Abstract
Psychologically stressful experiences evoke changes in cardiovascular physiology that may influence risk for cardiovascular disease (CVD). But what are the neural circuits and intermediate physiological pathways that link stressful experiences to cardiovascular changes that might in turn confer disease risk? This question is important because it has broader implications for our understanding of the neurophysiological pathways that link stressful and other psychological experiences to physical health. This review highlights selected findings from brain imaging studies of stressor-evoked cardiovascular reactivity and CVD risk. Converging evidence across these studies complements animal models and patient lesion studies to suggest that a network of cortical, limbic, and brainstem areas for central autonomic and physiological control are important for generating and regulating stressor-evoked cardiovascular reactivity via visceromotor and viscerosensory mechanisms. Emerging evidence further suggests that these brain areas may play a role in stress-related CVD risk, specifically by their involvement in mediating metabolically-dysregulated or extreme stressor-evoked cardiovascular reactions. Contextually, the research reviewed here offers an example of how brain imaging and health neuroscience methods can be integrated to address open and mechanistic questions about the neurophysiological pathways linking psychological stress and physical health.
Keywords: cardiovascular disease, central autonomic network, functional connectivity, stressor-evoked cardiovascular reactivity, visceral prediction errors
1. Introduction
How do stress-related processes instantiated in the brain relate to an individual’s risk for atherosclerotic cardiovascular disease (CVD) and related adverse cardiovascular outcomes that continue to be leading burdens to public health (Mozaffarian et al., 2016)? Addressing this open question is important: it has the potential to (i) advance our mechanistic understanding of the human neurophysiological substrates for psychological and behavioral influences on the development of CVD and (ii) inform novel and brain-based efforts to better predict and possibly reduce CVD risk. Historically, acute cardiovascular reactions (e.g., rapid and autonomically mediated rises in blood pressure [BP] and heart rate [HR]) to psychological stressors have been among the most heavily investigated stress-related parameters of CVD risk. Over the short-term, such stressor-evoked cardiovascular reactions may be adaptive, insofar as they provide hemodynamic and metabolic support for contextually appropriate behaviors that confer survival advantage (e.g., fight-or-flight behaviors). Over the long-term, however, stressor-evoked cardiovascular reactions that are exaggerated, prolonged, and repeatedly expressed may initiate or exacerbate pathophysiological changes in the heart and vasculature. More precisely, there is longstanding and cumulative epidemiological evidence that individuals who exhibit a phenotype characterized by the expression of large-magnitude or metabolically-exaggerated stressor-evoked cardiovascular reactions are at elevated risk for clinical and preclinical endpoints of CVD (for reviews, see Gerin et al., 2000; Chida & Steptoe, 2010; Taylor, Kamarck, Dianzumba, 2003; Krantz & Manuck, 1984; Scwartz et al., 2003; Treiber et al., 2003). These endpoints include an accelerated progression of atherosclerosis (e.g., Barnett et al., 1997; Jennings et al., 2004); the premature development of hypertension (e.g., Carroll et al., 2011; Carroll et al., 2012); increased ventricular mass (e.g., Allen, Matthews, & Sherman, 1997; Georgiades et al., 1996); concentric remodeling of the heart (e.g., al’Absi et al., 2006); future coronary events (e.g, myocardial infarctions) (Schwartz et al., 2003, Trieber et al., 2003); and cardiovascular disease mortality (Carroll et al., 2012).
The brain has long been implicated in the control of cardiovascular function, particularly in linking stressful experiences to cardiovascular changes associated with clinical events and disease pathophysiology (for reviews see Dampney, 2015; Lane et al., 2009a; 2009b; Palma & Benarroch, 2014; Taggart et al., 2016; Esler, 2017). For example, Cannon originally proposed that intense emotions, such as fright, were generated in the brain and triggered peripheral physiological responses that could end one’s life in “voodoo death” (Cannon, 1928; 1942). It has also long been known that brain damage and neurological phenomena (e.g., epilepsy, stroke) can result in detrimental effects on circulatory control via the autonomic nervous system, including sudden cardiac death (Colivicchi et al., 2005; Oppenheimer, 2006; Abboud et al., 2006; Tomson, Nashef, & Ryvlin, 2008; Nagai, Hoshide, & Kario, 2010). It is in this historical and behavioral medicine context that a growing number of brain imaging studies have sought to explicate the neural circuits that are jointly (i) engaged by psychological stressors and (ii) involved in coordinating autonomic and neuroendocrine activity to proximally influence cardiovascular responding. From a health neuroscience perspective (Erickson et al., 2014), a guiding assumption of these brain-imaging studies is that a better understanding of these neural circuits will help define the mechanistic pathways by which psychological stress may confer CVD risk and result in adverse clinical events. The goal of this brief review is to highlight key and convergent findings from these studies, as well as describe salient methodological issues, interpretive caveats, and future directions inherent to brain-imaging studies of cardiovascular reactivity and CVD risk.
2. Stressor-evoked cardiovascular reactivity and CVD
Psychological stressors can be defined as perceived threats to well-being that tax or exceed an individual’s capacity to cope with such threats (Lazarus, 1966). Individuals differ appreciably, however, in the extent to which they ascribe threat-related and psychological meaning to events, contexts, and myriad other stimuli. They also differ in the extent to which they construe their available coping resources and options as adequate for managing potential sources of threat. These individual differences are thought to arise from psychological appraisal processes that usually operate outside of awareness and are instantiated in forebrain neural circuits that (i) process internal and external sources of information for their personal relevance and threat-related meaning and (ii) calibrate peripheral physiology with behavioral action to support stressor coping and responding (Cohen, Gianaros, & Manuck, 2016). From this perspective, individual differences in stressor-evoked cardiovascular reactions that are linked to CVD risk are believed to be accounted for in part by corresponding differences in the functionality of forebrain neural circuits that link stressor processing (psychological appraisals) with physiological regulation, especially autonomic and neuroendocrine regulation of the cardiovascular system (Gianaros & Wager, 2015). To elaborate, stressor-evoked cardiovascular reactions result from intermediate changes in sympathetic and parasympathetic nervous system and hypothalamic-pituitary-adrenal axis outflow to the heart and vasculature. These autonomic and neuroendocrine effector changes in turn influence parameters of cardiovascular physiology (e.g., cardiac output, peripheral vessel resistance) to redirect blood flow and perfuse tissues according to anticipated or ongoing behavioral needs. Accordingly, a major focus in human and nonhuman animal studies on the neurobiology of cardiovascular stress reactivity has focused on cortical and subcortical circuits that proximally influence autonomic and neuroendocrine function. An emerging conceptual perspective is that these circuits specifically generate anticipatory visceromotor commands to alter parameters of cardiovascular physiology that prepare individuals to behaviorally cope with appraised threats (psychological stressors) (Gianaros & Wager, 2015). Thus, these visceromotor commands can be conceptualized as ‘visceral predictions’, insofar as they are anticipatory to the metabolic and behavioral demands engendered by appraised stressors (cf., Clark et al., 2013; Chanes & Feldman Barrett, 2017). As noted above, some individuals who are vulnerable to CVD exhibit a phenotype that is typified by cardiovascular changes (e.g., increases in HR and BP) that are in excess of the metabolic and behavioral demands of a given stressor. An example would be an individual with a stable, trait-like phenotype to exhibit a rise in HR in excess of 30 beats per minute (bpm) and/or a rise in systolic blood pressure (SBP) in excess of 30 mmHg while anticipating the delivery of a public speech that requires minimal metabolic effort. In this example, such stressor-evoked changes in HR and BP reflect a metabolic mis-calibration between anticipatory cardiovascular function and actual behavioral needs, a so-called ‘visceral prediction error’. Such exaggerated stressor-evoked cardiovascular reactions can be contrasted with coordinated changes in cardiovascular physiology that occur with physical activity and exercise for leisure or sport, where energy and metabolic needs are calibrated, presumably by central visceromotor commands that are updated and fine-tuned according to viscerosensory feedback (Fisher, Young, & Fadel, 2015; Shoemaker et al., 2015). Metabolically-exaggerated, cardiovascular reactions to psychological stressors can be readily assessed using laboratory paradigms that integrate psychological (mental) stress testing with conventional exercise physiology methods (e.g., Carroll, Phillips, & Balanos, 2009; Balanos et al., 2010). In these paradigms, ‘exaggerated reactors’ are those individuals who exhibit stressor-evoked cardiovascular changes that well exceed their stressor-evoked oxygen consumption change (i.e., their cardiovascular system is working in excess of their metabolic system; Turner & Carroll, 1985). For example, in a study assessing metabolic and cardiovascular activity in healthy young adults, an ‘exaggerated reactor’ had a heart rate of ~115 beats per minute (bpm) during psychological stress, when the metabolically appropriate response, predicted by oxygen consumption and determined by exercise performance testing, was only 85 bpm (i.e., heart rate was 30 bpm in excess of what was predicted as ‘metabolically appropriate’; Turner & Carroll, 1985). A longstanding view is that the repeated and cumulative expression of such metabolically-disproportionate, stressor-evoked cardiovascular reactions contribute to or exacerbate pathophysiological changes that are conducive to CVD and CVD events among vulnerable individuals (Carroll, Phillips, & Balanos, 2009; Balanos et al., 2010; Turner & Carroll, 1985; Sherwood, Allen, Obrist, & Langer, 1986; Obrist, 1981). Likewise, there has been a recent surge of research focusing on different kind of ‘visceral prediction error’, involving failures to mount appropriate cardiovascular reactions to psychological stressors. Hence, some individuals exhibit a tendency to show blunted or minimal changes in cardiovascular physiology across a range of motivated behavioral states, including those related to psychologically stressful experiences. Compared with their more reactive counterparts, individuals expressing a phenotype for blunted reactivity are more likely to engage in disadvantageous health behaviors that confer CVD risk through pathways that are independent from those of exaggerated reactors (e.g., Ginty et al., 2016; Wiggert et al., 2016) and they exhibit motivational and psychological characteristics (e.g. substance use problems, depressive symptoms, impulsivity) that may heighten CVD risk (e.g., Carroll et al., in press; de Rooij, 2013; Bennett et al., 2014). Advances in brain-imaging have recently provided the necessary tools to characterize the putative neural circuits that contribute to these individual differences in stressor-evoked cardiovascular reactivity, particularly via intermediate autonomic and neuroendocrine pathways. Specifically, brain-imaging studies have shown that individuals who exhibit larger rises in blood pressure and heart rate (i.e., greater cardiovascular reactivity) during stress often exhibit concurrently greater increases in activity in several regions for visceral control, including the anterior cingulate cortex, medial prefrontal cortex, insula, hippocampus, basal ganglia, periacqueductal gray (PAG), and pons. There is also some evidence to suggest that people who exhibit greater cardiovascular reactivity also display greater activation in the amygdala and extended amygdala, but this has not been a reliable finding (Gianaros et al. 2008, Wager et al., 2009). In this regard, the increasing integration of multivariate and whole-brain pattern analyses with brain-imaging studes of stress will provide greater specificity with respect to network-level quantitative changes that predict individual differences in cardiovascular reactivity (Woo et al., 2017). For example, Eisenbarth et al., 2016 demonstrated that multivariate patterns of activity across the entire brain were reliably predictive of concurrent increases in heart rate and skin conductance during the anticipation of delivering a socially evaluated speech. Moreover, these multivariate patterns expressed some overlap within limbic areas, as well as some specificity (non-overlap) with respect to heart rate and skin conductance predictive associations (Eisenbarth et al., 2016). These findings suggest that a focus on particular brain regions or directional activity changes in particular brain regions during stress may ultimately have circumscribed utility in understanding the neural bases of stress physiology and that multivariate methods may better capture activity patterns across brain networks that are associated with different parameters of stress physiology. We next describe common paradigms and approaches used in these studies, and we highlight selected convergent findings on the neural correlates of stressor-evoked cardiovascular reactivity.
3. Brain-imaging studies of stressor-evoked cardiovascular reactivity
As detailed in recent meta-analyses and other reviews (e.g., Gianaros & Wager, 2015; Thayer et al., 2012; Myers 2016; Shoemaker & Goswami, 2015; Beissner et al. 2013, Muscatell & Eisenberger), functional divisions of the anterior cingulate cortex (ACC) and adjacent medial prefrontal cortex (mPFC), insula, hippocampus, and amygdala may be viewed as a core – albeit not exclusive – components of a broader network of forebrain systems involved in mediating stressor-evoked changes in cardiovascular activity. These forebrain systems are specifically viewed to play a role in stress appraisal processes, by ascribing personal and threat-related meaning to events, contexts, and other sources of information that are encoded and experienced (Gianaros & Wager, 2015). These forebrain systems are also viewed to play a dual role in peripheral physiological regulation via their functional interactions with subcortical circuits that proximally alter autonomic and neuroendocrine outflow to the periphery (e.g., heart and vasculature). Specifically, forebrain areas can functionally interact with one another as a network to modulate visceromotor and viscerosensory functions of cell groups within the thalamus, hypothalamus, PAG and medullary regions – which govern and monitor autonomic and neuroendocrine outflow to the heart and blood vessels in coordination with behavioral actions and motivated dispositions to act (Bandler, Keay, Floyd, & Price, 2000; Öngür & Price, 2000; Saper, 2002; Ulrich-Lai & Herman, 2009). Across several recent brain-imaging studies, stressor-evoked cardiovascular changes (e.g., in BP and HR) have been reliably associated with activity changes in these forebrain and subcortical regions, consistent with invasive animal work and patient lesion studies on central cardiovascular, autonomic, and neuroendocrine control (Critchely et al., 2003; Oppenheimer & Cechetto, 2016; Shoemaker et al., 2015; Shoemaker & Goswami, 2015). Conventionally, these forebrain and subcortical regions have been referred to as components or nodes of a central autonomic network (Bennarroch, 1993; Saper, 2002) or, more inclusively, a visceral control network.
A common methodological approach in brain-imaging studies that examine stressor-evoked cardiovascular and other physiological reactions to activity changes in visceral control regions is to employ behavioral task paradigms that involve processing conflicting stimuli, performing under time-pressure and negative social evaluation, and even anticipating electric shocks (see Table 1 for extended examples). During these task paradigms, changes in peripheral physiology (e.g., HR and BP) are often measured concurrently with ongoing functional activity across the whole brain or in selected brain regions. By this methodological approach, researchers seek to identify patterns of neural activity that may correspond to psychological threat appraisal processes, as well as visceromotor commands (e.g., the basis of presumptive visceral prediction errors) or viscerosensory processes linked to the efferent generation and afferent representation of changes in peripheral physiology, respectively. As one example, Wager and colleagues (2009) demonstrated that ventromedial PFC (vmPFC) and rostral ACC activity during the anticipation of a socially-evaluated speech predicted individual differences in the magnitude of HR reactivity. Moreover, they demonstrated that vmPFC and ACC associations with HR reactivity were mediated (statistically accounted for) by concurrent changes in the PAG and thalamus, thus potentially defining a forebrain-to-subcortical pathway for stressor-evoked cardiovascular (HR) reactivity. These findings agree with neuroanatomical tracing work in primates and other nonhuman animal models demonstrating projections from the medial prefrontal cortex to the PAG and hypothalamus (Bandler et al., 2000; Dampney, 2015), as well as primate anatomical work on the coritical control of the adrenal medulla (Dum et al., 2016). As noted above, this particular line of work was also extended recently to define a multivariate pattern of neural activity encompassing the vmPFC and other networked limbic regions that reliably predicted individual differences in stressor-evoked HR and sympathetic nervous system reactivity (skin conductance) using multivariate methods (Eisenbarth, Chang, & Wager, 2016). In parallel work, Gianaros et al. (2008) demonstrated that individual differences in stressor-evoked BP reactivity related to concurrently greater activation in the amygdala, as well as more strongly correlated activity of the amygdala with the rostral ACC and brainstem (pons). In aggregate, these and related studies employing other stressor paradigms are thus helping to define the forebrain and subcortical neural circuits that couple presumptive psychological threat appraisals with the peripheral expression of cardiovascular reactions implicated in CVD risk.
Table 1.
Example studies of stressor-evoked neural, autonomic, neuroendocrine, and cardiovascular reactivity.
| Citation | Stress task | Physiological measures |
|---|---|---|
| Critchley et al., 2000 | Mental arithmetic, isometric exercise | Heart rate, blood pressure |
| Dalton et al., 2005 | Electric shocks | Cardiac contractility |
| Dedovic et al., 2014 | Montreal Imaging Stress Task | Salivary cortisol |
| Eisenberger et al., 2007 | Cyberball (social exclusion) | Salivary cortisol |
| Gianaros et al., 2004 | Verbal working memory task; spatial working memory task | Heart rate, High-frequency heart rate variability |
| Gianaros et al., 2007 | Stroop interference task | Blood pressure |
| Gianaros et al., 2012 | Multisource Interference Task | Blood pressure, heart rate, baroreflex sensitivity |
| Ginty et al., 2012 | Multisource Interference Task | Heart rate |
| Holsen et al., 2012 | Visual stimuli | High-frequency heart rate variability |
| Hermens et al. 2011 | Aversive video clip | Salivary cortisol, heart rate |
| Wang et al., 2005 | Mental arithmetic | Salivary cortisol, heart rate |
| Wager et al., 2009 | Speech preparation | Heart rate |
It is important to note that efferent visceromotor and afferent viscerosensory processes that influence and represent stressor-evoked changes in peripheral (e.g., cardiovascular) physiology are thought to be represented by the functional interplay or network-level interactions among forebrain and subcortical neural circuits. In other words, it is implausible that any given brain area acts in isolation from networked areas to generate or control peripheral physiological stress reactions, including cardiovascular reactivity. Recent advances in brain-imaging analytical approaches have provided a basis for quantifying such network-level interactions and relating them to changes in peripheral physiology (e.g., Hermens et al., 2011; Quaedflieg et al., 2015). These approaches specifically provide for metrics of stressor-evoked changes in functional connectivity (i.e., cross-correlated activity between multiple areas of the brain), and have made use of three broad methodological techniques. Here, augmented stressor-evoked connectivity is represented by an overall increase in the positive correlations between two areas or within a given network as a whole. In contrast, weakened or inverse stressor-evoked connectivity would be represented by overall decreases or more negative correlations between two areas or within network as a whole. The first and most common approach in this line of work is to select a predetermined, anatomical region-of-interest or ‘seed’ (e.g., the amygdala) and then compare the relationships (correlations) between activity in that seed and activity in other brain regions at rest, during a stressor task, or during stressor recovery (e.g., Fan et al., 2015; Sinha et al., 2016). Studies using these network-based approaches typically examine how groups of brain areas are altered by stressor tasks, but they have rarely measured how individual differences in peripheral physiological (e.g., HR, BP) responses relate to network-level or connectivity changes. For example, using a seed-based approach Sinha and colleagues (2016) demonstrated that viewing aversive pictures increased the functional connectivity (cross-correlation) between vmPFC activity and the left anterior PFC, dorsolateral PFC, and inferior parietal lobe. As another example, using a seed-based approach, Gianaros and colleagues (2012) demonstrated that functional connectivity of a canonical visceral control area, the anterior insula (Oppenheimer & Cechetto, 2016), was increased during a psychological stressor that increased BP and decreased cardiovagal baroreflex sensitivity. Specifically, stressor-evoked changes were observed between the anterior insula and ACC, amygdala, PAG, and pons. A second approach to quantifying network-level properties involves the application of multivariate time-series analyses, such as independent component analyses (ICA) (Beckmann et al., 2005; Bullmore & Sporns, 2009; Calhoun et al., 2001). This approach involves identifying distributed brain networks that exhibit coherent patterns of activity across time, and examining how connectivity across the identified networks changes either during or after stressor tasks. As an example, Hermans and colleagues (2011) applied ICA to neural activity assessed while subjects viewed aversive film clips and identified a canonical “ventral attention” or “salience network”, primarily consisting of previously described forebrain and subcortical structures including the cingulate, insula, and amygdala. Across participants, connectivity within this network related to stressor-evoked cortisol and salivary alpha-amylase responses. A third and more recent approach first partitions the entire brain into multiple regions, and examines connectivity between every pair of regions across the whole brain, in turn treating the brain as a network in an unbiased manner. This approach is conceptually similar to seed-based analyses, with the distinction that it involves characterizing connectivity across every seed-to-seed pair rather than a few a priori selected pairs or regions. Recently, Maron-Katz and colleagues (2016) examined connectivity across 490 brain regions before and after a stress induction, and found that stress increased connectivity of the thalamus and cerebral cortex, and reduced connectivity between the parietal and temporal lobes. A more extended summary of this line of research bearing on stressor-evoked changes in network-level changes can be found in Tables 2 and 3.
Table 2.
Studies examining stressor-evoked functional connectivity changes.
| Citation | Stress task | Analysis | Seed Region | Stressor-evoked changes |
|---|---|---|---|---|
| Admon et al. 2009 | Backward masked photographs, prior to and following military service | Seed-based | Hippocampus | ↑Psychological stress, ↓vmPFC connectivity |
| Akdeniz et al. 2014 | Mental arithmetic and mental rotation | Seed-based | pgACC | ↑Perceived discrimination, ↑ connectivity with dACC |
| Fan et al. 2015 | Montreal Imaging Stress Task | Seed-based | Amygdala | ↑Connectivity with mPFC, posterior cingulate cortex, anterior insula, putamen, caudate, thalamus |
| Gianaros et al. 2012 | Multisource Interference Task | Seed-based | Insula | ↑Connectivity with ACC, amygdala, pons, midbrain PAG |
| Liston et al. 2009 | Attention-shifting task following 1 month of chronic psychosocial stress | Seed-based | Left and right dorsolateral prefrontal cortex | ↓Connectivity with contralateral dorsolateral prefrontal cortex, ventral prefrontal cortex, putamen, anterior and posterior cingulate, premotor, posterior parietal, and fusiform cortex, and cerebellum; ↑Connectivity with middle temporal lobe |
| Maron-Katz et al. 2016 | Serial subtraction task | Network-based | N/A (or many) | ↑Thalamo-cortical connectivity, ↓parietal-temporal connectivity |
| McMenamin et al. 2014 | Aversive shock | Network-based; graph theory | N/A (or many) | Initial ↑ in ventral-attention network efficiency, encompassing ACC, insula, followed by ↓connectivity in ventral-attention network |
| Sinha et al. 2016 | Aversive pictures | Seed-based | vmPFC | ↑Connectivity with left anterior prefrontal cortex, dorsolateral prefrontal cortex, and inferior parietal lobe |
| Van Marle et al. 2010 | Immediately following aversive movie | Seed-based | Amygdala | ↑Connectivity with dACC, anterior insula, dorso-rostral pontine |
| Veer et al. 2011 | Immediately following Trier Social Stress Task | Seed-based | Amygdala | ↑Connectivity with the posterior cingulate cortex, and vmPFC |
Table 3.
Studies examining stressor-evoked functional connectivity changes and physiological changes.
| Citation | Stress task | Seed Region | Stressor-evoked changes and physiology |
|---|---|---|---|
| Gianaros et al. 2008 | Stroop interference task | Amygdala | ↑Mean arterial pressure reactivity associated with ↑amygdala functional connectivity with the pgACC, pons, orbitofrontal cortex, insula, hippocampus, caudate, middle temporal lobe, occipital cortex, and cerebellum |
| Hermens et al. 2011 | Aversive pictures | Independent component analysis | ↑Cortisol and alpha-amyalse responses associated with stronger ‘salience network’ interconnectivity |
| Quaedflieg et al. 2015 | Imaging Maastricht Acute Stress Task | Amygdala | Cortisol responders had ↑amygdala connectivity with anterior hippocampal complex and parahippocampal gyrus |
| Wager et al. 2009 | Speech preparation task | PFC/ACC activity related to ↑PAG and thalamus activity and heart rate reactivity |
Despite the growth of work in this area, however, what remains incompletely understood are some of the more precise functions and actions encoded within network-level properties of visceral control circuits and how they proximally influence stressor-evoked cardiovascular reactions. For example, are these actions representing visceral prediction errors and efferent commands, afferent processing, or both? And, over what time scale? In these regards, methodological advances in brain-imaging and concurrent physiological monitoring have not yet enabled researchers to readily differentiate the neural correlates of afferent and efferent processes in the context of stressor-evoked cardiovascular reactions, as has been done with other physiological adjustments (e.g., with exercise or baroreceptor unloading ; Shoemaker & Goswami, 2015; Shoemaker et al., 2015; Shoemaker, Wong, & Cechetto, 2012). Another major frontier for future work on stressor-evoked cardiovascular reactivity will be to integrate emerging image acquisition methods for more precise functional assessments of brainstem circuits, which relay both descending stressor-evoked visceromotor commands from forebrain areas and ascending viscerosensory information from the periphery to these forebrain areas (Beissner, 2015; Beissner et al., 2014; Bar et al., 2016).
In addition to the latter future directions, another need in this area of research is an interpretive framework for conceptualizing the neurophysiological correlates (e.g., network-level metrics of visceral control areas) of stressor-evoked changes in physiology, particularly cardiovascular physiology in the context of risk for CVD. Specifically, there is not a uniform conceptual framework for understanding the mechanisms and processes by which psychological stressors engage or alter coordinated activity among central visceral control regions to influence cardiovascular reactivity, particularly via visceromotor and viscerosensory processes. Nor are there quantitative metrics that reflect the bidirectional relationships between stressor-evoked cardiovascular reactions and neural activity patterns that generate and represent these reactions. An emerging perspective, described in the next section, however, is that forebrain regions that are engaged by psychological stressors and involved in threat appraisal processes influence cardiovascular responding by alternating the operational characteristics of homeostatic feedback loops for circulatory control (visceral control loops) (Gianaros & Wager, 2015).
4. Central visceral control mechanisms for stressor-evoked cardiovascular reactivity
As noted earlier, changes in BP and HR are reliably evoked by psychological stressors, and these stressor-evoked cardiovascular changes have long been linked to CVD risk. BP itself is a circulatory parameter that is under the control of the baroreflex. The baroreflex constrains beat-to-beat variation in BP by adjusting sympathetic and parasympathetic outflow to the heart and vasculature to alter heart rate and vessel tone, and hence cardiac output and peripheral resistance. Rises in BP distort stretch-sensitive baroreceptors with free nerve endings situated most densely in the bulb of the carotid artery and the aortic arch. This distortion caused by rises in BP increases the transmission of pressure-related information encoded by the baroreceptors along vagal and glossopharyngeal pathways to the nucleus of the solitary tract (NTS) in the brainstem, which issues mono- and multi-synaptic projections not only to pre-autonomic source nuclei in the brainstem (dorsal vagal nucleus, nucleus ambiguous, caudal and ventrolateral medulla), but also to forebrain regions that are presumably involved in psychological threat appraisals (e.g., vmPFC, ACC, insula, amygdala). NTS projections to pre-autonomic source nuclei serve to proximally alter vagal and sympathetic outflow to adjust BP toward a homeostatic set point (e.g., by modulating HR, cardiac output, and peripheral resistance). By contrast, NTS projections to forebrain regions enable the afferent representation of BP (Dampney, 1994; Berntson, Sarter, & Cacioppo, 1998; Critchely & Harrison, 2013). In addition to these ascending projections of the NTS that transmit viscerosensory information, forebrain regions project back to the NTS. These descending projections provide a basis for forebrain regions to modify the normal operating characteristics of the baroreflex at the level of the brainstem across a range of behavioral states. Such modifications may well vary across behavioral states, individuals, and particular stages of lifespan development. They may also have differential implications for health and disease risk. In this regard, illustrative comparisons may be drawn between reflex modifications that that unfold during experiences of psychological stress and physical activity (e.g., exercise).
To elaborate, exercise increases both BP and heart rate as is the case for psychological stress. Yet despite some early suggestions to the contrary, the arterial baroreflex continues to function while its set-point is ‘reset’ to function around the prevailing BP (Lind et al., 1964; Raven, Fadel, & Ogoh, 2006; Potts, 2006; Fisher, Young, & Fadel, 2015). In fact it appears that by buffering increases in vasomotor tone, an appropriately functioning baroreflex is important in restraining the BP response to exercise (Joyner, 2006). In terms of carotid baroreflex control of heart rate, the maximum gain of the stimulus-response reflex function curve is preserved, however the operating point, which is located near the point of maximal gain at rest, is shifted away from the centering point and towards the threshold at a locus of reduced gain (i.e., sensitivity). As noted, psychological stress reliably reduces baroreflex sensitivity, specifically cardiovasgal sensitivity, is shown. However, the full stimulus-response relationship during experiences of stress has yet to be elucidated. The baroreflex alterations observed during exercise have been attributed to the interactive effects of visceromotor central neural commands and viscerosensory information arising from the exercise pressor reflex (for reviews see Raven et al., 2006; Potts, 2006; Fisher et al., 2015). In contrast to exercise, typical experiences of acute psychological stress are not accompanied by similar metabolic and mechanical viscerosensory changes, and thus no discernable exercise pressor reflex (Turner & Carroll, 1985). However, several areas of the brain associated with central command during exercise are also engaged by acute psychological stressors: ACC, insula, PAG (e.g., Green & Paterson, 2008; Green et al., 2007; Nowak et al., 2005; Thornton et al., 2001). Thus, forebrain regions involved in the appraisal of psychological stressors may evoke cardiovascular stress reactions (e.g., simultaneous rises in BP and HR) via visceral predictive processes described above that modify visceral or homeostatic control loops, such as the baroreflex. In extension, individuals with a phenotype for exaggerated cardiovascular reactivity may be at elevated risk for CVD because of the chronic expression of visceral prediction errors that repeatedly dampen the sensitivity of homeostatic control loops in ways that differ from behavioral states, such as exercise, and confer vulnerability for consequent pathology over time. What is more, to the extent that baroafferent traffic conveys viscerosensory information regarding the magnitude of BP changes to the NTS and forebrain regions for visceral control, it would appear that a phenotype for exaggerated stressor-evoked cardiovascular reactivity may reflect impairment in ‘visceral learning’. In other words, among individuals with this phenotype visceral prediction errors do not appear to be ‘corrected’ over time - insofar as exaggerated cardiovascular reactivity is reliably and repeatedly expressed in the laboratory and daily life (Zanstra & Johnston, 2011). This perspective can be extended and applied to what is referred to as prolonged cardiovascular responding, or responding that does not recover to resting or baseline levels. Impaired or delayed BP recovery, failure to return back to resting cardiovascular activity levels, to psychological stressors, for example, has been associated with hypertension (Steptoe & Marmot, 2005; Stewart, Janicki, & Kamarck, 2006). Here, it would appear that baroafferent traffic is failing to result in a homeostatic return of BP, possibly via input to brainstem baroreflex circuits by forebrain systems for stressor appraisal. In this interpretive framework, visceromotor and viscerosensory mechanisms appear to be key components of the brain-body pathways by which appraisal systems of the forebrain may link states of psychological stress and physiological responding to influence physical health (see Figure 1). However, the effect of psychological stress on baroreflex function is incompletely understood. The majority of the research examining baroreflex function during psychological stress has focused on cardiovagal sensitivity with limited consideration of baroreflex control of sympathetic nerve activity to the skeletal muscle vasculature. The scant research in the area suggests that there are individual differences in muscle sympathetic nerve activity responses to psychological stress and an attenuation of the sympathetic baroreflex at the onset of psychological stress (Carter & Ray, 2009; Durocher, Klein, & Carter, 2011). Similarly, the full-stimulus response characteristics of baroreflex control during psychological stress are not well understood. More work is needed to understand the regulation of baroreflex function during psychological stress and the implications for health and disease.
Figure 1.
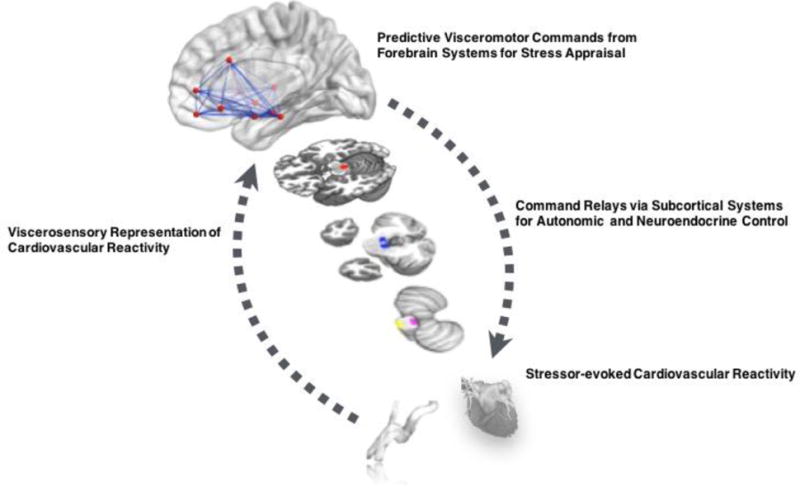
Conceptual illustration of brain-body pathways linking psychological stress to stressor-evoked cardiovascular reactions linked to cardiovascular disease risk. A network of forebrain areas appraise psychological stimuli as threats that tax or exceed coping capacities. These appraisals lead to visceromotor commands or ‘predictions’ for anticipated metabolic support for motivated behaviors. These commands are relayed via subcortical and brainstem cell groups to influence autonomic and neuroendocrine outflow to the heart and vasculature. Chronically exaggerated or metabolically disproportionate stressor-evoked cardiovascular (e.g., BP) reactions may exert shear or tensile stress on blood vessel walls over time, and they may accelerate atherosclerosis or influence risk for later cardiovascular disease endpoints. Vagal and other viscerosensory channels relay feedback signals from visceral organs and systems in the periphery, enabling the afferent representation of peripheral stressor-evoked physiological reactions by forebrain areas. Afferent feedback may influence the magnitude, duration, or general patterning of stressor-evoked reactions and may also affect appraisal-related neural activity.
5. Conclusions
The neurophysiological or ‘brain-body’ pathways linking psychological stress and CVD risk still remain largely uncertain (Lovallo, 2005; Lane et al., 2009). Arguably, delineating these pathways may not only aid in developing brain-based strategies for augmenting CVD risk stratification and prediction in the emerging field of neurocardiology (Shivkumar et al., 2016; Silvani et al., 2016), but also in furthering a mechanistic understanding of stress-related processes contributing to CVD vulnerability. To illustrate, a recent seminal study demonstrated that higher levels of resting amygdalar activity predicted development of CVD over a 3.7 year period (Tawakol et al., 2017). Additionally, increased amygdalar activity was associated with alterations in immune activity, arterial inflammation, and perceived stress providing evidence of potential mechanistic pathways underpinning CVD development (Tawakol et al., 2017). Such work represents the next generation of research on neurobiology of stress and disease risk. In view of these possibilities, a growing corpus of brain-imaging research is helping to better define these pathways. Existing findings are converging to suggest that individual differences in stressor-evoked cardiovascular reactivity are associated with activity and connectivity in brain systems that are jointly involved in processing (appraising) stressors and regulating the cardiovascular system via autonomic and neuroendocrine pathways. These systems encompass a distributed network of cortical and subcortical brain areas involved in mobilizing hemodynamic and metabolic support for stress-related behavioral responding via visceromotor commands. These systems also represent dimensions of cardiovascular physiology via viscerosensory pathways. An emerging perspective is that brain areas for visceral control calibrate the magnitude of physiological (e.g., cardiovascular) reactions to self-relevant stressors to support contextually-adaptive behavioral coping processes. An individual’s propensity to exhibit ‘mis-calibrations’ reflected by network-level interactions between these regions may reflect a dimension of individual differences that underlies the expression of ‘exaggerated’ (metabolically-excessive or pathophysiological) cardiovascular reactions, including stressor-evoked BP and HR reactions that are linked to preclinical and clinical CVD endpoints. Such ‘mis-calibrations’ may be conceptualized as visceral prediction errors, in that they reflect anticipatory visceromotor commands for putatively pathogenic changes in physiology that outstrip metabolic needs and arise from the suppression of homeostatic visceral control loops. What is needed are methods and metrics to better quantify network signaling (e.g., connectivity) characteristics among forebrain and brainstem visceral control areas to test hypotheses derived from this conceptual view and relate these network-level characteristics to markers of CVD pathology and vulnerability. Additionally, what is also needed is work determining the possible genetic and developmental origins of these individual differences in central circulatory control, as well as work on lifestyle and behavioral factors linked to stress and stress physiology (e.g., physical activity) that influence the neurobiology of CVD risk and are amenendable to intervention effort.
Highlights.
Cardiovascular reactions to stress may confer CVD risk.
Brain circuits for autonomic control generate and regulate cardiovascular stress reactions.
These circuits may be important for linking stress to CVD.
Acknowledgments
Funding
The authors would like to acknowledge the following funding sources: T32 HL07560, R01 HL089850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abboud H, Berroir S, Labreuche J, Orjuela K, Amarenco P. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Annals of Neurology. 2006;59:691–699. doi: 10.1002/ana.20806. [DOI] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Devereux RB, Rao DC, Kitzman D, Oberman A, Hopkins P, Arnett DK. Blood pressure stress reactivity and left ventricular mass in a random community sample of African American and Caucasian men and women. American Journal of Cardiology. 2006;97:240–244. doi: 10.1016/j.amjcard.2005.07.134. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. Journal of Physiology. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MT, Matthews KA, Sherman FS. Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension. 1997;30:782–787. doi: 10.1161/01.hyp.30.4.782. [DOI] [PubMed] [Google Scholar]
- Akdeniz C, Tost H, Streit F, Haddad L, Wüst S, Schäfer A, Meyer-Lindenberg A. Neuroimaging Evidence for a Role of Neural Social Stress Processing in Ethnic Minority–Associated Environmental Risk. JAMA psychiatry. 2014;71:672–680. doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- Balanos GM, Phillips AC, Frenneaux MP, McIntyre D, Lykidis C, Griffin HS, Carroll D. Metabolically exaggerated cardiac reactions to acute psychological stress: the effect of resting blood pressure status and possible underlying mechanisms. Biological Psychology. 2010;85:104–111. doi: 10.1016/j.biopsycho.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Research Bulletin. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. Journal of Hypertension. 1997;15:49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, De Luca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processings of autonomic function. Journal of Neuroscience. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F. Functional MRI of the Brainstem: Common problems and their solutions. Clinical Neuroradiology. 25:251–257. doi: 10.1007/s00062-015-0404-0. [DOI] [PubMed] [Google Scholar]
- Beissner F, Schumann A, Brunn F, Eisentrager D, Bar KJ. Advances in functional magnetic resonance imaging of the human brain. Neuroimage. 2014;86:91–98. doi: 10.1016/j.neuroimage.2013.07.081. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Bennett C, Blissett J, Carroll D, Ginty AT. Rated and measured impulsivity in children is associated with diminished cardiac reactions to acute psychological stress. Biological Psychology. 2014;102:68–72. doi: 10.1016/j.biopsycho.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Bernston GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behavior Brain Research. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, PEarlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent components analysis. Human Brain Mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. “Voodoo” death. American Journal of Antrhropology. 1942;44:169–181. [Google Scholar]
- Cannon WB. The mechanism of emotional disturbance of bodily functions. New England Journal of Medicine. 1928;198:877–884. [Google Scholar]
- Carroll D, Ginty AT, Der G, Hunt K, Benzeval M, Phillips AC. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology. 2012;49:1444–1448. doi: 10.1111/j.1469-8986.2012.01463.x. [DOI] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Painter RC, Roseboom TJ, Phillips AC, de Rooij SR. Systolic blood pressure reactions to acute stress are associated with future hypertension status in the Dutch Famine Birth Cohort Study. International Journal of Psychophysiology. 2012;85:270–273. doi: 10.1016/j.ijpsycho.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2017.02.025. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Balanos GM. Metabolically exaggerated cardiac reactions to acute psychological stress revisted. Psychophysiology. 2009;46:270–275. doi: 10.1111/j.1469-8986.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Der G, Hunt K, Benzeval M. Blood pressure reactions to acute mental stress and future blood pressure status: data from the 12-year follow-up of the West of Scotland Study. Psychosomatic Medicine. 2011;73:737–742. doi: 10.1097/PSY.0b013e3182359808. [DOI] [PubMed] [Google Scholar]
- Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. American Journal of Physiology Heart and Circulation Physiology. 2009;296:H847–H853. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanes L, Feldman Barrett L. Redefining the role of limbic areas in cortical processing. Trends in Cognitive Sciences. doi: 10.1016/j.tics.2015.11.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1033. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behavioral and Brain Sciences. 2013;36:181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gianaros PJ, Manuck SB. A stage model of stress and disease. Perspectives on Psychological Science. 2016;11:456–463. doi: 10.1177/1745691616646305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchely HD, Corfield DR, Chandler MP, Mathais CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. Journal of Physiology. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Critchely HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Colivicchi F, Bassi A, Santini M, Caltagirone C. Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke. Stroke. 2005;36:1710–1715. doi: 10.1161/01.STR.0000173400.19346.bd. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. J. Cogn. Neurosci. 2005;17:969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiology Reviews. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Amerian Journal of Physiology. Regulatory, Integrative, and Comparitive Physiology. 2015;309:R429–R443. doi: 10.1152/ajpregu.00051.2015. [DOI] [PubMed] [Google Scholar]
- Dedobic K, Duchesne A, Engert V, Lue SD, Andrews J, Efanov SI, Beaudry T, Pruessner JC. Psychological, endocrine and neural responses to social evaluation in subclinical depression. Social Cognitive & Affective Neuroscience. 2014;10:1632–1644. doi: 10.1093/scan/nst151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij SR. Blunted cardiovascular and cortisol reactivity to acute psychological stress: a summary of results from the Dutch Famine Birth Cohort Study. International Journal of Psychophysiology. 2013;90:21–27. doi: 10.1016/j.ijpsycho.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE. Psychological stress and cardiovascular disease. Journal of the American College of Cardiology. 2008;51:1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher JJ, Klein JC, Carter JR. Attenuation of the sympathetic baroreflex sensitivity during the onset of acute mental stress in humans. American Journal of Physiology – Heart and Circulatory Physiology. 2011;300:H1788–H1793. doi: 10.1152/ajpheart.00942.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proceedings of the National Academy of Sciences. 2016;113:9922–9927. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek S, Dietel A, Schandry R, Reyes Del Paso GA. Increased baroreflex sensitivity and reduced cardiovascular reactivity in individuals with chronic low blood pressure. Hypertension Research. 2008;31:1873–1878. doi: 10.1291/hypres.31.1873. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth H, Chang LJ, Wager TD. Multivariate brain prediction and skin conductance responses to social threat. Journal of Neuroscience. 2016;36:11987–11998. doi: 10.1523/JNEUROSCI.3672-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Creswell JD, Verstynen TD, Gianaros PJ. Health Neuroscience: Defining a new field. Current Directions in Psychological Science. 2014;23:446–453. doi: 10.1177/0963721414549350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M. Mental stress and human cardiovascular disease. Neuroscience and Biobehavioral Reviews. 2017;74:269–276. doi: 10.1016/j.neubiorev.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Fan Y, Pestke K, Feeser M, Aust S, Pruessner JC, Böker H, Grimm S. Amygdala–hippocampal connectivity changes during acute psychosocial stress: joint effect of early life stress and oxytocin. Neuropsychopharmacology. 2015;40:2736–2744. doi: 10.1038/npp.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Comprehensive Physiology. 2015;5:475–512. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- Georgiades A, Lemne C, de Faire U, Lindvall K, Frendrikson M. Stress-induced laboratory blood pressure in relation to ambulatory blood pressure and left ventricular mass among borderline hypertensive and normotensive individuals. Hypertension. 1996;28:641–646. doi: 10.1161/01.hyp.28.4.641. [DOI] [PubMed] [Google Scholar]
- Gerin W, Pickering TG, Glynn L, Christenfeld N, Schwartz A, Carroll D, Davidson K. A historical context for behavioral models of hypertension. Journal of Psychosomatic Research. 2000;48:369–377. doi: 10.1016/s0022-3999(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derybshire SW, Matthews KA. Heightend functional neural activiation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping. 2012;33:1700–1716. doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. The Journal of Neuroscience. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain-body pathways linking psychological stress and physical health. Current Directions in Psychological Sciences. 2015;24:313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Gianaros PJ, Derbyshire SW, Phillips AC, Carroll D. Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiology. 2012;50:219–229. doi: 10.1111/psyp.12017. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Williams SE, Jones A, Roseboom TJ, Phillips AC, Painter RC, Carroll D, de Rooij SR. Diminished heart rate reactivity to acute psychological stress is associated with enhanced carotid intima-media thickness through adverse health behaviors. Psychophysiology. 2016;53:769–775. doi: 10.1111/psyp.12640. [DOI] [PubMed] [Google Scholar]
- Green AL, Patterson DJ. Identification of neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: Implications for exercise control. Experimental Physiology. 2008;93:1022–1028. doi: 10.1113/expphysiol.2007.039461. [DOI] [PubMed] [Google Scholar]
- Green AL, Wang S, Owen SL, Xie K, Liu X, Paterson DJ, Stein JF, Bain PG, Aziz TZ. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16:1741–1745. doi: 10.1097/01.wnr.0000183904.15773.47. [DOI] [PubMed] [Google Scholar]
- Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Long-term stability of cardiovascular and catecholamine responses to stress tests: an 18-year follow-up study. Hypertension. 2010;55:131–136. doi: 10.1161/HYPERTENSIONAHA.109.143164. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Fernández G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Holsen LH, Lee JH, Spaeth SB, Ogden LA, Klibanski A, Whitfield-Gabrieli S, Sloan RP, Goldstein JM. Brain hypoactivation, autonomic nervous system dysregulation, and gonadal hormones in depression. A preliminary study. Neuroscience Letters. 2012;514:57–61. doi: 10.1016/j.neulet.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Baroreceptor function during exercise: resetting the record. Experimental Physiology. 2006;91:27–36. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiological reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychological Bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ, Rose RM, Dorssman DA, Schneiderman N, Thayer JF, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, part I: historical context, methods and relevant basic science. Psychosomatic Medicine. 2009a;71:117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Critchley HD, Derbyshire SD, Drossman DA, Wager TD, Schneiderman N, Chesney MA, Jennings JR, Lovallo WR, Rose RM, Thayer JF, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, part II: clinical applications and implications for research. Psychosomatic Medicine. 2009b;71:135–151. doi: 10.1097/PSY.0b013e318198a11f. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Psychological stress and the coping process. McGraw-Hill; New York, NY: 1966. [Google Scholar]
- Li C, Fitzgerald ME, Ledoux MS, Gong S, Ryan P, Del Mar N, Reiner A. Projections from the hypothalamic paraventricular nucleus and the nucleus of the solitary tract to prechordoidal neurons in the superior salivatory nucleus: Pathways controlling rodent choroidal blood flow. Brain Research. 2010;1358:123–139. doi: 10.1016/j.brainres.2010.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AR, Taylor SH, Humphreys PW, Kennelly BM, Donald KW. The circulatory effects of sustained voluntary muscle contraction. Clinical Science. 1964;27:229–244. [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences. 2009;106:912–917. doi: 10.1073/pnas.0807041106. https://doi.org/10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR. Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. International Journal of Psychophysiology. 2005;58:119–132. doi: 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Maron-Katz A, Vaisvaser S, Lin T, Hendler T, Shamir R. A large-scale perspective on stress-induced alterations in resting-state networks. Scientific reports. 2016;6 doi: 10.1038/srep21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Science. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. The Journal of Neuroscience. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ. Heart disease and stroke statics – 2016 update. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR, Eisenberger NI. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behavioral & Immunity. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Eisenberger NI. A social neuroscience perspective on stress and health. Social Personality & Psychology Compass. 2012;6:890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. Corticolimbic regulation of cardiovascular responses to stress. Physiology & Behavior. doi: 10.1016/j.physbeh.2016.10.015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into brain-heart axis. Journal of the American Society of Hypertension. 2010;4:174–182. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Nowak M, Holm S, Biering-Sorensen F, Secher NH, Friberg L. “Central command” and insular activation during attempted footlifting in paraplegic humans. Human Brain Mapping. 2005;25:259–265. doi: 10.1002/hbm.20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular Psychophysiology: A Perspective. New York, NY: Plenum Press; 1981. [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S. Cerebrogenic cardiac arrhythmias: cortical lateralization and clinical significance. Clinical Autonomic Research. 2006;16:6–11. doi: 10.1007/s10286-006-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S, Cechetto D. The insula cortex and the regulation of cardiac function. Comprehensive Physiology. 2016;6:1081–1133. doi: 10.1002/cphy.c140076. [DOI] [PubMed] [Google Scholar]
- Palma JA, Benarroch EE. Neural control of the heart: Recent concepts and clinical correlations. Neurology. 2014;83:261–271. doi: 10.1212/WNL.0000000000000605. [DOI] [PubMed] [Google Scholar]
- Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Experimental Physiology. 2006;91:59–72. doi: 10.1113/expphysiol.2005.032227. [DOI] [PubMed] [Google Scholar]
- Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Experimental Physiology. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- Quaedflieg CWEM, van de Ven V, Meyer T, Siep N, Merckelbach HLGJ, Smeets T. Temporal dynamics of stress-induced alternations of intrinsic amygdala connectivity and neuroendocrine levels. PloS one. 2015;10:e0124141. doi: 10.1371/journal.pone.0124141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Reviews of Neroscience. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. http://dx.doi.org/10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Gerin W, Davidson KW, Pickering TG, Brosschot JF, Thayer JF, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosomatic Medicine. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Obrist PA, Langer AW. Evaluation of beta-adrenergic influences on cardiovascular and metabolic adjustments to physical and psychological stress. Psychophysiology. 1986;23:89–104. doi: 10.1111/j.1469-8986.1986.tb00602.x. 1986. [DOI] [PubMed] [Google Scholar]
- Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, et al. Clinical neurocardiology defining the values of neuroscience-based cardiovascular therapeutics. Journal of Physiology. 2016;594:3911–3954. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Goswami R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Frontiers of Physiology. 2015;6:240. doi: 10.3389/fphys.2015.00240. http://dx.doi.org/10.3389/fphys.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Norton KN, Baker J, Luchyshyn T. Forebrain organization for autonomic cardiovascular control. Autonomic Neuroscience. 2015;188:5–9. doi: 10.1016/j.autneu.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Wong SW, Cechetto DF. Cortical circuitry associated with reflex cardiovascular control in humans: does the cortical autonomic network “speak” or “listen” during cardiovascular arousal. Ant Rec (Hoboken) 2012;295:1375–1384. doi: 10.1002/ar.22528. [DOI] [PubMed] [Google Scholar]
- Silvani A, Calandra-Buonaura G, Dampney RA, Cortelli P. Brain-heart interactions: physiology and clinical implications. Philosophical transactions. Series A, Mathematical, physical, and engineering Sciences. 2016:374. doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proceedings of the National Academy of Sciences. 2016;113:8837–8842. doi: 10.1073/pnas.1600965113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Fieldman G, Evans O, Perry L. Control over work pace job strain and cardiovascular responses in middle-aged men. Journal of Hypertension. 1993;11:751–759. doi: 10.1097/00004872-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. Journal of Hypertension. 2005;23:529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Janicki DL, Kamarck TW. Cardiovascular reactivity to and recovery from psychological challenge as predictors of 3-year change in blood pressure. Health Psychology. 2006;25:111–118. doi: 10.1037/0278-6133.25.1.111. [DOI] [PubMed] [Google Scholar]
- Taggart P, Critchley H, van Duijvendoden S, Lambiase PD. Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Autonomic Neuroscience: Basic & Clinical. 2016;199:54–65. doi: 10.1016/j.autneu.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Taylor TR, Kamarck TW, Dianzumba S. Cardiovascular reactivity and left ventricular mass: an integrative review. Annals of Behavioral Medicine. 2003;26:182–193. doi: 10.1207/S15324796ABM2603_03. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurology. 2008;7:1021–1031. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Pitman RK. Relation between resting amygdalar activity and cardiovascular revents: a longitudinal and cohort study. Lancet. 2017 doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. Journal of Physiology. 2001;533:823–836. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Turner JR, Carroll D. Heart rate and oxygen consumption during mental arithmetic, a video game, and graded exercise: further evidence of metabolically-exaggerated cardiac adjusetments? Psychophysiology. 1985;22:261–267. doi: 10.1111/j.1469-8986.1985.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic resposnes. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Marle HJ, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57:1534–1541. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Wiggert N, Wihlem FH, Nakajima M, al’Absi M. Chronic smoking, trait anxiety, and the physiological response to stress. Substance Use and misuse. 2016;51:1619–1628. doi: 10.1080/10826084.2016.1191511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Tyalor SF. Brain mediators of cardiovascular responses to social threat: part 1: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academicy of Sciences. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Chang LI, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nature Neuroscience. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, Raz G, Everaerd D, Beckmann CF, Tendolkar I, Hendler T, Hermans EJ. Dynamic shifts in large-scale brain network balance as a function of arousal. Journal of Neuroscience. 2016:1759–16. doi: 10.1523/JNEUROSCI.1759-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanstra YJ, Johnston DW. Cardiovascular reactivity in real life settings: measurement, mechanisms and meaning. Biological Psychology. 2011;86:98–105. doi: 10.1016/j.biopsycho.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


