Abstract
Photodynamic therapy (PDT) employs a photosensitizer (PS) and visible light in the presence of oxygen, leading to production of cytotoxic reactive oxygen species, which can damage the cellular organelles and cause cell death. In dermatology PDT has usually taken the form of topical application of a precursor in the heme biosynthesis pathway, called 5-aminolevulinic acid (or its methyl ester), so that an active PS, protoporphyrin IX accumulates in the skin. As PDT enhances dermal remodeling and resolves chronic inflamation, it has been used to treat cutaneous disorders include actinic keratoses, acne, viral warts, skin rejuvenation, psoriasis, localized scleroderma, some non-melanoma skin cancers and port-wine stains. Efforts are still needed to mitigate the side effects (principally pain) and improve the overall procedure.
Keywords: Photodynamic therapy, cosmetic, dermatology
1. Introduction
The use of photodynamic therapy (PDT) in dermatology is gaining remarkable interest all over the world. Although most cutaneous disorders are still not considered to be life-threatening, there is increasing demand from patients for improvements in their appearance, self-perception and quality of life. This is especially evident in advanced societies (both Western and Eastern) as disposable incomes rise. The major treatments in dermatology include pharmacotherapy, biologics, lasers/light sources, PDT and other non-surgical approaches. PDT employs a photosensitizer (PS) and visible light in the presence of oxygen, leading to production of cytotoxic reactive oxygen species, which can damage the cellular organelles and cause cell death. PDT has been used to treat cutaneous disorders since the early 20th century. However, the range of indications has recently significantly. expanded The easy access of the skin to light-based therapy, the ongoing introduction of new light sources and novel PS, have worked together to encourage the application of PDT to a variety of cutaneous diseases. Improved effects have been demonstrated in a variety of dermatologic conditions. This article will review the clinical application of PDT in dermatology beyond non-melanoma cancer from 2013 to 2017.
2. Mechanisms of PDT
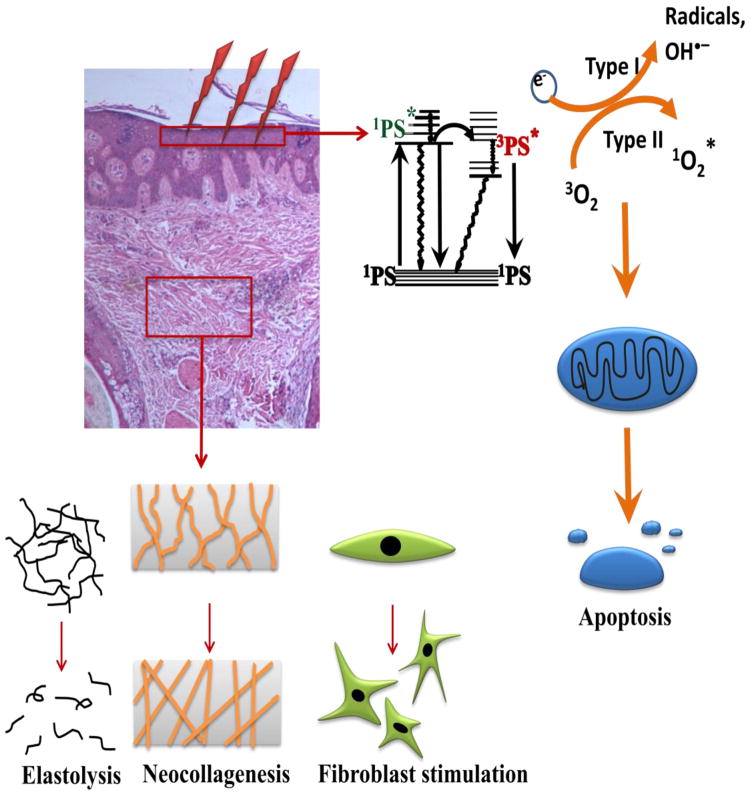
PDT basically requires three separate components: a non-toxic dye, termed a photosensitizer (PS); a low intensity of visible light; and a diseased “target cell or tissue” containing sufficient oxygen. A photochemical reaction takes place between these three components leading to the generation of cytotoxic reactive oxygen species (ROS) and consequently to cell and tissue destruction (Fig. 1). Two complementary photochemical reactions can take place from the long-lived triplet excited-state of the PS (3PS*). Firstly, in a Type 1 reaction, the 3PS can react directly with a substrate (often oxygen) undergoing an electron transfer reaction initially producing free radicals that may further react with oxygen to produce ROS (including hydroxyl radicals). Alternatively in Type 2 reaction, the PS can transfer its energy directly to ground state (triplet) molecular oxygen to form excited-state singlet oxygen (also a ROS). Both Type 1 and Type 2 reactions can occur simultaneously. On the cellular level, the mitochondrial pathway is the most likely mechanism by which PDT induces oxidative damage in the target cells, leading to induction of apoptosis [1–3].
Figure 1.
Mechanisms of PDT for cutaneous diseases
Current data also suggest that the application of PDT to the skin can enhance dermal remodeling through the production of type I and III procollagen thereby increasing dermal collagen density and epidermal thickness, and making it possible to improve aged skin (Fig. 1) [4]. Another in vivo study employed biochemical and immunohistochemical analyses after photodynamic therapy using topical 5-ALA and pulsed-dye laser treatment, and it demonstrated marked elevations in types I and III procollagen that lasted for at least 6 months [5].
3. Photosensitizers
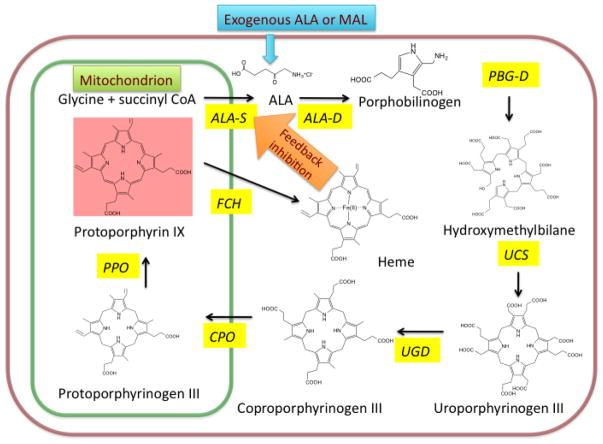
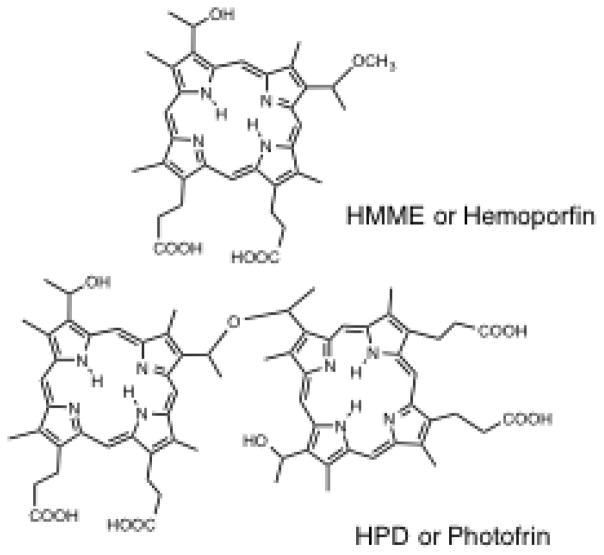
PS can be delivered to patients by several routes (topical, oral, or intravenous). The introduction of the photosensitizer-pro-drug called 5-aminolevulinic acid (5-ALA) was a breakthrough for PDT in dermatology. Topically applied 5-ALA is metabolized to protoporphyrin IX (PPIX) through the heme biosynthetic pathway. This pathway is common to all nucleated mammalian cells and leads from succinyl CoA and glycine through several intermediates to PPIX and finally to heme. There is a natural feedback control mechanism, whereby excess heme inhibits the ALA-synthase enzyme and prevents accumulation of possible phototoxic intermediates in cells. However when exogenous ALA (or the easily hydrolyzed methyl ester of ALA called MAL) is added to the system, PPIX accumulates in the cels since the final enzyme (ferrochelatase) is then rate-limiting (Fig. 2) [6]. The accumulated excess of PPIX generates ROS after illumination leading to apoptosis and necrosis of targeted tissue. The 5-ALA molecule is small enough to penetrate the skin barrier and can accumulate within pilosebaceous units and hyperproliferative keratinocytes compared to normal skin, making the treatment selective. Moreover in many cutaneous pathologic lesions, the barrier function of the stratum corneum is compromised, so this further increases the selective penetration of ALA. An additional advantage is that PPIX is used up (photobleached) during the irradiation, which makes the risk of PDT overtreatment relatively low [7, 8]. Another frequently used PS prodrug (especially in Europe) is methyl aminolevulinate (MAL), which is an esterified form of ALA, with more lipophilic properties, allowing for deeper penetration into the skin compared to ALA. A wide range of studies have been performed with the MAL in cosmetic dermatology. A relatively new product is the porphyrin-based PS called hematoporphyrin mono-methyl ether (HMME, Hemoporfin) [9]. Hemoporfin has shown strong photodynamic effects, and hemoporfin-based PDT induces significant cell death with lower toxicity and a shorter-term skin phototoxictiy, compared with a previous photosensitizer with similar structure called Photofrin (Fig. 3).
Figure 2. Use of exogenous ALA to bypass regulation of heme biosynthetic cycle to produce excess PPIX that acts as a potent PS within mitochondria.
ALA-S = 5-aminolevulinic acid synthase
ALA-D = 5-aminolevulinic acid dehydratase
PBG-D = porphobilinogen deaminase
UCS = uroporphyrinogen co-synthase
UGD = uroporphyrinogen decarboxylase
CPO = coproporphyrinogen oxidase
PPO = protoporphyrinogen oxidase
FCH = ferrochelatase
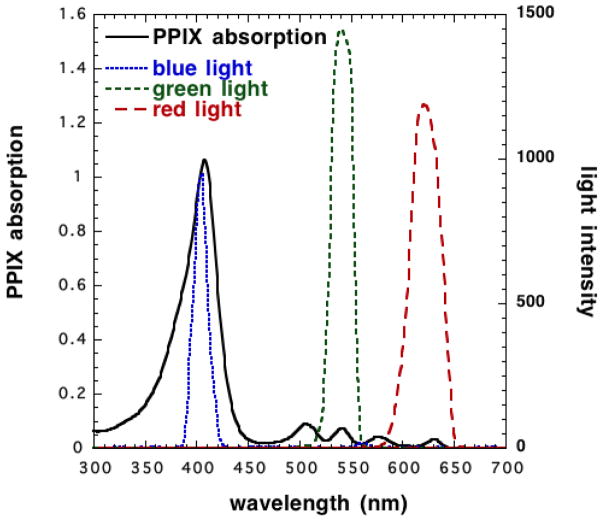
Figure 3.
Absorption spectra of PPIX and emission spectra of various light sources
4. Light sources
The absorption spectrum of PPIX is maximally activated at 410 nm (Soret band) with significantly lower peaks at 505, 540, 580 and 635 nm (Fig. 4), so many different light sources can be used in PDT, including broad-spectrum continuous-wave light sources or lamps (white, blue, red, or green light), lasers, intense pulsed light sources (IPL), filtered xenon arc lamps, metal halide or fluorescent lamps, and more recently light emitting diodes (LED). Currently, a new trend has emerged towards the use of daylight as a light source (both at home and under clinical monitoring) termed daylight PDT (dPDT). Daylight is the combination of direct and diffuse sunlight that predominates outdoors during the daylight hours. The idea that all PPIX absorption peaks are within the visual spectrum of daylight led to the use of dPDT with many benefits to patients in terms of convenience and reduced pain. Since the power density of sunlight is much lower than artificial red light sources used in the clinics, the overall treatment takes much longer, but the patients are compensated by much less pain, and the opportunity to sit pleasantly outside for some hours reading a book.
Figure 4.
Chemical structures of HMME or Hemoporfin along with HPD or Photofrin
5. Temperature and PDT
The critical role of skin temperature on the efficacy of PDT is just starting to be appreciated. A one-year follow-up study demonstrate a significant increase in the efficacy of thermally modulated PDT on the extremities for actinic keratoses, and suggested that warming the skin after the application of ALA was well tolerated and increased the long-term efficacy of PDT [10]. The underlying mechanisms of the temperature effect include a better PS uptake into cells, and/or a higher PpIX production rate inside the cells, together with vasodilatation of skin blood vessels leading to an improved oxygen supply during PDT [11].
6. Clinical Applications
PDT is a widely approved therapy in dermatology. Many applications have been evaluated, including both cosmetic and clinical indications. The recommendations contained in the European dermatology forum guidelines on topical PDT [12], including actinic keratoses, acne, viral warts, photorejuvenation, psoriasis, hypertrophic and keloid scars will be reviewed, as well as the use of systemic PDT (intravenously injected photosensitziers) on port wine stains.
6.1 Actinic keratoses (Strength of Recommendation A, Quality of Evidence 1)
Actinic keratoses (AKs), are frequently caused by long-term sun exposure, and are considered to be precursor lesions that can go on to develop into invasive squamous cell carcinoma. AKs respond well to PDT; in a network meta-analysis for 5-ALA PDT, cryotherapy, diclofenac 3% in 2.5% hyaluronic acid (DCF/HA), 5-fluorouracil (5-FU) 0.5% or 5.0%, imiquimod 5%, ingenol mebutate (IMB) 0.015–0.05%, MAL-PDT and placebo, the relative efficacy rankings were as follows: 5-FU > ALA-PDT ≈ IMI ≈ IMB ≈ MAL-PDT > cryotherapy > DCF/HA > placebo [13]. Another meta-analysis including 9 randomized PDT clinical trials compared ALA-PDT or MAL-PDT with cryotherapy, PDT had a 14% better chance of lesion clearance compared with cryosurgery for thin AKs on the face or scalp [14]. Efficacy, cosmetic outcome and patient overall satisfaction with MAL-PDT were reported to be superior in comparison with DCF/HA in a recent randomized clinical trial [15].
However, the efficacy of PDT on distal extremities was lower than for those situated on the face and scalp. Recently, Willey et al [16] reported the efficacy and tolerability of temperature-modulated PDT for the treatment of AKs on the extremities. The upper and/or lower extremities of 20 subjects were treated with 20% ALA-PDT. The side of the body that was heated during incubation with ALA (1h, 38.8°C) responded significantly better than the control (non-heated) side at 2 and 6 months. Both procedures were well tolerated. PDT as a treatment for AKs has been tested in different races and skin colors. It was reported that ALA-PDT was safe and effective for Chinese AK patients [17]. PDT has also been shown to be an efficient and well tolerated treatment modality for AKs in immunosuppressed patients [18]. Researchers have made a variety of efforts to improve the efficacy of PDT against AKs. Pulsed lasers that create micro-channels may facilitate ALA delivery thus improving PDT response. Choi et al [19] applied Erbium: yttrium-aluminum-garnet (Er:YAG) fractional laser-assisted MAL-PDT (FL-PDT) with 2 and 3h incubation times, compared with MAL-PDT alone for the treatment of facial and scalp AKs. 3h-FL-PDT had a higher efficacy and yielded a lower recurrence rate than 2h-AFL-PDT and 3h-MAL-PDT. Another prospective, randomized, non-blinded 3h long incubation study also demonstrated that FL-PDT was significantly more effective than MAL-PDT [20]. Other treatment modalities that can be combined with PDT to enhance the efficacy of PDT have been reported. Haedersdal et al compared the efficacy of a single treatment with either ablative fractional laser (AFXL)-assisted PDT vs. AFXL alone for difficult-to-treat AKs in organ transplant recipients. Complete response rates were significantly higher for AFXL-PDT (73%) compared with AFXL alone (31%) [21]. In addition, topical 5% 5-fluorouracil (5-FU) cream has been reported to enhance the treatment efficacy of PDT in 24 patients with Aks on the hands [22]. Moreover, a new stable nanoemulsion-based gel formulation of 5-ALA for PDT of AKs was reported to be more efficacious when compared with MAL cream [23].
Most recently, studies with daylight-PDT have been reviewed elsewhere. Daylight-PDT has been reported to be “not inferior” to conventional PDT in most of the studies with high tolerability and greater patient satisfaction, that was demonstrated by a systemic review and meta-analysis [24]. The activation of PPIX by daylight exposure means that the power density is much lower than that of traditional light sources, and this means that the pain suffered by patients during PDT is significantly less. So far daylight-PDT has been used to treat different grades of AKs in different geographical locations (Table 2).
Table 2.
Use of PDT in the treatment of acne
| Reference | N | Type of trial; Study population | Light source (dose); Session number (interval); Follow-up | Results |
|---|---|---|---|---|
| Asayama-Kosaka et al.[71] | 11 | Prospective, investigator-blinded; Fitzpatrick skin types III to IV | 5% ALA-PDT with broadband (600 to 1100 nm, 15 J/cm2) light; Once; 3 months | Decrease in global acne grading score. |
| Yang et al.[72] | 75 | Prospective, parallel-arm, open; facial acne conglobata | 5% ALA-PDT with red light (633 ± 10 nm, 100 mW/cm2 and 50 J/cm2) versus Chinese herbal medicine mask with red light; 3 courses (10 days); 34–35 days | ALA-PDT > control group. |
| Chen et al.[73] | 50 | Prospective, randomized, controlled; moderate to severe facial acne | 5% ALA-PDT with red light (633 ± 10 nm, 10 mW/cm2 and 120 J/cm2) versus red light alone; 3 courses (1 week); 6 weeks | ALA-PDT > red light alone at weeks 4 and 6. |
| Calzavara-Pinton et al.[74] | 92 | Retrospective, observational, open study; | MAL-PDT with red light (635 ± 18 nm, 37 J/cm2); 2–4 treatments (2 to 4 weeks); 2.1 ± 1.8 months | Improvement in acne: marked (77.2%), moderate (13.0%), poor (9.8%). The marked response was maintained at follow-up by 72.8% patients. |
| Kwon et al.[75] | 46 | Prospective, double blinded, randomized, vehicle-controlled; facial acne | ALA-bu (1.5% 3-butenyl ALA-bu gel)-PDT with daylight; every day; 12 weeks | Inflammatory and non-inflammatory lesions reduction: 58.0%, 34.1% respectively. No significant change in the control group. |
| Liu et al.[76] | 150 | Controlled, randomized; moderate to severe acne | 5% ALA-PDT with red light (633 ± 6 nm, 105 mW/cm2 and 126 J/cm2) versus Intense pulse light (420 nm, 30–40 ms, 11–15 J/cm2) versus LED (combination of 415 ± 5 nm blue light with 40 mW/cm2 and 633 ± 6 nm red light with 105 mW/cm2) versus control; Until the inflammatory lesion count reduced by ≥90% (1 week versus 1week versus 2–3 days); 3 months after the end of treatment | At 1 month, PDT>IPL>LED. Mean number of sessions required, PDT: 3 ± 1.52; IPL: 6 ± 2.15; LED: 9 ± 3.34. After 3 months, patients with recurred lesions PDT<IPL<LED. |
| Tao et al.[77] | 125 | Prospective; Severe facial acne vulgaris, Fitzpatrick skin types III to IV | 3.6% ALA-PDT with LED light (633 nm, 66 mW/cm2 and 126 J/cm2); 3 courses (2 weeks); 12weeks | The total effective rates after the initial treatment were 1.6%, 24.8%, 68.8%, 89.6% and 88.8% at the 2- 4- 6- 8-and 12-week respectively. |
| Song et al.[78] | 24 | Randomized, single-blinded, controlled, split face; equivalent acne severity between the 2 sides of face | Topical chlorophyll-a-PDT versus LED only (430 ± 10 nm blue light with 600 mW/cm2, 1170 J/cm2, 660 ± 10 nm red light with 650 mW/cm2, 1080 J/cm2); 8 sessions (twice per week); 6 weeks | Chlorophyll-a-PDT > LED in reducing acne lesion counts, acne severity grades, and sebum levels. |
| Dessinioti et al.[79] | 12 | Prospective; inflammatory acne vulgaris on the face | 4% MAL-PDT with LED (635 nm, 37 J/cm2); 2 treatments (1 week); 4 weeks | Reduced acne lesion counts: 35% at week 1 and 30% at week 4. |
| Dong et al. [80] | 46 | Prospective; moderate to severe acne vulgaris | 10% ALA-PDT with LED (red and green light at a peak wavelength of 630 ± 6 nm, 543–548 nm, 250 mW/cm2, 230 J/cm2); 2–3 treatments (30 days); 12 weeks | Overall effectiveness rate: 89.13%, maximum efficacy was shown at the 12 weeks follow up. |
| Hong et al.[81] | 20 | Prospective, controlled, split face; Fitzpatrick skin types III to IV and active acne lesions | MAL plus red light (wavelength not mentioned, 34 mW/cm2, 22 J/cm2) versus MAL plus IPL (530–750 nm, 2.5 ms, duration with 10 ms delay, 8–10 J/cm2); 3 treatments (2 weeks); 8 weeks | Red light and IPL were both effective with a faster response time for red light. |
| Ma et al.[82] | 21 | Prospective; Adolescents patients with Pillsbury III to IV severe facial acne | 5% ALA-PDT with LED (633 nm, 75–80 mW/cm2, 90–96 J/cm2);3 courses; 8 weeks | ALA-PDT 3 sessions > ALA-PDT 2 sessions > ALA-PDT 1 session. |
| Moftah et al.[83] | 35 | Prospective, randomized, comparative, split body; truncal acne vulgaris | MAL-PDT with IPL (550–1200 nm, pulse duration of 30 ms, 8 cm spot size, 13–16 J/cm2) versus IPL alone (same parameters); 3 treatments (1 week); 1 month | MAL-PDT > IPL. |
| Pariser et al. [84] | 19 | Double-blinded, randomized, vehicle-controlled multicenter; Fitzpatrick skin types I to VI and severe facial acne vulgaris | 80mg/g MAL-PDT with red light (635 nm, 37 J/cm2) versus vehicle PDT; 4 treatments (2 weeks); 12 weeks | MAL-PDT > vehicle PDT in reducing inflammatory lesions, with no significant improvement in non-inflammatory lesions. |
| Pinto et al. [85] | 36 | Prospective, controlled; mild to moderate facial acne | 160mg g-1MAL-PDT with red light (635 nm, 37 J/cm2, 70 mW/cm2) versus red light alone; 2 sessions (2 weeks); 10 weeks | Total response: MAL-PDT > red light alone, MAL-PDT had a rapid clinical improvement. |
| Seo et al. [86] | 47 | Prospective; Fitzpatrick skin types III–V | 0.1% ICG-PDT with diode laser (25% in 810 nm and 75% in 940 nm, 12 mm spot size, 200–300 ms pulse duration, 18–22 J/cm2); 3 or 5 sessions (2 weeks); 2 weeks after the last treatment | Significant reduction in acne lesions; ICG-PDT 5 sessions > ICG-PDT 3 sessions. |
| Qureshi et al.[87] | 3 | Case series; refractory inflammatory and cystic acne | Pre-treat with non-ablative fractional laser and followed by 20% ALA-PDT; single; 6 months | Two patients had remission of acne after treatment. |
| Yew et al.[88] | 15 | Prospective; truncal acne in Asians | 5% ALA-PDT with red light (630 nm, 37 J/cm2, 38 mW/cm2); single; 12 weeks | 64.2% reduction in the inflammatory lesions count and 24.3% reduction in the non-inflammatory lesions count. |
| Tao et al.[89] | 136 | Prospective; moderate to severe acne | 3.6% ALA-PDT with red light; three sessions (2 weeks); 12 weeks | The total effectiveness rate and cure rate was 92.65% and 47.06%, respectively. |
| Park et al.[90] | 1,213 | Retrospective; facial acne | Indocyanine green (ICG)-PDT and IPL; three or five treatments (2 weeks) | Marked to excellent improvement was 39.8%, minimal to moderate improvement was 60.2% with high patients’ satisfaction scores. |
PDT is generally preferred as a treatment modality for AKs verses other competing treatments because of its improved cosmetic effects. In the most recent expert consensus, dPDT has been positioned as a valuable option for patients with multiple AKs in small or large fields [25].
6.2 Acne (Strength of Recommendation B, Quality of Evidence I) and acne scarring
PDT has been widely used as a treatment modality for the most common (and one of the most distressing) dermatologic disorders, acne vulgaris (Fig. 5). On the basis of a systemic review including 36 clinical trials, it was shown that PDT is safe and effective for both inflammatory and non-inflammatory acne lesions, and can improve the severity of lesions from mild to severe [26]. Table 1 summarizes some of the important studies covering the use of ALA-PDT for acne. Blue light is the most potent wavelength for activation of the endogenous porphyrin components of Propionibacterium acnes because the 407–420 nm band possesses the highest absorption coefficient in the porphyrin spectrum. There have been studies of the use of blue light alone (without any application of ALA) to treat acne [27]. However, blue light has a poor depth of penetration into the skin (limited to only approximately 1mm) while red light can penetrate to about 3 mm (albeit it with much lower absorption by PPIX). The selective destruction of the sebaceous gland unit and eradication of P. acnes are thought to be the mechanisms of action of PDT in acne [28]. The rise in antibiotic-resistant strains of P. acnes makes alternative therapies mandatory. The main advantages of PDT for acne are its excellent results despite low invasiveness, especially in patients who have not responded to topical therapy and oral antibiotics, and are not good candidates for isotretinoin [29].
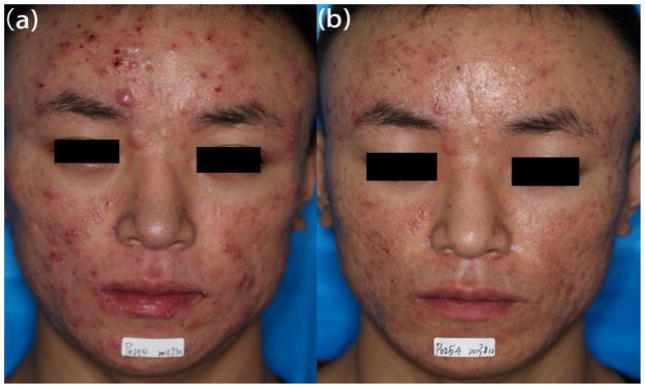
Figure 5. Improvement of skin lesions on a patient with acne vulgaris.
(a) Before treatment. (b) After 3 sessions of ALA-PDT.
Table 1.
Summary of studies on daylight-PDT in actinic keratoses (AKs)
| Reference | N | Type of trial; Study population | Light source (dose); Session number (interval); Follow-up | Results |
|---|---|---|---|---|
| Togsverd-Bo et al.[59] | 16 | Prospective, randomized, controlled, blinded; Scalp, chest, extremities | Ablative fractional laser (AFL) pretreatment 16% MAL-PDT with daylight (30 min after MAL application, 120 min) versus conventional MAL-PDT with LED (630 nm, 68 mW/cm2, 37 J/cm2); single Tx; 3 months | Efficacy and tolerability: AFL-pretreatment combined with daylight-PDT (dPDT) > MAL conventional PDT (cPDT). |
| Rubel et al. [60] | 100 | Randomized, controlled, investigator-blinded, multicenter, intra-individual; face, scalp | MAL-PDT with daylight (30 min after MAL application, 120 min) versus conventional MAL-PDT with red light; single Tx; 24 weeks | Mild AKs response rate: dPDT ≈ cPDT, dPDT was better tolerated, nearly painless and more convenient. |
| Neittaanm aki-Perttu et al. [61] | 13 | Randomized, split-face, prospective, observer-blinded; Grade I-III AKs | BF-200 ALA-dPDT versus MAL-dPDT; once for grade I AKs, twice for grade II-III AKs; 3 months | Clearance rate for grade I: BF-200 ALA-dPDT > MAL-dPDT; for grade II: BF-200 ALA-dPDT ≈ MAL-dPDT. |
| Lacour et al.[62] | 100 | Phase III, multicentre, randomized, investigator-blinded, controlled, intra-individual; mild to moderate AKs | MAL-PDT with daylight (30 min after MAL application, 120 min) versus MAL PDT with red light (37 J/cm2); single Tx; 12 weeks | Mild or moderate AKs response rate: dPDT ≈ cPDT, dPDT was better tolerated, nearly painless with high patient satisfaction. |
| Fargnoli et al.[63] | 35 | Prospective, intra-patient, left-right, comparison; face or scalp | MAL-PDT with daylight (30 min after MAL application, 120 min) versus conventional MAL-PDT with red light (37 J/cm2); single; 3–6 months | Clearance rate for grade I: dPDT ≈ cPDT; for grade II and III: dPDT < cPDT. dPDT was associated with lower pain and reduced severity of local adverse events. |
| Lane et al.[64] | 80 | Prospective; multiple AKs on the face, chest, arms and legs | ALA-PDT with daylight (shade 2.5 h for the first day and 15–30 min for the following day); one or two sessions; 3 months | Significant reduction in lesions and patients tolerated well. |
| Spelman et al.[65] | 100 | phase III, randomized, controlled; mild AKs on face and scalp in eight different geographical locations throughout Australia | MAL-PDT with daylight (direct light for 2 h, light intensity 305 W/m2) versus MAL-PDT with red light; | dPDT can be used to treat face and scalp AK in Australia effectively throughout the entire year as long as weather conditions permit daylight exposure and allow participants to remain under direct light for 2 h. |
| Grinblat et al.[66] | 14 | Prospective; Grade I to II AKs on the face | MAL-PDT with daylight (direct light for 60–90 min); 1 to 3 sessions; 3 months | The average reduction for a single session of dPDT was 87.9%; no recurrence after 3 months, none of patients had severe side effects. |
| Calzavara-pinton et al.[67] | 22 | Comparative, intra-patient, split face, randomized; Grade I to II AKs on the face and scalp | MAL-PDT with daylight (single session) versus ingenol mebutate gel with daylight (3 days’ treatment cycle); 3 months | Both treatments had a similar efficacy. |
| Genovese et al.[68] | 27 | Intraindividual comparative analysis; Grade I to II AKs on the face and scalp | dPDT versus ingenol mebutate (IM); single session; 6 months | Clearance rate was similar. |
| O’ Gorman et al. [69] | 22 | Prospective, randomized, single-blind, split-scalp; AKs on the forehead and scalp | MAL-PDT with daylight versus MAL-PDT with artificial white light (AWL) LED; treatment last for 3 months; 9 months | The reduction in total AK count per treatment field was similar in all timepoints. |
| Neittaanm aki et al.[70] | 14 | Prospective, controlled, single-blind; grade I to III AKs on the face or scalp | dPDT with 0.2% hexylaminolaevulinate (HAL) versus dPDT with 16% MAL; 3 months; 12 months | The mean lesion clearance was 67% with HAL and 66% with MAL. |
Acne scarring is a common complication of acne, and is both disfiguring and challenging despite various currently available treatments. One single-center, double-blinded, randomized, pilot study (n=6) used 20% ALA or vehicle solution alone to either the right or left sides of the face for a 60-minute incubation followed by 417 nm blue light after full-face treatment with microdermabrasion, and showed 80% of patients displayed a greater improvement in scarring on the ALA-treated side after five sessions [30]. Another prospective, single-arm, pilot study enrolled forty subjects with severe acne. The patients were treated with 15% ALA-PDT for four times, then they received ablative fractional Er:YAG laser five times, an 80% overall improvement in acne scars was noted [31].
6.3 Viral warts (Strength of Recommendation B, Quality of Evidence I)
Warts consist of benign cutaneous hyper-proliferative lesions resulting from infection with human papilloma viruses (HPV). The treatment of warts poses a therapeutic challenge; warts that are recalcitrant to therapy and recurrent warts remain a problem. Burtica et al. [32] initially treated a man with recalcitrant facial warts with topical 80% trichloroacetic acid and 0.05% isotretinoin solution for 1 month to reduce the thickness of the warts. Subsequently, MAL-PDT was used with red light and showed 60% and 100% clearance after the first and second session respectively. Complications were mild. A biopsy showed no recurrence at 1-month follow-up.
Li et al. [33] treated a total of 6146 lesions in 55 patients. ALA-PDT with 633 nm red light at concentrations of 5%, 10% and 20% were used and applied for 4 h. Three sessions were repeated at 2-week intervals. The mean overall clearance was 74.1%, 68.8% and 64.6% at 4, 8 and 12 weeks respectively. Complete response rate was the lowest in the 5% ALA group, while hyperpigmentation was more developed in the 20% group. The overall recurrence rate was 16.7%.
Qian et al. [34] enrolled 30 patients into a step-up regimen (dose escalation). The first session included 5% ALA for 1.5 h with 10 min of LED irradiation. The second session used 10% ALA for 3 h with 20 min of irradiation. The third session used 20% ALA for 3 h with 20 min of irradiation. The treatment interval was 2 weeks. Patients with fulminant lesions (n=10) exhibited 100% cure rate at day 15, with no recurrence at 6-month follow-up. 18 patients received second session with complete remission response in three patients. 15 patients underwent the third session and numbers of complete resolution, partial resolution and no response were 10, 1, and 4 respectively. Patients reported fewer adverse events with subsequent treatments even though the concentration of ALA was increased.
Yang et al. [35] treated 12 patients with recalcitrant flat facial warts. 10% ALA gel was applied topically to the lesions and the incubation time was 3h. LED light at 630 ± 10 nm at an irradiance of 60–100 mW/cm2 was used. Ten patients completed three sessions and of these five had complete lesion clearance while the other five were significantly improved. At the 24-week follow-up, the average success rate was 88.8% with no recurrences.
Combination therapy using a super pulsed carbon dioxide (CO2) laser together with ALA-PDT in patients with recalcitrant flat facial warts was reported [36]. All lesions in 6 patients showed complete response after two to four treatment courses in 3 month follow-up with no relapsed cases after 3–18 months.
PDT is a treatment that offers good results in the treatment of viral warts, especially for those patients who are resistant to routine treatment.
6. 4 Skin rejuvenation (Strength of Recommendation A, Quality of Evidence1)
Clinical observations have shown that PDT is associated with high level of improvement in the signs of photo-aging such as fine lines, wrinkles, dyspigmentation, sallowness, roughness and telangiectasia. In one study, Torezan [37] et al reported treating ten patients with facial photodamage and actinic keratoses with MAL-PDT or microneedling-assisted PDT. In this prospective, randomized, split face study, they were able to demonstrate the effectiveness of both modalities in the treatment of photodamage, mottled pigmentation, roughness and sallowness, with the superior efficacy of microneedling-assisted PDT compared to MAL-PDT alone on fine lines at day 30. At day 90, facial erythema and coarse wrinkles also improved in the microneedling-assisted PDT side of the face, in addition to fine lines for the MAL-PDT side. Side effects were more common and intense on the microneedling-assisted PDT side. Ji [38] et al reported the results of 10% ALA-PDT and red light alone in the treatment of forearm extensor photoaging in 14 patients. Impressively, the change of appearance of photoaging lesions, stratum corneum hydration and transepidermal water loss (TEWL) were more obvious in the ALA-PDT group. The signs of typical damage due to solar elastosis were improved in both groups. Zhang [39] et al evaluated a single treatment with red light, red light-PDT, IPL or IPL-PDT for photodamaged neck skin. In this randomized, single-blinded study, the appearance of photoaging lesions was improved. The stratum corneum hydration, L* value, elasticity and thickness increased whereas the TEWL and melanin index value decreased with superior changes in the red light-PDT and IPL-PDT group. The a* and erythema index value increased in the red-light PDT group. No significant change was observed in the red light group. A randomized, controlled, split face, single blinded study used a 0.5% 5-ALA liposomal spray for periorbital wrinkles in Asians [40]. One half of the face was treated with IPL-PDT and the other half with long pulsed Nd:YAG laser. Lateral periorbital wrinkles treated with PDT showed better results than the control (Nd-YAG) side without serious adverse effects. Sanclemente [41]et al assessed sixty patients with symmetric facial photodamage with different treatments, MAL and daylight vs. matching placebo and daylight. They found that dPDT with MAL was safe and effective for the treatment of facial photodamage.
PDT can both rejuvenate their skin and also treat their visible or incipient UV-induced lesions, it shows excellent efficacy and tolerability.
6. 5 Psoriasis (Strength of Recommendation D, Quality of Evidence 1)
There have been a large number of studies devoted to the treatment of psoriasis with PDT. Initial research [42] showed that PDT might be helpful, but later studies could not confirm these expectations. A systematic review and meta-analysis searched Medline, Embase, and Cochrane databases during the period of January 1980 to June 2012, including 765 studies, and found that the pooled effect estimate of the efficacy of topical PUVA, targeted UVB and PDT were 77%, 61%, 22% respectively. PDT has high percentage of side effects in treating localized psoriasis [43]. Moreover, another systematic search of the PubMed Medline database disclosed that topical ALA-PDT failed to demonstrate a consistent, efficacious response and frequently suffered from intolerable adverse reactions (severe pain and burning sensations) [44]. Current evidence of PDT remains unclear for psoriasis with disappointing efficacy and unwanted side effects.
6. 6 Emerging indications
6.6.1 Localized scleroderma
Localized scleroderma is a cutaneous fibrotic disorder, characterized by increased dermal collagen accumulation. Five patients with localized scleroderma responded well to PDT [45]. In these patients, the sclerosis was found to have regressed significantly following a course of treatment that lasted between 3 and 6 months. This finding attracted great interest, as the direct effect of PDT cannot reach the layers of dermis where the pathological changes occur in scleroderma. It was hypothesized that PDT may influence dermal fibroblasts into producing increased amounts of collagen-degrading matrix-metalloproteinase (MMP1 and MMP3) [46].
6.6.2 keloids (Strength of Recommendation C, Quality of Evidence II–iii)
This mechanism may also be used to explain the recent findings of PDT for treatment of keloid disease. Keloids are a type of scar formed from fibroproliferative dermal tissue characterized by the excessive proliferation of fibroblasts. Mendoza et al. [47] evaluated the cytotoxic effects of PDT using MAL and ALA on KD fibroblasts compared to normal skin fibroblasts. Li et al. [48] investigated the effects of ALA-PDT and showed that hypertrophic scar-derived fibroblasts accumulated more PpIX. A clinical study used MAL and red light (37J/cm2) to treat keloids with PDT once per week for 3 weeks [49]. They found that blood flow, collagen, and hemoglobin levels significantly decreased from week 1 to 3 and pliability increased significantly (p = 0.001). Only one patient with a keloid in a stress-prone anatomical location experienced recurrence of KD. All other patients had no recurrence at 9-month follow-up. Larger studies are needed to confirm the efficacy of PDT for these two conditions.
6. 7 Port-wine stains
Port-wine stains (PWS) are common congenital and progressive vascular lesions appearing mainly on the face and neck that can represent a serious disfigurement. Pulsed-dye laser (PDL) is the current standard treatment for PWS. However, about 20% of PWS are resistant to PDL [50]. Vascular-targeted PDT with an intravenous injection of a hematoporphyrin derivative followed after a short time by irradiation might be an alternative for the treatment of PWS (Fig. 6). Gao et al [51] compared the effect of PDT and PDL in two adjacent flat areas of PWS lesions from 15 patients in a randomized, controlled, single-blind study. Patients received either single-session PDL or PDT and were followed up colorimetrically and visually at least two months. The study demonstrated that PDT was as effective as PDL and even superior in some cases. The similar comparison was conducted in pediatric patients [52]. A total of 260 patients between 3 and 10 years old with facial PWS received either 585 nm PDL or Hemoporfin-PDT were analyzed in this retrospective study. The rates of excellent response in PDT and PDL group were 25.0% and 10.9% respectively. The overall response rate of PDT was superior to PDL for purple lesions (93.0% vs. 75.6%), whereas the pigmentation and scar formation in PDT group were lower. Recently, Yu et al [53] reported a case of long-term (18 years) follow-up of PWS lesions after PDT. The lesions presented stable and observed no obvious recurrence. Histological data of residual PWS revealed smaller vessels post-treatment. Hemoporfin has been used in PDT to treat port-wine stain. One randomized clinical trial compared PDT-Hemoporfin group with PDT-placebo group. It was reported that HMME-mediated PDT and 532 nm continuous wave laser was effective and safe for adolescent and adult patients with port-wine stains [54]. Tang et al [55] recruited 50 patients with PWS and assigned them randomly to the HMME-PDT group or the placebo group in session 1, both groups received HMME-PDT in the second session. Two sessions of treatments failed to show accumulation effects on treatment reactions. And positive correlation between treatment efficiency and the severity of scab or pain might be positive. PDT using the systemic porphyrin-related photosensitizer appears to represent an effective approach for treating PWS.
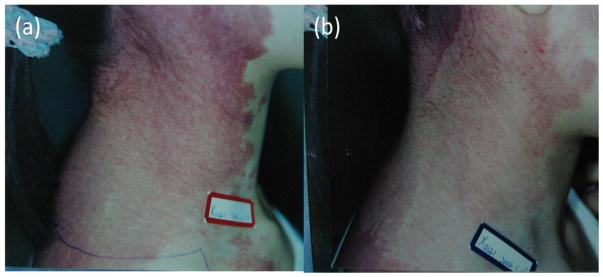
Figure 6. Patients with port-wine stains treated with PDT.
(a) Before treatment. (b) Excellent response after 2 sessions of PDT.
7. Adverse effects
The goal of using PDT in cosmetic disorders is to improve the overall aesthetic appearance, but it is possible that PDT could result in poor or even undesirable outcomes due to production of adverse effects (Fig. 7). These phototoxic reactions may be variable in intensity and duration (Table 3), and can also vary markedly from patient to patient and even from lesion to lesion. Even in dPDT studies, erythema and crusting were the most common adverse findings. Ways to make PDT more acceptable should be considered. Recent studies revealed that superpotent corticosteroid before and just after PDT reduced the erythema 24 h after treatment of multiple AKs[56]. The further clinical trail found that pulse-PDT (short incubation time) significantly reduced post-treatment erythema [57]. Both the ways did not compromise efficacy. Recently, Gerber reported a case with post-treatment erythema treated with Brimonidine tartrate (BT) 0.33% gel. The observation demonstrates that BT effectively reduces erythema and improve the tolerability of dPDT [58].
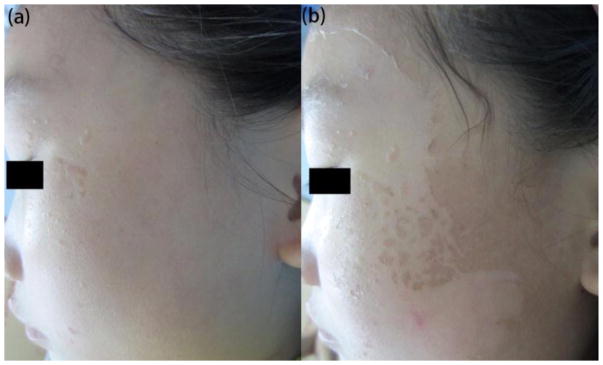
Figure 7. Adverse effect of PDT.
(a) A patient with viral warts. (b) Peeling after 1 session of ALA-PDT.
Table 3.
Adverse effects of photodynamic therapy
| Time point | Adverse effects |
|---|---|
| During the treatment | Pain, burning, stinging, prickling sensation |
| Post treatment | Erythema |
| Short-term and medium-term | Edema, scabbing or crusting, peeling Vesicle, blister, infection, contact dermatitis, [91] bruise, [92] urticaria, [93] skin |
| Long-term | Phototoxicity, scar formation, hyperpigmentation, hypopigmentation |
8. Concluding remarks
PDT is a therapeutic procedure used with increasing frequency in cosmetic dermatology, as it has advantageous features both for clinical application and lowering the costs of treatment., However in some circumstances non-healthcare professionals may perform the treatment, which could increase hazards to both patients and care providers. Care must be taken to ensure the qualifications and training of healthcare providers.
PDT has been widely used for many off-label purposes in cosmetic dermatology with great success. Although the therapeutic protocols regarding use of PDT in many cosmetic cutaneous diseases have not yet been standardized, and vary significantly between the different literature studies, PDT will likely continue to be explored thoroughly for cosmetic use. Nevertheless it is recommended that physicians should always employ careful assessment of protocols and individual patients, and educate patients with regard to realistic outcomes and proper precautions.
Highlights.
ALA-mediated PDT is growing in popularity in cosmetic dermatology
PDT can cause collagen remodeling and can resolve acute inflammation
PDT has been used to treat acne and daylight PDT is becoming used
Skin rejuvenation, viral warts, psoriasis, localized scleroderma, keloids.
Pain is the main side-effect of ALA-PDT
Acknowledgments
Xiang Wen was supported by West China Hospital of Sichuan University.
Michael R Hamblin was supported by US NIH grants R01AI050875 and R21AI121700.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn Ther. 2005;2:1–23. doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostinis P, Buytaert E, Breyssens H, Hendrickx N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem Photobiol Sci. 2004;3:721–729. doi: 10.1039/b315237e. [DOI] [PubMed] [Google Scholar]
- 4.Lv T, Huang ZF, Wang HW, Lin JQ, Chen GN, Chen XW, et al. Evaluation of collagen alteration after topical photodynamic therapy (PDT) using second harmonic generation (SHG) microscopy--in vivo study in a mouse model. Photodiagnosis Photodyn Ther. 2012;9:164–169. doi: 10.1016/j.pdpdt.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Orringer JS, Hammerberg C, Hamilton T, Johnson TM, Kang S, Sachs DL, et al. Molecular effects of photodynamic therapy for photoaging. Arch Dermatol. 2008;144:1296–1302. doi: 10.1001/archderm.144.10.1296. [DOI] [PubMed] [Google Scholar]
- 6.Thunshelle C, Yin R, Chen Q, Hamblin MR. Current Advances in 5-Aminolevulinic Acid Mediated Photodynamic Therapy. Curr Dermatol Rep. 2016;5:179–190. doi: 10.1007/s13671-016-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci. 2008;7:283–289. doi: 10.1039/b712847a. [DOI] [PubMed] [Google Scholar]
- 8.van Veen RL, Aalders MC, Pasma KL, Siersema PD, Haringsma J, van de Vrie W, et al. In situ light dosimetry during photodynamic therapy of Barrett’s esophagus with 5-aminolevulinic acid. Lasers Surg Med. 2002;31:299–304. doi: 10.1002/lsm.10129. [DOI] [PubMed] [Google Scholar]
- 9.Lei TC, Glazner GF, Duffy M, Scherrer L, Pendyala S, Li B, et al. Optical properties of hematoporphyrin monomethyl ether (HMME), a PDT photosensitizer. Photodiagnosis Photodyn Ther. 2012;9:232–242. doi: 10.1016/j.pdpdt.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Willey A, Anderson RR, Sakamoto FH. Temperature-Modulated Photodynamic Therapy for the Treatment of Actinic Keratosis on the Extremities: A One-Year Follow-up Study. Dermatol Surg. 2015;41:1290–1295. doi: 10.1097/DSS.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 11.Mordon S. A commentary on the role of skin temperature on the effectiveness of ALA-PDT in Dermatology. Photodiagnosis Photodyn Ther. 2014;11:416–419. doi: 10.1016/j.pdpdt.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Morton C, Szeimies RM, Sidoroff A, Wennberg AM, Basset-Seguin N, Calzavara-Pinton P, et al. European Dermatology Forum Guidelines on topical photodynamic therapy. Eur J Dermatol. 2015;25:296–311. doi: 10.1684/ejd.2015.2570. [DOI] [PubMed] [Google Scholar]
- 13.Gupta AK, Paquet M. Network meta-analysis of the outcome ‘participant complete clearance’ in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250–259. doi: 10.1111/bjd.12343. [DOI] [PubMed] [Google Scholar]
- 14.Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:1281–1288. doi: 10.1001/jamadermatol.2014.1253. [DOI] [PubMed] [Google Scholar]
- 15.Zane C, Facchinetti E, Rossi MT, Specchia C, Calzavara-Pinton PG. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol. 2014;170:1143–1150. doi: 10.1111/bjd.12844. [DOI] [PubMed] [Google Scholar]
- 16.Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094–1102. doi: 10.1097/01.DSS.0000452662.69539.57. [DOI] [PubMed] [Google Scholar]
- 17.Cai H, Wang YX, Sun P, Yang ZY, Tian R, Liu XY, et al. Photodynamic therapy for facial actinic keratosis: a clinical and histological study in Chinese patients. Photodiagnosis Photodyn Ther. 2013;10:260–265. doi: 10.1016/j.pdpdt.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Basset-Seguin N, Baumann Conzett K, Gerritsen MJ, Gonzalez H, Haedersdal M, Hofbauer GF, et al. Photodynamic therapy for actinic keratosis in organ transplant patients. J Eur Acad Dermatol Venereol. 2013;27:57–66. doi: 10.1111/j.1468-3083.2011.04356.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598–1605. doi: 10.1111/jdv.12953. [DOI] [PubMed] [Google Scholar]
- 20.Ko DY, Jeon SY, Kim KH, Song KH. Fractional erbium: YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529–1539. doi: 10.1111/jdv.12334. [DOI] [PubMed] [Google Scholar]
- 21.Helsing P, Togsverd-Bo K, Veierod MB, Mork G, Haedersdal M. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087–1092. doi: 10.1111/bjd.12507. [DOI] [PubMed] [Google Scholar]
- 22.Nissen CV, Heerfordt IM, Wiegell SR, Mikkelsen CS, Wulf HC. Pretreatment with 5-Fluorouracil Cream Enhances the Efficacy of Daylight-mediated Photodynamic Therapy for Actinic Keratosis. Acta Derm Venereol. 2017 doi: 10.2340/00015555-2612. [DOI] [PubMed] [Google Scholar]
- 23.Dirschka T, Radny P, Dominicus R, Mensing H, Bruning H, Jenne L, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168:825–836. doi: 10.1111/bjd.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomas-Velazquez A, Redondo P. Switching From Conventional Photodynamic Therapy to Daylight Photodynamic Therapy For Actinic Keratoses: Systematic Review and Meta-analysis. Actas Dermosifiliogr. 2017 doi: 10.1016/j.ad.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Calzavara-Pinton P, Haedersdal M, Barber K, Basset-Seguin N, Del Pino Flores ME, Foley P, et al. Structured Expert Consensus on Actinic Keratosis: Treatment Algorithm Focusing on Daylight PDT. J Cutan Med Surg. 2017:1203475417702994. doi: 10.1177/1203475417702994. [DOI] [PubMed] [Google Scholar]
- 26.Keyal U, Bhatta AK, Wang XL. Photodynamic therapy for the treatment of different severity of acne: A systematic review. Photodiagnosis Photodyn Ther. 2016;14:191–199. doi: 10.1016/j.pdpdt.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Bagherani N. Efficacy of blue light in treatment of acne. Dermatol Ther. 2016;29:210. doi: 10.1111/dth.12291. [DOI] [PubMed] [Google Scholar]
- 28.Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145–163. doi: 10.2147/CCID.S35334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boen M, Brownell J, Patel P, Tsoukas MM. The Role of Photodynamic Therapy in Acne: An Evidence-Based Review. Am J Clin Dermatol. 2017 doi: 10.1007/s40257-017-0255-3. [DOI] [PubMed] [Google Scholar]
- 30.Linkner RV, Jim On S, Haddican M, Singer G, Shim-Chang H. Evaluating the Efficacy of Photodynamic Therapy with 20% Aminolevulinic Acid and Microdermabrasion as a Combination Treatment Regimen for Acne Scarring: A Split-face, Randomized, Double-blind Pilot Study. J Clin Aesthet Dermatol. 2014;7:32–35. [PMC free article] [PubMed] [Google Scholar]
- 31.Yin R, Lin L, Xiao Y, Hao F, Hamblin MR. Combination ALA-PDT and ablative fractional Er:YAG laser (2,940 nm) on the treatment of severe acne. Lasers Surg Med. 2014;46:165–172. doi: 10.1002/lsm.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burtica EC, Magnano M, Loi C, Bardazzi F, Patrizi A. Photodynamic therapy with 5-methylaminolevulinic acid in the treatment of multiple warts of the face. J Dermatolog Treat. 2013;24:137–138. doi: 10.3109/09546634.2011.595773. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Jiao B, Zhou F, Tan Q, Ma Y, Luo L, et al. Comparative study of photodynamic therapy with 5%, 10% and 20% aminolevulinic acid in the treatment of generalized recalcitrant facial verruca plana: a randomized clinical trial. J Eur Acad Dermatol Venereol. 2014;28:1821–1826. doi: 10.1111/jdv.12319. [DOI] [PubMed] [Google Scholar]
- 34.Qian G, Wang S, Deng D, Yang G. Is the step-up therapy of topical 5-aminolevulinic acid photodynamic therapy effective and safe for the patients with recalcitrant facial flat wart? Dermatol Ther. 2014;27:83–88. doi: 10.1111/dth.12060. [DOI] [PubMed] [Google Scholar]
- 35.Yang YL, Sang J, Liao NX, Wei F, Liao W, Chen JH. Off-label photodynamic therapy for recalcitrant facial flat warts using topical 5-aminolevulinic acid. Lasers Med Sci. 2016;31:929–936. doi: 10.1007/s10103-016-1925-8. [DOI] [PubMed] [Google Scholar]
- 36.Gao J, Shen C, Ko R, Zheng X, Wang Z, Liu S, et al. Combination effect of super pulsed carbon dioxide laser and photodynamic therapy for recalcitrant facial flat warts: A preliminary study. J Cosmet Laser Ther. 2016;18:56–57. doi: 10.3109/14764172.2015.1063664. [DOI] [PubMed] [Google Scholar]
- 37.Torezan L, Chaves Y, Niwa A, Sanches JA, Jr, Festa-Neto C, Szeimies RM. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39:1197–1201. doi: 10.1111/dsu.12233. [DOI] [PubMed] [Google Scholar]
- 38.Ji J, Zhang LL, Ding HL, Wang HW, Huang Z, Wang XX, et al. Comparison of 5-aminolevulinic acid photodynamic therapy and red light for treatment of photoaging. Photodiagnosis Photodyn Ther. 2014;11:118–121. doi: 10.1016/j.pdpdt.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhang HY, Ji J, Tan YM, Zhang LL, Wang XJ, Wang PR, et al. Evaluation of 5-aminolevulinic acid-mediated photorejuvenation of neck skin. Photodiagnosis Photodyn Ther. 2014;11:498–509. doi: 10.1016/j.pdpdt.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Shin HT, Kim JH, Shim J, Lee JH, Lee DY, Lee JH, et al. Photodynamic therapy using a new formulation of 5-aminolevulinic acid for wrinkles in Asian skin: A randomized controlled split face study. J Dermatolog Treat. 2015;26:246–251. doi: 10.3109/09546634.2014.933163. [DOI] [PubMed] [Google Scholar]
- 41.Sanclemente G, Mancilla GA, Hernandez G. A double-blind randomized controlled trial to assess the efficacy of daylight photodynamic therapy with methyl-aminolevulinate vs. Placebo and daylight in patients with facial photodamage. Actas Dermosifiliogr. 2016;107:224–234. doi: 10.1016/j.ad.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Boehncke WH, Elshorst-Schmidt T, Kaufmann R. Systemic photodynamic therapy is a safe and effective treatment for psoriasis. Arch Dermatol. 2000;136:271–272. doi: 10.1001/archderm.136.2.271. [DOI] [PubMed] [Google Scholar]
- 43.Almutawa F, Thalib L, Hekman D, Sun Q, Hamzavi I, Lim HW. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5–14. doi: 10.1111/phpp.12092. [DOI] [PubMed] [Google Scholar]
- 44.Choi YM, Adelzadeh L, Wu JJ. Photodynamic therapy for psoriasis. J Dermatolog Treat. 2015;26:202–207. doi: 10.3109/09546634.2014.927816. [DOI] [PubMed] [Google Scholar]
- 45.Karrer S, Abels C, Landthaler M, Szeimies RM. Topical photodynamic therapy for localized scleroderma. Acta Derm Venereol. 2000;80:26–27. doi: 10.1080/000155500750012469. [DOI] [PubMed] [Google Scholar]
- 46.Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Keratinocyte-derived cytokines after photodynamic therapy and their paracrine induction of matrix metalloproteinases in fibroblasts. Br J Dermatol. 2004;151:776–783. doi: 10.1111/j.1365-2133.2004.06209.x. [DOI] [PubMed] [Google Scholar]
- 47.Mendoza J, Sebastian A, Allan E, Allan D, Mandal P, Alonso-Rasgado T, et al. Differential cytotoxic response in keloid fibroblasts exposed to photodynamic therapy is dependent on photosensitiser precursor, fluence and location of fibroblasts within the lesion. Arch Dermatol Res. 2012;304:549–562. doi: 10.1007/s00403-012-1264-y. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Zhou ZP, Hu L, Zhang WJ, Li W. Apoptotic cell death induced by 5-aminolaevulinic acid-mediated photodynamic therapy of hypertrophic scar-derived fibroblasts. J Dermatolog Treat. 2014;25:428–433. doi: 10.3109/09546634.2012.697987. [DOI] [PubMed] [Google Scholar]
- 49.Ud-Din S, Thomas G, Morris J, Bayat A. Photodynamic therapy: an innovative approach to the treatment of keloid disease evaluated using subjective and objective non-invasive tools. Arch Dermatol Res. 2013;305:205–214. doi: 10.1007/s00403-012-1295-4. [DOI] [PubMed] [Google Scholar]
- 50.Savas JA, Ledon JA, Franca K, Chacon A, Nouri K. Pulsed dye laser-resistant port-wine stains: mechanisms of resistance and implications for treatment. Br J Dermatol. 2013;168:941–953. doi: 10.1111/bjd.12204. [DOI] [PubMed] [Google Scholar]
- 51.Gao K, Huang Z, Yuan KH, Zhang B, Hu ZQ. Side-by-side comparison of photodynamic therapy and pulsed-dye laser treatment of port-wine stain birthmarks. Br J Dermatol. 2013;168:1040–1046. doi: 10.1111/bjd.12130. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Zhang TH, Huang Z, Li Q, Yuan KH, Hu ZQ. Comparison of pulsed dye laser (PDL) and photodynamic therapy (PDT) for treatment of facial port-wine stain (PWS) birthmarks in pediatric patients. Photodiagnosis Photodyn Ther. 2014;11:491–497. doi: 10.1016/j.pdpdt.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Yu W, Ma G, Qiu Y, Chen H, Jin Y, Yang X, et al. 18 years long-term results of facial port-wine stain (PWS) after photodynamic therapy (PDT)--a case report. Photodiagnosis Photodyn Ther. 2015;12:143–145. doi: 10.1016/j.pdpdt.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Y, Tu P, Zhou G, Zhou Z, Lin X, Yang H, et al. Hemoporfin Photodynamic Therapy for Port-Wine Stain: A Randomized Controlled Trial. PLoS One. 2016;11:e0156219. doi: 10.1371/journal.pone.0156219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang Y, Xie H, Li J, Jian D. The association between treatment reactions and treatment efficiency of HMME-photodynamic therapy on port wine stains: a prospective double blind randomized controlled trial. Photodiagnosis Photodyn Ther. 2017 doi: 10.1016/j.pdpdt.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Wiegell SR, Petersen B, Wulf HC. Topical corticosteroid reduces inflammation without compromising the efficacy of photodynamic therapy for actinic keratoses: a randomized clinical trial. Br J Dermatol. 2014;171:1487–1492. doi: 10.1111/bjd.13284. [DOI] [PubMed] [Google Scholar]
- 57.Wiegell SR, Petersen B, Wulf HC. Pulse photodynamic therapy reduces inflammation without compromising efficacy in the treatment of multiple mild actinic keratoses of the face and scalp: a randomized clinical trial. Br J Dermatol. 2016;174:979–984. doi: 10.1111/bjd.14465. [DOI] [PubMed] [Google Scholar]
- 58.Gerber PA. Topical brimonidine tartrate 0.33% gel effectively reduces the post-treatment erythema of daylight-activated photodynamic therapy. Br J Dermatol. 2016;174:1422–1423. doi: 10.1111/bjd.14384. [DOI] [PubMed] [Google Scholar]
- 59.Togsverd-Bo K, Lei U, Erlendsson AM, Taudorf EH, Philipsen PA, Wulf HC, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients - a randomized controlled trial. Br J Dermatol. 2015;172:467–474. doi: 10.1111/bjd.13222. [DOI] [PubMed] [Google Scholar]
- 60.Rubel DM, Spelman L, Murrell DF, See JA, Hewitt D, Foley P, et al. Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: a randomized controlled trial. Br J Dermatol. 2014;171:1164–1171. doi: 10.1111/bjd.13138. [DOI] [PubMed] [Google Scholar]
- 61.Neittaanmaki-Perttu N, Karppinen TT, Gronroos M, Tani TT, Snellman E. Daylight photodynamic therapy for actinic keratoses: a randomized double-blinded nonsponsored prospective study comparing 5-aminolaevulinic acid nanoemulsion (BF-200) with methyl-5-aminolaevulinate. Br J Dermatol. 2014;171:1172–1180. doi: 10.1111/bjd.13326. [DOI] [PubMed] [Google Scholar]
- 62.Lacour JP, Ulrich C, Gilaberte Y, Von Felbert V, Basset-Seguin N, Dreno B, et al. Daylight photodynamic therapy with methyl aminolevulinate cream is effective and nearly painless in treating actinic keratoses: a randomised, investigator-blinded, controlled, phase III study throughout Europe. J Eur Acad Dermatol Venereol. 2015;29:2342–2348. doi: 10.1111/jdv.13228. [DOI] [PubMed] [Google Scholar]
- 63.Fargnoli MC, Piccioni A, Neri L, Tambone S, Pellegrini C, Peris K. Conventional vs. daylight methyl aminolevulinate photodynamic therapy for actinic keratosis of the face and scalp: an intra-patient, prospective, comparison study in Italy. J Eur Acad Dermatol Venereol. 2015;29:1926–1932. doi: 10.1111/jdv.13076. [DOI] [PubMed] [Google Scholar]
- 64.Lane KL, Hovenic W, Ball K, Zachary CB. Daylight photodynamic therapy: the Southern California experience. Lasers Surg Med. 2015;47:168–172. doi: 10.1002/lsm.22323. [DOI] [PubMed] [Google Scholar]
- 65.Spelman L, Rubel D, Murrell DF, See JA, Hewitt D, Foley P, et al. Treatment of face and scalp solar (actinic) keratosis with daylight-mediated photodynamic therapy is possible throughout the year in Australia: Evidence from a clinical and meteorological study. Australas J Dermatol. 2016;57:24–28. doi: 10.1111/ajd.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grinblat BM, Festa Neto C, Sanches JA, Jr, Szeimies RM, Oliveira AP, Torezan LA. Daylight photodynamic therapy for actinic keratoses in Sao Paulo, Brazil. Photodermatol Photoimmunol Photomed. 2015;31:54–56. doi: 10.1111/phpp.12127. [DOI] [PubMed] [Google Scholar]
- 67.Moggio E, Arisi M, Zane C, Calzavara-Pinton I, Calzavara-Pinton P. A randomized split-face clinical trial analyzing daylight photodynamic therapy with methyl aminolaevulinate vs ingenol mebutate gel for the treatment of multiple actinic keratoses of the face and the scalp. Photodiagnosis Photodyn Ther. 2016;16:161–165. doi: 10.1016/j.pdpdt.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Genovese G, Fai D, Fai C, Mavilia L, Mercuri SR. Daylight methyl-aminolevulinate photodynamic therapy versus ingenol mebutate for the treatment of actinic keratoses: an intraindividual comparative analysis. Dermatol Ther. 2016;29:191–196. doi: 10.1111/dth.12334. [DOI] [PubMed] [Google Scholar]
- 69.O’Gorman SM, Clowry J, Manley M, McCavana J, Gray L, Kavanagh A, et al. Artificial White Light vs Daylight Photodynamic Therapy for Actinic Keratoses: A Randomized Clinical Trial. JAMA Dermatol. 2016;152:638–644. doi: 10.1001/jamadermatol.2015.5436. [DOI] [PubMed] [Google Scholar]
- 70.Neittaanmaki-Perttu N, Karppinen TT, Tani T, Snellman E, Gronroos M. Long-term Outcome of Low-concentration Hexyl-5-aminolaevulinate Daylight Photodynamic Therapy for Treatment of Actinic Keratoses. Acta Derm Venereol. 2017;97:120–121. doi: 10.2340/00015555-2484. [DOI] [PubMed] [Google Scholar]
- 71.Asayama-Kosaka S, Akilov OE, Kawana S. Photodynamic Therapy with 5% delta-Aminolevulinic Acid is Safe and Effective Treatment of Acne Vulgaris in Japanese Patients. Laser Ther. 2014;23:115–120. doi: 10.5978/islsm.14-OR-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang GL, Zhao M, Wang JM, He CF, Luo Y, Liu HY, et al. Short-term clinical effects of photodynamic therapy with topical 5-aminolevulinic acid for facial acne conglobata: an open, prospective, parallel-arm trial. Photodermatol Photoimmunol Photomed. 2013;29:233–238. doi: 10.1111/phpp.12059. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, Song H, Chen S, Zhang J, Niu G, Liu X. Clinical efficacy of 5-aminolevulinic acid photodynamic therapy in the treatment of moderate to severe facial acne vulgaris. Exp Ther Med. 2015;10:1194–1198. doi: 10.3892/etm.2015.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calzavara-Pinton PG, Rossi MT, Aronson E, Sala R Italian Group For Photodynamic T. A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 1: inflammatory and aesthetic indications. Photochem Photobiol Sci. 2013;12:148–157. doi: 10.1039/c2pp25124h. [DOI] [PubMed] [Google Scholar]
- 75.Kwon HH, Moon KR, Park SY, Yoon JY, Suh DH, Lee JB. Daylight photodynamic therapy with 1.5% 3-butenyl 5-aminolevulinate gel as a convenient, effective and safe therapy in acne treatment: A double-blind randomized controlled trial. J Dermatol. 2016;43:515–521. doi: 10.1111/1346-8138.13191. [DOI] [PubMed] [Google Scholar]
- 76.Liu LH, Fan X, An YX, Zhang J, Wang CM, Yang RY. Randomized trial of three phototherapy methods for the treatment of acne vulgaris in Chinese patients. Photodermatol Photoimmunol Photomed. 2014;30:246–253. doi: 10.1111/phpp.12098. [DOI] [PubMed] [Google Scholar]
- 77.Tao SQ, Xia RS, Li F, Cao L, Fan H, Fan Y, et al. Efficacy of 3.6% topical ALA-PDT for the treatment of severe acne vulgaris. Eur Rev Med Pharmacol Sci. 2016;20:225–231. [PubMed] [Google Scholar]
- 78.Song BH, Lee DH, Kim BC, Ku SH, Park EJ, Kwon IH, et al. Photodynamic therapy using chlorophyll-a in the treatment of acne vulgaris: a randomized, single-blind, split-face study. J Am Acad Dermatol. 2014;71:764–771. doi: 10.1016/j.jaad.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 79.Dessinioti C, Masouri S, Drakaki E, Katsambas A, Antoniou C. Short-contact, low-dose methyl aminolaevulinate photodynamic therapy for acne vulgaris. Br J Dermatol. 2016;175:215. doi: 10.1111/bjd.14460. [DOI] [PubMed] [Google Scholar]
- 80.Dong Y, Zhou G, Chen J, Shen L, Jianxin Z, Xu Q, et al. A new LED device used for photodynamic therapy in treatment of moderate to severe acne vulgaris. Photodiagnosis Photodyn Ther. 2016;13:188–195. doi: 10.1016/j.pdpdt.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Hong JS, Jung JY, Yoon JY, Suh DH. Acne treatment by methyl aminolevulinate photodynamic therapy with red light vs. intense pulsed light. Int J Dermatol. 2013;52:614–619. doi: 10.1111/j.1365-4632.2012.05673.x. [DOI] [PubMed] [Google Scholar]
- 82.Ma Y, Liu Y, Wang Q, Ren J, Xiang L. Prospective study of topical 5-aminolevulinic acid photodynamic therapy for the treatment of severe adolescent acne in Chinese patients. J Dermatol. 2015;42:504–507. doi: 10.1111/1346-8138.12836. [DOI] [PubMed] [Google Scholar]
- 83.Moftah NH, Ibrahim SM, Wahba NH. Intense pulsed light versus photodynamic therapy using liposomal methylene blue gel for the treatment of truncal acne vulgaris: a comparative randomized split body study. Arch Dermatol Res. 2016;308:263–268. doi: 10.1007/s00403-016-1639-6. [DOI] [PubMed] [Google Scholar]
- 84.Pariser DM, Eichenfield LF, Bukhalo M, Waterman G, Jarratt M, Group PDTS. Photodynamic therapy with methyl aminolaevulinate 80 mg g(-1) for severe facial acne vulgaris: a randomized vehicle-controlled study. Br J Dermatol. 2016;174:770–777. doi: 10.1111/bjd.14345. [DOI] [PubMed] [Google Scholar]
- 85.Pinto C, Schafer F, Orellana JJ, Gonzalez S, Hasson A. Efficacy of red light alone and methyl-aminolaevulinate-photodynamic therapy for the treatment of mild and moderate facial acne. Indian J Dermatol Venereol Leprol. 2013;79:77–82. doi: 10.4103/0378-6323.104673. [DOI] [PubMed] [Google Scholar]
- 86.Seo HM, Min HG, Kim HJ, Shin JH, Nam SH, Han KS, et al. Effects of repetitive photodynamic therapy using indocyanine green for acne vulgaris. Int J Dermatol. 2016;55:1157–1163. doi: 10.1111/ijd.13258. [DOI] [PubMed] [Google Scholar]
- 87.Qureshi S, Lin JY. Utilizing non-ablative fractional photothermolysis prior to ALA-photodynamic therapy in the treatment of acne vulgaris: a case series. Lasers Med Sci. 2017;32:729–732. doi: 10.1007/s10103-016-2029-1. [DOI] [PubMed] [Google Scholar]
- 88.Yew YW, Lai YC, Lim YL, Chong WS, Theng C. Photodynamic Therapy With Topical 5% 5-Aminolevulinic Acid for the Treatment of Truncal Acne in Asian Patients. J Drugs Dermatol. 2016;15:727–732. [PubMed] [Google Scholar]
- 89.Tao SQ, Li F, Cao L, Xia RS, Fan H, Fan Y, et al. Low-Dose Topical 5-Aminolevulinic Acid Photodynamic Therapy in the Treatment of Different Severity of Acne Vulgaris. Cell Biochem Biophys. 2015;73:701–706. doi: 10.1007/s12013-015-0627-3. [DOI] [PubMed] [Google Scholar]
- 90.Park KY, Kim JY, Hyun MY, Oh WJ, Jeong SY, Han TY, et al. 1,213 Cases of Treatment of Facial Acne Using Indocyanine Green and Intense Pulsed Light in Asian Skin. Biomed Res Int. 2015;2015:596161. doi: 10.1155/2015/596161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cordey H, Ibbotson S. Allergic contact dermatitis to topical prodrugs used in photodynamic therapy. Photodermatol Photoimmunol Photomed. 2016 doi: 10.1111/phpp.12252. [DOI] [PubMed] [Google Scholar]
- 92.Yuan KH, Gao JH, Huang Z. Adverse effects associated with photodynamic therapy (PDT) of port-wine stain (PWS) birthmarks. Photodiagnosis Photodyn Ther. 2012;9:332–336. doi: 10.1016/j.pdpdt.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Miguelez A, Martin-Santiago A, Bauza A, Gilaberte Y. Urticaria-like reaction secondary to photodynamic therapy in 2 pediatric patients. Actas Dermosifiliogr. 2013;104:727–729. doi: 10.1016/j.adengl.2012.12.010. [DOI] [PubMed] [Google Scholar]