Abstract
Background
Stroke mechanisms and the risk of recurrent thromboembolism are incompletely understood in patients with primary brain tumors. We sought to better delineate these important clinical features.
Methods
We performed a retrospective cohort study of adults with primary brain tumors diagnosed with MRI-confirmed acute ischemic stroke at Memorial Sloan Kettering Cancer Center from 2005 to 2015. Study neurologists collected data on patients’ cancer history, stroke risk factors, treatments, and outcomes. Stroke mechanisms were adjudicated by consensus. The primary outcome was recurrent thromboembolism (arterial or venous) and the secondary outcome was recurrent ischemic stroke. Kaplan-Meier statistics were used to calculate cumulative outcome rates, and Cox hazards analysis was used to evaluate the association between potential risk factors and outcomes.
Results
We identified 83 patients with primary brain tumors and symptomatic acute ischemic stroke. Median survival after index stroke was 2.2 years (interquartile range, 0.5–7.0). Tumors were mostly gliomas (72%) and meningiomas (13%). Most strokes were from unconventional mechanisms, particularly radiation vasculopathy (36%) and surgical manipulation (18%). Small-or large-vessel disease or cardioembolism caused 13% of strokes, while 29% were cryptogenic. Cumulative recurrent thromboembolism rates were 11% at 30 days, 17% at 180 days, and 27% at 365 days; while cumulative recurrent stroke rates were 5% at 30 days, 11% at 180 days, and 13% at 365 days. We found no significant predictors of outcomes.
Conclusions
Patients with primary brain tumors generally develop strokes from rare mechanisms and their risk of recurrent thromboembolism, including stroke, is high.
Keywords: Stroke, thromboembolism, cancer and stroke, brain tumor, cerebrovascular disease
Introduction
Primary brain tumors are diagnosed in 77,000 people each year in the United States, and their incidence may be increasing.(1) Compared to other cancer types, primary brain tumors are associated with especially high morbidity and mortality.(2) However, advances in cancer treatments have led to longer survival in patients with malignant brain tumors.(3) Stroke, an important cardiovascular complication of cancer and a leading cause of adult disability, becomes more clinically relevant as these patients are living longer.(4)
Ischemic stroke portends a poor prognosis in patients with active systemic cancer. Roughly one-third of cancer patients with stroke develop a recurrent thromboembolic event by 3 months, and 13% develop recurrent ischemic stroke, which is approximately three times the expected rate in non-cancer patients.(5, 6) Moreover, the median survival after ischemic stroke in this population is 84 days.(5) The high risk of recurrent thromboembolism and death after stroke in the systemic cancer population is partly due to cancer-associated coagulopathy, a well-recognized non-traditional stroke mechanism correlating with heightened cancer activity.(7) It is uncertain whether patients with primary brain tumors and ischemic stroke also face an increased risk of recurrent thromboembolism, as prior small studies have suggested that stroke in this population is more often from non-coagulopathic mechanisms such as radiation vasculopathy and surgery.(8–10) However, childhood cancer survivors with prior cranial radiation face an elevated stroke risk.(11–13) Additionally, some primary brain tumors have been associated with hypercoagulability.(14, 15)
We analyzed a large, contemporary cohort of patients with primary brain tumors and acute ischemic stroke to better characterize stroke mechanisms in this population. In addition, we sought to evaluate the risk and predictors of recurrent thromboembolism, stroke, and death among these patients. Our prespecified hypothesis was that rates of recurrent stroke and other thromboembolism in patients with primary brain tumors and acute ischemic stroke would be elevated when compared to the general stroke population, similar to systemic cancer patients.
Materials and Methods
Study Design
This was a retrospective cohort study of adult patients with primary brain tumors diagnosed with acute ischemic stroke at Memorial Sloan Kettering Cancer Center (MSKCC). Patients were included if their stroke was diagnosed in the inpatient or outpatient setting at an MSKCC facility, including Memorial Hospital, a 470-bed academic, quaternary-care cancer hospital in Manhattan, and several satellite ambulatory care clinics throughout New York, New Jersey, and Connecticut. Patients treated at MSKCC are actively followed for clinical outcomes, particularly thrombotic events and death, regardless of where the outcome occurs. Therefore, medical records from outside institutions are systematically collected and archived electronically to facilitate comprehensive care. Consequently, patients hospitalized for stroke at non-MSKCC hospitals who then saw MSKCC providers as an outpatient were also included as long as adequate clinical information and brain imaging were available for review. The MSKCC Institutional Review Board approved this study and granted a waiver of informed consent because of minimal risk to patients.
Study Subjects
Patients aged 18 years or older with a primary brain tumor and subsequent diagnosis of acute ischemic stroke at MSKCC between January 1, 2005 and December 31, 2014 were included. These patients were identified by searching the Department of Neurology’s comprehensive clinical database for any patient diagnosed with “primary brain tumor,” “primary central nervous system tumor,” “stroke,” “ischemic stroke,” or “transient ischemic attack,” and by reviewing MSKCC’s administrative claims database for patients with International Classification of Diseases-9-Clinical Modification diagnosis codes for primary brain tumors (191.x, 192.0, 192.1, 192.8, 192.9, 225.0, 225.1, 225.2, 225.8, 225.9) and acute ischemic stroke (433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 436). These codes were selected based on use in prior studies on similar topics.(5, 16) “Transient ischemic attack” was included in the query of the departmental clinical database; however, only patients with MRI-confirmed acute arterial ischemic stroke were included in the study cohort.
Query results were reviewed manually. Patients met eligibility criteria if they had an MRI-confirmed arterial ischemic stroke, a preceding diagnosis of primary brain tumor, and adequate clinical information regarding stroke presentation and diagnostic evaluation. Ischemic stroke was defined as a new neurologic deficit with corresponding evidence of acute infarction on brain MRI in the absence of clinical or radiologic indication of a non-cerebrovascular etiology. Because patients with primary brain tumors undergo routine surveillance imaging, which may lead to incidental findings including stroke, we also collected information on patients with MRI evidence of acute infarction in the absence of new neurologic deficits. These patients were classified as having silent brain infarctions and were analyzed in a separate secondary analysis.
Patients with stroke diagnosed by CT alone were excluded given the lack of imaging specificity. Primary brain tumor was defined as any primary tumor, whether intra-axial or extra-axial, malignant or benign, occurring within the cranium. We excluded patients with active malignancies outside of the central nervous system metastatic to the brain, as we were interested specifically in the primary brain tumor patient population.
Measurements
Data were collected regarding patients’ demographics, vascular risk factors, oncologic history, antithrombotic medication use, stroke evaluation and treatment, hospital discharge disposition, modified Rankin Scale (mRS) at discharge, and post-discharge follow-up. Brain tumors were categorized as glioma versus non-glioma. Gliomas were classified as high-grade and low-grade by WHO criteria.(17) Investigators certified in mRS ascertainment(18) utilized all available information from clinical and physical therapy notes to determine the discharge mRS. Abiding by guidelines for the proper conduct of retrospective research,(19) variables were defined in a data dictionary by investigator consensus and several mock abstraction sessions were performed before official chart reviews. In addition, data were collected on standardized data abstraction forms, which were also designed iteratively by investigator consensus.
Patients’ strokes were categorized by the modified TOAST (Trial of Org 10172 in Acute Stroke Treatment) study criteria.(20) Stroke mechanisms, including those unique to the cancer population, were predefined in the study data dictionary. Perioperative stroke was defined as stroke occurring within 30 days of a major or minor operation. For these patients, when the stroke was found immediately following surgery and was in or contiguous with the surgical field, and no alternative cause was identified or suspected, the mechanism of stroke was classified as surgical manipulation. In keeping with prior studies,(8) stroke from radiation vasculopathy was defined as stroke ipsilateral to the patient’s site of prior brain radiation, with no other identified mechanism of stroke. Imaging evidence of intracranial stenosis was not required for the diagnosis of radiation vasculopathy because radiation can cause small vessel vasculopathy,(21, 22) and lacunar-appearing strokes have been reported in patients after radiation.(23) Each patient’s stroke mechanism was independently adjudicated by two neurologists (NSP and JEB) with a board-certified stroke neurologist (BBN) serving as the final arbitrator.
Outcomes
All available inpatient and outpatient medical records, including transmitted outside institution records and telephone encounters, were reviewed from the time of the index stroke through December 31, 2015. Our primary outcome was any recurrent thromboembolic event, defined as in our prior studies as a composite of any recurrent ischemic stroke, transient ischemic attack, myocardial infarction, deep venous thrombosis, pulmonary embolism, or systemic arterial thrombosis or embolism.(5) Secondary outcomes were recurrent ischemic stroke, intracranial hemorrhage, symptomatic intracranial hemorrhage, major bleeding, and death.
Statistical Analysis
Patient characteristics were evaluated with standard descriptive statistics. Cumulative outcome rates were determined by Kaplan-Meier methods, and patients were censored at the time of last follow-up, death, or end of study on December 31, 2015. Multivariable Cox proportional hazard modeling was used to evaluate the association between several prespecified covariates and clinical outcomes. The following covariates were chosen based on data from prior studies and biological plausibility: age at time of index stroke, sex, nature of tumor (glioma versus non-glioma), prior stroke or TIA, and radiation-associated vasculopathy as the underlying index stroke mechanism.(13, 24, 25) The number of variables was determined based on the anticipated sample size. Variables were entered and kept in the multivariable model regardless of significance at the univariate level. The threshold of statistical significance was p <0.05.
Several prespecified secondary analyses were performed. We evaluated the effect of recurrent thromboembolism on survival while adjusting for age, sex, and high-grade glioma tumor type. We examined outcome rates only among patients with gliomas and, separately, those patients who had received vascular endothelial growth factor inhibitors within 30 days prior to their index stroke. Lastly, recurrent thromboembolism rates were measured among the secondary cohort of patients with silent brain infarctions, and these rates were compared using the log-rank test to the primary cohort of patients with symptomatic strokes. All statistical analyses were performed using Stata MP Version 12 (StataCorp, College Station, TX).
Results
Patient Characteristics
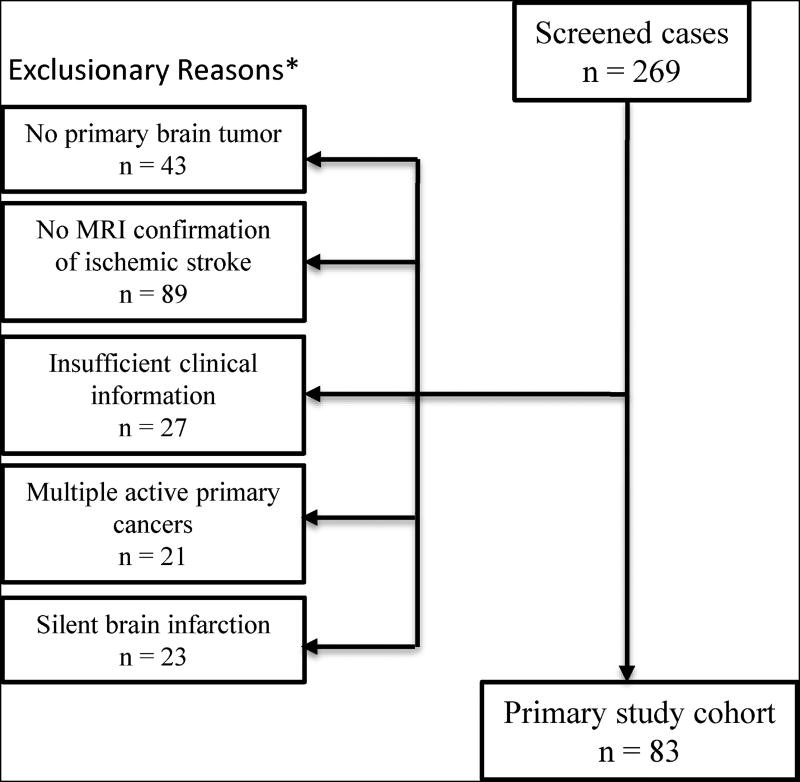
Of 269 patients identified for review, 83 patients with primary brain tumors and symptomatic acute ischemic stroke met our eligibility criteria and were included in the final analysis (Figure 1). These 83 patients constituted 0.8% of the 10,468 unique patients seen at MSKCC for primary brain tumor during the study period. Their median age was 60 years (interquartile range, 51–67) and 53% were women (Table 1). Vascular risk factors were common, particularly hypertension (53%) and hyperlipidemia (40%). Most patients had gliomas (n=60, 72%), particularly high-grade gliomas (n=49, 59%), or meningiomas (n=11, 13%). Other primary brain tumor types included medulloblastoma (n=2), ependymoma (n=2), pituitary adenoma (n=2), and primary central nervous system lymphoma (n=1). Most patients (71%) had received head or neck radiation therapy, and 31% had received non-surgical cancer treatment within 30 days of their stroke, including 12 with vascular endothelial growth factor inhibitor therapy and 20 with other chemotherapy. There were 16 patients (19%) who had their index stroke within 30 days of surgery. The median number of days between surgery and stroke diagnosis was 1 day (IQR, 0.25–1.75).
Figure 1. Study Cohort Eligibility Flowsheet.
Flow chart diagram describing patient selection for study. *Some patients had multiple reasons for exclusion.
Table 1.
Characteristics of Patients with Primary Brain Tumors and Ischemic Stroke, Stratified by Recurrent Thromboembolism
| Characteristica | Total (n = 83) |
Recurrent Thromboembolism (n=21) |
No recurrent Thromboembolism (n=62) |
P- value |
|---|---|---|---|---|
| Age, years, median (IQR) | 60 (51–67) | 59 (45–64) | 61 (53–68) | 0.34 |
| Women | 44 (53%) | 9 (43%) | 35 (56%) | 0.20 |
| Race-ethnicity | 1.00 | |||
| White | 72 (87%) | 18 (86%) | 54 (87%) | |
| Black | 4 (5%) | 1 (5%) | 3 (5%) | |
| Other | 7 (8%) | 2 (10%) | 5 (8%) | |
| Comorbidities | ||||
| Hypertension | 44 (53%) | 10 (48%) | 34 (55%) | 0.35 |
| Hyperlipidemia | 33 (40%) | 5 (24%) | 28 (45%) | 0.07 |
| Diabetes | 9 (11%) | 1 (5%) | 8 (13%) | 0.28 |
| Coronary disease | 6 (7%) | 2 (10%) | 4 (6%) | 0.48 |
| Atrial fibrillation | 1 (1%) | 0 (0%) | 1 (2%) | 0.75 |
| Prior stroke or TIA | 7 (8%) | 1 (5%) | 6 (10%) | 0.43 |
| History of smoking | 26 (31%) | 6 (29%) | 20 (32%) | 0.49 |
| Oncological history | ||||
| Glioma | 60 (72%) | 14 (67%) | 46 (74%) | 0.34 |
| High-grade glioma | 49 (59%) | 11 (52%) | 38 (61%) | 0.32 |
| Low-grade glioma | 11 (13%) | 3 (14%) | 8 (13%) | 0.57 |
| Meningioma | 11 (13%) | 3 (14%) | 8 (13%) | 0.57 |
| Other | 12 (14%) | 4 (19%) | 8 (13%) | 0.36 |
| Recent cancer treatmentb | 26 (31%) | 2 (10%) | 24 (39%) | 0.01 |
| Prior head or neck RT | 59 (71%) | 17 (81%) | 42 (68%) | 0.19 |
| Antiplatelet at discharge | 52 (63%) | 12 (57%) | 40 (65%) | 0.36 |
| Anticoagulant at discharge | 7 (8%) | 1 (5%) | 6 (10%) | 0.43 |
Abbreviations: IQR, interquartile range; TIA, transient ischemic attack; RT, radiotherapy.
Data are presented as number (%) unless otherwise specified.
Non-surgical treatment within the last 30 days.
The median time from primary brain tumor diagnosis to the index stroke was 2.0 years (interquartile range [IQR], 0.7–8.0). At the time of the index stroke, 13 patients (16%) were taking an antithrombotic medication. Diagnostic stroke evaluations included intracranial or extracranial vessel imaging in 69%, an echocardiogram in 69%, and inpatient cardiac rhythm evaluation in 81%. After excluding patients who died during hospitalization and those with strokes due to surgical manipulation, 77% had vessel imaging, 77% had an echocardiogram, and 77% had inpatient cardiac rhythm evaluation.
Demographics, stroke characteristics, and tumor characteristics were similar between patients who developed a recurrent thromboembolism after index stroke and those who did not except that cancer treatment in the 30 days before index stroke was less common in patients ultimately diagnosed with recurrent thromboembolism (10% vs. 39%, p=0.01) (Table 1).
Index Stroke Mechanisms and Treatments
Conventional stroke mechanisms were rarely identified, with 48 patients (58%) diagnosed with a known but unconventional etiology and 24 (29%) diagnosed with cryptogenic stroke (Table 2). By TOAST criteria, 9 patients (11%) had small-vessel disease, 1 patient (1%) had cardiac embolism, and 1 patient (1%) had large-artery atherosclerosis. The most common unconventional stroke mechanisms were radiation vasculopathy (36%) and surgical manipulation (18%). The median time from radiation therapy completion to the index stroke was 2.1 years (IQR, 1.0–8.1). The discharge mRS could be determined in 72 patients (87%), and the median was 3 (IQR, 3–4). An antithrombotic medication was prescribed at discharge to 74% of patients, and 56% were prescribed a statin; prescription rates were higher when excluding patients with stroke due to surgical manipulation—81% were prescribed an antithrombotic medication and 62% a statin. Of those discharged on an antithrombotic medication, 52 (88%) received an antiplatelet medication and 7 (12%) an anticoagulant medication.
Table 2.
Specific Stroke Mechanisms in Patients with Primary Brain Tumors and Acute Ischemic Stroke
| Specific stroke mechanism | No. (%) |
|---|---|
| Radiation vasculopathy | 30 (36%) |
| Cryptogenic | 24 (29%) |
| Surgical manipulation | 15 (18%) |
| Small-vessel disease | 9 (11%) |
| Extrinsic compression of vessel by tumor | 3 (4%) |
| Intracranial large-artery atherosclerosis | 1 (1%) |
| Atrial fibrillation | 1 (1%) |
Primary Analysis
The median study follow-up time was 1.1 years (IQR, 0.4–3.1), and the median number of documented patient follow-up encounters during this period was 10 (IQR, 5–20). Over this period, 48 (58%) patients died. Median survival from the index stroke was 2.2 years (IQR, 0.5–7.0). For patients who were alive at last follow-up, the median study follow-up time was 2.7 years (IQR, 1.1–4.3). After the index stroke, 12 patients were diagnosed with subsequent ischemic stroke, 2 with TIA, and 8 with venous thromboembolism. Cumulative rates of any recurrent thromboembolism were 11% at 30 days, 14% at 90 days, 17% at 180 days, and 27% at 365 days (Figure 2). Cumulative rates of recurrent ischemic stroke were 5% at 30 days, 8% at 90 days, 11% at 180 days, and 13% at 365 days. Major bleeding was diagnosed in 6 patients (7%), all with intracranial hemorrhage; of these, 3 patients were on aspirin. Recurrent thromboembolism or stroke (ischemic or hemorrhagic) was the cause of death for 5 (10%) of the 48 patients who died during follow-up. Specifically, 2 patients died from a recurrent ischemic stroke, 2 died from an intracranial hemorrhage, and one died from pulmonary embolism.
Figure 2. Cumulative Rates of Recurrent Thromboembolism and Ischemic Stroke in Patients with Primary Brain Tumors and Acute Ischemic Stroke.
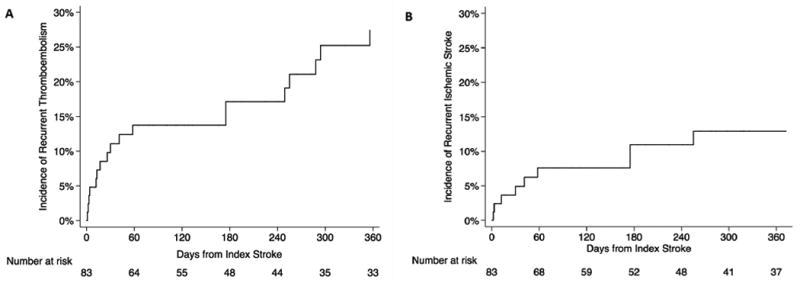
Cumulative rates of recurrent thromboembolism (Panel A) and recurrent ischemic stroke (Panel B) in patients with primary brain tumors after acute ischemic stroke.
In our prespecified univariable and multivariable Cox analyses, we found no significant predictors of recurrent thromboembolism (Table 3) or recurrent stroke (Table 4). A nonsignificant trend towards increased recurrent stroke risk was observed in patients with radiation vasculopathy as the mechanism of their index stroke (hazard ratio 2.4, 95% confidence interval 0.7–7.8).
Table 3.
Univariable and Multivariable Cox Proportional Hazards Models of Factors Associated with Recurrent Thromboembolism
| Variable | Univariable HR | 95% CI | Multivariable HR | 95% CI |
|---|---|---|---|---|
| Age | 1.23 | 0.51–2.97 | 1.21 | 0.48–3.03 |
| Female gender | 0.69 | 0.29–1.64 | 0.64 | 0.26–1.56 |
| Prior stroke or TIA | 0.52 | 0.70–3.89 | 0.50 | 0.06–3.86 |
| Radiation vasculopathya | 1.27 | 0.54–2.99 | 1.34 | 0.55–3.26 |
| Glioma tumor type | 0.97 | 0.39–2.43 | 0.97 | 0.37–2.54 |
Abbreviations: HR, hazard ratio; CI, confidence interval; TIA, transient ischemic attack.
Radiation vasculopathy refers to a patient’s index stroke mechanism.
Table 4.
Univariable and Multivariable Cox Proportional Hazards Models of Factors Associated with Recurrent Ischemic Stroke
| Variable | Univariable HR | 95% CI | Multivariable HR | 95% CI |
|---|---|---|---|---|
| Age | 1.04 | 0.31–3.41 | 1.06 | 0.31–3.65 |
| Female gender | 0.45 | 0.13–1.50 | 0.41 | 0.11–1.44 |
| Prior stroke or TIA | 0.97 | 0.12–7.54 | 1.00 | 0.12–8.50 |
| Radiation vasculopathya | 1.98 | 0.62–6.28 | 2.41 | 0.74–7.80 |
| Glioma tumor type | 0.71 | 0.22–2.27 | 0.73 | 0.21–2.54 |
Abbreviations: HR, hazard ratio; CI, confidence interval; TIA, transient ischemic attack.
Radiation vasculopathy refers to a patient’s index stroke mechanism.
Secondary Analyses
In exploratory multivariable analysis, recurrent thromboembolism was non-significantly associated with reduced survival after adjusting for age, sex, and high-grade glioma tumor type (hazard ratio, 1.59; 95% confidence interval, 0.80–3.14). Cumulative rates of recurrent thromboembolism (log-rank test: p=0.95) and recurrent ischemic stroke (log-rank test: p=0.56) were similar for patients with gliomas and those with other brain tumors. Of 12 patients who had received vascular endothelial growth factor inhibitor therapy within 30 days prior to the index stroke, none had recurrent thromboembolism.
We identified an additional 23 patients with silent brain infarction in addition to the 83 in the primary analysis. This group did not differ significantly in patient demographics, clinical characteristics, or stroke mechanisms. After silent brain infarction, cumulative recurrent thromboembolism rates were 9% at 90 days, 19% at 180 days, and 19% at 365 days; these cumulative incidence rates were not significantly different compared to patients with symptomatic strokes (log-rank test: p=0.91). Similarly, cumulative rates of recurrent ischemic stroke were 4% at 90 days, 15% at 180 days, and 15% at 365 days, which again were not significantly different compared to patients with symptomatic strokes (log-rank test: p=0.48). Median survival was nonsignificantly longer in patients with silent brain infarction compared to those with symptomatic strokes (3.9 years versus 2.2 years; log-rank test: p = 0.54).
Discussion
In a contemporary cohort of patients with primary brain tumors and acute ischemic stroke, we found high rates of recurrent thromboembolism and stroke, similar to what has been reported in patients with systemic cancer.(5, 26, 27) Patients with primary brain tumors experienced a short-term recurrent stroke rate that was approximately double the recurrent stroke rate observed in the general population.(6) We did not find any significant independent predictors of recurrent thromboembolism. Patients with radiation vasculopathy appeared to face double the risk of recurrent stroke, although this trend was nonsignificant. Additionally, we found a nonsignificant trend towards reduced survival in patients who developed recurrent thromboembolism. These associations did not reach statistical significance likely due to the relatively small number of patient outcomes.
In our study, stroke mechanisms in patients with primary brain tumors were often unique to the specific treatments they received, particularly cranial radiotherapy and neurosurgical manipulation, which together accounted for 54% of strokes. These results are consistent with prior smaller studies that have also reported radiation vasculopathy and surgical injury to be common causes of stroke in the primary brain tumor population.(8, 10) However, the perioperative stroke rate in our cohort was less than in previous studies, perhaps because we included all primary brain tumor types (not just gliomas) and we excluded incidental strokes from our primary analysis.(8–10) We also found that patients with radiation vasculopathy had a non-significant trend towards a higher recurrent stroke rate. Prior research has shown that cervicocephalic radiation is a strong risk factor for cerebrovascular complications in children and adults.(8, 11–13) In our population, that risk was realized at a median of only two years after completion of radiotherapy, emphasizing that most patients with brain tumors will survive into the period of risk. Interestingly, stroke mechanisms in this cohort of patients with primary brain tumors differed markedly when compared to previous studies of patients with systemic cancer, in whom cryptogenic strokes are common.(5) In the systemic cancer population, many of these cryptogenic strokes are thought to arise from non-bacterial endocarditis,(28, 29) a form of cancer-related hypercoagulability.(30, 31) The paucity of cryptogenic and radiographically cardioembolic-appearing strokes in our cohort suggests that this mechanism is uncommon in patients with primary brain tumors.
We are unaware of any previously published studies reporting thromboembolism and stroke recurrence rates in patients with primary brain tumors. In a prior study of patients with systemic cancer, the cumulative 6-month recurrent thromboembolism rate was 37%, and the cumulative 6-month recurrent stroke rate was 16%.(5) This is in contrast to the general stroke population, which has a 1-year cumulative recurrent stroke rate of approximately 4.8%.(6) The unconventional mechanisms of stroke in systematic cancer and primary brain tumor patients likely contribute to the high risk of recurrent events. Alternatively, it is possible that patients with cancer are not treated as aggressively for their stroke as patients without cancer; however, this is unlikely to fully explain the disparity in stroke rates between groups, as patients in this and prior studies generally had thorough diagnostic evaluations and regularly received secondary stroke prevention.(5, 32)
In secondary analyses, we found similarly high rates of recurrent thromboembolism and stroke in patients with silent brain infarctions as compared to those with symptomatic strokes. Emerging data in non-cancer patients suggest that silent brain infarctions are associated with a two-fold increased risk of subsequent stroke.(33) Therefore, identification of a silent brain infarction in a primary brain tumor patient might serve as an opportunity to prevent a disabling stroke through initiation of targeted stroke prevention therapies.(34)
This study has several limitations. First, this was a retrospective study, so some outcomes of interest may have been missed. In addition, MSKCC is not a certified stroke center; therefore, some patients with acute stroke may have been taken elsewhere and the data not captured. These limitations would result in an underestimation of recurrence risks. Second, the study was performed at an urban, quaternary care, cancer center such that the results may not generalize to other settings. Third, although our cohort was relatively large for this topic, our multivariable regression analyses may have been underpowered as evidenced by the wide confidence intervals. Finally, we did not collect long-term functional outcome data, so we are unable to determine the impact of recurrent thromboembolism and ischemic stroke on patient-centered outcomes such as disability and quality of life.
Conclusions
Stroke mechanisms in patients with primary brain tumors are unique when compared to both patients with systemic cancer and the general population. These patients survive for years after their index stroke but face an increased risk for recurrent thromboembolism and ischemic stroke. Improving secondary stroke prevention in patients with primary brain tumors may reduce disability.
Acknowledgments
This study was supported by NIH grants K23NS091395 (BN), R01NS097443 (HK), K23NS082367 (HK), and the Florence Gould Endowment for Discovery in Stroke (BN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouse C, Gittleman H, Ostrom QT, et al. Years of potential life lost for brain and CNS tumors relative to other cancers in adults in the United States, 2010. Neuro Oncol. 2016;18:70–7. doi: 10.1093/neuonc/nov249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DR, Ma DJ, Buckner JC, et al. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118:5608–13. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 5.Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. doi: 10.1212/WNL.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navi BB, Kamel H, Sidney S, et al. Validation of the Stroke Prognostic Instrument-II in a large, modern, community-based cohort of ischemic stroke survivors. Stroke. 2011;42:3392–6. doi: 10.1161/STROKEAHA.111.620336. [DOI] [PubMed] [Google Scholar]
- 7.Navi BB, Singer S, Merkler AE, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke. 2014;45:2292–7. doi: 10.1161/STROKEAHA.114.005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreisl TN, Toothaker T, Karimi S, et al. Ischemic stroke in patients with primary brain tumors. Neurology. 2008;70:2314–20. doi: 10.1212/01.wnl.0000314648.82924.6f. [DOI] [PubMed] [Google Scholar]
- 9.Seidel C, Hentschel B, Simon M, et al. A comprehensive analysis of vascular complications in 3,889 glioma patients from the German Glioma Network. J Neurol. 2013;260:847–55. doi: 10.1007/s00415-012-6718-9. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya-Matsuoka C, Cachia D, Yust-Katz S, et al. Ischemic stroke in patients with gliomas at The University of Texas-M.D. Anderson Cancer Center. J Neurooncol. 2015;125:143–8. doi: 10.1007/s11060-015-1880-4. [DOI] [PubMed] [Google Scholar]
- 11.Fullerton HJ, Stratton K, Mueller S, et al. Recurrent stroke in childhood cancer survivors. Neurology. 2015;85:1056–64. doi: 10.1212/WNL.0000000000001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:643–8. doi: 10.1016/j.ijrobp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris B, Partap S, Yeom K, et al. Cerebrovascular disease in childhood cancer survivors: A Children's Oncology Group Report. Neurology. 2009;73:1906–13. doi: 10.1212/WNL.0b013e3181c17ea8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoron L, Arbit E. Hemostatic changes in patients with brain tumors. J Neurooncol. 1994;22:87–100. doi: 10.1007/BF01052885. [DOI] [PubMed] [Google Scholar]
- 15.Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer. 2000;89:640–6. doi: 10.1002/1097-0142(20000801)89:3<640::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Murthy SB, Moradiya Y, Shah S, et al. In-hospital outcomes of thrombolysis for acute ischemic stroke in patients with primary brain tumors. J Clin Neurosci. 2015;22:474–8. doi: 10.1016/j.jocn.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 18.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Roongpiboonsopit D, Kuijf HJ, Charidimou A, et al. Evolution of cerebral microbleeds after cranial irradiation in medulloblastoma patients. Neurology. 2017;88:789–96. doi: 10.1212/WNL.0000000000003631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy ES, Xie H, Merchant TE, et al. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015;122:421–9. doi: 10.1007/s11060-015-1732-2. [DOI] [PubMed] [Google Scholar]
- 23.Fouladi M, Langston J, Mulhern R, et al. Silent lacunar lesions detected by magnetic resonance imaging of children with brain tumors: a late sequela of therapy. J Clin Oncol. 2000;18:824–31. doi: 10.1200/JCO.2000.18.4.824. [DOI] [PubMed] [Google Scholar]
- 24.Brada M, Burchell L, Ashley S, et al. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 1999;45:693–8. doi: 10.1016/s0360-3016(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 25.Burger PC, Mahley MS, Dudka L, et al. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44:1256–72. doi: 10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Jung KH, Park KH, et al. Clinical manifestation of cancer related stroke: retrospective case-control study. J Neurooncol. 2013;111:295–301. doi: 10.1007/s11060-012-1011-4. [DOI] [PubMed] [Google Scholar]
- 27.Lau KK, Wong YK, Teo KC, et al. Stroke patients with a past history of cancer are at increased risk of recurrent stroke and cardiovascular mortality. PLoS One. 2014;9:e88283. doi: 10.1371/journal.pone.0088283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta T, Karas MG, Segal AZ, et al. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol. 2006;97:894–8. doi: 10.1016/j.amjcard.2005.09.140. [DOI] [PubMed] [Google Scholar]
- 29.Seok JM, Kim SG, Kim JW, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. 2010;68:213–9. doi: 10.1002/ana.22050. [DOI] [PubMed] [Google Scholar]
- 30.el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518–23. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 31.Rogers LR, Cho ES, Kempin S, et al. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med. 1987;83:746–56. doi: 10.1016/0002-9343(87)90908-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim SG, Hong JM, Kim HY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke. 2010;41:798–801. doi: 10.1161/STROKEAHA.109.571356. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, Giambrone AE, Gialdini G, et al. Silent Brain Infarction and Risk of Future Stroke: A Systematic Review and Meta-Analysis. Stroke. 2016;47:719–25. doi: 10.1161/STROKEAHA.115.011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith EE, Saposnik G, Biessels GJ, et al. Prevention of Stroke in Patients With Silent Cerebrovascular Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. doi: 10.1161/STR.0000000000000116. [DOI] [PubMed] [Google Scholar]