Abstract
The global prevalence of obesity highlights the importance of understanding on regulation of energy homeostasis. The central melanocortin system is an important intersection connecting the neural pathways controlling satiety and energy expenditure to regulate energy homeostasis by sensing and integrating the signals of external stimuli. In this system, neural melanocortin receptors, melanocortin-3 and -4 receptors (MC3R and MC4R), play crucial roles in the regulation of energy homeostasis. Recently, multiple intracellular signaling pathways and biased signaling at neural MCRs have been discovered, providing new insights into neural MCR signaling. This review attempts to summarize biased signaling including biased receptor mutants (both naturally occurring and lab-generated) and biased ligands at neural MCRs, and to provide a better understanding of obesity pathogenesis and new therapeutic implications for obesity treatment.
Keywords: Melanocortin-3 receptor, melanocortin-4 receptor, obesity, energy homeostasis, biased signaling, AgRP
1. Introduction
Obesity is defined as a state of abnormal or excessive fat accumulation with risk of developing chronic conditions associated with several adverse health consequences such as type 2 diabetes mellitus, hypertension, cardiovascular disease, and certain type of cancer [1]. Obesity has become a global health issue, with 1.46 billion adults overweight and over 200 million men and 300 million women obese worldwide [2]. In the United States, 8.1% of infants and toddlers have abnormally high weight for recumbent length, and 16.9% of 2- to 19-year-olds and 34.9% of adults aged 20 years or older are obese [3]. Obesity is commonly caused by imbalanced energy intake and expenditure. Studies indicated that genetic factors are involved in obesity pathogenesis [4].
Neural melanocortin receptors (MCRs), including melanocortin-3 and melanocortin-4 receptors (MC3R and MC4R), two of the five melanocortin receptors (MC1R to MC5R), are highly expressed in central nervous system (CNS) [5-8]. Neural MCRs have been shown to be involved in regulating various physiological processes, such as energy homeostasis, cardiovascular function, reproduction and sexual function, anti-inflammation, and other functions [9-12]. MC3R and MC4R are components in leptin-melanocortin pathway and have been extensively studied in obesity pathogenesis. Targeted deletion of Mc3r and Mc4r in mice causes obesity [13-15]. These two neural MCRs have non-redundant roles in regulation of energy homeostasis as emphasized by mice lacking both receptors exhibiting more severe obesity phenotypes than mice lacking either receptor alone [14]. Subsequent studies have shown that MC3R and MC4R exert different functions in regulation of energy homeostasis. MC3R primarily regulates feed efficiency [14-16] and feeding rhythm [17-19], whereas MC4R plays an essential role in controlling both food intake and energy expenditure [11, 20]. Human genetic studies provided further evidence for the function of these two receptors in regulating energy homeostasis. MC3R and MC4R were shown to be involved in human obesity pathogenesis, although the role of MC3R in human obesity pathogenesis is more controversial [21-27] (reviewed in [10, 11, 28-31].
Neural MCRs share the same endogenous agonists and antagonists. There are four endogenous agonists, including α-, β-, and γ-melanocyte-stimulating hormone (MSH), and adrenocorticotropic hormone (ACTH). They are post-translational processing products of the precursor polypeptide pro-opiomelanocortin (POMC) which is primarily produced by arcuate nucleus of the hypothalamus (ARC) in CNS and the anterior pituitary as well as the skin [32]. The post-translational processing of POMC is tissue specific and only MSHs are produced in the CNS [33]. As the major endogenous agonist in CNS, α-MSH activates neural MCRs to induce a negative energy balance, consisting the catabolic arm of melanocortin system [34]. Among numerous G protein-coupled receptors (GPCRs), of which MCRs belong to, MCRs are quite unique to have endogenous antagonists Agouti and Agouti-related peptide (AgRP). Agouti binds to MC1R with high affinity [35], whereas AgRP selectively binds to neural MCRs [36, 37]. AgRP principally antagonizes α-MSH binding to neural MCRs and stimulates a long-lasting increase in food intake to result in a positive energy balance, representing the anabolic arm of melanocortin system [34, 38]. However, recent studies in vivo using mouse model lacking neuronal MSH suggested that the delayed and long-lasting effects of AgRP on appetite control are independent of melanocortin signaling [39, 40], and basal intracellular cAMP levels in cells with MC3R or MC4R expression are decreased by AgRP [39, 41-44], indicating AgRP to be an inverse agonist independent of antagonizing α-MSH.
The conventional signaling pathways of neural MCRs are mediated by the stimulatory G protein, Gαs. Most of the earlier studies on neural MCRs focused on Gs-mediated signaling. However, increasing number of recent studies have demonstrated that similar to other GPCRs, neural MCRs can couple to other G proteins or signaling mediators to trigger the downstream signaling pathways, in addition to the conventional Gαs-cAMP signaling pathway. Biased signaling was also proposed for neural MCRs since we and others identified some biased mutant neural MCRs which selectively trigger signaling pathways upon ligand stimulation, and some biased agonists which can selectively induce signaling pathways through neural MCRs [45-56].
In this review, we will first briefly summarize the central melanocortin system and its importance in regulation of energy homeostasis. Then, the multiple intracellular signaling pathways, biased signaling, and therapeutic relevance of biased signaling at neural MCRs will be reviewed. Considering the physiological roles of MC4R in regulation of energy homeostasis have been well established and MC3R in human obesity pathogenesis is controversial, we will primarily focus on MC4R. Insights gained from these studies will enhance our understanding on neural MCR signaling and lead to new therapeutic strategies for obesity as well as other neural MCR-related diseases.
2. The central melanocortin system in energy homeostasis
The central melanocortin system is defined as a collection of central nervous system circuits including different types of neurons in CNS that express wither the ligands or the receptors. There are two subsets of neurons within the ARC that express endogenous ligands POMC and AgRP for neural MCRs, respectively [57]. These two subsets of neurons are described as “first order” neurons since they are able to sense and integrate the external stimuli (humoral and nutrient cues) including leptin, insulin, ghrelin, serotonin, orexin, and glucose [34, 58-63]. Among these external stimuli, leptin is the most significant ligand, circulating at levels proportional to the body fat mass to act on the full-length leptin receptor (LepRb) highly expressed in first order neurons [58, 64], thus stimulating the POMC neurons to increase α-MSH production and suppressing AgRP neurons to inhibit the release of AgRP. Neurons expressing MC3R and MC4R within multiple areas of brain are the target of α-MSH and AgRP, which are defined as “second order” neurons [34]. MC3R and MC4R in these neurons can be activated by α-MSH or inactivated by AgRP to exhibit negative or positive energy balance, respectively [34].
The MC4R is the most intensively studied target in central melanocortin system. In Mc4r knockout mouse model, it was demonstrated that MC4R is required for inhibitory effect of leptin on food intake [65] and involved in regulation of metabolism [66]. Notably, control of food intake and energy expenditure is exerted by different neurons, as demonstrated using loxTB Mc4r mice (transcription of Mc4r gene was blocked by loxTB sequence), with MC4R in paraventricular nucleus of hypothalamus (PVH) and amygdala regulating food intake while MC4R on neurons outside of PVH and amygdala controlling energy expenditure [20]. Restoration of MC4R expression in PVH/amygdala using Cre-lox technique attenuates 60% of weight gain in loxTB Mc4r mice [20]. The increased food intake is completely rescued since 100% of the hyperphagia is prevented in those mice, while reduced energy expenditure is not altered, thus indicating the divergence of melanocortin pathways in controlling food intake and energy expenditure [20]. Subsequent studies showed that MC4R regulates energy expenditure via neurons located in the intermediolateral nucleus of the spinal cord (IML) [67] and dorsomedial nucleus of the hypothalamus (DMH) [68]. Moreover, cardiovascular functions, linear growth, and cholesterol metabolism are mediated by MC4R within the PVN, whereas glucose metabolism and thermogenesis are mediated by MC4R outside of the PVN [68, 69]. Another recent study demonstrated that MC4R in POMC neurons may act as an auto-excitatory and/or an auto-potentiation mechanism enhancing POMC neuron activation to regulate energy homeostasis [70].
Although studies on functional roles of MC3R in energy homeostasis are less extensive compared with that of the MC4R, MC3R is also important in regulation of energy homeostasis [14, 15]. Different from Mc4r deletion causing hyperphagia in mice, Mc3r deleted mice exhibit increased fat mass and reduced lean mass despite normal food intake or hypophagia [14, 15], suggesting that MC3R is primarily involved in regulation of feed efficiency. Another important role of MC3R in maintaining energy homeostasis is regulation of circadian rhythm: Mc3r deleted mice display abnormal rhythmic expression of clock genes [19], impaired behavioral adaption [18], and anomalous metabolic adaption to restricted feeding [17]. Also, MC3R expressed by POMC neurons was identified and proposed to serve as auto-inhibitory role inhibiting POMC neuron activation to regulate energy homeostasis [71, 72].
Collectively, the central melanocortin system serves as the intersection point which connects the neural pathways controlling satiety and metabolism to maintain energy homeostasis by sensing and integrating the signals of external stimuli (Fig. 1).
Fig. 1. The central melanocortin system.
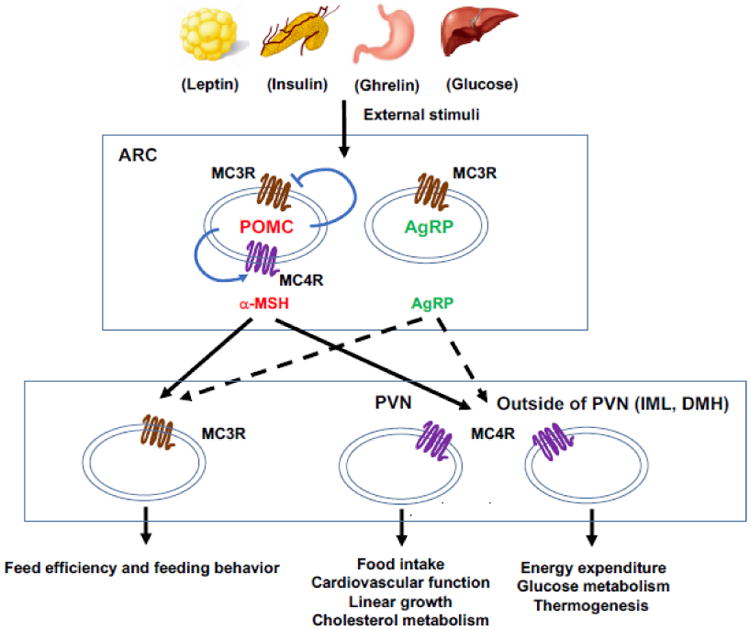
POMC and AgRP neurons in the ARC are defined as “first order” neurons which integrate external stimuli and project to “second order” neurons with neural MCR expression. Neurons in PVN and IML mediate different physiological functions in energy homeostasis. MC3R and MC4R in POMC neurons can also serve auto-inhibitory and auto-excitatory roles to regulate energy homeostasis.
3. Intracellular signaling pathways of neural MCRs
On the cell membrane, upon agonist binding, neural MCRs are activated and undergo conformational change, thereby generating intracellular signaling, including both G protein-dependent and -independent signaling.
Like other Gαs-coupled GPCR family members, once neural MCRs are activated, the α-subunit of Gs protein will disassociate from the βγ heterodimer and then activate adenylyl cyclase (AC) to increase the intracellular cAMP levels and subsequently activate protein kinase A (PKA). This Gαs-protein mediated signaling is the conventional intracellular signaling and also the most commonly investigated in MCR intracellular signaling studies.
In addition to Gαs, the MC4R can also couple to other G proteins, such as Gαi protein which inhibits AC activity to decrease cAMP level and Gαq protein which activates phospholipase C (PLC) and downstream kinase protein kinase C (PKC) [47, 73]. Büch et al. observed that in hypothalamic cell line GT1-7 cells, α-MSH-induced incorporation of GTPγS35 (a measure of G protein activation) can be partially (∼50%) blocked by pertussis toxin (PTX) and α-MSH-induced cAMP accumulation is increased due to the PTX treatment by 53% (PTX is a Gi inhibitor), suggesting that both Gαs and Gαi proteins are activated through MC4R upon α-MSH stimulation [47]. Newman et al. confirmed that MC4R mediates increase of intracellular Ca2+ level through Gαq-PLC dependent signaling in another hypothalamic cell line, GT1-1 cells [73]. Other signaling pathways are also identified in cells expressing MC4Rs, including extracellular signal–regulated kinases 1/2 (ERK1/2), c-Jun N-terminal kinases (JNK), 5′ AMP-activated protein kinase (AMPK), and protein kinase B (PKB or AKT) [56, 74-79].
ERK1/2 pathway is one of three mitogen-activated protein kinases (MAPK) pathways, and the other two pathways are JNK and p38 [80]. ERK1/2 activation through MC4R varies based on cell types and ligands used. In GT1-7 cells, ERK1/2 activation through MC4R induced by α-MSH is only PKA-dependent [79]. In rat solitary nucleus neurons, the PKA-dependent ERK1/2 activation through MC4R is increased after central administration of MCR agonist, Melanotan II (MTII) [76]. NDP-α-MSH induces ERK1/2 activation via Gi protein in HEK293 cells expressing MC4Rs, but through Ca2+/PKC pathway in GT1-1 cells [81]. In CHO-KI cells, ERK1/2 activation through MC4R is mediated by phosphoinositide 3-kinase (PI3K) rather than PKA [75]. The diversity of ERK1/2 activation through MC4R makes this signaling pathway complicated. Although β-arrestin mediated ERK1/2 activation was observed in MC5R [82] and other GPCRs such as β2-adrenergic receptor (β2-AR), V2 vasopressin receptor (V2R), parathyroid hormone receptor, subtype 1 (PTH1R), and angiotensin II receptor type 1A (AT1R) [83], and even β-arrestin mediated MC4R endocytosis was demonstrated [46, 84], there is still no evidence of β-arrestin-mediated ERK1/2 activation in MC4R.
For the JNK pathway, NDP-α-MSH does-dependently inhibits JNK in HEK293 cells stably expressing MC4R [78]. AMPK phosphorylation level was demonstrated to be decreased by MC4R activation with MTII in PVH neurons and NDP-α-MSH stimulation in MC4R-transfected HEK293T cells [56, 74]. AKT activation is induced by NDP-α-MSH in GT1-7 cells [56]. Ion channels on the cell membrane are thought to be effectors in a variety of signaling pathways initiated by GPCRs. Regulation of ion channels by G protein can be indirect (via second messengers and activation of downstream kinase) or direct (via G protein βγ subunits) [85]. One recent study showed that MC4R in PVH neurons can couple to potassium channel Kir7.1 independent of G protein [54], further expanding the intracellular signaling effectors of MC4R.
Intracellular signaling of MC3R, compared to that of MC4R, is less extensively studied. In addition to the Gαs signaling pathway, MC3R can couple to Gαi protein and activate ERK1/2 signaling via a Gαi protein-PI3K signaling system in HEK293 cells upon NDP-α-MSH stimulation, which is independent of PKA, PKC and Ca2+ [77]. However, data obtained from our lab showed that AgRP induces ERK1/2 activation through MC3R in HEK293T cells independent of PKA and PI3K [56], suggesting the mechanism of ERK1/2 activation through MC3R is also different based on cell types and ligands. As with MC4R, it is still unclear on the role of β-arrestin in ERK1/2 activation, although MC3R endocytosis and desensitization are mediated by β-arrestin [46, 86]. MC3R activation was also demonstrated to activate AKT [56, 86], to increase intracellular Ca2+ levels dependent or independent of inositol 1,4,5 triphosphate (IP3) [87, 88], to induce PKC pathway [89], and to induce sustained inhibition of AMPK activity [56].
So far, several studies have identified the physiological functions of multiple intracellular signaling pathways initiated by neural MCRs. The conventional Gαs signaling of MC4R has been shown to be crucial in inducing anorexigenic signaling in hypothalamus to result in a negative energy balance. However, paradoxical evidences that some constitutively active mutant MC4Rs with increased cAMP generation were identified from obese individuals [11] suggest that Gαs signaling pathway through MC4R is not the only pathway involved in regulation of energy homeostasis, and other pathways through MC4R independent of Gαs protein may also be involved. Further studies confirmed that only energy expenditure mediated by MC4R is through Gαs signaling pathway since CNS-specific Gαs deficiency results in a specific defect in energy expenditure without effect on food intake [90, 91]. Food intake, as another aspect of maintaining energy homeostasis through MC4R, is shown to be controlled by several signaling pathways. Regulation of food intake mediated by MC4R is controlled by Gαq signaling pathway with the evidence that PVN-specific Gαq deletion causes hyperphagic obesity without affecting energy expenditure [69].
MC4R modulation of the AMPK pathway in PVH neurons is also involved in regulating food take. MC4R activation decreases AMPK phosphorylation thereby inhibiting food intake [74]. ERK1/2 signaling through MC4R is thought to be involved in regulating energy homeostasis by mediating inhibition of food intake [76, 79] in addition to its function on mediating cell proliferation and inhibiting apoptosis [81]. A recent study showed that food intake inhibition caused by MC4R activation is dependent on coupling to closure of potassium channel Kir7.1 to cause PVH neuron depolarization [54]. Although MC3R primarily regulates energy homeostasis by controlling feed efficiency and maintaining circadian rhythm, the relationship between its G protein-mediated intracellular signaling pathways and the physiological effects has not been widely investigated. Additionally, MC3R-mediated ERK1/2 signaling cascade is involved in regulation of feeding behaviors [18] other than its function on mediating cell proliferation [77] and anti-inflammation [55].
4. Biased signaling at neural MCRs
4.1. Multi-state model of receptor activation and biased signaling
The models for GPCR activation and signaling has been debated over several decades. Initially, receptor activation was modeled with the ternary complex model in which ligand-receptor-G protein (or transducer) complex triggers the downstream signaling [92]. Then, the revised and extended ternary complex model of GPCR was developed due to the discovery of constitutively active mutants which exhibit capability of triggering G protein signaling even in the absence of ligand [93]. This model explains constitutive activation as an alteration of the normal equilibrium between the inactive and active state of receptor, and constitutive activation shifts a higher proportion of receptor in active state [94-96]. At present, the multi-state model of receptor activation has been widely accepted due to the evidences obtained from many different pharmacological studies [95]. Different from the previous ternary complex models suggesting receptor has a single signaling-competent conformation resulting in activation of all signaling pathways, this multi-state model highlights that receptor activation is a highly dynamic process in which multiple active conformations can be induced by different molecules to mediate different signaling pathways [95, 97], thus introducing the concept of biased signaling: activated receptor selectively triggers a distinct signaling pathway rather than others [94]. Biased signaling, as an important concept in GPCR intracellular signaling, has been identified in many members of GPCR family [97, 98]. Data from us and others [45, 47, 51, 52, 56] suggested that neural MCRs are able to selectively stabilize particular receptor active conformations and preferentially trigger distinct signaling pathways, consistent with the multi-state model of receptor activation and biased signaling in other GPCRs.
4.2. Biased mutants identified in neural MCRs
Biased mutants are the receptor mutants which selectively adopt particular receptor active conformation upon normal ligand stimulation, resulting in differential signaling to the different signaling pathways [97]. In recent years, biased mutants have been identified with an increasing number in neural MCRs. The first MC4R biased mutant associated with severe early-onset obesity is D90N [47, 99]. This receptor mutant is unable to initiate Gαs signaling even though it can bind agonists normally [99]. Subsequently, D90N was demonstrated to couple to Gαi protein rather than Gαs protein to decrease cAMP levels in pertussis toxin (PTX)-sensitive manner [47]. Both inactivated Gαs signaling and activated Gαi signaling might account for the obesity phenotype of this mutant [100]. We recently observed biased signaling in 25 naturally occurring mutant MC4Rs [51]. These mutants initiate biased signaling in ligand-induced Gαs-cAMP level and ERK1/2 phosphorylation level [51]. Among these 25 biased mutants, five Class V variants (C40R, V50M, T112M, A154D and S295P) have defective ERK1/2 signaling despite normal cell surface expression, ligand binding, and agonist-stimulated cAMP generation [51]. This defective ERK1/2 signaling might account for obesity in patients harboring these mutations [51]. In structure-function studies, many artificially generated mutant MC4Rs also exhibit biased signaling in Gαs-cAMP and ERK1/2 activation [48, 49, 101]. Additionally, some naturally occurring and artificially generated mutant MC3Rs are also biased in Gαs-cAMP and ERK1/2 signaling pathways [52, 53, 102].
Inactive or partially active MC4R mutants were considered as the crucial genetic factors leading to obesity. Conventionally, in many previous studies, only ligand-induced Gαs-cAMP signaling pathway was taken into consideration in MC4R functional analyses, whereas the roles of other signaling pathways in obesity pathogenesis were neglected. Discovery of biased mutants provides us new understanding on causal relationship between mutant neural MCRs and the obesity phenotype. Not only conventional Gαs signaling accounts for the regulation of energy homeostasis through neural MCRs, other signaling pathways are also involved in this process. Defects in signaling pathways other than Gαs (such as Gαi or ERK1/2) might also result in abnormal regulation of energy homeostasis, leading to obesity.
4.3. Biased agonists identified in neural MCRs
Compared to balanced agonists which induce signaling pathways through receptor with similar efficacy, biased agonists can activate receptor in selective active conformation and trigger some signaling pathways over others [97]. Table 1 lists the biased ligands that have been identified in neural MCRs, including both endogenous and artificial ligands.
Table 1. Biased ligands of neural MCRs.
| Biased ligand | Receptor | Pathway | Cell system | Reference |
|---|---|---|---|---|
| AgRP | MC4R | Inhibiting Gs Activating Gi, Kir7.1, ERK1/2, and Akt |
GT1-7 (Gi, Akt) PVH neurons (Kir7.1) HEK293T (ERK1/2) |
[47, 50, 54, 56] |
| MC3R | Inhibiting Gs Activating ERK1/2 |
HEK293T | [56] | |
|
MCL0020 ML00253764 Ipsen 5i |
MC4R | Inhibiting Gs Activating ERK1/2 |
HEK293T | [50] |
| AP1189 | MC3R | Not increasing Gs Activating ERK1/2 and calcium mobilization |
HEK293A | [55] |
|
THIQ NBI55886 NBI56453 NBI58702 NBI58704 |
MC4R | Activating Gs Impaired in calcium mobilization and receptor internalization |
HEK293 | [45] |
4.3.1 AgRP as a biased ligand in neural MCR signaling
The AgRP is an orexigenic signaling molecule consisting of 112 amino acids (derived from a precursor of 132 amino acids), which was independently identified by three teams [36, 37, 103], with the synthetic fragment AgRP (83-132), which is produced endogenously, still possessing potent activity as receptor antagonist [104, 105]. This orexigenic peptide is co-expressed with neuropeptide Y in the ARC, projecting to the “second order” neurons where neural MCRs are expressed, generating an orexigenic effect in contrast to anorexigenic effect of α-MSH. In addition to its antagonism of α-MSH, evidences suggested that synthetic fragments of AgRP, AgRP (83-132) and smaller fragment AgRP (87–120), can also act as inverse agonists to decrease the basal activity of MC4R independent of melanocortins [41, 42]. Recent studies demonstrated that indeed AgRP is a biased agonist for neural MCRs.
The first study that demonstrated AgRP as a biased agonist to selectively activate Gαi protein was performed in GT1-7 cells. It was demonstrated that AgRP decreases cAMP accumulation in this type of cells, accounting for the inverse agonism of AgRP [47]. However, when G protein activation initiated by AgRP was investigated by measuring incorporation of GTPγS35, they found AgRP could promote the incorporation of GTPγS35, and AgRP-induced incorporation of GTPγS35 can be blocked by PTX, suggesting AgRP activates Gαi protein in addition to simultaneously blocking Gαs signaling [47]. Thus, they proposed a new MC4R activation model, in which α-MSH-induced anorexigenic stimulus through MC4R is initiated by one MC4R active conformational state which triggers Gαs signaling, whereas AgRP-induced orexigenic stimulus through MC4R is initiated by another MC4R active conformational state which triggers Gαi signaling [100]. This model is also supported by some evidences demonstrating that regulation of ion channel activity through MC4R-initiated Gαi signaling could impact appetite. Melanocortins and AgRP can modulate the excitability of hypothalamic neurons by alterations of resting potassium conductance [106]. The inward-rectifier potassium channels are regulated by Gβγ subunits released from Gαi protein [107, 108]. Moreover, AgRP was shown to inhibit excitation of hypothalamic neurons in a PTX-sensitive manner, which further demonstrated that Gαi signaling is involved in regulating excitation of hypothalamic neurons [109]. Hence, AgRP on appetite control is potentially through a process including activation of Gαi protein, releasing of Gβγ subunits from Gαi protein, regulation of potassium channels by Gβγ, and modulation of excitability of hypothalamic neurons. This study reveals AgRP as a biased agonist for Gαi signaling, and AgRP-induced Gαi activation also accounts for the orexigenic effect.
Another study showed AgRP biased agonism on Gαs signaling and regulation of potassium channels in PVH neurons. In CNS regions outside of PVH, depolarization is dependent on G protein supported by the fact that activity of potassium channels is regulated by Gβγ subunits released from Gαi protein [107, 108]. However, MC4R-mediated PVH neuron depolarization was demonstrated to be independent of G protein signaling since the treatment of GDPβS, an inhibitory GDP analogue, does not inhibit MC4R-mediated depolarization [54]. Other inhibitors of G protein signaling such as gallein, a Gβγ blocker, is also ineffective in blocking this depolarization [54]. These results demonstrated that activity of potassium channels in PVH neurons is not regulated by G protein signaling. Furthermore, endogenous agonist α-MSH activates MC4R resulting in an increased intracellular cAMP level through Gαs signaling and inhibition of K+ efflux through potassium channel Kir7.1, which have depolarizing effect. AgRP increases K+ efflux but does not trigger Gαs signaling, therefore AgRP exhibits pronounced bias for coupling MC4R to inward rectifier potassium channel Kir7.1 over Gαs signaling to generate a hyperpolarization [54]. β-arrestin, an adaptor protein downstream of GPCRs, is thought to be able to mediate G-protein independent signaling and is a key regulator of ion channels [110]. In this study, β-arrestin cannot be recruited to the receptor upon AgRP treatment [54], although other study demonstrated AgRP induces β-arrestin recruitment through MC4R in HEK293 cells [46]. Thus, AgRP induced Kir7.1 activation in PVH is both G-protein and β-arrestin independent. Further studies are still needed to investigate whether AgRP directly activates Kir7.1 through MC4R or any other adaptors are involved upstream of Kir7.1.
Studies in our lab identified AgRP as a biased agonist at kinase signaling pathways. When neural MCR-mediated Gαs-cAMP and ERK1/2 activation were investigated, we discovered that AgRP selectively increases phosphorylation of ERK1/2 but decreases cAMP levels in HEK293T cells expressing MC3Rs or MC4Rs [50, 56]. Notably, the alterations in these two pathways are independent, suggesting AgRP-induced biased activation on ERK1/2 pathway is parallel to Gαs-cAMP pathway [56]. Although AgRP induced β-arrestin recruitment through neural MCRs and β-arrestin mediated ERK1/2 activation through MC5R were demonstrated [46, 82], little is known on whether β-arrestins serve as adaptors linking neural MCRs to the downstream signaling molecules. In other GPCRs, β-arrestins mediate slower and persistent ERK1/2 activation compared with rapid and transient ERK1/2 activation mediated by G protein [111]. However, AgRP-induced ERK1/2 activation through neural MCRs is transient [50, 56]. Therefore, AgRP-induced ERK1/2 activation through neural MCRs may not be β-arrestin-dependent. Moreover, AgRP induces significant AKT phosphorylation in GT1-7 cells but not in HEK293T cells expressing MC3Rs or MC4Rs, indicating that AgRP is also a biased agonist for MC4R-mediated AKT activation [56].
In summary, AgRP acts as a biased ligand to inhibit Gαs-cAMP signaling but induces Gαi signaling and opening of potassium channel Kir7.1, ERK1/2 signaling or AKT signaling through neural MCRs in different cell types. These discoveries on biased agonism of AgRP expand our understanding of the long-term action of AgRP on regulating appetite beyond its antagonism and inverse agonism which were previously demonstrated.
4.3.2 Other biased ligands in neural MCRs signaling
In addition to AgRP, other inverse agonists are also shown to be biased ligands. Small molecule ligands including MCL0020, ML00253764, and Ipsen 5i exhibit their agonistic properties on activating ERK1/2 but not Gαs-cAMP at some natural occurring constitutively active mutant MC4Rs in HEK293T cells [50]. Small molecule ligand AP1189 is characterized as biased agonist at MC3R to induce ERK1/2 activation and calcium mobilization but not Gαs-cAMP levels in HEK293A cells [55].
When some MC4R agonists, including peptide and nonpeptide agonists, were investigated in HEK293 cells expressing MC4Rs, the results showed that the peptide agonists α-MSH, β-MSH, Des-acetyl-α-MSH, and NDP-α-MSH are potent agonists among cAMP accumulation, calcium mobilization, and receptor internalization, and γ-MSH is a partial agonist among these three processes [45]. However, other five nonpeptide agonist, THIQ, NBI 55886, NBI 56453, NBI 58702, and NBI 58704, are significantly impaired in calcium mobilization and receptor internalization compared with their action on cAMP accumulation [45]. Given the fact that intracellular calcium mobilization through MC4R is mediated by Gαq protein in hypothalamic neurons [73] and neural MCR internalization is mediated by β-arrestin in HEK293 and COS-7 cells [46, 84], we assume that these nonpeptide agonists are biased agonists at Gαs-cAMP accumulation and Gαq-calcium mobilization/ β-arrestin-receptor internalization signaling pathways. However, more studies are still required to clarify the mechanism by which these ligands induce biased activation on Gαs-AMP and calcium mobilization/receptor internalization.
5. Therapeutic relevance of biased signaling at neural MCRs
Over the past decade, with the increasing discoveries and studies on biased signaling in GPCRs, especially β-arrestin mediated biased signaling, the physiological functions and therapeutic potential of biased signaling in GPCRs have been delineated. A variety of receptors have been investigated, and their β-arrestin mediated processes have also been identified in different therapeutic areas such as cardiovascular, renal, metabolic, and reproductive systems [112]. Some of β-arrestin mediated signaling pathways are thought to mediate beneficial physiologic responses such as those in β1-adrenergic receptor (β1-AR) with cardioprotective effect, AT1R with maintenance of cardiovascular homeostasis, and PTH1R with anabolic bone formation, whereas their G protein mediated signaling are detrimental [112]. Moreover, opposite to the beneficial physiological response mediated by G protein signaling through GPR109A in treatment of dyslipidemia, the detrimental physiological response, significantly increased cutaneous flushing, is mediated by β-arrestin signaling through this receptor [113]. Therefore, biased agonists that are developed to selectively activate G protein or β-arrestin mediated signaling with maximal beneficial effects and minimal adverse effects can be more effective and safer than unbiased agonists which equally activate both signaling pathways. Several biased agonists have been demonstrated to be improved therapeutic agents with improved efficacy and fewer side effects, such as carvedilol targeting β2-AR [114], Sar1,Ile4,Ile8-AngII (SII) targeting AT1R [115], D-Trp12,Tyr34 PTH targeting PTH1R [116], and MK-0354 targeting GPR109A [113]. However, therapeutic potential of biased signaling at neural MCRs was poorly studied and only a few studies reported biased agonists used in pharmacological and physiological studies on neural MCRs.
5.1. MC4R activation and cardiovascular dysfunction
MC4R activation increases sympathetic activity to induce satiety thereby leading to a reduction in food intake [117, 118]. Also, sympathetic activation can result in an enhanced metabolic rate to increase energy expenditure [118]. However, MC4R-mediated sympathetic activation also results in cardiovascular side effects including increased blood pressure and heart rate [119-121]. Homozygous and heterozygous Mc4r-deficient mice have resistance to development of hypertension despite significant obesity [122]. Heart rate and mean arterial pressure do not change in Mc4r-deficient mice but increase in wild-type mice after acute administration of α-MSH [120]. Central blockade of MC4R in rats by chronic injection of AgRP decreases both mean arterial pressure and heart rate, although increased food intake and body weight can be observed as expected [123, 124].
A clinical study on MC4R-deficient patients showed that although the patients with MC4R haploinsufficiency are obese, the prevalence of hypertension is significantly lower in these subjects compared to the control subjects with normal MC4R and similar degree of obesity [125]. This study also showed that heart rate is significantly higher in control subjects [125]. Additionally, short-term administration of a synthetic peptide MC4R agonist LY2112688 can cause increased mean blood pressure in healthy overweight or obese subjects [125]. However, a subsequent study reported that short-term administration of another small peptide agonist, RM-493, can increase resting energy expenditure and fat oxidation without increasing blood pressure in obese patients [126]. However, whether this agonist preferentially activates specific signaling pathway to exert the beneficial effects but do not generate adverse side effects remains unknown.
5.2. Therapeutic potential of MC4R biased agonists in treatment of obesity
Previous studies have demonstrated that MC4R activation mediates its diverse physiological effects via multiple signaling pathways including Gαs, Gαi, αq, ERK1/2, AMPK, JNK, and regulation of potassium channel Kir7.1. The physiological effects of some signaling pathways have also been clarified. The conventional Gαs signaling mediates effects on energy expenditure, glucose metabolism and thermogenesis outside of PVH [34, 69] [68] and regulates cardiovascular functions (blood pressure and heart rate) within PVH [69, 127]. However, the appetite control is mediated via Gαq and regulation of Kir7.1 in PVH [54, 69]. Many current MC4R agonists, serving as unbiased agonists, activate all or multiple intracellular signaling pathways [45, 50]. MC4R activation by these agonists can lead to increased energy expenditure via Gαs signaling and decreased food intake via Gαq signaling or closure of potassium channel Kir7.1, resulting in a negative energy balance and matching the therapeutic purpose for treatment of obesity. However, activated Gαs signaling can also cause the adverse side effects on cardiovascular functions with high blood pressure and heart rate [69, 127]. Based on knowledge of biased signaling, we can propose a new potential therapeutic strategy for obesity. Biased ligands that potently trigger Gαq signaling or closure of Kir7.1 to suppress food intake but not activate Gαs signaling with cardiovascular side effects would be better drugs for targeting MC4R (Fig. 2).
Fig. 2. Therapeutic relevance of biased signaling at neural MC4R.
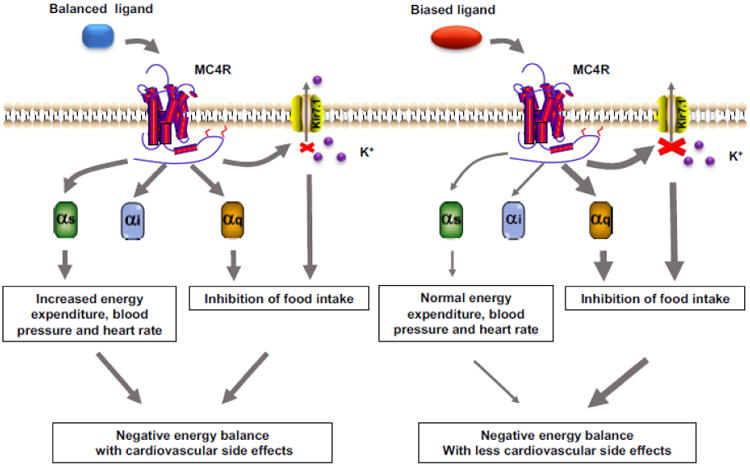
The MC4R could couple to Gs, Gq and Kir7.1. Normal balanced agonist activates MC4R and non-selectively induces Gs and Gq signaling and closure of Kir7.1, resulting in a negative energy balance with side effects. However, biased ligand activates MC4R and selectively induces Gq signaling and closure of Kir7.1, resulting a negative energy balance without side effects.
A small peptide agonist, RM-493, was demonstrated to reduce food intake and lead to persistent weight loss without alteration of cardiovascular functions in a diet-induced obese nonhuman primate model [128]. Although the exact mechanism for the observed effects of RM-493 remains unknown, a potential explanation is that RM-493 is a MC4R biased agonist which selectively couples MC4R to the particular signaling pathways. α-MSH analogue MC4-NN2-0453 is a long-acting MC4R agonist investigated for obesity treatment [129]. Clinical trial showed that MC4-NN2-0453 exhibits a neutral effect on blood pressure in contrast to other MC4R agonists [130]. Currently, this peptide was identified to preferentially couple MC4R to potassium channel Kir7.1 over Gαs protein to depolarize PVH neurons in vitro [54]. It is unable to induce cAMP-mediated intestinal peptide YY (anorexigenic peptide released by ileum and colon) release in vivo by intraperitoneal administration of this ligand, whereas it can potently inhibit food intake in mice similar as other α-MSH analogues [54]. Given its effect on Gαs signaling and regulation of Kir7.1, MC4-NN2-0453 might be a potent biased agonist to inhibit food intake without the pressor response in the treatment of human obesity.
5.3. Therapeutic potential of neural MCR biased agonists in the treatment of other disorders
Several studies suggested that MC4R is involved in modulating reproductive function, and MC4R modulates penile erectile function likely through neuronal circuitry in spinal cord centers and somatosensory afferent nerve terminals of penis [11, 131]. MC4R agonists, including MTII, PT-141, and LY2112688, are effective in induce penile erection in men with erectile dysfunction [132, 133]. However, another MC4R agonist, MK-0493, does not induce erectile response [134], indicating the structure of the particular agonist is significant in determining erectile response [11]. Therefore, it is possible for treatment of erectile dysfunction without the cardiovascular side effects, if biased agonists could be identified to induce the particular signaling pathway resulting only erectile response but not alteration of other metabolic responses.
In addition to the role of neural MC3R in regulation of energy homeostasis, it was also shown that peripheral MC3R plays a role in anti-inflammation. Further, a recent study suggested that ERK1/2 phosphorylation initiated by activated MC3R leads to anti-inflammatory actions [55]. AP1189, one orally bioavailable ligand, is effective in inducing ERK1/2 phosphorylation to exhibit anti-inflammatory response through MC3R without altering cAMP activation [55].
Collectively, biased agonists at MC3R or MC4R provide us new opportunities for design of therapeutics with improved therapeutic effects and avoiding those associated with side effects.
6. Conclusions and future directions
Over the last two decades, the critical roles of neural MCRs on energy homeostasis have been elucidated by a series of investigations, with ongoing studies focusing on the detailed mechanisms and neuronal network in the regulation of energy homeostasis. Discovery of multiple intracellular signaling pathways at neural MCRs facilitates the dissection of signaling pathways mediating different physiological functions of the neural MCRs. Moreover, biased signaling including biased receptor mutants and biased ligands at neural MCRs provides us new insight into obesity pathogenesis and pharmacology of neural MCRs. So far, neural MCRs have been known as the targets for treatment of obesity, but available therapeutic strategies targeting neural MCRs are far from ideal due to the side effects, especially associated with increased blood pressure and heart rate. Biased signaling at the neural MCRs provides us a new and potentially better way to screen and develop drugs as biased agonist in obesity treatment. Long-term studies in obese individuals are required to determine whether these identified biased agonists are effective and safe in treatment of obesity.
Highlights.
Neural melanocortin receptors are critical regulators of energy homeostasis.
Neural melanocortin receptors initiate signaling to multiple pathways.
There are biased mutant receptors and biased ligands at the neural melanocortin receptors.
Biased ligands might be better therapeutics for obesity and other diseases associated with neural melanocortin receptors.
Acknowledgments
Our original studies on the neural melanocortin receptors were supported by following grants: National Institutes of Health grant R15DK077213, American Diabetes Association Grant 1-12-BS212, Intramural Grant Program of Auburn University, and Animal Health and Disease Research Program and Interdisciplinary Grant of Auburn University College of Veterinary Medicine. We thank former graduate students, Xiu-Lei Mo, Hui Huang, Fan Yang, Shan He, and Zhao Yang, for their contributions to the original studies summarized in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tao YX, Yuan ZH, Xie J. G protein-coupled receptors as regulators of energy homeostasis. Prog Mol Biol Transl Sci. 2013;114:1–43. doi: 10.1016/B978-0-12-386933-3.00001-7. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens G, Cowan M, Danaei G, Lin J, Paciorek C, Singh G, Gutierrez H, Lu Y, Bahalim A. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'rahilly S, Farooqi IS, Yeo GS, Challis BG. Minireview: human obesity—lessons from monogenic disorders. Endocrinology. 2003;144:3757–3764. doi: 10.1210/en.2003-0373. [DOI] [PubMed] [Google Scholar]
- 5.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 6.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 8.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 9.Getting SJ, Riffo-Vasquez Y, Pitchford S, Kaneva M, Grieco P, Page CP, Perretti M, Spina D. A role for MC3R in modulating lung inflammation. Pulm Pharmacol Ther. 2008;21:866–873. doi: 10.1016/j.pupt.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Tao YX. Mutations in melanocortin-4 receptor and human obesity. Prog Mol Biol Transl Sci. 2009;88:173–204. doi: 10.1016/S1877-1173(09)88006-X. [DOI] [PubMed] [Google Scholar]
- 11.Tao YX. The melanocortin-4 receptor: Physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begriche K, Girardet C, McDonald P, Butler AA. Melanocortin-3 receptors and metabolic homeostasis. Prog Mol Biol Transl Sci. 2013;114:109–146. doi: 10.1016/B978-0-12-386933-3.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 15.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW, Mynatt RL, Gettys TW. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J. 2005;19:1482–1491. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 17.Sutton GM, Begriche K, Kumar KG, Gimble JM, Perez-Tilve D, Nogueiras R, McMillan RP, Hulver MW, Tschop MH, Butler AA. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010;24:862–872. doi: 10.1096/fj.09-142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begriche K, Marston OJ, Rossi J, Burke LK, McDonald P, Heisler LK, Butler AA. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes Brain Behav. 2012;11:291–302. doi: 10.1111/j.1601-183X.2012.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girardet C, Butler AA. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim Biophys Acta. 2014;1842:482–494. doi: 10.1016/j.bbadis.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 22.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 23.Lee YS, Poh LK, Loke KY. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J Clin Endocrinol Metab. 2002;87:1423–1426. doi: 10.1210/jcem.87.3.8461. [DOI] [PubMed] [Google Scholar]
- 24.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 25.Tao YX, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab. 2004;89:3936–3942. doi: 10.1210/jc.2004-0367. [DOI] [PubMed] [Google Scholar]
- 26.Feng N, Young SF, Aguilera G, Puricelli E, Adler-Wailes DC, Sebring NG, Yanovski JA. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes. 2005;54:2663–2667. doi: 10.2337/diabetes.54.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao YX. Functional characterization of novel melanocortin-3 receptor mutations identified from obese subjects. Biochim Biophys Acta. 2007;1772:1167–1174. doi: 10.1016/j.bbadis.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Tao YX. Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol. 2005;239:1–14. doi: 10.1016/j.mce.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Tao YX. Mutations in the melanocortin-3 receptor (MC3R) gene: Impact on human obesity or adiposity. Curr Opin Investig Drugs. 2010;11:1092–1096. [PubMed] [Google Scholar]
- 30.Hinney A, Volckmar AL, Knoll N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci. 2013;114:147–191. doi: 10.1016/B978-0-12-386933-3.00005-4. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Tao YX. Mutations in melanocortin-3 receptor gene and human obesity. Prog Mol Biol Transl Sci. 2016;140:97–129. doi: 10.1016/bs.pmbts.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Smith AI, Funder JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 33.Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- 34.Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci. 2016;19:206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanchard SG, Harris CO, Ittoop OR, Nichols JS, Parks DJ, Truesdale AT, Wilkison WO. Agouti antagonism of melanocortin binding and action in the B16F10 murine melanoma cell line. Biochemistry. 1995;34:10406–10411. doi: 10.1021/bi00033a012. [DOI] [PubMed] [Google Scholar]
- 36.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 37.Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, Van der Ploeg LH. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem Biophys Res Commun. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- 38.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 39.Tolle V, Low MJ. In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes. 2008;57:86–94. doi: 10.2337/db07-0733. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci. 2008;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nijenhuis WA, Oosterom J, Adan RA. AgRP(83-132) acts as an inverse agonist on the human melanocortin-4 receptor. Mol Endocrinol. 2001;15:164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- 42.Haskell-Luevano C, Monck EK. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Pept. 2001;99:1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- 43.Chai BX, Neubig RR, Millhauser GL, Thompson DA, Jackson PJ, Barsh GS, Dickinson CJ, Li JY, Lai YM, Gantz I. Inverse agonist activity of agouti and agouti-related protein. Peptides. 2003;24:603–609. doi: 10.1016/s0196-9781(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 44.Tao YX, Huang H, Wang ZQ, Yang F, Williams JN, Nikiforovich GV. Constitutive activity of neural melanocortin receptors. Methods Enzymol. 2010;484:267–279. doi: 10.1016/B978-0-12-381298-8.00014-9. [DOI] [PubMed] [Google Scholar]
- 45.Nickolls SA, Fleck B, Hoare SR, Maki RA. Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. J Pharmacol Exp Ther. 2005;313:1281–1288. doi: 10.1124/jpet.105.083337. [DOI] [PubMed] [Google Scholar]
- 46.Breit A, Wolff K, Kalwa H, Jarry H, Buch T, Gudermann T. The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin-3 and -4 receptors. J Biol Chem. 2006;281:37447–37456. doi: 10.1074/jbc.M605982200. [DOI] [PubMed] [Google Scholar]
- 47.Büch TR, Heling D, Damm E, Gudermann T, Breit A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. J Biol Chem. 2009;284:26411–26420. doi: 10.1074/jbc.M109.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H, Tao YX. Pleiotropic functions of the transmembrane domain 6 of human melanocortin-4 receptor. J Mol Endocrinol. 2012;49:237–248. doi: 10.1530/JME-12-0161. [DOI] [PubMed] [Google Scholar]
- 49.Mo XL, Yang R, Tao YX. Functions of transmembrane domain 3 of human melanocortin-4 receptor. J Mol Endocrinol. 2012;49:221–235. doi: 10.1530/JME-12-0162. [DOI] [PubMed] [Google Scholar]
- 50.Mo XL, Tao YX. Activation of MAPK by inverse agonists in six naturally occurring constitutively active mutant human melanocortin-4 receptors. Biochim Biophys Acta. 2013;1832:1939–1948. doi: 10.1016/j.bbadis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 51.He S, Tao YX. Defect in MAPK signaling as a cause for monogenic obesity caused by inactivating mutations in the melanocortin-4 receptor gene. Int J Biol Sci. 2014;10:1128–1137. doi: 10.7150/ijbs.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang F, Huang H, Tao YX. Biased signaling in naturally occurring mutations in human melanocortin-3 receptor gene. Int J Biol Sci. 2015;11:423–433. doi: 10.7150/ijbs.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z, Huang ZL, Tao YX. Functions of DPLIY motif and helix 8 of human melanocortin-3 receptor. J Mol Endocrinol. 2015;55:107–117. doi: 10.1530/JME-15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, Denton JS, Cone RD. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520:94–98. doi: 10.1038/nature14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montero-Melendez T, Gobbetti T, Cooray SN, Jonassen TE, Perretti M. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J Immunol. 2015;194:3381–3388. doi: 10.4049/jimmunol.1402645. [DOI] [PubMed] [Google Scholar]
- 56.Yang Z, Tao YX. Biased signaling initiated by agouti-related peptide through human melanocortin-3 and -4 receptors. Biochim Biophys Acta. 2016;1862:1485–1494. doi: 10.1016/j.bbadis.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 58.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 59.Cowley MA. Hypothalamic melanocortin neurons integrate signals of energy state. Eur J Pharmacol. 2003;480:3–11. doi: 10.1016/j.ejphar.2003.08.087. [DOI] [PubMed] [Google Scholar]
- 60.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 61.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 66.Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen M, Shrestha YB, Podyma B, Cui Z, Naglieri B, Sun H, Ho T, Wilson EA, Li YQ, Gavrilova O. Gsα deficiency in the dorsomedial hypothalamus underlies obesity associated with Gsα mutations. J Clin Invest. 2017;127:500–510. doi: 10.1172/JCI88622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li YQ, Shrestha Y, Pandey M, Chen M, Kablan A, Gavrilova O, Offermanns S, Weinstein LS. Gq/11α and Gsα mediate distinct physiological responses to central melanocortins. J Clin Invest. 2016;126:40–49. doi: 10.1172/JCI76348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol. 2013;305:R359–368. doi: 10.1152/ajpregu.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 73.Newman EA, Chai BX, Zhang W, Li JY, Ammori JB, Mulholland MW. Activation of the melanocortin-4 receptor mobilizes intracellular free calcium in immortalized hypothalamic neurons. J Surg Res. 2006;132:201–207. doi: 10.1016/j.jss.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 75.Vongs A, Lynn NM, Rosenblum CI. Activation of MAP kinase by MC4-R through PI3 kinase. Regul Pept. 2004;120:113–118. doi: 10.1016/j.regpep.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 76.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 77.Chai B, Li JY, Zhang W, Ammori JB, Mulholland MW. Melanocortin-3 receptor activates MAP kinase via PI3 kinase. Regul Pept. 2007;139:115–121. doi: 10.1016/j.regpep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Chai B, Li JY, Zhang W, Wang H, Mulholland MW. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides. 2009;30:1098–1104. doi: 10.1016/j.peptides.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Damm E, Buech TR, Gudermann T, Breit A. Melanocortin-induced PKA activation inhibits AMPK activity via ERK-1/2 and LKB-1 in hypothalamic GT1-7 cells. Mol Endocrinol. 2012;26:643–654. doi: 10.1210/me.2011-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 81.Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. 2006;27:2846–2857. doi: 10.1016/j.peptides.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Rodrigues AR, Almeida H, Gouveia AM. Melanocortin 5 receptor signaling and internalization: role of MAPK/ERK pathway and β-arrestins 1/2. Mol Cell Endocrinol. 2012;361:69–79. doi: 10.1016/j.mce.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 83.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-Arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 84.Shinyama H, Masuzaki H, Fang H, Flier JS. Regulation of melanocortin-4 receptor signaling: agonist-mediated desensitization and internalization. Endocrinology. 2003;144:1301–1314. doi: 10.1210/en.2002-220931. [DOI] [PubMed] [Google Scholar]
- 85.Dascal N. Ion-channel regulation by G proteins. Trends Endocrinol Metab. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- 86.Nyan DC, Anbazhagan R, Hughes-Darden CA, Wachira SJ. Endosomal colocalization of melanocortin-3 receptor and β-arrestins in CAD cells with altered modification of AKT/PKB. Neuropeptides. 2008;42:355–366. doi: 10.1016/j.npep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Konda Y, Gantz I, Del Valle J, Shimoto Y, Miwa H, Yamada T. Interaction of dual intracellular signaling pathways activated by the melanocortin-3 receptor. J Biol Chem. 1994;269:13162–13166. [PubMed] [Google Scholar]
- 88.Mountjoy KG, Kong PL, Taylor JA, Willard DH, Wilkison WO. Melanocortin receptor-mediated mobilization of intracellular free calcium in HEK293 cells. Physiological Genomics. 2001;5:11–19. doi: 10.1152/physiolgenomics.2001.5.1.11. [DOI] [PubMed] [Google Scholar]
- 89.Wachira SJ, Hughes-Darden CA, Taylor CV, Ochillo R, Robinson TJ. Evidence for the interaction of protein kinase C and melanocortin 3-receptor signaling pathways. Neuropeptides. 2003;37:201–210. doi: 10.1016/s0143-4179(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 90.Chen M, Wang J, Dickerson KE, Kelleher J, Xie T, Gupta D, Lai EW, Pacak K, Gavrilova O, Weinstein LS. Central nervous system imprinting of the G protein Gsα and its role in metabolic regulation. Cell Metab. 2009;9:548–555. doi: 10.1016/j.cmet.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen M, Berger A, Kablan A, Zhang J, Gavrilova O, Weinstein LS. Gsα deficiency in the paraventricular nucleus of the hypothalamus partially contributes to obesity associated with Gsα mutations. Endocrinology. 2012;153:4256–4265. doi: 10.1210/en.2012-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Lean A, Stadel J, Lefkowitz R. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 93.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the β2-adrenergic receptor: extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- 94.Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 95.Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- 96.Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture) Angew Chem Int Ed Engl. 2013;52:6366–6378. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- 97.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Gruters A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52:2984–2988. doi: 10.2337/diabetes.52.12.2984. [DOI] [PubMed] [Google Scholar]
- 100.Breit A, Büch TR, Boekhoff I, Solinski HJ, Damm E, Gudermann T. Alternative G protein coupling and biased agonism: new insights into melanocortin-4 receptor signalling. Mol Cell Endocrinol. 2011;331:232–240. doi: 10.1016/j.mce.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 101.Patten CS, Daniels D, Suzuki A, Fluharty SJ, Yee DK. Structural and signaling requirements of the human melanocortin 4 receptor for MAP kinase activation. Regul Pept. 2007;142:111–122. doi: 10.1016/j.regpep.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 102.Huang H, Tao YX. Functions of the DRY motif and intracellular loop 2 of human melanocortin 3 receptor. J Mol Endocrinol. 2014;53:319–330. doi: 10.1530/JME-14-0184. [DOI] [PubMed] [Google Scholar]
- 103.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 104.Quillan JM, Sadee W, Wei ET, Jimenez C, Ji L, Chang JK. A synthetic human Agouti-related protein-(83-132)-NH2 fragment is a potent inhibitor of melanocortin receptor function. FEBS Lett. 1998;428:59–62. doi: 10.1016/s0014-5793(98)00487-6. [DOI] [PubMed] [Google Scholar]
- 105.Creemers JW, Pritchard LE, Gyte A, Le Rouzic P, Meulemans S, Wardlaw SL, Zhu X, Steiner DF, Davies N, Armstrong D, Lawrence CB, Luckman SM, Schmitz CA, Davies RA, Brennand JC, White A. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology. 2006;147:1621–1631. doi: 10.1210/en.2005-1373. [DOI] [PubMed] [Google Scholar]
- 106.Smith MA, Hisadome K, Al-Qassab H, Heffron H, Withers DJ, Ashford ML. Melanocortins and agouti-related protein modulate the excitability of two arcuate nucleus neuron populations by alteration of resting potassium conductances. J Physiol. 2007;578:425–438. doi: 10.1113/jphysiol.2006.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leaney JL. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur J Neurosci. 2003;18:2110–2118. doi: 10.1046/j.1460-9568.2003.02933.x. [DOI] [PubMed] [Google Scholar]
- 108.Milovic S, Steinecker-Frohnwieser B, Schreibmayer W, Weigl LG. The sensitivity of G protein-activated K+ channels toward halothane is essentially determined by the C terminus. J Biol Chem. 2004;279:34240–34249. doi: 10.1074/jbc.M403448200. [DOI] [PubMed] [Google Scholar]
- 109.Fu LY, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 112.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin-and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. β-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. β-Arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A β-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 118.Greenfield JR. Melanocortin signalling and the regulation of blood pressure in human obesity. J Neuroendocrinol. 2011;23:186–193. doi: 10.1111/j.1365-2826.2010.02088.x. [DOI] [PubMed] [Google Scholar]
- 119.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43:370–375. doi: 10.1161/01.HYP.0000111836.54204.93. [DOI] [PubMed] [Google Scholar]
- 120.Ni XP, Butler AA, Cone RD, Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24:2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 121.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 123.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43:1312–1317. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- 124.Tallam LS, Kuo JJ, da Silva AA, Hall JE. Cardiovascular, renal, and metabolic responses to chronic central administration of agouti-related peptide. Hypertension. 2004;44:853–858. doi: 10.1161/01.HYP.0000148993.47498.b2. [DOI] [PubMed] [Google Scholar]
- 125.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 126.Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, Zhao X, Ring M, Psota TL, Cone RD, Panaro BL, Gottesdiener KM, Van der Ploeg LH, Reitman ML, Skarulis MC. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J Clin Endocrinol Metab. 2015;100:1639–1645. doi: 10.1210/jc.2014-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li P, Cui BP, Zhang LL, Sun HJ, Liu TY, Zhu GQ. Melanocortin 3/4 receptors in paraventricular nucleus modulate sympathetic outflow and blood pressure. Exp Physiol. 2013;98:435–443. doi: 10.1113/expphysiol.2012.067256. [DOI] [PubMed] [Google Scholar]
- 128.Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Conde-Frieboes K, Thøgersen H, Lau JF, Sensfuss U, Hansen TK, Christensen L, Spetzler J, Olsen HB, Nilsson C, Raun K. Identification and in vivo and in vitro characterization of long acting and melanocortin 4 receptor (MC4-R) selective α-melanocyte-stimulating hormone (α-MSH) analogues. Journal of medicinal chemistry. 2012;55:1969–1977. doi: 10.1021/jm201489a. [DOI] [PubMed] [Google Scholar]
- 130.Royalty JE, Konradsen G, Eskerod O, Wulff BS, Hansen BS. Investigation of safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of a long-acting α-MSH analog in healthy overweight and obese subjects. The Journal of Clinical Pharmacology. 2014;54:394–404. doi: 10.1002/jcph.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci U S A. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dorr RT, Lines R, Levine N, Brooks C, Xiang L, Hruby VJ, Hadley ME. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996;58:1777–1784. doi: 10.1016/0024-3205(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 133.Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000;56:641–646. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 134.Krishna R, Wong P, Stevens C, De Lepeleire I, Van Dyck K, Rosen RC, Gendrano IN, 3rd, Peeters M, Wagner JA, Herman GA. Lack of erectogenic activity of a novel selective melanocortin-4 receptor agonist in a clinical experimental model. J Clin Pharmacol. 2008;48:1237–1241. doi: 10.1177/0091270008320925. [DOI] [PubMed] [Google Scholar]


