Abstract
Objectives
We sought to evaluate the efficacy, efficiency and physiological consequences of automated, end-point directed resuscitation systems and compare them to formula-based bolus resuscitation.
Design
Experimental human hemorrhage and resuscitation
Setting
Clinical Research Laboratory
Subjects
Healthy volunteers
Interventions
Subjects (n=7) were subjected to hemorrhage and underwent a randomized fluid resuscitation scheme on separate visits 1) formula-based bolus resuscitation (BR) 2) semi-autonomous (decision-assist, DA) fluid administration and 3) fully autonomous (closed-loop, CL) resuscitation. Hemodynamic parameters, volume shifts, fluid balance, and cardiac function were monitored during hemorrhage and resuscitation. Treatment modalities were compared based on resuscitation efficacy and efficiency.
Measurements and Main Results
All approaches achieved target BP by 60 min. Following hemorrhage, the total amount of infused fluid (BR: 30ml/kg, DA: 5.6±3 ml/kg, CL: 4.2±2ml/kg, p<0.001), plasma volume, extravascular volume (BR: 17±4ml/kg, DA: 3±1ml/kg, CL: -0.3±0.3ml/kg, p<0.001), body weight and urinary output remained stable under DA and CL and were significantly increased under bolus resuscitation. Mean arterial pressure initially decreased further under bolus resuscitation (-10mmHg, p<0.001) and was lower under BR than CL at 20min (BR: 57±2mmHg CL: 69±4mmHg, p=0.036). Colloid-osmotic pressure (BR: 19.3±2mmHg DA, CL: 24±0.4mmHg, p<0.05) and hemoglobin concentration were significantly decreased after bolus fluid administration.
Conclusions
We define efficacy of decision-assist and closed-loop resuscitation in human hemorrhage. In comparison to formula-based bolus resuscitation, both semiautonomous and autonomous approaches were more efficient in goal-directed resuscitation of hemorrhage. They provide favorable conditions for the avoidance of over-resuscitation and its adverse clinical sequelae. Decision-assist and closed-loop resuscitation algorithms are promising technological solutions for constrained environments and areas of limited resources.
Keywords: closed-loop resuscitation, decision-assist resuscitation, ATLS resuscitation, human hemorrhage
Introduction
Intravenous fluid therapy remains the cornerstone of treating hypovolemia, but the non-systematic use of endpoints to guide volume resuscitation efforts results in heterogeneous scientific conclusions (1–3). Both hypo and hypervolemia result in damage, dysfunction and failure of tissue and end organs, albeit by different mechanisms (4–7). Precise fluid management is favorable to reduce sequelae of under- and over-resuscitation (6,7). Thus, optimizing resuscitation of hypovolemia secondary to hemorrhage remains a challenge (8).
For hemorrhage, several teaching manuals, including the American College of Surgeons (ACS), recommends a fixed-formula-based regimen that replaces 3 mL crystalloid fluid per 1 mL estimated blood loss (3:1 rule)(9). However, most recently, the ATLS working group takes general steps towards recommending less aggressive resuscitation with lower initial volumes (10). Still, while clinical presentation and vital signs initially influence the assessment of hypovolemic severity and recommended fluid amount, the monitoring of resuscitation success remains open-ended, inconclusively defined and subject to wide interpretation (10–11).
Recent concepts introduced end-point or goal directed volume therapy, in which fluid is titrated proportional to the measured value of a specific physiological variable (6,12). Non-invasive blood pressure (NIBP) is one of the most readily accessible surrogate indicator of intravascular volume and can reliably detect hypovolemia (13). While NIBP has limitations, it remains the primary endpoint chosen by the ACS. The advent of precise, portable and reliable measuring devices also make it a promising variable for endpoint resuscitation (14–15). Computed algorithms that analyze endpoint data and continuously adapt fluid therapy through automated infusion pumps have been tested in various animal experiments and for common resuscitation fluids (17–20).
In the present study, we assess, the efficacy and efficiency of two main concepts: closed-loop (CL) resuscitation, an autonomously operating and recursive circuit of endpoint measurement and therapy adjustment; and Decision-assist (DA) resuscitation, which, based on endpoint measurements, recommends therapy adjustments that have to be confirmed and entered manually. We compared both systems to formula-based fluid resuscitation in humans undergoing hemorrhage.
Methods
This study was approved by the Institutional Review Board (IRB) of the University of Texas Medical Branch at Galveston (IRB# 07-371). Seven healthy volunteers underwent 3 different randomized fluid resuscitation regimens for experimentally induced hemorrhage of 10ml/kg bodyweight separated by at least 4 weeks. The resuscitation regimens were (supplemental Figure 1):
BR; bolus resuscitation: A fixed 30 mL/kg fluid bolus was administered over 20 min based on previous ATLS 3:1 resuscitation guidelines(9).
DA; Semi-automated care: Resuscitation was based on NIBP measurements obtained through a Wireless Vital Signs (WVSM, AthenaGTX, Johnson IA) monitor, routed to a computerized algorithm decision interface, which prompted the user to confirm or override the calculated infusion rate facilitated through a Zoll powerinfusor (Zoll Medical Chelmsford MA).
CL; Autonomous care: Fluid therapy was based on intra-arterial blood pressure measurements obtained through an Impact Lightweight Trauma Monitor (LTM, Impact – Zoll Medical Chelmsford MA) processed through a computerized algorithm interface and the calculated autonomous infusion facilitated through a Zoll Powerinfuser.
The computerized algorithm for DA used a decision table linking deficit in blood pressure to infusion rate; while the CL algorithm used a non-linear equation linking blood pressure to infusion rate. Both algorithms automatically administered or recommended aggressive fluid resuscitation volumes when blood pressure was furthest from target, whereas, when target–pressures were approached, fluid volumes were reduced towards zero to facilitate a “soft landing approach”. The algorithm was developed for tight blood pressure control for head injury – thus a target MAP of 70 mmHg was used; we appreciate that lower MAP endpoints could also be chosen for more hypotensive resuscitation (MAP=50 mmHg).
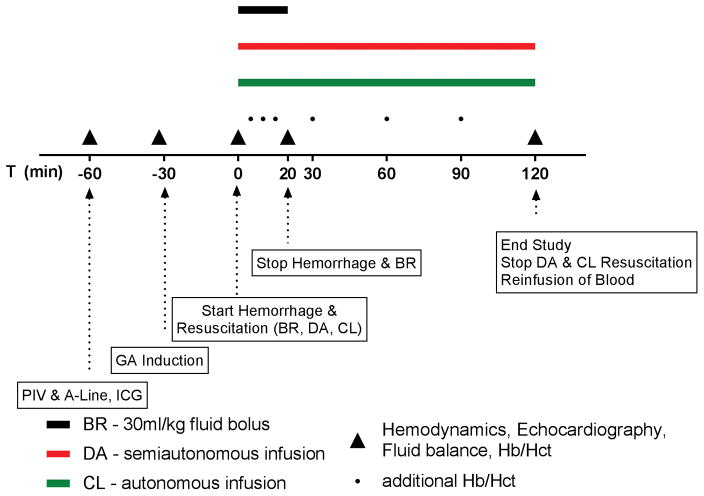
Figure 1 illustrates the experiment. On the day of the experiment, subject weight was recorded. A peripheral IV, arterial line and hemodynamic monitors were placed. Subjects were induced and maintained under general anesthesia (GA, (T-30 = Tminus30)), while spontaneously breathing via a laryngeal mask airway (LMA, size 4 female and size 5 male). After thirty minutes of GA, 10 mL/kg of blood were removed over 20 minutes (T0–T20). After onset of hemorrhage, the respective fluid infusion was initiated. The effects of fluid resuscitation on volume spaces, hemodynamic parameters, urinary output and cardiac function were followed for 120 minutes (T120). At T120, the blood collected during the hemorrhage from the subject was re-infused over 10–20 min. Body weight was recorded and the subject discharged when safety criteria were met.
Figure 1.
Study design. Subjects are treated for induced hemorrhage with 3:1 resuscitation formula (BR), decision-assist infusion (DA) and autonomous closed-loop (CL) infusion at separate time points (min. 4 weeks apart). Endpoint measurements are taken at various time points before and after hemorrhage. A-Line: arterial line. PIV: peripheral intravenous line. ICG: Indocyanide Green. GA: general anesthesia. Hb: Hemoglobin. Hct: Hematocrit.
General Anesthesia
Propofol (2–3 mg/kg) was used to induce general anesthesia. During induction, a patent airway was confirmed and the LMA was inserted into the oral pharynx. General anesthesia was maintained with a continuous infusion of propofol (75–150 μg/kg/min). A bispectral index (BIS) monitor (Covidien, Minneapolis MN) was used to titrate anesthesia depth to a BIS index of 50 throughout the study (21). BIS and propofol infusion rate were recorded every 15 minutes.
Calculation of dilution of fluid spaces and volume changes
Total fluid infused was recorded. Plasma volume (PV) was determined using an indocyanine green (ICG, Akorn Inc., Buffalo Grove, IL, USA) dilution and mass balance approach as previously described (22–24). Hct was corrected for the F-cell ratio (0.91); blood volume (BV) and red cell volume (RBCV) were derived as:
BV and PV at any given time t (BVt and PVt) were calculated by indicator dilution accordingly.
Change in extravascular volume (ΔEVV) was calculated by subtracting changes in plasma volume and cumulative urinary output from volume infused at each time point:
Urinary output
As previously published, urinary output throughout the study was measured by bladder ultrasound (Bladderscan, Diagnostic Ultrasound Corporation, Bothell, WA) (24).
Blood sampling
Baseline hemoglobin (Hb) and hematocrit (Hct) samples were taken during the stabilization period (T-60 to T-30) prior to GA and hemorrhage. Arterial blood samples were measured in duplicate for Hb and Hct at T0, T5, T10, T15, T20, T30, T60, T90 and T120. Samples for plasma protein concentrations (total protein and albumin) to calculate colloid osmotic pressure (24) were taken at similar time points. The total blood for plasma volume determination and for blood samples was 55 mL.
Echocardiography
End-diastolic (EDV) and end-systolic volume (ESV) measurements were obtained from a 3.5 MHz transducer and ultrasound system (Vivid 7 PRO BT04, GE Medical Systems, Milwaukee, WI) in two-chamber, apical parasternal long-axis view at T-30, T0, T20, T60 and T120. The modified Simpson’s rule was applied for calculating EDV, ESV, stroke volume (SV) and ejection fraction (EF%).
Hemodynamic Monitoring
Specific hemodynamic measurements included mean arterial pressure (MAP, measured by arterial catheter), cardiac output (CO, measured by echocardiography), heart rate (measured by ECG) and systemic vascular resistance (calculated from MAP/CO*80).
All data are presented as Mean ± SD unless noted otherwise. Statistical analyses were performed using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla CA). Paired repeated parametric measurements were analyzed with 2-way ANOVA and Tukey’s correction for multiple comparisons. Statistical significance was accepted at p<0.05.
Results
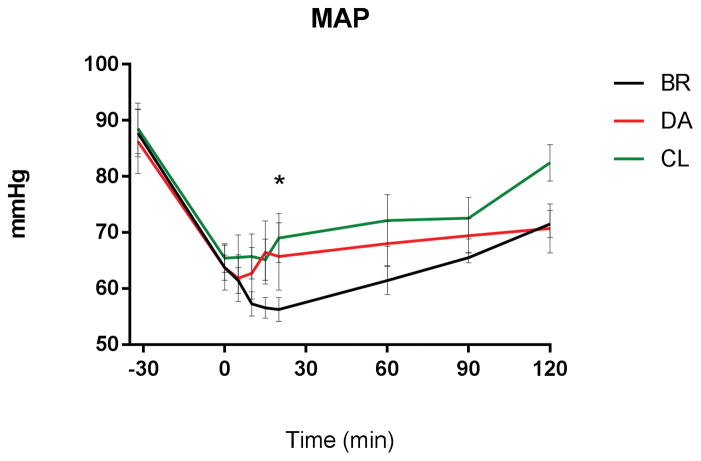
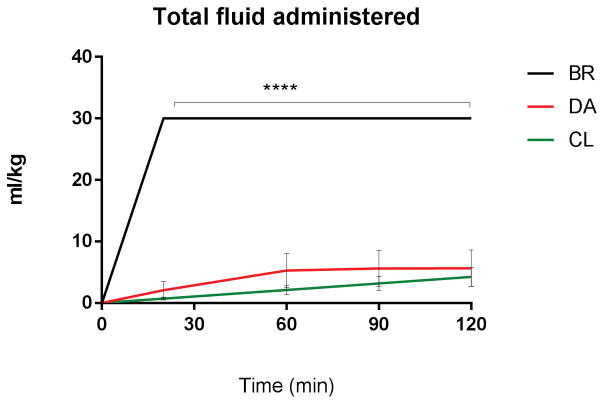
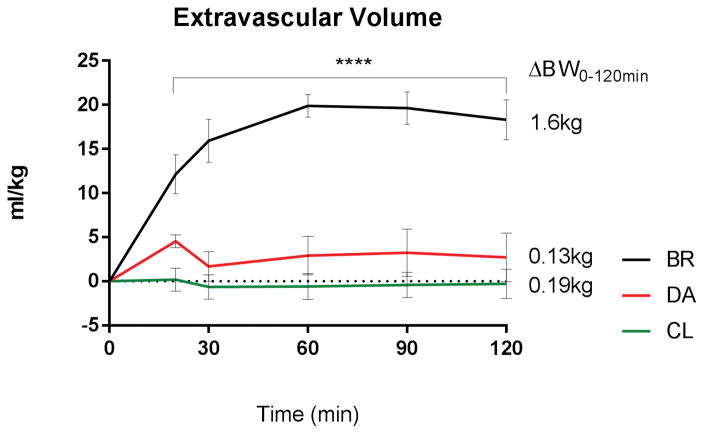
DA and CL infusion reached and maintained target MAP of 70±5 mm Hg after a median 15 and 10 minutes respectively, BR after 60 minutes, without significant differences between the groups through log rank comparison. Area under the curve (AUC) analysis of MAP showed significantly lower AUC for BR versus CL (AUC BR: 2173±144 AUC CL: 3034±198, p<0.01, supplemental Figure 4), representing less time at target blood pressure. During administration of BR bolus fluid, MAP paradoxically decreased significantly from 66±2mmHg (T-15/0; post anesthesia/pre-hemorrhage) to 56±2 mmHg (T15/20; post hemorrhage/complete bolus administration; p < 0.001, Figure 4). MAP did not decrease following initiation of CL or DA resuscitation. At 20 minutes after hemorrhage and resuscitation, MAP was significantly lower in the BR group versus CL fluid group (BR: 57±2mmHg CL: 69±4mmHg, p=0.036, Figure 4). The total amount of infused fluid (Figure 2), plasma volume (supplemental Figure 2) and extravascular volume expansion (Figure 3) and urinary output (supplemental Figure 3) remained steady throughout DA and CL infusion and were significantly increased in the BR group. Hemoglobin concentration was unchanged during DA and CL infusion, but transiently decreased following BR fluid administration, before returning towards normal values at T 30 (supplemental Figure 5). Colloid-osmotic pressure remained unchanged under DA and CL infusion and was significantly and persistently reduced following hemorrhage and resuscitation in the BR group (Figure 5). Systemic vascular resistance and heart rate decreased following the induction of anesthesia and hemorrhage, but showed no significant differences between the groups. Echocardiographic evaluation showed a significant increase in EDV at 20 minutes (end of hemorrhage and BR resuscitation) in the BR group compared to DA and CL (BR: 143±4ml DA: 107±4ml CL: 120±5ml, pBR:DA <0.0001, pBR:CL = 0.0018, supplemental Figure 6), before returning to baseline at 60 minutes. ESV and CO did not differ significantly between the groups. Supplemental Table 1 shows detailed echocardiographic function results.
Figure 4.
MAP during resuscitation: Significant drop of MAP at T20 under BR compared to baseline (T0) and to DA or CL (p<0.05).
Figure 2.
Fluid requirements after hemorrhage. Comparable, stable volumes between DA and CL; increased total volume of fluid administered with BR (p<0.0001).
Figure 3.
Fluid balance: Stable extravascular volume after resuscitation with DA and CL, increased under BR without return to baseline (p<0.0001). Mean difference in body weight (ΔBW) between T0 and T120 increased under BR, no significant changes under DA or CL.
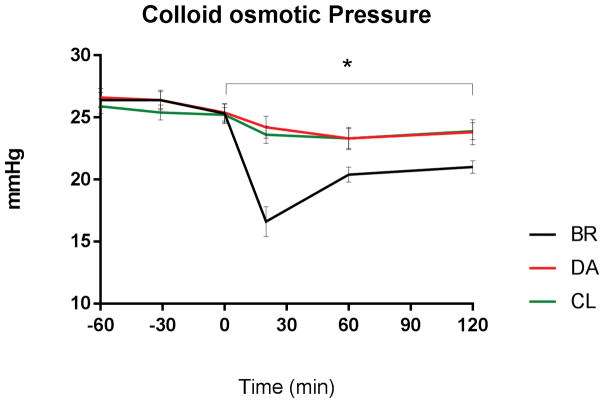
Figure 5.
Dilution effects of bolus resuscitation. Decreased colloid osmotic pressure after BR vs. DA or CL without return to baseline (p<0.05). No differences between DA and CL.
Discussion
Herein we demonstrate for the first time the efficacy and efficiency of semiautonomous (decision-assist) and autonomous (closed-loop) NIBP-controlled resuscitation in a setting of human hemorrhage under general anesthesia.
Effectiveness of resuscitation is expressed by successful maintenance of vital organ perfusion in order to preserve their function. Whether a certain MAP can be restored and maintained following hemorrhage could serve as a surrogate parameter for the success of resuscitation efforts. Our systems of DA and CL infusion achieved normotensive MAP after median 10 and 15 minutes, respectively, and maintained it thereafter. DA and CL systems maintained a normal plasma volume, extravascular volume, urinary output, hemoglobin concentration and colloid-osmotic pressure, suggesting that feedback using MAP as an end-point, adequately replaces the physiologic volume deficit over the course of resuscitation.
Various clinical situations similar to our experimental approach could initially or definitively benefit from this form of end-point directed fluid replacement: elective or emergency surgical procedures under general anesthesia, in which estimated blood loss has been shown to be both poorly reproducible and underestimated (21,22); sustained head injury, in which avoidance of hypotension is one of the most powerful predictors of outcome (23,24); subclinical gastrointestinal bleeding in sedated ICU patients who require tight continuous blood pressure control and resuscitation (25); conservatively treated blunt abdominal trauma (26), or the stationary phase following initial burn shock resuscitation (27). We assert that volume-intensive settings such as acute septic and burn shock resuscitation, penetrating trauma with substantial blood loss, uncontrolled hemorrhage of any kind or other similar events are explicitly not a target for the specific approach we used. Nonetheless, we believe that the efficiency and simplicity of (semi-) automated end-point resuscitation can be advantageous under certain circumstances. Conservation of limited resources is of importance in field care, transportation of critically ill patients, or in an event of mass casualty. Furthermore, autonomous resuscitation systems with built in algorithms provide the ability to export expertise. This could be advantageous in treating large numbers of patients by fewer and less experienced personnel, as required characteristically following natural disasters, terrorist attacks or military emergencies (28–30).
Our experimental model confirms a major shortcoming of formula-based resuscitation, in which adherence to the recommended ratio of lost-to-infused volume inevitably entails over-resuscitation of smaller deficits. We demonstrate that, under formula-based 3:1-resuscitation, total volume administered, plasma volume, extravascular volume and urinary output are significantly increased while parameters indicating hemodilution, such as hemoglobin concentration and colloid-osmotic pressure are reduced throughout the experiment. Temporary cardiac strain, although clinically irrelevant in a population of healthy volunteers, was evident through increased EDV following bolus infusion. Interestingly, the small increase in cardiac output for the BR group was offset by a drop in hemoglobin, which would leave effective oxygen delivery unchanged.
The proven detrimental sequelae of fluid overload such as generalized and pulmonary edema (31,32), exacerbation of congestive heart failure (33), organ perfusion deficits (34), as well as dilution of clotting factors, electrolytes and administered medication (35) lead to increased morbidity and mortality in critically ill patients (36). Therefore, the critical care community has grown increasingly sensitive to the advantages of less aggressive resuscitation approaches as proposed for instance by the Surviving Sepsis Campaign and many others (36–38). In light of these developments, another critical and little described finding of this experiment is the further decrease in blood pressure of 10–15 mmHg that persisted for 20 minutes after and despite administration of a large fluid bolus. We hypothesize this effect could be mediated by capillary shear stress and nitric oxide release (39), a reduction in blood viscosity (40), or the release of atrial natriuric peptide (ANP) following the volume-induced stretching of the atrial wall (41). In a clinical scenario, in which ongoing resuscitation measures are based on blood pressure improvement, this paradoxical effect could induce a feedback loop in which “fluid begets more fluid” and the consequences of over-infusion exacerbate.
There are limitations to this experiment. We acknowledge that few clinicians would actually resuscitate the hemorrhage volume proposed in this experiment with the recommended 3:1 infusion ratio; at the same time, we stress that ATLS continues to assert this rule regardless of hemorrhage extent (9). Further, we wanted to test our algorithms that were designed to treat patients with traumatic brain injury, and thus more aggressive as episodic hypotension worsens outcomes (23,24). Therefore, formula based resuscitation was chosen as a control group for this experiment not primarily to emphasize superiority of (semi)autonomous approaches, but to provide perspective towards its physiological implications.
The use of general anesthesia itself accounted for a blood pressure decrease of 20% in all groups before hemorrhage. Blood pressure in conscious subjects does not decrease significantly enough following similar hemorrhage due to preserved compensatory sympathetic responses (42). Furthermore, anesthesia attenuates the hemodynamic response to blood loss by increasing the unstressed venous volume, thereby reducing venous return (43). The combination of both effects made anesthesia a unique and realistic model to study the physiology of human hemorrhage under these exact conditions. While we did not elect to study a non-resuscitation group in the present study, we previously showed that mild hemorrhage (7 mL/kg) under general anesthesia in a similar model results in the inability to restore blood pressure to pre-hemorrhage levels by 120 min (44). Thus, we show that small amounts of fluid are needed to achieve target MAP following hemorrhage. We cannot, from current data, conclude that the observed further drop in MAP following fluid bolus administration is unrelated to anesthesia-induced dysregulation of endogenous MAP feedback control. We are thus bound to describe the effect which warrants further research.
NIBP is often deemed a non–optimal target for resuscitation and surrogate parameter for fluid load (45). There is, however, ample evidence suggesting that non-invasively measured MAP is a better indicator of invasive blood pressure than non-invasive systolic blood pressure alone (45–47). While to solve this partial discrepancy is beyond the scope of this study, it has been shown recently that the difference of NIBP and invasive arterial blood pressure is clinically negligible in both adults and children with few exceptions (48,49); NIBP continues to be widely used by ICU physicians (50), and NIBP is proportional to intravascular volume during hemorrhage (12). It is furthermore readily and feasibly available to professionals of all levels of experience and causes few complications (51). While blood pressure is a robust and reliable parameter for most cases, there is an age-dependence regarding its readings and circumstances such as severe occlusive arterial disease, missing extremities, or mismatches between cuff and patient size may lead to erroneous readings and infusion, warranting additional caution (52).
The concept of subjecting humans to experimental hemorrhage may seem cavalier, but is well documented in the literature (53,54). The induced hemorrhage was large enough to produce a realistic clinical scenario of mild-to-moderate hemorrhage (up to 1 Liter under anesthesia), yet small enough to ensure subject safety. Non-resuscitative methods of studying volume shifts during hypovolemia in humans involve implementing lower body negative pressure (55), which does not require the physical removal of blood from subjects to simulate hypovolemia. We assert that our model offers more realistic experimental conditions as true fluid shifts occur since red blood cell mass is removed and actual resuscitation with realistic quantities of fluid is performed. Models of extensive hemorrhage in humans remain ethically questionable and difficult to design.
In conclusion, we believe that the gradual replacement of indiscriminate formula-based resuscitation with end-point directed semiautonomous or autonomous infusion systems may be one means of providing standardized and individualized fluid therapy at the same time to patients undergoing hemorrhage.
Supplementary Material
Table 1. Results of echocardiography, systemic resistance and heart rate over the duration of the study. EDV: end-diastolic volume. ESV: end-systolic volume. CO: cardiac output. SVR: systemic vascular resistance. HR: heart rate. All data Mean ± SEM.
Supplemental figure 1: Resuscitation schemes. LR: Lactated Ringer’s electrolyte solution. UTMB: University of Texas Medical Branch.
Supplemental figure 2: increased plasma volume after bolus resuscitation vs. DA or CL (p<0.001). No differences between DA and CL.
Supplemental figure 3: Increase in urinary output after T60 under BR vs. DA and CL (p<0.01). No differences between DA and CL.
Supplemental figure 4: Area under the curve (AUC) of MAP over time. Increased AUC under CL vs. BR resuscitation, no differences between BR and DA or CL and DA.
Supplemental figure 5. Dilution effects of bolus resuscitation. Decrease of Hemoglobin concentration between T10–30 after BR vs. DA or CL (p<0.01). Stable Hb under DA and CL.
Supplemental figure 6. End-diastolic volume (EDV) during hemorrhage and resuscitation. Transient increase of EDV at T20 after BR compared to DA or CL (p<0.01). No differences between DA and CL.
Acknowledgments
Financial Support: Office of Naval Research: Award Number: N00014-08-1-1056 (2012)
This study was conducted with the support of the Institute for Translational Sciences - Clinical Research Center (ITS-CRC) at the University of Texas Medical Branch (UTMB) at Galveston, TX. Supported in part by a Clinical Translational Science Award (# UL1TR000071) from the National Center for Research Resources, National Institutes of Health. The authors also thank volunteers and ITS-CRC staff of the research team for their contribution.
Footnotes
All experiments and work were performed at the University of Texas Medical Branch, Galveston, Texas.
Copyright form disclosure: Dr. Hundeshagen’s institution received funding from the National Institutes of Health (NIH) Clinical Translational Science Award (# UL1TR000071), and he received support for article research from the NIH. Dr. Kramer’s institution received funding from the Office of Naval Research; he received funding from Acros (stock); he received support for article research from the Office of Naval Research; and he disclosed he is the Chief Science Officer for two Startups, Arcos and Resuscitation Solutions, Inc. Mr. Seeton’s institution received funding from the Department of Defense (DoD) - Office of Naval Research. Ms. Henkel’s institution received funding from the DoD - Office of Naval Research. Dr. Kinsky’s institution received funding from the DoD, Office of Naval Research (as PI), and he received support for article research from the DoD, Office of Naval Research. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Velmahos GC, Demetriades D, Shoemaker WC, et al. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Ann Surg. 2000 Sep;232(3):409–18. doi: 10.1097/00000658-200009000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickell WH, Wall MJ, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 3.Dutton RP, Mackenzie CF, Scalea TM, et al. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma Acute Care Surg. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Tisherman SA, Barie P, Bokhari F, et al. Clinical practice guideline: endpoints of resuscitation. J Trauma. 2004;57(4):898–912. doi: 10.1097/01.ta.0000133577.25793.e5. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman BS, Rackow EC, Falk JL, et al. The Relationship Between Oxygen Delivery and Consumption during Fluid Resuscitation of Hypovolemic and Septic Shock. Chest. 1984;85:336–340. doi: 10.1378/chest.85.3.336. [DOI] [PubMed] [Google Scholar]
- 6.Pearse RM, Harrison DA, Mac Donald N, et al. Effect of a Perioperative, Cardiac Output–Guided Hemodynamic Therapy Algorithm on Outcomes Following Major Gastrointestinal Surgery: A Randomized Clinical Trial and Systematic Review. JAMA. 2014;311:2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 7.Boyd JH, Forbes J, Nakada T, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 8.Lira A, Pinsky MR. Choices in fluid type and volume during resuscitation: impact on patient outcomes. Ann Intensive Care. 2014;4:38. doi: 10.1186/s13613-014-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kortbeek JB, Al Turki SA, Ali J, et al. Advanced trauma life support, 8th edition, the evidence for change. J Traum. 2008;64:1638–50. doi: 10.1097/TA.0b013e3181744b03. [DOI] [PubMed] [Google Scholar]
- 10.ATLS, Subcommittee, and International ATLS working group. Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg. 2013;74(5):1363. doi: 10.1097/TA.0b013e31828b82f5. [DOI] [PubMed] [Google Scholar]
- 11.Afessa B, Gajic O, Keegan MT, et al. Impact of introducing multiple evidence-based clinical practice protocols in a medical intensive care unit: a retrospective cohort study. BMC Emerg Med. 2007;7:10. doi: 10.1186/1471-227X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern SA, Dronen SC, Birrer P, et al. Effect of blood pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann Emerg Med. 1993;22:155–163. doi: 10.1016/s0196-0644(05)80195-7. [DOI] [PubMed] [Google Scholar]
- 13.Lakhal K, Ehrmann S, Runge I, et al. Tracking hypotension and dynamic changes in arterial blood pressure with brachial cuff measurements. Anesth Analg. 2009;109:494–501. doi: 10.1213/ane.0b013e3181a8d83a. [DOI] [PubMed] [Google Scholar]
- 14.Michell MW, Rafie AD, Shah A, et al. Hypotensive and normotensive resuscitation of hemorrhagic shock with Hextend or lactated Ringers (LR) Crit Care Med. 2003:A41–A41. [Google Scholar]
- 15.Vaid SU, Shah A, Mitchell AD, et al. Normotensive and hypotensive closed-loop resuscitation using 3. 0% NaCl to treat multiple hemorrhages in sheep. Crit Care Med. 2006;34:1185–1192. doi: 10.1097/01.CCM.0000207341.78696.3A. [DOI] [PubMed] [Google Scholar]
- 16.Vaid SU, Shah A, Mitchell AD, et al. Hemodynamic and metabolic consequences of hypotensive resuscitation using hypertonic 3. 0% NaCl. Shock. 2003;170:19–58. [Google Scholar]
- 17.Chaisson NF, Kramer GC, Deyo DJ, et al. Comparison of different targets for endpoint resuscitation of a severe uncontrolled hemorrhage. Shock. 2001;130:15–44. [Google Scholar]
- 18.Chaisson NF, Kirschner RA, Deyo DJ, et al. Near-infrared spectroscopy-guided closed-loop resuscitation of hemorrhage. J Trauma Acute Care Surg. 2003;54:S183–S192. doi: 10.1097/01.TA.0000064508.11512.28. [DOI] [PubMed] [Google Scholar]
- 19.Salinas J, Drew G, Gallagher J, et al. Closed-loop and decision-assist resuscitation of burn patients. J Trauma Acute Care Surg. 2008;64:S321–S332. doi: 10.1097/TA.0b013e31816bf4f7. [DOI] [PubMed] [Google Scholar]
- 20.Rafie AD, Rath PA, Mitchel MW, et al. Hypotensive resuscitation of multiple hemorrhages using crystalloid and colloids. Shock. 2004;22:262–269. doi: 10.1097/01.shk.0000135255.59817.8c. [DOI] [PubMed] [Google Scholar]
- 21.Smetannikov Y, Hopkins D. Intraoperative bleeding: a mathematical model for minimizing hemoglobin loss. Transfusion. 1996;36:832–835. doi: 10.1046/j.1537-2995.1996.36996420764.x. [DOI] [PubMed] [Google Scholar]
- 22.Brecher ME, Monk T, Goodnough LT. A standardized method for calculating blood loss. Transfusion. 1997;37:1070–1074. doi: 10.1046/j.1537-2995.1997.371098016448.x. [DOI] [PubMed] [Google Scholar]
- 23.Carney NA, Ghajar J. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24:S1–S2. [Google Scholar]
- 24.Søreide E, Deakin CD. Pre-hospital fluid therapy in the critically injured patient—a clinical update. Injury. 2005;36:1001–1010. doi: 10.1016/j.injury.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Zuccaro G. Management of the adult patient with acute lower gastrointestinal bleeding. Am J Gastroenterol. 1998;93(8):1202–1208. doi: 10.1111/j.1572-0241.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 26.McKinley BA, Marvin RG, Cocanour CS, et al. Blunt trauma resuscitation: the old can respond. Arch Surg. 2000;135(6):688–695. doi: 10.1001/archsurg.135.6.688. [DOI] [PubMed] [Google Scholar]
- 27.Pham TN, Leopoldo CC, Gibran NS. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29(1):257–266. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 28.Kano M, Siegel JM, Bourque LB, et al. First-Aid Training and Capabilities of the Lay Public: A Potential Alternative Source of Emergency Medical Assistance Following a Natural Disaster. Disasters. 2005;29:58–74. doi: 10.1111/j.0361-3666.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 29.Quarantelli EL. Disaster crisis management: A summary of research findings. J Manag Stud. 1988;25:373–385. [Google Scholar]
- 30.Bagg MR, Covey DC, Powell ET. Levels of Medical Care in the Global War on Terrorism. J Am Acad Orthop Surg. 2006;14:S7–9. doi: 10.5435/00124635-200600001-00003. [DOI] [PubMed] [Google Scholar]
- 31.Maslove DM, Chen BTM, Wang H, et al. The diagnosis and management of pleural effusions in the ICU. J Intensive Care Med. 2013;28:24–36. doi: 10.1177/0885066611403264. [DOI] [PubMed] [Google Scholar]
- 32.Gaar KA, Taylor AE, Owens LJ, et al. Effect of capillary pressure and plasma protein on development of pulmonary edema. Am J Physiol. 1967;213:79–82. doi: 10.1152/ajplegacy.1967.213.1.79. [DOI] [PubMed] [Google Scholar]
- 33.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, et al. Fluid overload in acute heart failure—Re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10(2):165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Oda J, Yamashita K, Inoue T, et al. Resuscitation fluid volume and abdominal compartment syndrome in patients with major burns. Burns. 2006;32:151–154. doi: 10.1016/j.burns.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Mehrotra R, Gaudio RD, Palazzo M, et al. Antibiotic pharmacokinetic and pharmacodynamic considerations in critical illness. Intensive Care Med. 2004;30:2145–2156. doi: 10.1007/s00134-004-2428-9. [DOI] [PubMed] [Google Scholar]
- 36.Kelm DJ, Perrin JT, Cartin-Ceba R, et al. Fluid Overload in Patients with Severe Sepsis and Septic Shock Treated with Early-Goal Directed Therapy is Associated with Increased Acute Need for Fluid-Related Medical Interventions and Hospital Death. Shock. 2015;43:68–73. doi: 10.1097/SHK.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–80. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]
- 38.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.70. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballermann BJ, Dardik A, Eng E, et al. Shear stress and the endothelium. Kidney Int. 1998;54:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 40.Letcher RL, Chien S, Pickering TG, et al. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects: role of fibrinogen and concentration. Am J Med. 1981;70:1195–1202. doi: 10.1016/0002-9343(81)90827-5. [DOI] [PubMed] [Google Scholar]
- 41.Ballermann BJ, Brenner BM, George E, et al. Brown memorial lecture. Role of atrial peptides in body fluid homeostasis. Circ Res. 1986;58:619–630. doi: 10.1161/01.res.58.5.619. [DOI] [PubMed] [Google Scholar]
- 42.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991;260:H305–318. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 43.Shen T, Baker K. Venous return and clinical hemodynamics: how the body works during acute Hemorrhage. Adv Physiol Educ. 2015;39:267–271. doi: 10.1152/advan.00050.2015. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Heyne CC, Kinsky MP, et al. Effects of Isoflurane on Hemodynamics in Hemorrhaged Awake- and Isoflurane Anesthetized Human. Anesthesiology. 2008;109:A663. [Google Scholar]
- 45.McMahon N, Hogg LA, Corfield AR, et al. Comparison of non-invasive and invasive blood pressure in aeromedical care. Anaesthesia. 2012;67(12):1343–7. doi: 10.1111/j.1365-2044.2012.07302.x. [DOI] [PubMed] [Google Scholar]
- 46.Saugel B, Dueck R, Wagner JY. Measurement of blood pressure. Best Pract Res Clin Anaesthesiol. 2014;28(4):309–22. doi: 10.1016/j.bpa.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Petersen NH, Ortega-Gutierrez S, Reccius A, et al. Comparison of non-invasive and invasive arterial blood pressure measurement for assessment of dynamic cerebral autoregulation. Neurocrit care. 2014 Feb;20(1):60–8. doi: 10.1007/s12028-013-9898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romagnoli S, Ricci Z, Quattrone D, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18:644. doi: 10.1186/s13054-014-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joffe R, Duff J, Garcia Guerra G, et al. The accuracy of blood pressure measured by arterial line and non-invasive cuff in critically ill children. Crit Care. 2016;20:177. doi: 10.1186/s13054-016-1354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatterjee A, DePriest K, Blair R, et al. Results of a survey of blood pressure monitoring by intensivists in critically ill patients: a preliminary study. Crit Care Med. 2010;38:2335–2338. doi: 10.1097/CCM.0b013e3181fa057f. [DOI] [PubMed] [Google Scholar]
- 51.Sakka SG. Blood pressure monitoring in the critically ill patient: A place for noninvasive assessment? Crit Care Med. 2012;40:1366–1367. doi: 10.1097/CCM.0b013e3182413a07. [DOI] [PubMed] [Google Scholar]
- 52.Liu B, Li Q, Qiu P. Comparison between invasive and non-invasive blood pressure in young, middle and old age. Blood press. 2016;25(3):155–61. doi: 10.3109/08037051.2015.1110935. [DOI] [PubMed] [Google Scholar]
- 53.Drobin D, Hahn RG. Volume Kinetics of Ringer’s Solution in Hypovolemic Volunteers. J Am Soc Anesthesiol. 1999;90:81–91. doi: 10.1097/00000542-199901000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Riddez L, Hahn RG, Brismar B, et al. Central and regional hemodynamics during acute hypovolemia and volume substitution in volunteers. Crit Care Med. 1997;25:635–640. doi: 10.1097/00003246-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Convertino VA. Lower body negative pressure as a tool for research in aerospace physiology and military medicine. J Gravitational Physiol. 2001;8:1–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Results of echocardiography, systemic resistance and heart rate over the duration of the study. EDV: end-diastolic volume. ESV: end-systolic volume. CO: cardiac output. SVR: systemic vascular resistance. HR: heart rate. All data Mean ± SEM.
Supplemental figure 1: Resuscitation schemes. LR: Lactated Ringer’s electrolyte solution. UTMB: University of Texas Medical Branch.
Supplemental figure 2: increased plasma volume after bolus resuscitation vs. DA or CL (p<0.001). No differences between DA and CL.
Supplemental figure 3: Increase in urinary output after T60 under BR vs. DA and CL (p<0.01). No differences between DA and CL.
Supplemental figure 4: Area under the curve (AUC) of MAP over time. Increased AUC under CL vs. BR resuscitation, no differences between BR and DA or CL and DA.
Supplemental figure 5. Dilution effects of bolus resuscitation. Decrease of Hemoglobin concentration between T10–30 after BR vs. DA or CL (p<0.01). Stable Hb under DA and CL.
Supplemental figure 6. End-diastolic volume (EDV) during hemorrhage and resuscitation. Transient increase of EDV at T20 after BR compared to DA or CL (p<0.01). No differences between DA and CL.