Abstract
Objectives
Acetate and lactate are important cariogenic acids produced by oral bacteria. They produced different residual dentin structures in artificial lesions of similar depth. We evaluated if such lesions responded in the same way to a polymer-induced-liquid-precursor(PILP) remineralization.
Design
Dentin blocks obtained from human third molars, divided into 6 groups(n=3). Blocks were demineralized with acetate(66 hours) or lactate(168 hours) buffer at pH 5.0 to create 140 μm target lesion depths. A-DEM and L-DEM groups received no remineralization. Other groups were remineralized for 14 days. 100 μg/mL polyaspartate was added into the remineralizing buffer for A-PIL and L-PIL, whereas A-CAP and L-CAP were treated with the same solution but without polyaspartate. Cross-sectioned blocks were examined for shrinkage and AFM-topography. Line profiles of reduced elastic modulus(Er) were obtained by AFM-based nanoindentation across the lesion. Ultrastructures were examined with TEM.
Results
A-PIL and L-PIL recovered in shrinkage to the original height of the dentin and it appeared normal with tubules, with increases in Er at both outer flat and inner sloped zones. At the sloped zone, acetate lesions lost more Er but recovery rate after PILP was not statistically different from lactate lesions. A-CAP and L-CAP showed surface precipitates, significantly less recovery in shrinkage or Er as compared to PILP groups. TEM-ultrastructure of PILP groups showed similar structural and mineral components in the sloped zone for lesions produced by either acid.
Conclusions
The PILP process provided significant recovery of both structure and mechanical properties for artificial lesions produced with acetate or lactate.
Keywords: Dentin caries models, Remineralization, Demineralization, PILP mineralization, Nanomechanical property, Atomic force microscopy
1. Introduction
Oral bacteria break down ingested carbohydrates and produce organic acids that induce demineralization of tooth tissues and lead to dental caries. Two of the most important of these acids are acetic and lactic acids (Distler & Kroncke, 1986). Standardized artificial caries lesions offer many advantages over efforts that utilize natural lesions because demineralization protocols can be reproducible and lesions of different size can be readily made based on demineralization kinetics of a given acid. Natural coronal dentin lesions have been characterized using nanoindentation, and have an outer relatively flat zone of low modulus in the most active lesions and an inner gradient zone as the modulus values increase with depth until normal dentin values are reached (Zheng, Hilton, Habelitz, Marshall, & Marshall, 2003). Mclntyre used both acetate and lactate to create artificial root dentin caries with similar zones and micro-hardness values to natural lesions (McIntyre, Featherstone, & Fu, 2000). The 2 acids used in McIntyre’s study both demineralized dentin by diffusion-dominated mechanisms. In previous work we measured the kinetics of demineralization of coronal dentin using acetate and lactate buffers at pH = 5 and found that dentin demineralization is a diffusion-dominated process for both acids, but proceeded much more slowly using lactate as compared to acetate and the mineral content, mechanical property profiles and ultrastructures were significantly different in lesions of the same depth (Chien et al., 2016). These results suggest that lesions produced using the different acids may respond differently to remineralization treatments.
Remineralization of dentin lesions and demineralized dentin matrix using calcium and phosphate containing solutions has proven to be significantly more difficult than enamel lesions. Several related processes have been introduced that have the capacity to remineralize collagen fibrils and bone and dentin matrices (Chen et al., 2015; L. B. Gower, 2008; Olszta, Douglas, & Gower, 2003; Tay & Pashley, 2008). The capacity to provide intrafibrillar mineralization of the collagen has been suggested to be critical to restoration of the mechanical properties of dentin (Bertassoni, Habelitz, Kinney, Marshall, & Marshall, 2009; Kinney, Habelitz, Marshall, & Marshall, 2003; Kinney et al., 2001). The system developed by Gower et al. (L. Gower & Odom, 2000; L. B. Gower, 2008; Olszta et al., 2003) has been shown to be particularly effective at remineralization of collagen matrices (Jee, Thula, & Gower, 2010), so we have applied this polymer-induced liquid precursor (PILP) mineralization process to artificial caries lesions in coronal dentin prepared from acetate buffer at pH = 5, and have shown that it provided complete recovery of mineral content throughout the lesion depth (as seen in micro-CT), while nanomechanical testing found the reduced elastic modulus (Er) recovered to 50–60% of normal dentin in the severely demineralized outer zone and fully recovery of the inner zone (Burwell et al., 2012).
However, the differences in residual dentin structure, such as more residual minerals left in acetate lesions, while lactate dissolved minerals much more slowly and along collagen fibers after their independent demineralizations (Chien et al., 2016), suggest that there may also be important differences in remineralization behavior, and therefore we undertook a comparative evaluation using lesions of similar depth produced from lactate and acetate buffers at pH = 5.
The hypothesis tested in this study was that artificial lesions created by lactate respond similarly to PILP remineralization treatment as acetate lesions of the same depth.
2. Materials and Methods
2.1. Human teeth
Fresh human 3rd molars were obtained from subjects who required extraction according to protocols approved by the UCSF Committee on Human Research. After extraction the teeth were sterilized by gamma radiation for 24 hours (Brauer, Saeki, Hilton, Marshall, & Marshall, 2008; White, Goodis, Marshall, & Marshall, 1994) then stored in DI water at 4°C until used.
2.2. Artificial caries lesions
2.2.1. Demineralization
Dentin blocks measuring 6 (length) × 3(width) × 2.5(height) (mm) were prepared from the coronal regions of the teeth. The occlusal surface of each dentin block was cut and ground so that the surface was just below the dentin enamel junction (DEJ) to simulate progression of the natural caries in coronal dentin. The occlusal surface was polished with SiC abrasive papers and a series of aqueous diamond solutions on polishing cloths to 0.25 μm (Buehler, Lake Bluff, IL). Two coats of nail varnish (Revlon #270, New York, NY) were painted on the polished surface except for a window 3 × 3 mm that was left to create an artificial lesion upon acid exposure. The nail varnish protected area served as a reference after the treatments rendered (as shown in Fig.1). Demineralization buffers containing 2.2 mM calcium phosphate and either 0.05 M acetic acid or lactic acid, pH adjusted to 5.0, were used to create artificial caries lesions similar to those reported previously (McIntyre et al., 2000). Each nail varnish coated dentin block was placed in a 50 ml tube with 40 ml of the selected buffer. Demineralization time was calculated from the kinetics curve obtained from a previous study (Chien et al., 2016), 66 hours for acetate buffer, 168 hours for lactate buffer to create lesions with a target depth of approximately 140 μm. After demineralization, samples were harvested, rinsed thoroughly with DI water and stored in 100% humidity to maintain hydration until studied.
Fig.1.
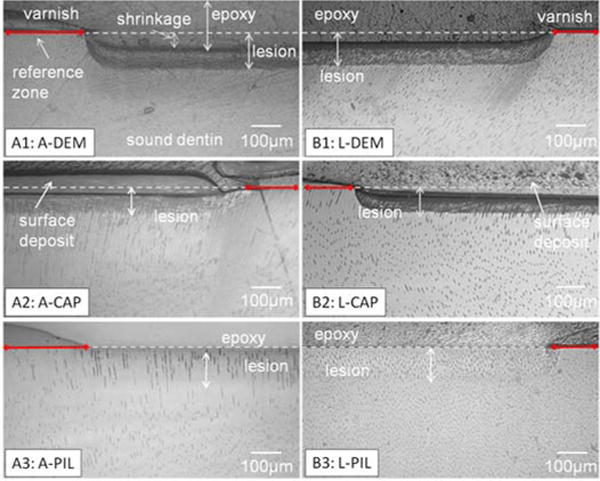
Reflective light microscopy images of cross sections of artificial lesions before and after remineralization. Lactate took longer than acetate to create similar lesion depth. Collapse of severely demineralized substance was shown as a shrinkage when the specimens were embedded in dehydrating conditions (A1, B1). Artificial lesions created by both acetate and lactate showed full recovery of shrinkage after PILP remineralization for 14 days(A3,B3) whereas without PILP, there was less recovery (A2, B2).
2.2.2. Remineralization
Demineralized dentin blocks were randomly assigned to 6 groups (n=3/group) as shown in Table 1. For group 3~6, each block was placed in 40 ml of remineralizing buffer containing Tris, 4.5 mM calcium and 2.1 mM phosphate, at pH 7.4 for 14 days in an incubator at 37°C, with agitation provided by a rocker platform for remineralizing treatment. 100 μg/ ml of Poly-L-aspartic acid (PASP) sodium salt, with molecular weight of 27 kDa (Alamanda polymers, Huntsville, AL) was added to A-PIL and L-PIL groups (Burwell et al., 2012; Jee, Thula, et al., 2010). Negative control groups (A-CAP and L-CAP) were treated in the remineralizing buffer prepared in the same way except without PASP. The “no” treatment groups (A-DEM and L-DEM) did not receive any remineralizing treatment and served to show the characteristics of demineralized lesions.
Table 1.
Treatments rendered to each group (n=3).
| Steps | Buffer | Group 1 A-DEM |
Group 2 L-DEM |
Group 3 A-CAP |
Group 4 L-CAP |
Group 5 A-PIL |
Group 6 L-PIL |
|---|---|---|---|---|---|---|---|
| Demineralizati on ~140μm lesion | 2.2mM CaHPO4, (pH 5.0) | Acetate | Lactate | Acetate | Lactate | Acetate | Lactate |
|
| |||||||
| Remineralizati on 14 days | Tris/Tris-HCl (pH 7.4) | – | – | Ca: 4.5mM P: 2.1mM |
Ca: 4.5mM P: 2.1mM |
Ca: 4.5mM P: 2.1mM PASP: 100μg/m L |
Ca: 4.5mM P: 2.1mM PASP: 100μg/m L |
2.3. AFM and Mechanical property
2.3.1. Embedding and cross sectioning
The degree of remineralization can be assessed using different methods such as by recovery of mechanical properties (hardness, modulus), mineral content evaluated by x-ray attenuation, and changes in microstructure, often using TEM. Remineralization solutions are supersaturated and if lesions are shallow, surface precipitation may alter properties and mineral content to give the misleading suggestion that remineralization has occurred. Such spontaneous precipitation of calcium-phosphate tends to occur, which can increase mineral density at near surface depths of 10 to 20 μm. However, portions of deeper lesions may not be remineralized. Thus it is important to use deeper lesion depths. In addition, evaluating only from the surface can mask the challenge of delivering the mineral into the main body of the lesions.
Evaluating the remineralization treatment efficacy according to an increase of mineral content alone may not allow differentiation of extra- and intra-fibrillar mineral deposits because mechanical properties of the dentin are sensitive to the restoration of intrafibrillar mineral of collagen (Bertassoni et al., 2009; Kinney et al., 2003). As noted above, the lesion depths evaluated by mineral density and mechanical properties do not always match (Burwell et al., 2012; Chien et al., 2016). Thus we take the approach of doing a series of nanoindentations along the cross sectioned lesions which cover all the regions, starting with the embedding material, fully demineralized outer zone, to the transition zone and finishing on the non-affected dentin, and while in hydrated conditions. This is in addition to the structure and mineral density analysis to fully understand the reconstruction of dentin.
Excess moisture was removed by blotting the dentin blocks containing the treated lesions prior to embedding them in room temperature curing epoxy (Epoxycure, Buehler, Lake Bluff, IL). The embedded blocks were cut with a slow speed saw under water (Buehler, Lake Bluff, IL), perpendicular to the treated occlusal surface to reveal the lesion profile. A thin slice obtained from the center of the specimen (~1200 μm) was glued onto the AFM specimen discs (Ted Pella, Redding, CA) with a small amount of cyanoacrylate (QX-4, MDS Products, Laguna Hills, CA), then polished to 0.25 μm with diamond as described in the dentin block preparation section above.
2.3.2. AFM based nanoindentation
An atomic force microscope (Nanoscope IIIA, Digital Instrument, Santa Barbara, CA) equipped with a Triboscope load-displacement transducer (Hysitron, Minneapolis, MN) and nanopositioner (nPoint, Middleton, WI) was used for nanomechanical property testing. A diamond Berkovich tip (fluid cell, 100 nm tip radius) was used and the system was calibrated with a fused silica standard in wet and dry conditions. Site specific reduced elastic modulus (Er) was obtained with loads of 200 μN to 500 μN with a 3 second trapezoidal loading curve as described previously (Burwell et al., 2012). Each indentation yielded a load-displacement curve, from which Er was determined as previously described (Balooch et al., 1998; Oliver & Pharr, 1992). An AFM topographic image was acquired with the indentation tip along the nanoindentation measurement to ensure the indentation was made into intertubular dentin). Er values that were more than 24 GPa were assumed to be from subsurface peritubular dentin and excluded from further analysis. Following the indentations, cross sectioned specimens were examined under reflective light microscopy with x10 magnification to measure the shrinkage from the embedding procedure and accounted for in the distance from the reference layer position as described elsewhere (Burwell et al., 2012).
2.4. Transmission electron microscope (TEM) for ultrastructure of lesions
Selected samples from the artificial lesions before and after remineralization treatment from the two different acid groups were evaluated by transmission electron microscopy (TEM). 70 nm thick sections were used to determine the ultrastructure of remineralized collagen and minerals. Slices from artificial lesions from the different acids were embedded in Spurr’s resin (Ted Pella, Redding, CA) after ethyl alcohol and then acetone dehydration. Selected regions were trimmed, and ultrathin sections (~70 nm) were cut in the occlusal or sagittal plane of the tissue with a diamond knife on an ultramicrotome (Reichert-Jung Ultracut E, Leica, Wetzlar, Germany). Tissue sections were placed on Formvar™ copper grids and examined in a JEOL JEM 1400 TEM (JEOL Ltd, Tokyo, Japan) at an accelerating voltage of 120 kV. Images were recorded using a CCD camera (Gatan Inc., Pleasanton, CA).
2.5. Statistics
Er values more than 24 GPa may be related to the peritubular dentin and were excluded in the further analysis. Two-way ANOVA was used to evaluate 2 × 2 interactions between acids (acetate/lactate) and treatments (CaP or PILP) on Er AUC recovery analysis. One-way ANOVA were used after confirming that there were no interactions. Tukey-Kramer HSD was used to account for multiple comparisons.
3. Results
3.1. Shrinkage and microstructure
Shrinkage is related to the collapse of the demineralized dentin and recession of demineralized dentin upon dehydration (Marshall, Marshall, Kinney, & Balooch, 1997; Marshall et al., 1998; Saeki, Marshall, Gansky, & Marshall, 2001). When the mineral is removed from the dentin it is replaced by water, and therefore the dentin shrinks upon dehydration. Embedding specimens in epoxy was necessary to preserve the delicate demineralized structure at the topmost surface during sectioning and polishing, and dehydration was unavoidable during this process. Light microscopy images that show reduced shrinkage serve as a quick indicator of structural recovery after treatments (Burwell et al., 2012). Micrographs with light microscopy, Fig.1 A1: A-DEM and B1: L-DEM show the typical lesion depth created by acetate and lactate respectively. After 14 days of the PILP-remineralizing treatment, shrinkage decreased for both acidic treatments (A3: A-PIL and B3: L-PIL). Groups that received remineralization with Ca-P solution showed surface precipitates and less recovery in shrinkage (A2: A-CAP, B2: L-CAP). PILP-Remineralization also recovered some of the original structure such as dental tubules and some of peritubular dentin in sloped zone for both acetate and lactate lesions, although some of the tubules seemed to remain widened or filled (Fig.2). The appearance of enlarged tubules is related to the lack of peritubular dentin. In A-PIL, the area most severely exposed to acid (closer to the surface, ~20 μm) had less distinct peritubular dentin structure compared to the deeper part of the lesion. Due to the orientation of cross-sectioning, it was difficult to see peritubular dentin in L-PIL.
Fig.2.
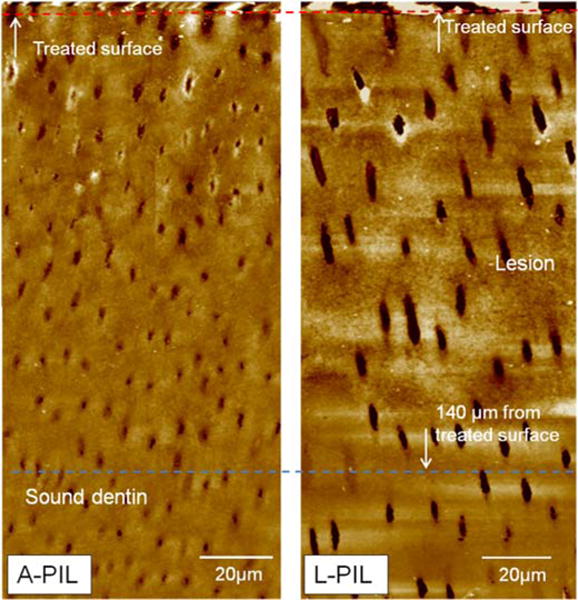
AFM images of artificial dentin lesions created by acetate or lactate after PILP-remineralization for 14 days. Structures of both lesions were recovered, including some peritubular dentin. Dentin tubules in L-PIL look larger due to the tubule orientation in the cross section.
3.2. TEM ultrastructure observation
TEM results are shown in Figures 3 and 4. The outer zone of lactate seemed to have mineralized more after PILP remineralization than for the acetate lesions. L-PIL showed interesting over growth of mineral at the treated surface and tubule’s wall. Near the margins of tubules, long thin crystals have grown out of the dentin matrix without confinement of collagen. Crystallites grown within dentin matrix are hydroxyapatite as shown by electron diffraction (data not shown), as shown in Burwell et al., 2012 (Fig.3). Inner sloped zone after remineralization showed very similar ultrastructure (Fig.4) for both remineralized acid groups.
Fig. 3.
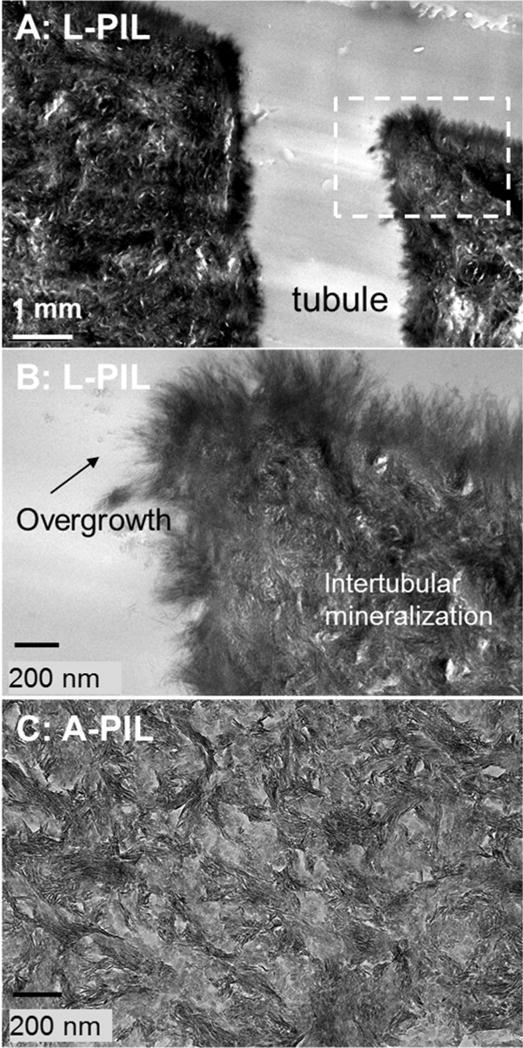
Outer zones (flat region in the Er profiles) of lactate and acetate lesions after PILP remineralization process. Ultrastructure under TEM shows the lactate lesion was more mineralized.
Fig.4.
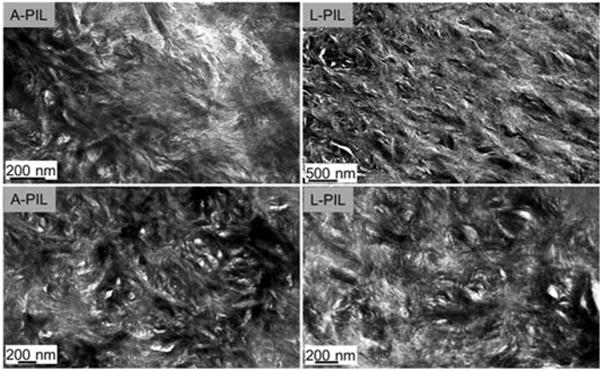
Graded zones (slope region in the Er profiles) of lactate and acetate lesions after PILP remineralization process, resulting in a very similar ultrastructure as seen by TEM.’
3.3. Nanomechanical property profiles
Nanomechanical property profiles through the lesion depths created by either acetic or lactic acid and their recovery after the remineralization treatment are shown in Fig.5 A. The 140 μm target lesion depth was determined from kinetic curves for both acids (Chien et al., 2016). Depth measurements based on polarized light microscopy (PLM), and mineralization profiles agree, whereas mechanical property measurements generally show somewhat deeper demineralization as was seen in our prior studies (Burwell et al., 2012; Chien et al., 2016). Lactate showed less shrinkage, followed by ~60 to 70 μm wide flat zone and relatively steep sloped zone with increased depth until Er reached the range of sound dentin. Acetate lesions had more shrinkage, a flat zone width of 50–60 μm, and a broader sloped zone that had a shallower slope than the lactate lesions, similar traits to those of the previous study with 180 μm target depth (Chien et al., 2016). Remineralization treatment without PILP showed some recovery in the sloped zone, but less recovery in the flat zone in Er for both acids (Fig.5 B1,B2). The treated surfaces of these dentin blocks were covered with precipitated material (n=3/3 for both acetate and lactate). Mechanical properties of dentin adjacent to the precipitation material can be increased (width ranged 0 to 40 μm), which was evident as increased mechanical properties with large S.D. that was observed at about 20 μm near the treated surface in Fig.5 B1.
Fig.5.
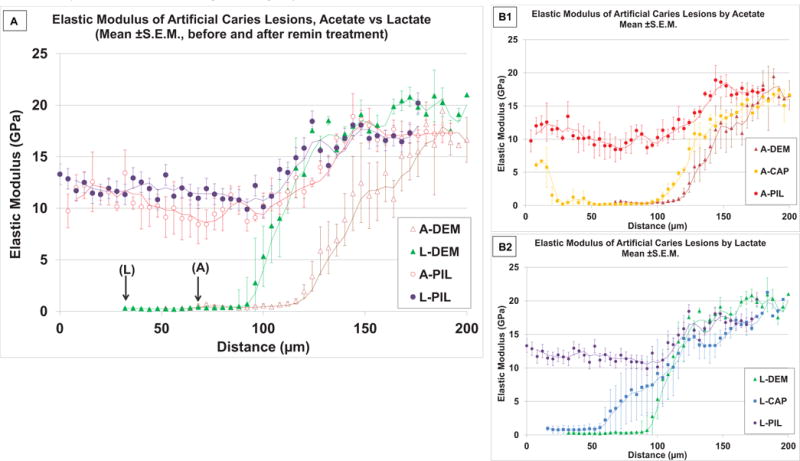
Nanoindentation showing reduced elastic modulus (Er, n = 3) of artificial lesions from cross-sectioned specimens before and after PILP remineralization treatments. A) Comparison of Er along the lesion depth for two acids, (Acetate vs. Lactate), with arrows showing the actual measurement onset point due to shrinkage for each acid. Both acids had outer surface flat zone and inner sloped zone from the region of partial demineralization. After PILP remineralization, both lesions showed increases in Er in similar profiles. B1 and B2 show effects of PILP remineralization compared to the treatment without PASP in Er for each acid. Both acid lesions showed improved recovery at the outer flat zone when PASP was added to the remineralization solution. (Trendlines : moving averages).
3.4. Recovery of reduced elastic modulus
To compare the extent of remineralization after the different treatments, we determined the area from the Er vs. distance curves from Fig.5. The lost mechanical properties were determined as the area between the demineralization curve and sound dentin value for each tooth to standardize the differences between the teeth used (Fig.6 A). After remineralization the area between the demineralization curve and the remineralization curve were used to quantify the amount of recovery of mechanical properties (Fig.6 B). Thus Recovery (%) was defined as
Fig.6.
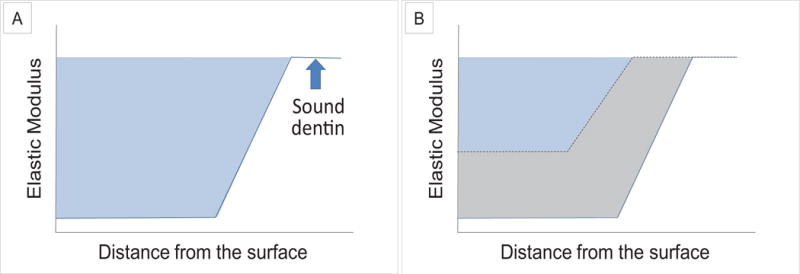
A measure of the lost mechanical properties from the reduced elastic modulus (Er) vs. distance curves after demineralization with an upper boundary provided by the value of sound dentin (light blue region; A). This represented the Recovery Goal that was sought for remineralization. The Recovery Gained was defined as the area between the demineralization curve (blue line) and the Er curve (black dotted line) after remineralization (gray region; B). Recovery (%) was ratio of the Gain/Goal × 100.
Fig.7 shows the recovery after 14 days remineralization. In considering the different change point between the two acids, area values were segmented into outer and inner zones at 120 μm for acetate and at 90 μm for lactate, respectively. There were no statistical interactions between treatments (CaP or PILP) and acids (acetate or lactate) for either segment or in total recovery. For both acids, the outer surface flat zone recovered about 55% without significant difference between acids (p=0.40), but there was significant difference between treatments (p< 0.001). At the inner sloped zone, there was a difference in recovery between acids (p=0.008). A-PIL and L-PIL recovered Er of sloped zone with no significant difference (p=0.100), while L-DEM sloped zone retained more Er than A-DEM after demineralization, due to a steeper sloped zone, as shown in Fig.5A. There was no significant difference between CaP and PILP groups for lactate lesions (p=0.786) or acetate lesions (p=0.353), L-CAP had less recovery than A-PIL (p=0.014) in the sloped zone. In total, there were significant differences between treatments (p < 0.001). Both lesions were remineralized and regained AUC values without significant difference (p = 0.459) with PILP (acetate 59.7 ± 6.0, lactate 48.2 ± 9.2%). There was no difference (p = 0.981) without PILP in recovery (acetate 13.5±3.0, lactate 10.5 ± 24.2%).
Fig.7.
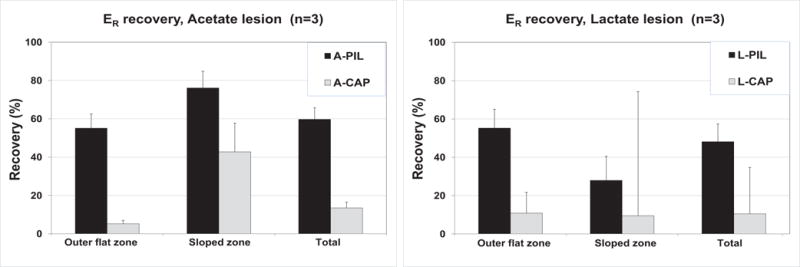
Recovery of each of the zones are shown for each acid. There was no difference between the two acids in PILP recovery at the outer flat zone, for both acids recovered in the same way up to 55 %. This is in stark contrast to the CaP treated sample (P<0.001), which only recovered to 5 % (acetate) or 11% (lactate). Inner sloped zone showed significant difference in acids(p=0.008),but not by treatments. In total (0 – 180 μm), mechanical property loss was larger for acetate, but there was no significant difference in recovery by acids. Both lesions recovered better when PASP was added (P<0.001).
4. Discussion
The goal of this research is recreating the original biological structure and function of the dentin matrix through reintroduction of apatite mineral into artificial lesions demineralized with cariogenic acids with clinically meaningful lesion depths. The question to be answered in this paper is if the same remineralization approach is applicable to lesions created by different cariogenic acids, which possibly possess different demineralization process and residual characteristics as described previously (Chien et al., 2016). We have characterized our artificial dentin lesions and shown that they have outer severely demineralized zones and inner zones with different mineral densities and mechanical properties. The outer lesion lost most of the inter-and intrafibrillar minerals (Chien et al., 2016), resembling the characteristics of the most active natural lesions and inner sloped zones that resemble less active lesions (Pugach et al., 2009; Zheng et al., 2003).
We did not change the acid buffer during the demineralization process, but Qi et al. showed the mineral density of 300 μm depth artificial lesions created with pH cycling of acetate had a similar 2 zone profile with a gently sloped external portion and a more steep internal portion (Qi et al., 2012). Both acid lesions responded to PILP-remineralization treatments and recovered substantially in their elastic modulus when measured by nanoindentation. Both of them recovered 55 % in the outer zone while the inner sloped zone showed 76 % recovery in acetate and 28 % in lactate, the difference at inner slope zone is related to the steeper slope in lactate lesions (Fig. 5 and 7). The incomplete recovery in Er may mean the incorporation of mineral into intrafibrillar space of collagen was not fully achieved in the outer zone. The difference between before and after PILP treatment is smaller in lactate because of its narrower sloped zone (Fig.5 B2).
The outcomes of PILP remineralization reports have varied by the evaluation methods used, the dimension and the type of collagen scaffolds (Jee, Thula, et al., 2010; Olszta et al., 2007 ; Qi et al., 2012; Thula, Rodriguez, et al., 2011; Thula, Svedlund, et al., 2011). The methods used for evaluating mineral density such as EDS and XCT do not always match mechanical property observations in our dentin cross-sectioned study (Burwell et al., 2012). When cross-sections were examined in their reports, specimens from the same biological origin with similar thickness revealed that inner regions were not remineralized or were unclear on defining the extent of remineralization (Jee, Culver, Li, Douglas, & Gower, 2010; Thula, Rodriguez, et al., 2011; Thula, Svedlund, et al., 2011).
The type of scaffolds seemed to have greater impact than the dimension to the outcome. Jee et al. (Jee, Culver, et al., 2010) have shown penetration of the liquid precursor formed by adding PASP infiltrates at up to 500 μm into turkey tendon, and hypothesized that PILP reaches to the bottom of the lesion based on capillary action, not simply by diffusion. It was proposed that the fluidic character of the precursor phase enables it to be drawn into the spaces in collagen fibrils that are swollen with imbibed water (Jee, Culver, et al., 2010; Olszta et al., 2007). Thula et al. reported that 3 mm thickness type I collagen sponge could be remineralized after 6 days whereas solidification occurred and blocked further remineralization of demineralized bone (200~500 μm in thickness) and only surface 100μm was remineralized. From these findings they suggested that if dense matrix materials such as rat tail or turkey tendons or bone were used as scaffolds, the scaffold responds less to PILP - remineralization than spongy materials because the diffusion of PILP droplets in solution cannot reach the interior as readily as they can in a porous sponge matrix (Thula, Svedlund, et al., 2011). However, when collagen films made by condensing reconstituted collagen gels were used, they showed compressing and cross-linking the gel resulted in increased water permeability and higher mechanical properties after PILP remineralization (Li et al., 2012). So density and cross-linking may actually aid remineralization, and artificial matrices, such as collagen sponges, have less limitation of PILP penetration compared to the scaffolds which maintained original biological structure.
Although many of these studies used thicker scaffolds than the dentin specimens used in this research, we observed inner sloped zone’s recovery with both acid lesions; thus PILP seemed to reach to the bottom of the 140 μm lesion depth. In our dentin lesions, the inner zone, which is denser and presumably has more intact cross-linked collagen, responded better to PILP-remineralization, which is consistent with Li et al.’s report, but the outer flat zones of the lesions were less remineralized than the inner sloped zones, which differs from other matrices that had poorly remineralized depths (Jee, Culver, et al., 2010; Olszta et al., 2007 ; Thula, Rodriguez, et al., 2011). This probably is because collagen matrices studied were naturally unmineralized or contained less residual mineral, unlike the lesions evaluated here which are comprised of residual intrafibrillar and extrafibrillar apatite crystals as previously shown (Burwell et al., 2012). Instead of acids, the prior studies used EDTA to demineralize bone (Thula, Rodriguez, et al., 2011; Thula, Svedlund, et al., 2011), EDTA can remove minerals from dentin but leave the collagen intact when it is used for short periods (Sauro et al., 2010). It can also alter the collagen depending on the pH and length of time it is used (Serper & Calt, 2002). Since bone and dentin are similar (Goldberg, Kulkarni, Young, & Boskey, 2011; Veis, 1993), it may be possible that EDTA demineralized bone may have non-homogeneous characteristics similar to our demineralized dentin with two zones, where the outer zone surface mineralization seemed to occur, suggesting that solidification of the precursor may have blocked further remineralization.
Artificial lesions created by acetate or lactate showed different kinetics and different lesion profiles in structure and mechanical properties. To create the same depth lesion, lactate required a much longer acid exposure, but the lesions ended abruptly with narrower sloped zones. As shown previously, at the bottom of the lesions, acetate led to more dissolved peritubular dentin and the lactate stripped more minerals, and had more exposed collagen fibers than acetate (Chien et al., 2016). Hence, in recovery, PILP remineralization allowed repair of these different features of demineralization to provide a similar level of mechanical property and structure in the intertubular matrix (Fig.4 and 5) at the sloped zone after the 14 day remineralization treatment. Penetration depths of PILP into lesions produced by the different acids were basically the same for dentin lesions. This is of importance since natural lesions undergo changes in which the predominant bacteria may be altered over time. Thus the observation that both lactate and acetate based lesions can be remineralized suggests that the PILP approach may be efficacious in the functional remineralization of lesions with varying bacterial profiles. Future work should include evaluation of mixed acid demineralization or sequential acid demineralization prior to remineralization.
This raises the question of why the outer flat zone showed only partial recovery in Er. Demineralization of dentin occurs in 2 steps. If the dentin is exposed to acid, external minerals between collagen fibrils are removed first. If the exposure to the acid continues, intra-fibrillar minerals are removed (Balooch, Habelitz, Kinney, Marshall, & Marshall, 2008). When recovering, it occurs in reverse order: fibrils are first infiltrated with amorphous precursor in the PILP process that transforms to apatite (Jee, Thula, et al., 2010). If appropriate conditions are used, then extrafibrillar (interfibrillar) mineralization between the fibrils occurs after a certain degree of intrafibrillar mineralization has been achieved (Thula, Rodriguez, et al., 2011). However, if intrafibrillar inducing proteins or agents (such as PILP) are unavailable, then extrafibrillar precipitation can block intrafibrillar mineralization leading to poor mechanical properties (Kinney et al., 2003). In addition to the penetration depth, understanding the difference between outer and inner zones may lead to understanding the key factors of successful mineralization. Our artificial lesions with two zones mimicked natural dentin lesions which are non-homogeneous and unique (Pugach et al., 2009), compared to homogeneous scaffolds such as reconstituted collagen sponges or tendons. Intrafibrillar mineral is critical for maintaining mechanical properties (Kinney et al., 2003). The outer flat zone showed incomplete recovery in Er while mineral content was fully recovered (Burwell et al., 2012), which suggested partial recovery of intrafibrillar mineral at the outer zone or excess extrafibrillar mineral. Surface precipitation may impede further remineralization of the outer severely remineralized zone, or some of the collagen in that zone might have been degraded and can no longer undergo intrafibrillar mineralization. Finally it is possible that the amounts of intra-versus extrafibrillar mineral may not be balanced to achieve native dentin properties.
The severely demineralized outer zone had significant shrinkage when not mineralized, but the volume and structure, including some peritubular dentin, could be recovered after PILP remineralization (Fig.1 and Fig.2). Because the collagen network was still retained after the demineralization, the remineralization process rebuilt the intertubular dentin to restore much of the structure and mechanical properties. Tubule widening is related to the demineralization of peritubular dentin, which does not contain collagen (Goldberg et al., 2011), thus the region close to the exposed surface did not rebuild the peritubular dentin, leaving widened tubules. As remineralization proceeds a superficial precipitation may occur that occludes the tubules near the exposed surface. Peritubular dentin is also different from intertubular dentin in composition of NCPs (Goldberg et al., 2011; Habelitz et al., 2007). The external portion of the outer zone has been severely demineralized and lesions with such significant demineralization may not be completely remineralized or may need other analogs to mimic the missing NCPs for full remineralization. However, such severe demineralization may not be characteristic of the bulk of carious dentin lesions, so that effective remineralization might be achievable in many clinical lesions if a suitable delivery method can be devised. When we included the CaP-only treatment (Figs.1, 5 and 6), functional recovery was not likely to occur without the addition of PASP unless the lesion consisted only of the sloped zone; such lesions would be represented mainly by early limited lesions. Thus polyaspartate appears to be behaving as a biomimic for some of the proteins needed for remineralization of dentin. It should be noted that the direction of tubules may influence the access of the mineral precursor droplets to all regions of the dentin, and the recovery of peritubular dentin may be related to the different mineral preferences that were also observed around osteons of bone (Thula, Rodriguez, et al., 2011). Because the demineralization of dentin by acetate and lactate occurred under diffusion control (Chien et al., 2016), the region closer to the surface should have been exposed longer and affected more by the acids, resulting in a harder to remineralize scaffold. When dentin was treated with non-buffered, 0.1M acetate (pH4 .5) at 4°C, no collagen degradation was detected (Van Strijp, Klont, & Ten Cate, 1992), but Pashley et al reported denuded collagen in demineralized dentin created by brief phosphate etching can be degraded by endogenous MMPs. It is possible these MMPs were released during our demineralizing treatment (Pashley et al., 2004), then degraded some of the collagen or NCPs necessary for the intrafibrillar remineralization. It is also possible that NCPs diffused away during our treatment in buffered solutions, since soaking the EDTA treated demineralized dentin in NaCl solution can remove the residual NCPs (Chen et al., 2015). In our previous study it was shown that acetate treatment left significant mineral nanoparticles at the outer zone (Chien et al., 2016), which one might assume would promote nucleation of more mineral; but instead, remineralized lactate lesions yielded more mineral in TEM (Fig.3), thus suggesting that the infiltration mechanism depends more on unobstructed transport pathways than on classical crystallization mechanisms.
5. Conclusions
The PILP process provided significant recovery of both the structure and mechanical properties of artificial lesions produced using acetate or lactate buffers of the same approximate depth. These cariogenic acids produced lesion structure and properties which varied slightly in the initial lesions, and these differences were reflected in minor differences in the degree of recovery. Both lesion types had two zones: a severely demineralized outer zone (i.e., partial demineralization) that was incompletely recovered in mechanical properties and an inner sloped zone that completely recovered their mechanical properties. At the outer flat zone, incomplete recovery occurred, possibly due to degradation of the collagen matrix, or because PILP could no longer deliver mineral into intrafibrillar space due to solidification kinetics of the phase. This mineral precipitation could block the further delivery of mineral into the inner sloped zone. Overall, the results suggest that PILP may be successful in remineralizing dentin matrices that have been demineralized with two of the main acids that are produced by cariogenic bacteria, and therefore may provide a feasible approach for remineralization of complex natural dentin lesions that are subject to demineralization by a variety of cariogenic bacteria. Current work is focused on methods to optimize the recovery of the demineralized dentin matrix by using a combination of additives.
Highlights.
Acetate and lactate made different residual dentin structures in artificial lesions.
PILP process recovered both structure and mechanical properties for both lesions.
Ethics statement.
The study was approved by UCSF CHR (UCSF Committee on Human Research). Teeth were collected in accordance with internal review board policy, was not identifiable with personal data. The authors have no conflicts of interest associated with any materials used in this study.
Acknowledgments
This research publication was supported by the National Institute of Dental and Craniofacial Research of National Institutes of Health under grant number R01 DE016849.
Abbreviations
- PASP
poly-aspartic acid
- PILP
polymer-induced liquid-precursor
- DEJ
dentin enamel junction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. Mechanical properties of mineralized collagen fibrils as influenced by demineralization. J Struct Biol. 2008;162(3):404–410. doi: 10.1016/j.jsb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balooch M, Wu-Magidi IC, Balazs A, Lundkvist AS, Marshall SJ, Marshall GW, Kinney JH. Viscoelastic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J Biomed Mater Res. 1998;40(4):539–544. doi: 10.1002/(sici)1097-4636(19980615)40:4<539::aid-jbm4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW., Jr Biomechanical perspective on the remineralization of dentin. Caries Res. 2009;43(1):70–77. doi: 10.1159/000201593. Epub 000202009 Feb 000201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer DS, Saeki K, Hilton JF, Marshall GW, Marshall SJ. Effect of sterilization by gamma radiation on nano-mechanical properties of teeth. Dent Mater. 2008;24(8):1137–1140. doi: 10.1016/j.dental.2008.02.016. Epub 2008 Apr 1123. [DOI] [PubMed] [Google Scholar]
- Burwell AK, Thula-Mata T, Gower LB, Habelitz S, Kurylo M, Ho SP, Marshall GW. Functional remineralization of dentin lesions using polymer-induced liquid-precursor process. PLoS One. 2012;7(6):e38852. doi: 10.1371/journal.pone.0038852. doi: 38810.31371/journal.pone.0038852. Epub 0032012 Jun 0038813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Cao S, Wang H, Li Y, Kishen A, Deng X, Zhang X. Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLoS One. 2015;10(1):e0116553. doi: 10.1371/journal.pone.0116553. doi: 0116510.0111371/journal.pone.0116553. eCollection 0112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YC, Burwell AK, Saeki K, Fernandez-Martinez A, Pugach MK, Nonomura G, Marshall GW. Distinct decalcification process of dentin by different cariogenic organic acids: Kinetics, ultrastructure and mechanical properties. Arch Oral Biol. 2016;63:93–105. doi: 10.1016/j.archoralbio.2015.1010.1001. Epub 2015 Oct 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler W, Kroncke A. Formic acid in human single-site resting plaque–quantitative and qualitative aspects. Caries Res. 1986;20(1):1–6. doi: 10.1159/000260913. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;3:711–735. doi: 10.2741/e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower LB, Odom D. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (pilp) process. J Crystal Growth. 2000;210:719–734. [Google Scholar]
- Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 2008;108(11):4551–4627. doi: 10.1021/cr800443h. doi: 4510.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habelitz S, Rodriguez BJ, Marshall SJ, Marshall GW, Kalinin SV, Gruverman A. Peritubular dentin lacks piezoelectricity. J Dent Res. 2007;86(9):908–911. doi: 10.1177/154405910708600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee SS, Culver L, Li Y, Douglas EP, Gower LB. Biomimetic mineralization of collagen via an enzyme-aided PILP process. Journal of Crystal Growth. 2010;312:1249–1256. [Google Scholar]
- Jee SS, Thula TT, Gower LB. Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process. Part 1: influence of polymer molecular weight. Acta Biomater. 2010;6(9):3676–3686. doi: 10.1016/j.actbio.2010.03.036. doi: 3610.1016/j.actbio.2010.3603.3036. Epub 2010 Mar 3630. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res. 2003;82(12):957–961. doi: 10.1177/154405910308201204. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Pople JA, Driessen CH, Breunig TM, Marshall GW, Marshall SJ. Intrafibrillar mineral may be absent in dentinogenesis imperfecta type II (DI-II) J Dent Res. 2001;80(6):1555–1559. doi: 10.1177/00220345010800061501. [DOI] [PubMed] [Google Scholar]
- Li Y, Thula TT, Jee S, Perkins SL, Aparicio C, Douglas EP, Gower LB. Biomimetic mineralization of woven bone-like nanocomposites: role of collagen cross-links. Biomacromolecules. 2012;13(1):49–59. doi: 10.1021/bm201070g. Epub 202011 Dec 201071. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25(6):441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Wu-Magidi IC, Watanabe LG, Inai N, Balooch M, Kinney JH, Marshall SJ. Effect of citric acid concentration on dentin demineralization, dehydration, and rehydration: atomic force microscopy study. J Biomed Mater Res. 1998;42(4):500–507. doi: 10.1002/(sici)1097-4636(19981215)42:4<500::aid-jbm4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- McIntyre JM, Featherstone JD, Fu J. Studies of dental root surface caries. 1: Comparison of natural and artificial root caries lesions. Aust Dent J. 2000;45(1):24–30. doi: 10.1111/j.1834-7819.2000.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1583. [Google Scholar]
- Olszta MJ, Cheng X, Jee S, Kumar R, Kim Y, Kaufman M, Gower LB. Bone structure and formation: A new perspective. Materials Science and Engineering: R: Reports. 2007;58:77–116. [Google Scholar]
- Olszta MJ, Douglas EP, Gower LB. Scanning electron microscopic analysis of the mineralization of type I collagen via a polymer-induced liquid-precursor (PILP) process. Calcif Tissue Int. 2003;72(5):583–591. doi: 10.1007/s00223-002-1032-7. Epub 2003 Mar 2006. [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83(3):216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- Pugach MK, Strother J, Darling CL, Fried D, Gansky SA, Marshall SJ, Marshall GW. Dentin caries zones: mineral, structure, and properties. J Dent Res. 2009;88(1):71–76. doi: 10.1177/0022034508327552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YP, Li N, Niu LN, Primus CM, Ling JQ, Pashley DH, Tay FR. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater. 2012;8(2):836–842. doi: 10.1016/j.actbio.2011.10.033. doi: 810.1016/j.actbio.2011.1010.1033. Epub 2011 Oct 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K, Marshall SJ, Gansky SA, Marshall GW. Etching characteristics of dentin: effect of ferric chloride in citric acid. J Oral Rehabil. 2001;28(4):301–308. doi: 10.1046/j.1365-2842.2001.00715.x. [DOI] [PubMed] [Google Scholar]
- Sauro S, Toledano M, Aguilera FS, Mannocci F, Pashley DH, Tay FR, Osorio R. Resin-dentin bonds to EDTA-treated vs. acid-etched dentin using ethanol wet-bonding. Dent Mater. 2010;26(4):368–379. doi: 10.1016/j.dental.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serper A, Calt S. The demineralizing effects of EDTA at different concentrations and pH. J Endod. 2002;28(7):501–502. doi: 10.1097/00004770-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29(8):1127–1137. doi: 10.1016/j.biomaterials.2007.11.001. Epub 2007 Nov 1119. [DOI] [PubMed] [Google Scholar]
- Thula TT, Rodriguez DE, Lee MH, Pendi L, Podschun J, Gower LB. In vitro mineralization of dense collagen substrates: a biomimetic approach toward the development of bone-graft materials. Acta Biomater. 2011;7(8):3158–3169. doi: 10.1016/j.actbio.2011.04.014. doi: 3110.1016/j.actbio.2011.3104.3014. Epub 2011 Apr 3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thula TT, Svedlund F, Rodriguez DE, Podschun J, Pendi L, Gower LB. Mimicking the nanostructure of bone: Comparison of polymeric process-directing agents. Polymers (Basel) 2011;3(1):10–35. doi: 10.3390/polym3010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strijp AJ, Klont B, Ten Cate JM. Solubilization of dentin matrix collagen in situ. J Dent Res. 1992;71(8):1498–1502. doi: 10.1177/00220345920710080701. [DOI] [PubMed] [Google Scholar]
- Veis A. Mineral-matrix interactions in bone and dentin. J Bone Miner Res. 1993;8(Suppl 2):S493–497. doi: 10.1002/jbmr.5650081312. [DOI] [PubMed] [Google Scholar]
- White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma radiation. J Dent Res. 1994;73(9):1560–1567. doi: 10.1177/00220345940730091201. [DOI] [PubMed] [Google Scholar]
- Zheng L, Hilton JF, Habelitz S, Marshall SJ, Marshall GW. Dentin caries activity status related to hardness and elasticity. Eur J Oral Sci. 2003;111(3):243–252. doi: 10.1034/j.1600-0722.2003.00038.x. [DOI] [PubMed] [Google Scholar]


