Abstract
Objectives
Late-life depression is associated with cognitive deficits and increased risk for cognitive decline. The purpose of the study was to determine whether clinical characteristics could serve as phenotypes informative of subsequent cognitive decline. We examined age of depression onset and antidepressant remission at 3 months (acute response) and 12 months (chronic response).
Design
A longitudinal study of late-life depression in an academic center.
Participants
273 depressed and 164 never-depressed community dwelling elders aged 60 years or older were followed on average for over five years.
Measurements
Participants completed annual neuropsychological testing. Neuropsychological measures were converted to z-scores derived from the baseline performance of all participants. Cognitive domain scores at each time were then created by averaging z-scores across tests, grouped into domains of episodic memory, attention-working memory, verbal fluency, and executive function.
Results
Depressed participants exhibited poorer performance at baseline and greater subsequent decline in all domains. Early-onset depressed individuals exhibited a greater decline in all domains than the late-onset or nondepressed groups. For remission, remitters and nonremitters at both 3- and 12-months exhibited greater decline in episodic memory and attention-working memory than nondepressed subjects. 3-month remitters also exhibited a greater decline in verbal fluency and executive function, while 12-month nonremitters exhibited greater decline in executive function than other groups.
Conclusions
Consistent with past studies, depressed elders exhibit greater cognitive decline than nondepressed subjects, particularly individuals with early depression onset. This supports that repeated depressive episodes may contribute to decline. Clinical remission is not associated with less cognitive decline.
Keywords: depression, Cognition, longitudinal, memory, executive function, onset, remission, response, outcomes
INTRODUCTION
Late-life depression (LLD) is commonly associated with impaired cognition and increased risk of dementia,1,2 but the underlying relationship between depression and dementia is unclear. Nondemented elders with major depressive disorder exhibit cognitive dysfunction across multiple domains including executive function, episodic memory, visuospatial ability, and processing speed.3–5 Both retrospective and prospective studies demonstrate that LLD is associated with a two-fold increased risk of Alzheimer’s disease and vascular dementia.2,6,7 Similar to nondepressed populations, cognitive decline is linked to worsening cerebrovascular disease,8 hippocampal volume loss,9 and amyloid deposition.10 However, others report that the effect of depression on cognition is independent of underlying neuropathology.11,12 Thus pathological brain aging contributes to cognitive decline, but depression may have an independent effect on cognition, although the mechanism is unclear. It is also unclear whether there are clinical phenotypes within LLD that inform clinicians about risk of future decline.
Age of depression onset has previously distinguished phenotypes within LLD. This approach utilizes the age of the initial depressive episode to dichotomize older adults into late-onset depression (LOD) or early-onset depression (EOD), typically using an age cutoff between 50–60 years. The distinction developed from cross-sectional studies reporting that LOD is associated with greater neuroanatomical differences and cognitive impairment than EOD,13,14 although others have not observed similar relationships.3,4,15 Subsequent longitudinal studies found that both EOD and LOD are associated with increased dementia risk 16 and that individuals with both midlife and late-life depressive symptoms are at greater risk of dementia than those with only midlife or late-life symptoms.17 Thus greater numbers of lifetime depressive episodes are associated with increased dementia risk.18 Individuals with EOD may be at particular risk as they would be expected to have a greater number of depressive episodes than individuals with LOD.
Individuals with LOD and EOD may be at increased risk of cognitive decline through multiple complementary pathways.11,19 First, a “shared pathology” hypothesis is relevant for LOD. In this model, underlying neurodegenerative or vascular processes contribute to both cognitive decline and also negatively affect neural circuits involved in mood regulation.20 Damage to such circuits may predispose individuals to depression and diminish the ability of antidepressants to modify brain function and connectivity, resulting in poorer clinical response.21 Thus clinically relevant depressive symptoms in context of mild cognitive symptoms may be an early marker of brain pathology. Second, a “depression neurotoxicity” hypothesis proposes that repeated depressive episodes contribute to pathological brain aging and cognitive decline.19 On imaging, this may be characterized by white matter hyperintensities or regional atrophy. Mechanistically, depression may have this effect through altered hypothalamic-pituitary-adrenal (HPA) axis function or proinflammatory processes.19 Recurrent immune system dysregulation during depressive episodes may contribute to pathological brain aging and cognitive decline.22,23 Other hypotheses focusing on environmental, behavioral, or genetic differences are also viable and may interact with these pathways. For example, behavioral changes seen in depression, such as poor nutritional intake, reduced physical activity, and impaired sleep also influence vascular risk and proinflammatory processes. These factors may exacerbate neurotoxic effects of repeated depressive episodes.
These theories suggest that poor antidepressant responses may represent a clinical phenotype characterized by greater cognitive decline. Deficits in executive dysfunction and other cognitive domains are associated with poorer acute antidepressant medication responses and persistent long-term depressive symptoms. 20,24–26 Successful antidepressant treatment may improve cognitive performance acutely 27 although performance may not reach age-adjusted levels and deficits often persist despite successful treatment.5,28–30 Moreover, individuals with persistent depressive symptoms exhibit more rapid pathological brain aging,31,32 although it is unclear whether persistent depression hastens brain aging or whether brain aging influences the clinical antidepressant response.
The purpose of the study was to examine whether clinical characteristics of age of onset or achieving antidepressant remission serve as phenotypes informative of subsequent cognitive decline. We compared longitudinal change in cognitive performance between clinical LLD phenotypes and a cohort of never-depressed elders. We tested for differences based on age of depression onset, hypothesizing that individuals with EOD would exhibit a greater decline in neuropsychological test performance.17,18 Similarly, we characterized depressed participants based on remission status at clinical milestones of 3 months (acute remission) and 12 months (chronic remission). We hypothesized that nonremitted individuals would exhibit greater decline in performance over time.
METHODS
Sample
All participants were age 60 years or older and enrolled in the Duke University Neurocognitive Outcomes of Depression in the Elderly (NCODE) Study. Depressed participants had acute Major Depressive Disorder per DSM-IV criteria with a Center for Epidemiologic Studies – Depression (CES-D)33 score > 15. Diagnosis was based on the NIMH Diagnostic Interview Schedule (DIS)34 and confirmed by clinical interview with a geriatric psychiatrist. Exclusion criteria included: (1) another major psychiatric illness; (2) alcohol or drug abuse or dependence; (3) primary neurologic illness, including dementia. Individuals with comorbid anxiety disorders were not excluded as long as MDD was the primary diagnosis. Depressed participants were recruited through clinical referrals and limited advertisements. As previously reported,35 at entry depressed participants were often on antidepressant medications from prior treatment. Seventy percent of individuals were taking a selective serotonin reuptake inhibitor (SSRI) but only 18% were on SSRI alone. Other medications included tricyclic antidepressants (16%), monoamine oxidase inhibitors (1%), and other antidepressants (e.g., bupropion, venlafaxine, mirtazapine, trazodone, and duloxetine; 68%). Only 9% of individuals were not taking antidepressant medications at enrollment.
A smaller proportion of depressed participants took psychotropic medications from other drug classes. Twenty-one percent (N=58) of depressed participants were on an anxiolytic medication (such as lorazepam or alprazolam), 2.6% (N=7) on hypnotics specifically for sleep (including flurazepam, zolpidem, or zaleplon), and 3.6% (N=10) on antipsychotic medications.
Non-depressed community-dwelling participants were recruited from the Duke University Center for Aging Subject Registry. Eligible nondepressed participants had a non-focal neurological examination, no history of neurological or psychiatric disease, and no evidence of a lifetime depression diagnosis based on the DIS. All participants provided informed consent and the study was approved by the Duke University Institutional Review Board.
We have previously reported data from the NCODE study examining cognitive outcomes of depression.8,9,35–37 Data used in those analyses are also included in the current study. This study differs from those prior reports in various ways, including examining a larger sample size, examining change in cognition over a longer period of time, and in some cases examining change in cognitive performance across multiple domains.
Clinical Evaluation and Treatment
Demographic data and age at the time of the initial depressive episode were assessed using a structured interview. Comorbid medical problems (diabetes, hypertension, and heart disease) were assessed during that interview using self-report questions derived from the NIMH Epidemiological Catchment Area program.38 The clinician-rated Montgomery-Asberg Depression Rating Scale (MADRS) 39 was also used to assess severity in depressed participants, but MADRS data were not available for the nondepressed participants.
Depressed subjects were treated according to the Duke Somatic Treatment Algorithm for Geriatric Depression.40 This stepwise algorithm allows broad use of commercially available antidepressant medications. The majority of depressed subjects began sertraline on study entry, but the antidepressant regimen differed across the sample based on depression severity, past history, and medication tolerability. Switching antidepressant medications and augmentation strategies were allowed as indicated. Participants were evaluated at least every three months and more frequently if clinically indicated.
Neuropsychological Assessment
Individuals were screened and excluded for dementia at enrollment on the basis of an established protocol that included a comprehensive clinical evaluation and consultation with referring physicians. Additionally, participants were excluded for a score of 24 or less on the Mini-Mental State Examination. Following standardized procedures, participants completed neuropsychological testing at baseline and annually thereafter. Using our previously published strategy,35 we created z-scores for each neuropsychological measure derived from baseline performance across all participants. We then created cognitive domain scores by averaging z-scores across tests, grouped into rational constructs on the basis of clinical and research literature. The Trail Making Test (Part B) was reverse-scored prior to z-score conversion so that higher scores represented better performance for all variables. Cronbach's coefficient alpha (α) was computed for each domain.
Episodic memory: Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Learning, CERAD Word List Delayed Recall, Logical Memory I and II subtests from the Wechsler Memory Scale—Revised, and the Benton Visual Retention Test. Cronbach's α was 0.80.
Executive Function: The Symbol Digit Modalities Test and Part B of the Trail Making Test. Cronbach's α was 0.74.
Verbal fluency: Animal Naming and the Controlled Oral Word Association Test. Cronbach's α was 0.83.
Attention-Working memory: Forward and backward subtests of Digit Span and Ascending Digit Span. Cronbach's α was 0.84.
Definition of Depression Phenotypes
We dichotomized the depressed group based on clinically relevant factors in order to examine differences in longitudinal course of neuropsychological performance between subgroups. We first dichotomized the depressed group based on age of onset of their first depressive episode, defined as early onset depression (EOD, onset before age 50 years) or late onset depression (LOD, onset at age 50 years or later). Although others used an older cutoff for the definition of LOD, we selected a younger cutoff as this is an age range where significant medical comorbidity can develop, is an age where preclinical Alzheimer’s disease pathology can develop,41 and is consistent with our past approaches.42–44 This lower cutoff also allowed us to maximize power for longitudinal group comparisons.
We also dichotomized the depressed group based on whether they did or did not achieve clinical remission, using 3-months as a marker of acute remission and 1-year as a marker of remission with chronic treatment. Remission was dichotomized based on the clinician-rated MADRS at each time point, defined as a MADRS of 6 or less. Although this is a lower cutoff than used in some other studies that may define remission as a MADRS of 8 or less, we desired to identify individuals with minimal depressive symptoms at these times.
Statistical Analyses
For neuropsychological data, we created Z-scores for each measure based on the baseline performance of all participants as described above. These neuropsychological domain Z-scores were used as a repeated measures dependent variable. We utilized all available neuropsychological test data. If data were missing for a specific task, we did not calculate a Z-score for the associated domain for that individual but used other domains where all data were available. This resulted in differences in sample sizes across the four domains. To be included in analyses, participants needed to have completed at least two separate neuropsychological testing sessions.
Statistical analyses were conducted using SAS 9.4 (Cary, NC, USA). All statistical models shared similar independent variables and were distinguished by the definition used to define the group variable (depression diagnosis, age of onset, or remission status). We used general linear models to assess for baseline differences in neuropsychological domain z-scores between groups while controlling for age, sex, race, and education (in years). When the group variable in these analyses were statistically significant, we then conducted pairwise group comparisons of the cognitive domain z-score using the LSMEANS option in SAS.
Longitudinal analyses utilized mixed models specifying random intercepts by subject identifier with an unstructured covariance structure. In these models, cognitive domain z-score was a repeated measure, with independent variables of age, sex, education, race, study time and baseline neuropsychological domain Z-score. Initial longitudinal analyses additionally tested for interaction terms between time and vascular risk factor medical morbidity. The primary variable of interest was an interaction term between time and diagnostic group, allowing us to determine whether there was a different effect of time on neuropsychological domain performance across groups. For models with a statistically significant interaction term between time and group, we then examined the effect size of the interaction term and group differences in slope (representing change in z-transformed cognitive domain score over one year of time).
RESULTS
We examined data on 437 older adults. The 273 depressed and 164 nondepressed participants differed significantly on age, education, and report of vascular risk factor morbidity (Table 1). Depressed participants had an average age of initial depression onset of 40.8y (SD=20.6y, range 4y – 86y). Participants were in the study for an average of 68.0 months (SD = 49.3, range 9.8 – 182 months), with the nondepressed group participating for an overall longer duration. Not all participants had data across the entire study period. Data on the proportion of participants who had neuropsychological data at each study time point is detailed in Supplemental Table 1.
Table 1.
Demographic group differences
| Depressed (N=273) |
Nondepressed (N=164) |
Test Statistic | p Value | |
|---|---|---|---|---|
| Age (y) | 69.8 (6.3) | 68.0 (6.5) | 435 df, t = 2.86 | 0.0044 |
| Sex (% women) | 61.5% (168) | 68.9% (113) | x2=2.42 | 0.1198 |
| Race (% white) | 82.1% (224) | 84.1% (138) | x2 = 0.32 | 0.5738 |
| Education (y) | 14.4 (2.5) | 15.5 (1.8) | 416.94 df, t = 5.27 | < 0.0001 |
| Duration of Participation (months) | 62.8 (44.4) | 78.4 (56.3) | 284.28 df, t = 3.08 | 0.0023 |
| Self-Report of Medical Morbidity | ||||
| Diabetes | 15.0% (41) | 5.5% (9) | x2 = 8.75 | 0.0031 |
| Heart Disease | 20.8% (57) | 10.4% (17) | x2 = 7.96 | 0.0048 |
| Hypertension | 51.6% (141) | 25.0% (41) | x2 = 29.78 | < 0.0001 |
| Cognitive Domain Z-Scores | ||||
| Episodic Memory (N = 430) | N = 268 −0.05 (0.76) | N = 162 0.36 (0.61) | 395.43 df, t = 6.26 | < 0.0001 |
| Psychomotor-Executive Function (N = 423) | N = 260 −0.04 (0.79) | N = 163 0.41 (0.51) | 420.66 df, t = 7.22 | < 0.0001 |
| Verbal Fluency (N = 435) | N=271 −0.11 (0.85) | N = 164 0.41 (0.72) | 389.07 df, t = 6.79 | < 0.0001 |
| Attention-Working Memory (N = 399) | N = 253 − 0.07 (0.81) | N = 146 0.22 (0.79) | 397 df, t = 3.49 | 0.0005 |
All comparisons of categorical variables used chi-square tests with 1 degree of freedom, presented as percent (N). Comparison of continuous measures used pooled two-tailed t-tests, or Satterthwaite t-tests if variances were unequal, presented as mean (SD). Baseline cognitive data were missing for some participants, resulting in reduced sample sizes as presented. Cognitive domain data presented are z-transformed averages across multiple tests.
Comparisons in cognition between diagnostic groups
Initial univariate analyses demonstrated that depressed individuals performed more poorly across all four cognitive domains (Table 1; scores on individual tests are included in Supplemental Table 2). Models controlling for age, sex, education, and race likewise found statistically significant group differences in baseline neuropsychological test performance for episodic memory (N = 437; F1,431 = 24.64, p <0.0001), attention-working memory (N = 409; F1,403 = 7.33, p = 0.0071), verbal fluency (N = 437; F1,431 = 25.36, p <0.0001), and executive function (N = 432; F1,426 = 42.49, p <0.0001). In these analyses, the depressed group performed more poorly than the nondepressed group.
In longitudinal repeated measures analyses, we tested for the effects of medical morbidity on change in cognitive performance by incorporating an interaction term examining time and self-report of vascular medical morbidity (presence of hypertension, diabetes, or heart disease). These terms were not statistically significant in any model, so measures of medical morbidity were removed from analyses. the final models we found statistically significant group by time interactions in all domains, with the depressed group exhibiting a greater decline in performance over time (Figure 1A). This was true for episodic memory (one-year difference in z-score domain change = −0.0235, SE = 0.0049; F = 23.38, 1,1793 df, p <0.0001; effect size [ES] = 0.014), attention-working memory (difference = −0.0337, SE = 0.0061; F = 30.20, 1,1591 df, p < 0.0001; ES = 0.012), verbal fluency (difference = −0.0130, SE = 0.0057; F = 5.15, 1,1817 df, p = 0.0234; ES = 0.003) and executive function (difference = −0.0147, SE = 0.0054; F = 7.29, 1,1750 df, p = 0.0070; ES = 0.011).
Figure 1.
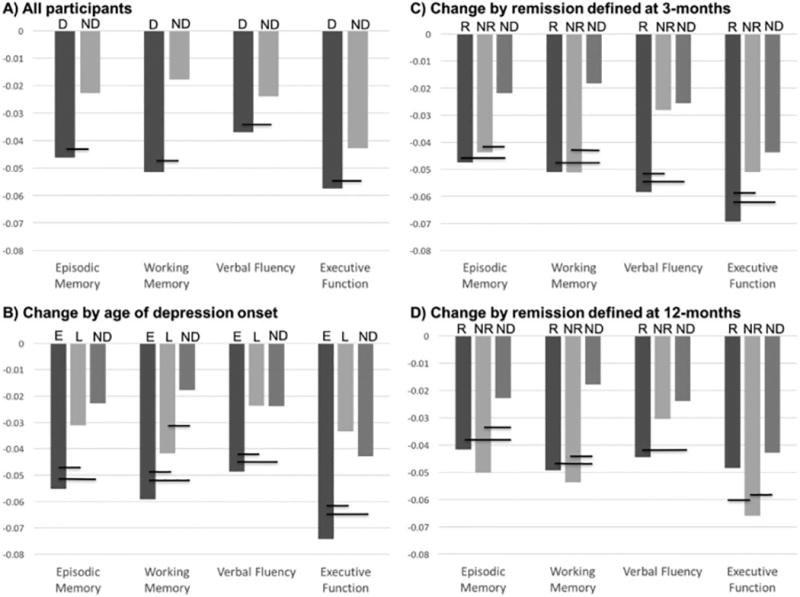
Group differences in annual change in neuropsychological domain z-scores
Figures compare annual change in z-scored neuropsychological domain performance by group, controlling for age, sex, race, and education level. Statistically significant group differences are indicated with a black horizontal bar covering the vertical group bars. Statistical results are presented in the text and in Tables 2–4. Abbreviations: D = Depressed; ND = Nondepressed; E = Early onset depression; L = Late onset depression; R = Remitted; NR = Nonremitted
Comparisons based on age of depression onset
In addition to 164 nondepressed participants, analyses included 267 depressed elders (6 individuals were missing age of onset data), 167 with EOD (first episode prior to age 50y) and 100 with LOD (first episode at age 50y or older). Group comparisons between the EOD and LOD groups demonstrated that the LOD group was older, had slightly fewer years of education, and a higher representation of men (Supplemental Table 3). There were no significant group differences in depression severity or medical morbidity. They were in the study for comparable amounts of time (EOD: mean 64.8 months; LOD: mean 63.8 months).
Univariate analyses of z-transformed cognitive domain scores suggested that the LOD group performed more poorly at baseline on episodic memory and verbal fluency (Supplemental Table 3). In models including nondepressed participants and controlling for demographic differences, we found statistically significant group differences in baseline test performance for episodic memory (F2,422 = 16.49, p <0.0001), attention-working memory (F2,389 = 3.98, p = 0.0194), verbal fluency (F2,422 = 14.40, p <0.0001) and executive function (F2,413= 23.31, p <0.0001). In pairwise group comparisons (Supplemental Table 4), we did not observe significant difference in baseline performance in any domain between depressed groups. The nondepressed group performed significantly better in all domains than both depressed groups, except for attention-working memory, where the difference between the nondepressed and EOD group was not significantly different.
In longitudinal repeated measures analyses, we found statistically significant group by time interactions in all four domains (Table 2, Figure 1B). For domains of episodic memory, verbal fluency, and executive function, the EOD group exhibited a greater decline in performance over time than either the LOD or nondepressed groups, who did not significantly differ. For the attention-working memory domain, we observed that the EOD exhibited a significantly greater decline than the LOD group, who in turn exhibited a greater decline than the nondepressed group.
Table 2.
Change over time in z-scored neuropsychological domains between groups defined by age of onset of initial depressive episode
| Domain | Difference in 1-Year Change |
Standard Error | Test Statistic and Effect Size |
p value |
|---|---|---|---|---|
| Episodic Memory | F2,1761 = 17.35 ES = 0.018 | < 0.0001 | ||
| • EOD - Nondepressed | −0.0323 | 0.0055 | t = −5.84 | < 0.0001 |
| • LOD – Nondepressed | −0.0084 | 0.0065 | t = −1.31 | 0.1913 |
| • EOD – LOD | −0.0239 | 0.0069 | t = −3.46 | 0.0006 |
| Attention-Working Memory | F2,1558 = 17.35 ES = 0.018 | <0.0001 | ||
| • EOD - Nondepressed | −0.0414 | 0.0071 | t = −5.80 | < 0.0001 |
| • LOD – Nondepressed | −0.0241 | 0.0082 | t = −2.94 | 0.0033 |
| • EOD – LOD | −0.0173 | 0.0088 | t = −1.96 | 0.0500 |
| Verbal Fluency | F2,1779 = 8.15 ES = 0.007 | 0.0003 | ||
| • EOD - Nondepressed | −0.0248 | 0.0065 | t = −.78 | 0.0002 |
| • LOD – Nondepressed | 0.0003 | 0.0077 | t = 0.03 | 0.9740 |
| • EOD – LOD | −0.0250 | 0.0082 | t = −3.06 | 0.0023 |
| Executive Function | F2,1718 = 17.59 ES = 0.022 | < 0.0001 | ||
| • EOD - Nondepressed | −0.0314 | 0.0063 | t = −5.02 | < 0.0001 |
| • LOD – Nondepressed | 0.0094 | 0.0073 | t = 1.30 | 0.1952 |
| • EOD – LOD | −0.0408 | 0.0079 | t = −5.20 | < 0.0001 |
Reported data are statistical results of the interaction between onset of depression (EOD or early-onset before age 50y; LOD or late-onset age 50y or later; or nondepressed) and study time, examining Z-transformed cognitive domain scores as the dependent variable. Models controlled for age, sex, race, education, and baseline domain score. Degrees of freedom for F statistics displayed as 2 (for the three groups), model error. T values determined from group comparisons within the models. ES = effect size
Comparisons based on 3-month remission status
At three months, 193 depressed subjects were nonremitted and 77 were remitted. Three depressed individuals did not have 3-month clinical data so were not included in these analyses. In models controlling for demographics, there were statistically significant group differences in baseline performance for episodic memory (F2,417 = 11.62, p <0.0001), attention-working memory (F2,386 = 3.64, p = 0.0272), verbal fluency (F2,422 = 11.64, p <0.0001) and executive function (F2,407= 21.26 , p <0.0001). In pairwise group comparisons (Supplemental Table 5), the remitted and nonremitted subjects did not differ in baseline performance in any domain. Both depressed groups also performed worse than the nondepressed groups in episodic memory, verbal fluency, and executive function. For attention-working memory, the remitted group performed comparably to the nondepressed group, while the nonremitted group performed worse.
In longitudinal repeated measures analyses, we found statistically significant group by time interactions in all four domains (Table 3, Figure 1C). For domains of episodic memory and attention-working memory, both depressed groups exhibited a greater decline in performance than did the nondepressed group, but there was no significant difference between the depressed groups. For executive function and verbal fluency, the 3-month remitters exhibited greater decline than either the nonremitting or nondepressed groups, but change in performance for nonremitters did not differ from nondepressed subjects.
Table 3.
Change over time in z-scored neuropsychological domains between groups defined after three months of antidepressant treatment
| Domain | Difference in Slope |
Standard Error |
Test Statistic and Effect Size |
p value |
|---|---|---|---|---|
| Episodic Memory | F2,1761 = 11.16 ES = 0.013 | < 0.0001 | ||
| • Nonremitted Depressed - Nondepressed | −0.0219 | 0.0055 | t = −3.95 | <0.0001 |
| • Remitted Depressed – Nondepressed | −0.0259 | 0.0069 | t = −3.73 | 0.0002 |
| • Nonremitted Depressed – Remitted Depressed | 0.0037 | 0.0073 | t = 0.66 | 0.5081 |
| Attention-Working Memory | F2,1560 = 15.15 ES = 0.019 | <0.0001 | ||
| • Nonremitted Depressed - Nondepressed | −0.0329 | 0.0069 | t = −5.10 | <0.0001 |
| • Remitted Depressed – Nondepressed | −0.0328 | 0.0091 | t = −3.61 | 0.0003 |
| • Nonremitted Depressed – Remitted Depressed | −0.0018 | 0.0095 | t = −0.19 | 0.8484 |
| Verbal Fluency | F2,1783= 10.13 ES = 0.010 | <0.0001 | ||
| • Nonremitted Depressed - Nondepressed | −0.0026 | 0.0062 | t = −0.41 | 0.6786 |
| • Remitted Depressed – Nondepressed | −0.0365 | 0.0084 | t = −4.37 | <0.0001 |
| • Nonremitted Depressed – Remitted Depressed | 0.0328 | 0.0084 | t = 3.89 | 0.0001 |
| Executive Function | F2,1718 = 5.18 ES = 0.010 | 0.0057 | ||
| • Nonremitted Depressed - Nondepressed | −0.0073 | 0.0062 | t = −1.44 | 0.1489 |
| • Remitted Depressed – Nondepressed | −0.0256 | 0.0077 | t = −3.19 | 0.0015 |
| • Nonremitted Depressed – Remitted Depressed | 0.0146 | 0.0084 | t = 1.96 | 0.0500 |
Reported data are statistical results of the interaction between diagnosis (remitted, nonremitted, or nondepressed) and study time, examining Z-transformed cognitive domain scores as the dependent variable. Models controlled for age, sex, race, education, and baseline domain score. Degrees of freedom for F statistics displayed as 2 (for the three groups), model error. T values determined from group comparisons within the models. ES = effect size
Comparisons based on twelve-month remission status
At twelve months, 145 depressed subjects were nonremitted and 128 were remitted. In models controlling for demographics, we found statistically significant group difference in baseline neuropsychological test performance between nondepressed, 12-month remitters, and 12-month nonremitting groups for episodic memory (F2,424 = 14.33, p <0.0001), attention-working memory (F2,390= 4.01, p = 0.0189), verbal fluency (F2,428 = 13.08, p <0.0001) and executive function (F2,414 = 22.13, p <0.0001). In pairwise group comparisons (Supplemental Table 6), the remitted and nonremitted subjects did not differ in baseline performance in any domain. Both depressed groups also performed worse than the nondepressed groups in all domains.
In longitudinal repeated measures analyses, we found statistically significant group by time interactions in all domains (Table 4, Figure 1D). For episodic memory and attention-working memory domains, both depressed groups exhibited greater decline over time than did the nondepressed cohort but there was no significant difference in change between the depressed cohorts. For executive function, the nonremitting group exhibited a greater decline in performance over time when compared with both the remitting and nondepressed groups, but there was no significant difference in change over time between the remitting and nondepressed groups. For verbal fluency, the remitted group exhibited significantly greater change than did the nondepressed group, but other group comparisons were not significantly different.
Table 4.
Change over time in z-scored neuropsychological domains between groups defined after twelve months of antidepressant treatment
| Domain | Difference in Slope |
Standard Error |
Test Statistic | p value |
|---|---|---|---|---|
| Episodic Memory | F2,1792 = 12.53 ES = 0.013 | <0.0001 | ||
| • Nonremitted Depressed - Nondepressed | −0.0275 | 0.0058 | t = −4.76 | <0.0001 |
| • Remitted Depressed – Nondepressed | −0.0189 | 0.0060 | t = −3.14 | 0.0017 |
| • Nonremitted Depressed – Remitted Depressed | −0.0085 | 0.0067 | t = −1.27 | 0.2030 |
| Attention-Working Memory | F2,1590 = 15.25 ES = 0.018 | <0.0001 | ||
| • Nonremitted Depressed - Nondepressed | −0.0360 | 0.0074 | t = −4.86 | <0.0001 |
| • Remitted Depressed – Nondepressed | −0.0315 | 0.0075 | t = −4.19 | 0.0001 |
| • Nonremitted Depressed – Remitted Depressed | −0.0045 | 0.0084 | t = −0.53 | 0.5945 |
| Verbal Fluency | F2,1816 = 4.15 ES = 0.005 | 0.0160 | ||
| • Nonremitted Depressed - Nondepressed | −0.0066 | 0.0068 | t = −0.97 | 0.3309 |
| • Remitted Depressed – Nondepressed | −0.0206 | 0.0072 | t = −2.88 | 0.0040 |
| • Nonremitted Depressed – Remitted Depressed | 0.0140 | 0.0079 | t = 1.77 | 0.0770 |
| Executive Function | F2,1748 = 6.34 ES = 0.010 | 0.0018 | ||
| • Nonremitted Depressed - Nondepressed | −0.0231 | 0.0065 | t = −3.54 | 0.0004 |
| • Remitted Depressed – Nondepressed | −0.0056 | 0.0067 | t = −0.82 | 0.4098 |
| • Nonremitted Depressed – Remitted Depressed | −0.0175 | 0.0076 | t = −2.31 | 0.0212 |
Reported data are statistical results of the interaction between diagnosis (remitted, nonremitted, or nondepressed) and study time, examining Z-transformed cognitive domain scores as the dependent variable. Models controlled for age, sex, race, education, and baseline domain score. Degrees of freedom for F statistics displayed as 2 (for the three groups), model error. T values determined from group comparisons within the models. ES = effect size
DISCUSSION
This long-term longitudinal study, following participants over an average of 5.7 years, has several key findings. First, depressed participants exhibited a greater decline than did nondepressed elders in domains of episodic memory, attention-working memory, verbal fluency and executive function. Second, EOD subjects declined more rapidly than either nondepressed or LOD subjects across all domains, while LOD subjects declined more rapidly than nondepressed subjects only in attention-working memory. Finally, remission status at 3 or 12 months was not associated with a benign cognitive course. Both remitters and nonremitters declined at comparable rates and faster than the nondepressed group in measures of episodic memory and working memory, with group differences also be observed in verbal fluency and executive function. However, it should be acknowledged that the degree of change observed across groups was low, with small effect sizes.
These results support past work associating LLD with cognitive decline.2–7 Supporting past negative studies,3,4,15 we did not observe any baseline differences in cognitive domain performance between the EOD and LOD groups. In longitudinal analyses, the EOD group exhibited greater decline than other groups, while aside from working memory, the LOD exhibited a rate of change comparable to the nondepressed subjects. These findings differ from past studies reporting that LOD individuals exhibit greater cognitive decline and greater risk of dementia over time 37,45 or complementary studies associating brain pathology in LOD with greater cognitive decline.46,47 This report methodologically differs from prior studies by examining participants over a longer period of time with a broader range of neuropsychological tests, however our findings should be tempered as we did not examine measures of brain pathology. Our findings are concordant with studies associating risk of cognitive decline with repeated depressive episodes.17,18 Although we did not quantify the number of lifetime depressive episodes, as participants were depressed at study entry, by definition all EOD participants had at least one prior episode. In contrast, late-onset individuals would be expected to have fewer episodes. Our findings support a hypothesis where repeated episodes contribute to cognitive decline.
Unfortunately, remission at key milestones is not a phenotype indicating reduced risk for cognitive decline. This is concordant with past work demonstrating that cognitive difficulties persist with antidepressant treatment 28–30 and bridges the gap with longitudinal studies demonstrating that depression is a risk factor for developing dementia.2,6,7 In this study, greater declines in cognitive performance was consistently observed for both remitters and nonremitters in episodic memory and attention-working memory. However, specific group differences observed in other domains (Figure 1, C and D) should also be highlighted. First, the 3-month remitters exhibited greater decline than nondepressed subjects across all four domains (Figure 1.C). Thus acute remission is not a marker of a benign future cognitive course. In contrast, when compared with nondepressed subjects, 3-month nonremitters did not exhibit a difference in verbal fluency or executive function change. Thus we see variability across domains, and although the implications are unclear, nonremitters defined at 3-months exhibit less deterioration in some domains than do remitting subjects. Second, in 12-month analyses, executive function declined faster in nonremitters, while 12-month remitters were comparable to nondepressed subjects. Executive dysfunction is persistently associated with poorer acute response to antidepressant treatment and longer time to remission.20,24 The difference in executive function findings between 3- and 12-month analyses may be related to individuals with executive deficits having a longer time to remission or, if they remit early, greater likelihood of relapse over that year.
Studies examining the interrelationship between depression and cognitive decline should consider that depression may directly contribute to or worsen cognitive decline. This is supported by a clinical-pathological study 11 reporting that higher levels of depressive symptoms were related to a faster rate of cognitive decline independent of neuropathology at autopsy. Several biological processes may mediate the relationship between depression and cognitive decline, including HPA axis alterations, proinflammatory processes, or vascular disease.19 These processes may interact with one another 48 but also interact with amyloid or tau pathology to affect cognition.
These findings have implications for clinical care. The message is clear: monitoring for cognitive changes is needed as even individuals exhibiting a robust antidepressant response are at risk for decline. Moreover, we need interventions to treat cognitive deficits in individuals with depression. This is an active area of research with evidence supporting non-pharmacological interventions including cognitive remediation 49 and exercise, while some psychotherapies appear to benefit performance on specific cognitive measures.50 Simply using current medications will not address the problem and new therapeutic approaches are needed.51
Despite the strength of a large longitudinal sample, this study also has limitations. Some limitations are common to longitudinal studies, such as subjects being lost to follow-up over time. For example, only half the sample has five-year neuropsychological data, with only one-quarter of the sample having data beyond seven years (Supplemental Table 1). Other limitations are specific to the current study. First, observed changes are modest across groups with small annual group changes in domain z-scores. Use of z-transformation of neuropsychological test scores allows us to combine tests but it complicates the clinical translation of our findings. Second, our cognitive battery has few assessments of executive function and language ability. Broader assessments would allow us to test for other specific findings relevant to LLD, such as response inhibition, set-shifting or semantic fluency. Additional limitations include using a self-report for age of onset, although this was obtained through a structured interview. Similarly, we have limited data on medical comorbidity and available data were obtained through self-report. Next, while we present data on psychotropic medication use at study entry, given the wide heterogeneity in medications used we are unable to examine for effects of medication use on cognitive course. Such questions are important to understand whether different classes of antidepressants differentially affect cognitive course, but is equally important for use of other psychotropic medications such as benzodiazepines, where use may be associated with increased risk of dementia.52 Finally, we examined change in cognitive domain performance over conversion to dementia in order to capture changes that did not meet the threshold for dementia but that still negatively affect function.
This study also did not include any imaging measures of brain pathology such as measures of hyperintensities or hippocampal atrophy. Such measures are associated with both depression and cognitive decline. These data were not available for a large portion of the study population, so were not included in analyses. Future work should incorporate imaging-based measures of brain aging while examining cognitive outcomes.
In conclusion, this demonstrates that depressed elders exhibit accelerated cognitive decline over time, particularly individuals with an earlier life onset. Antidepressant remission does not appear to be protective. More work is needed to understand the pathophysiology behind this relationship and to identify pharmacological and non-pharmacological treatments that benefit both mood and cognition.
Supplementary Material
Acknowledgments
This research was supported by NIH grants R21 MH099218, R01 MH102246, R01 MH054846, and K24 MH110598
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data were presented at the 2015 Meeting of the American College of Neuropsychopharmacology and the 2016 Meeting of the American Association for Geriatric Psychiatry.
No disclosures to report.
References
- 1.Taylor WD. Clinical practice. Depression in the elderly. N Engl J Med. 2014;371:1228–1236. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- 2.Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 4.Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Koenig AM, DeLozier IJ, Zmuda MD, et al. Neuropsychological functioning in the acute and remitted States of late-life depression. J Alzheimers Dis. 2015;45:175–185. doi: 10.3233/JAD-148006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byers AL, Vittinghoff E, Lui LY, et al. Twenty-year depressive trajectories among older women. Arch Gen Psychiatry. 2012;69:1073–1079. doi: 10.1001/archgenpsychiatry.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devanand DP, Sano M, Tang M-X, et al. Depressed mood and incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 8.Steffens DC, Potter GG, McQuoid DR, et al. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007;15:839–849. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- 9.Steffens DC, McQuoid DR, Payne ME, et al. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2011;19:4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweet RA, Hamilton RL, Butters MA, et al. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29:2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Capuano AW, Boyle PA, et al. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology. 2014;83:702–709. doi: 10.1212/WNL.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdelho A, Madureira S, Moleiro C, et al. Depressive symptoms predict cognitive decline and dementia in older people independently of cerebral white matter changes: the LADIS study. J Neurol Neurosurg Psychiatry. 2013;84:1250–1254. doi: 10.1136/jnnp-2012-304191. [DOI] [PubMed] [Google Scholar]
- 13.Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 14.Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodaty H, Luscombe G, Parker G, et al. Early and late onset depression in old age: different aetiologies, same phenomenology. J Affect Disord. 2001;66:225–236. doi: 10.1016/s0165-0327(00)00317-7. [DOI] [PubMed] [Google Scholar]
- 16.Geerlings MI, den Heijer T, Koudstaal PJ, et al. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 17.Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69:493–498. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byers AL, Yaffe K. Depression and risk of developing dementia. Nature reviews. Neurology. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneriya SH, Robbins-Welty GA, Smagula SF, et al. Predictors and Moderators of Remission With Aripiprazole Augmentation in Treatment-Resistant Late-Life Depression: An Analysis of the IRL-GRey Randomized Clinical Trial. JAMA psychiatry. 2016 doi: 10.1001/jamapsychiatry.2015.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor WD, Kudra K, Zhao Z, et al. Cingulum bundle white matter lesions influence antidepressant response in late-life depression: a pilot study. J Affect Disord. 2014;162:8–11. doi: 10.1016/j.jad.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 24.Alexopoulos GS, Kiosses DN, Heo M, et al. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Story TJ, Potter GG, Attix DK, et al. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16:752–759. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barch DM, D'Angelo G, Pieper C, et al. Cognitive improvement following treatment in late-life depression: relationship to vascular risk and age of onset. Am J Geriatr Psychiatry. 2012;20:682–690. doi: 10.1097/JGP.0b013e318246b6cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 29.Devanand DP, Pelton GH, Marston K, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry. 2003;18:123–130. doi: 10.1002/gps.802. [DOI] [PubMed] [Google Scholar]
- 30.Nebes RN, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treament: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 31.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 32.Taylor WD, McQuoid DR, Payne ME, et al. Hippocampus Atrophy and the Longitudinal Course of Late-life Depression. Am J Geriatr Psychiatry. 2014;22:1504–1512. doi: 10.1016/j.jagp.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 34.Robins LN, Helzer JE, Croughan J, et al. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 35.Potter GG, McQuoid DR, Steffens DC. Appetite loss and neurocognitive deficits in late-life depression. Int J Geriatr Psychiatry. 2015;30:647–654. doi: 10.1002/gps.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steffens DC, Welsh-Bohmer KA, Burke JR, et al. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. J Geriatr Psychiatry Neurol. 2004;17:202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 37.Sachs-Ericsson N, Corsentino E, Moxley J, et al. A longitudinal study of differences in late- and early-onset geriatric depression: depressive symptoms and psychosocial, cognitive, and neurological functioning. Aging Ment Health. 2013;17:1–11. doi: 10.1080/13607863.2012.717253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regier DA, Myers JK, Kramer M, et al. The NIMH Epidemiologic Catchment Area program: historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41:934–941. doi: 10.1001/archpsyc.1984.01790210016003. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 40.Steffens DC, McQuoid DR, Krishnan KRR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacol Bull. 2002;36:58–68. [PubMed] [Google Scholar]
- 41.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan KRR, McDonald WM. Arteriosclerotic depression. Med Hypotheses. 1995;44:111–115. doi: 10.1016/0306-9877(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 43.Burke J, McQuoid DR, Payne ME, et al. Amygdala volume in late-life depression: relationship with age of onset. Am J Geriatr Psychiatry. 2011;19:771–776. doi: 10.1097/JGP.0b013e318211069a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor WD, Steffens DC, Payne ME, et al. Influence of serotonin transporter promoter region polymorphisms on hippocampal volumes in late-life depression. Arch Gen Psychiatry. 2005;62:537–544. doi: 10.1001/archpsyc.62.5.537. [DOI] [PubMed] [Google Scholar]
- 45.Sachs-Ericsson N, Moxley JH, Corsentino E, et al. Melancholia in later life: late and early onset differences in presentation, course, and dementia risk. Int J Geriatr Psychiatry. 2014;29:943–951. doi: 10.1002/gps.4083. [DOI] [PubMed] [Google Scholar]
- 46.Sawyer K, Corsentino E, Sachs-Ericsson N, et al. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health. 2012;16:753–762. doi: 10.1080/13607863.2012.678478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hickie I, Naismith S, Ward PB, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 48.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morimoto SS, Wexler BE, Liu J, et al. Neuroplasticity-based computerized cognitive remediation for treatment-resistant geriatric depression. Nat Commun. 2014;5:4579. doi: 10.1038/ncomms5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackin RS, Nelson JC, Delucchi K, et al. Cognitive outcomes after psychotherapeutic interventions for major depression in older adults with executive dysfunction. Am J Geriatr Psychiatry. 2014;22:1496–1503. doi: 10.1016/j.jagp.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zurkovsky L, Taylor WD, Newhouse PA. Cognition as a therapeutic target in late-life depression: potential for nicotinic therapeutics. Biochemical pharmacology. 2013;86:1133–1144. doi: 10.1016/j.bcp.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pariente A, de Gage SB, Moore N, et al. The Benzodiazepine-Dementia Disorders Link: Current State of Knowledge. CNS Drugs. 2016;30:1–7. doi: 10.1007/s40263-015-0305-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


