(5, 6). When we normalized both groups to BSA, cardiac output remained lower in burned children, further supporting the notion that burned children have impaired cardiovascular responses to submaximal exercise.
The relative intensity of exercise is commonly used for exercise prescription purposes in clinical populations (24). For adults, relative HR-based prescriptions are used for exercise training given the linear relationship between HR response and oxygen uptake during exercise. However, no validated approaches currently exist for children, and we have shown the percent peak HR, peakVO2, and peak exercise work rate significantly differs between burned and non-burned healthy children, a finding that requires further study. We have also shown that burned children have an attenuated peak HR compared to non-burned children, suggesting that formulas developed by others (35)to predict peak HR values for the purpose of estimating exercise intensity (percent peak HR) may not be a valid approach in burned populations.
In summary, we show that children with severe burns have a reduced cardiovascular response to submaximal exercise relative to non-burned healthy children. Further examination of the cardiovascular response to exercise in larger samples and the exercise training response for improving cardiovascular function have important implications for rehabilitation exercise medicine. In addition, understanding these responses will offer insight into how to reduce long-term cardiovascular deficiencies and the risk of cardiovascular diseases in burned populations.
Acknowledgments
We would like to extend our sincere gratitude to the patients and their families who prolong their stay at our hospital to participate in rehabilitative exercise programs. We thank the skilled staff of the Wellness Center at Shriners Hospitals for Children®—Galveston for overseeing all patient testing, and the clinical research staff at Shriners Hospitals for Children®—Galveston for supporting patient recruitment and scheduling. Lastly, we would like to thank Dr. Kasie Cole for editorial assistance.
Funding: This work was supported by grants from the National Institutes of Health (P50-GM060338, R01-GM056687, and R01-HD049471),the National Institute for Disabilities, Independent Living and Rehabilitation Research (90DP00430100), and Shriners Hospitals for Children (84080 and 84090).
The authors have no competing interest to report. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. This work was supported by grants from the National Institutes of Health (P50-GM060338, R01-GM056687, and R01-HD049471), the National Institute for Disabilities, Independent Living and Rehabilitation Research (90DP00430100), and Shriners Hospitals for Children (84080 and 84090).
Footnotes
Competing interest: The authors have no competing interest to report. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Armstrong N, Welsman J, Winsley R. Is peak VO2 a maximal index of children's aerobic fitness? International journal of sports medicine. 1996;17(5):356–9. doi: 10.1055/s-2007-972860. [DOI] [PubMed] [Google Scholar]
- 2.Berndtsson G, Mattsson E, Marcus C, Larsson UE. Age and gender differences in VO2max in Swedish obese children and adolescents. Acta paediatrica. 2007;96(4):567–71. doi: 10.1111/j.1651-2227.2007.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen English Version. 2012 [Google Scholar]
- 4.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. Journal of applied physiology: respiratory, environmental and exercise physiology. 1984;56(3):628–34. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 5.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. Journal of the American College of Cardiology. 1995;25(5):1056–62. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 6.de Simone G, Devereux RB, Daniels SR, et al. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation. 1997;95(7):1837–43. doi: 10.1161/01.cir.95.7.1837. [DOI] [PubMed] [Google Scholar]
- 7.Desai MH, Mlcak RP, Robinson E, et al. Does inhalation injury limit exercise endurance in children convalescing from thermal injury? The Journal of burn care & rehabilitation. 1993;14(1):12–6. doi: 10.1097/00004630-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5(5):303–11. 1916. discussion 12-3. [PubMed] [Google Scholar]
- 9.Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Understanding the long-term impacts of burn on the cardiovascular system. Burns : journal of the International Society for Burn Injuries. 2016;42(2):366–74. doi: 10.1016/j.burns.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Ganio MS, Pearson J, Schlader ZJ, et al. Aerobic Fitness Is Disproportionately Low in Adult Burn Survivors Years After Injury. Journal of burn care & research : official publication of the American Burn Association. 2015;36(4):513–9. doi: 10.1097/BCR.0b013e3182a22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. The Journal of clinical endocrinology and metabolism. 2009;94(5):1656–64. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardee JP, Porter C, Sidossis LS, et al. Early rehabilitative exercise training in the recovery from pediatric burn. Medicine and science in sports and exercise. 2014;46(9):1710–6. doi: 10.1249/MSS.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart DW, Wolf SE, Chinkes DL, Lal SO, Ramzy PI, Herndon DN. Beta-blockade and growth hormone after burn. Annals of surgery. 2002;236(4):450–6. doi: 10.1097/00000658-200210000-00007. discussion 6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Annals of surgery. 2012;256(3):402–11. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyward VH, Gibson AL. Advanced fitness assessment and exercise prescription. Seventh. Champaign, IL: Human Kinetics; 2014. pp. xiv–537. pages p. [Google Scholar]
- 16.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PloS one. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. Journal of applied physiology. 2000;88(5):1650–8. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 18.Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation. 2010;122(8):790–7. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 19.Matecki S, Prioux J, Amsallem F, et al. [Maximal oxygen uptake in healthy children: factors of variation and available standards] Revue des maladies respiratoires. 2001;18(5):499–506. [PubMed] [Google Scholar]
- 20.McElroy K, Alvarado MI, Hayward PG, Desai MH, Herndon DN, Robson MC. Exercise stress testing for the pediatric patient with burns: a preliminary report. The Journal of burn care & rehabilitation. 1992;13(2 Pt 1):236–8. doi: 10.1097/00004630-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Mlcak RP, Desai MH, Robinson E, McCauley RL, Richardson J, Herndon DN. Increased physiological dead space/tidal volume ratio during exercise in burned children. Burns : journal of the International Society for Burn Injuries. 1995;21(5):337–9. doi: 10.1016/0305-4179(94)00017-4. [DOI] [PubMed] [Google Scholar]
- 22.O'Connell TD, Jensen BC, Baker AJ, Simpson PC. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacological reviews. 2014;66(1):308–33. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest : “cardiovascular deconditioning” or hypovolemia? Circulation. 2001;103(14):1851–7. doi: 10.1161/01.cir.103.14.1851. [DOI] [PubMed] [Google Scholar]
- 24.Pescatello LS. ACSM's guidelines for exercise testing and prescription. 9th. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. American College of Sports Medicine; pp. xxiv–456. p. p. [Google Scholar]
- 25.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 26.Porter C, Hardee JP, Herndon DN, Suman OE. The role of exercise in the rehabilitation of patients with severe burns. Exercise and sport sciences reviews. 2015;43(1):34–40. doi: 10.1249/JES.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter C, Tompkins RG, Finnerty CC, Sidossis LS, Suman OE, Herndon DN. The metabolic stress response to burn trauma: current understanding and therapies. Lancet. 2016;388(10052):1417–26. doi: 10.1016/S0140-6736(16)31469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds EM, Ryan DP, Sheridan RL, Doody DP. Left ventricular failure complicating severe pediatric burn injuries. Journal of pediatric surgery. 1995;30(2):264–9. doi: 10.1016/0022-3468(95)90572-3. discussion 9-70. [DOI] [PubMed] [Google Scholar]
- 29.Riddell MC. The endocrine response and substrate utilization during exercise in children and adolescents. Journal of applied physiology. 2008;105(2):725–33. doi: 10.1152/japplphysiol.00031.2008. [DOI] [PubMed] [Google Scholar]
- 30.Rivas E, McEntire SJ, Herndon DN, Mlcak RP, Suman OE. beta-adrenergic blockade does not impair the skin blood flow sensitivity to local heating in burned and non-burned skin under neutral and hot environments in children. Microcirculation. 2017 doi: 10.1111/micc.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowland T, Saltin B. Learning from children: the emergence of pediatric exercise science. Journal of applied physiology. 2008;105(1):322–4. doi: 10.1152/japplphysiol.90624.2008. [DOI] [PubMed] [Google Scholar]
- 32.Sayeed MM. Signaling mechanisms of altered cellular responses in trauma, burn, and sepsis: role of Ca2+ Archives of surgery. 2000;135(12):1432–42. doi: 10.1001/archsurg.135.12.1432. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan MJ, Cobb FR. Central hemodynamic response to exercise in patients with chronic heart failure. Chest. 1992;101(5 Suppl):340S–6S. doi: 10.1378/chest.101.5_supplement.340s. [DOI] [PubMed] [Google Scholar]
- 34.Suman OE, Thomas S, Beck KC, Mlcak RP, Herndon DN. Comparison of carbon monoxide (CO) single breath pulmonary diffusing capacity with non-rebreathing, open-circuit CO pulmonary diffusing capacity in healthy children. Pediatric pulmonology. 2006;41(11):1095–102. doi: 10.1002/ppul.20504. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology. 2001;37(1):153–6. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 36.Turley KR, Wilmore JH. Cardiovascular responses to submaximal exercise in 7- to 9-yr-old boys and girls. Medicine and science in sports and exercise. 1997;29(6):824–32. doi: 10.1097/00005768-199706000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Turley KR, Wilmore JH. Cardiovascular responses to treadmill and cycle ergometer exercise in children and adults. Journal of applied physiology. 1997;83(3):948–57. doi: 10.1152/jappl.1997.83.3.948. [DOI] [PubMed] [Google Scholar]
- 38.Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. American journal of clinical dermatology. 2003;4(4):245–72. doi: 10.2165/00128071-200304040-00004. [DOI] [PubMed] [Google Scholar]
- 39.Westby CM, Martin DS, Lee SM, Stenger MB, Platts SH. Left ventricular remodeling during and after 60 days of sedentary head-down bed rest. Journal of applied physiology. 2016;120(8):956–64. doi: 10.1152/japplphysiol.00676.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams FN, Herndon DN, Suman OE, et al. Changes in cardiac physiology after severe burn injury. Journal of burn care & research : official publication of the American Burn Association. 2011;32(2):269–74. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willis CE, Grisbrook TL, Elliott CM, Wood FM, Wallman KE, Reid SL. Pulmonary function, exercise capacity and physical activity participation in adults following burn. Burns : journal of the International Society for Burn Injuries. 2011;37(8):1326–33. doi: 10.1016/j.burns.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Wilmore DW, Long JM, Mason AD, Jr, Skreen RW, Pruitt BA., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Annals of surgery. 1974;180(4):653–69. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984;69(6):1079–87. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]