INTRODUCTION
Diarrhea is a leading cause of mortality in children, accounting for 9% of all deaths among children <5 years of age worldwide or 526,000 children in 2015 [1]. The majority of these deaths occur in Sub-Saharan Africa and Asia [1]. Globally, rotavirus is the most common cause of severe diarrhea among children <5 years of age, accounting for approximately 40% of severe diarrhea episodes in countries that have not yet introduced rotavirus vaccine [2]. In view of the high burden of rotavirus diarrhea mortality rates in developing countries and reassuring rotavirus vaccine efficacy and impact data from early introducing countries, the World Health Organization (WHO) recommends the inclusion of rotavirus vaccines in all national immunization programs [3].
In Zimbabwe, diarrhea is the second leading cause of childhood deaths, contributing to 10% to 15% of deaths in children <5 years of age in 2015 [1, 4]; rotavirus accounted for 41–56% of acute diarrhea hospitalizations prior to vaccine introduction [5]. In May 2014, the Government of Zimbabwe introduced the 2-dose, oral, monovalent, live attenuated human rotavirus vaccine, Rotarix (GlaxoSmithKline), into its Expanded Programme on Immunization (EPI). By the end of 2016, the reported administrative coverage of a full course of rotavirus vaccine had reached ~89% nationwide [6].
Although many countries have demonstrated substantial declines in all-cause diarrhea and rotavirus-related morbidity and mortality following rotavirus vaccine introduction, evaluations in low income African countries with high child mortality rates similar to Zimbabwe are limited. We evaluated the impact of rotavirus vaccine introduction on acute diarrhea and rotavirus-related healthcare visits among children <60 months of age to provide essential information to the government and other stakeholders regarding the benefits of rotavirus vaccination.
METHODS
Active surveillance of acute diarrhea and rotavirus-related hospitalizations and Accident and Emergency (A&E) Department visits
In anticipation of national rotavirus vaccine introduction, the Ministry of Health and Child Care (MOHCC), with support from WHO, established active rotavirus surveillance at three public hospitals located in and just outside of Harare: Chitungwiza Central Hospital (CCH), which has 94 beds allocated to pediatrics; Harare Central Hospital (HCH), which has 246 beds allocated to pediatrics; and Parirenyatwa Group Hospital (PGH), which has 175 beds allocated to pediatrics. These 3 institutions together serve a population of ~2.1 million urban and rural residents. For this evaluation, the three hospitals contributed active surveillance data from 1 January 2012 through 31 December 2016. Children <60 months of age who presented for treatment of acute diarrhea (defined as ≥3 episodes of non-bloody diarrhea within a 24 hour period of ≤7 days duration) were recruited for enrollment. After informed consent was obtained from the parent or caregiver of an eligible child, trained hospital staff collected demographic and clinical data using a case investigation form adapted from WHO rotavirus surveillance guidelines [7]. A stool specimen was also collected from each enrolled child within 48 hours of admission to the inpatient ward or the A&E Department oral rehydration corner. All stool specimens were transferred to the Zimbabwe National Virology Laboratory and tested for group A rotavirus by enzyme immunosorbent assay (EIA) (ProSpecT, Oxoid, UK). A subset of EIA test-positive specimens were then transported to the Medical Research Council (MRC) Diarrhoeal Pathogens Research Unit, University of Sefako Makgatho Health Sciences, Pretoria, South Africa, for quality control and genotyping.
Surveillance activities were enhanced in June 2014 for rotavirus vaccination impact evaluation activities. Enhanced activities included a more detailed case investigation form that retained the original surveillance questions but included more extensive clinical and vaccination history questions, hiring and training of additional surveillance team staff, and transfer of all EIA test-positive specimens to the MRC for genotyping.
Hospital register review
Since active surveillance activities at CCH, HCH, and PGH likely did not enroll 100% of children <60 months of age presenting for treatment of acute diarrhea, we retrospectively reviewed inpatient hospital registers at each hospital for 1 January 2012 through 31 December 2016. Monthly counts of acute diarrhea admissions were extracted from the hospital registers using any of the following terms that indicated acute diarrhea (including spelling permutations of the terms): “acute diarrhoea”, “diarrhoea”, “acute gastroenteritis”, “AG”, “AGE”, “acute GE”, “acute GE with dehydration”, “gastroenteritis”, “GE”, and “rotavirus”.
Passive surveillance of diarrhea-related outpatient visits
The Health Information and Surveillance Unit of the Department of Epidemiology and Disease Control (EDC) of the MOHCC coordinates and receives weekly reports of 17 conditions of public health importance which includes tallies of outpatient visits for acute watery diarrhea, with or without dehydration, among children <60 months of age from 1631 healthcare facilities, including and 7 provincial, 74 city health, and 6 central hospitals nationwide. The EDC provided data by district or hospital unit from 1 January 2012 through 31 December 2016 for this evaluation.
Data analyses
Using active surveillance data, we examined the monthly number and proportion of acute diarrhea and rotavirus EIA-positive hospitalizations and A&E visits among children <60 months of age at the three surveillance hospitals. We also examined the annual number and proportion of acute diarrhea and rotavirus EIA-positive hospitalizations and A&E visits by visit type (hospitalization, A&E visit) and age group (0–11 months, 12–23 months, and 24–59 months) and compared pre-vaccine introduction (2012–2013) data with post-vaccine introduction (2015–2016) data using Chi-square tests; we considered p-values of <0.05 to be significant. For the pre-/post-introduction analyses, 2014 was excluded as it was considered a transition year during which rotavirus vaccine was introduced, and PGH was excluded due to incomplete monthly reporting in 2012 and 2013.
Using extracted hospital register data from the three sentinel hospitals, we examined monthly trends in acute diarrhea hospitalizations and A&E visits by year and age group (0–11 months, 12–23 months, and 24–59 months). For this analysis, we excluded the year 2012 as some of the hospital registers were not available during that period.
National outpatient visit data were used to examine the number of outpatient visits for acute diarrhea with and without dehydration each month for children <60 months of age. Because the number of reporting districts and hospital units changed over time, we restricted this analysis to 61 districts and hospital units that reported data every month during the study period.
Data were analyzed using SAS version 9.3 and Excel version 2013. This evaluation was reviewed and approved by the Medicines Control Authority of Zimbabwe and determined to be public health non-research by the US Centers for Disease Control and Prevention and granted exception by WHO Ethical Review committee (ERC).
RESULTS
Monthly trends in acute diarrhea and rotavirus-related healthcare visits
Monthly active surveillance data from the three sentinel hospitals demonstrated annual seasonal peaks of hospitalizations and A&E visits (Figure 1). During 2012–2014, the number of visits peaked during the winter months in May and June; following rotavirus vaccine introduction, seasonal peaks in 2015 and 2016 shifted toward September and October and were substantially blunted. The percentage of rotavirus EIA-positive visits followed the same seasonal pattern. However, while the overall number of hospitalizations varied from year to year, the percentage of rotavirus EIA-positive specimens decreased during the post-vaccine introduction period as compared to the pre-introduction period.
Figure 1.

Acute diarrhea hospitalizations and accident & emergency (A&E) visits among children <60 months of age enrolled in active surveillance, by rotavirus test result — 3 sentinel surveillance hospitals, Zimbabwe, 1 January 2012—31 December 2016
Diarrhea hospitalizations recorded in registers from the three sentinel hospitals showed seasonal variation in the number of diarrhea hospitalizations, by month, in children 0–11 months and 12–23 months of age, with the highest numbers in May and June during 2013 and 2014 (Supplemental Digital Content 1, Panels A and B). In the post-vaccine introduction period, these higher numbers during May and June were not observed – the number of hospitalizations by month was relatively consistent in these two age groups, with a slight increase in October. There was no pattern of monthly variation observed in children 24–59 months of age during any time period (Supplemental Digital Content 1, Panel C).
Monthly variation in outpatient diarrhea-related visits from facilities nationwide among children <60 months of age mirrored that observed in the active surveillance data (Supplemental Digital Content 2). The highest number of visits was observed in May and June each year during 2012–2014, with a less pronounced increase in September and October. After rotavirus vaccine introduction, the highest number of diarrhea-related outpatient visits appeared in September.
Annual trends in acute diarrhea and rotavirus-related hospitalizations and A&E visits
Annual data on acute diarrhea and rotavirus-related hospitalizations and A&E visits among children <60 months of age at CCH and HCH showed increases in the total number of both visit types during 2015 and 2016, but an overall decline during 2015–2016 in the percentage of rotavirus test-positive visits (Supplemental Digital Content 3). The percentage of rotavirus EIA-positive hospitalizations declined from a median of 41% in 2012–2013 to 23% and 20% in 2015 and 2016, respectively. The percentage of rotavirus EIA-positive A&E visits declined from a median of 38% in 2012–2013 to 28% and 26% in 2015 and 2016, respectively.
Data by age group also showed increases in the absolute numbers of children enrolled in active surveillance and declines in the percentage of rotavirus positive visits during 2015–2016 at CCH and HCH (Figure 2). Overall for children <60 months of age, the percentage of rotavirus EIA-positive visits significantly declined from a pre-vaccine median of 41% to 26% in 2015 (P<.0001) and 24% in 2016 (P<.0001), a decline of 35% and 41%, respectively (Table). There was a noticeable decline in the percentage of rotavirus EIA-positive visits from children 0–11 months and 12–23 months of age; the decline was less among the 24–59 month age group. Among children 0–11 months of age, the percentage of rotavirus EIA-positive visits declined from a pre-vaccine median of 49% to 30% in 2015 (P<.0001) and 28% in 2016 (P<.0001), a decline of 40% and 43%, respectively (Table). Among children 12–23 months of age, the percentage of rotavirus EIA-positive visits declined from a pre-vaccine median of 37% to 29% in 2015 (P=.04) and 25% in 2016 (P=.0001), a decline of 21% and 33%, respectively. Among children 24–59 months of age, the percentage of rotavirus EIA-positive visits declined from a pre-vaccine median of 16% to 13% in 2015 (P=.19) and 15% in 2016 (P=.52), a decline of 22% and 5%, respectively.
Figure 2.
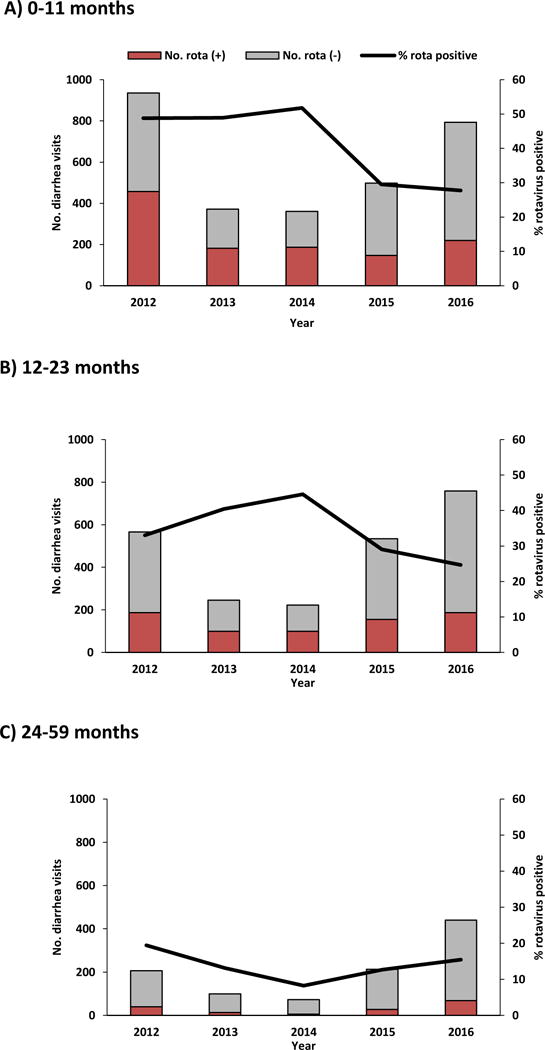
Annual acute diarrhea hospitalizations and A&E visits among children <60 months of age enrolled in active surveillance, by age group and rotavirus test result and proportion rotavirus test positive — Chitungwiza and Harare Central Hospitals, Zimbabwe, 1 January 2012—31 December 2016
Table.
Acute diarrhea, rotavirus hospitalizations and A&E cases enrolled in active surveillance before (2012–2013) and after (2015 and 2016) rotavirus vaccine introduction — Chitungwiza and Harare Central Hospitals, Zimbabwe, 1 January 2012—31 December 2016
| Age/time period | No. visits tested | No. rotavirus positive | % rotavirus positive | % decline in proportion rotavirus positive |
|---|---|---|---|---|
| All <60 months | ||||
| Median 2012–2013 | 1212 | 489 | 41 | ref |
| 2015 | 1245 | 329 | 26 | 35 (P<.0001) |
| 2016 | 1992 | 475 | 24 | 41 (P<.0001) |
| 0–11 months | ||||
| Median 2012–2013 | 654 | 320 | 49 | ref |
| 2015 | 498 | 147 | 30 | 40 (P<.0001) |
| 2016 | 793 | 220 | 28 | 43 (P<.0001) |
| 12–23 months | ||||
| Median 2012–2013 | 406 | 143 | 37 | ref |
| 2015 | 534 | 155 | 29 | 21 (P=.04) |
| 2016 | 759 | 187 | 25 | 33 (P=.0001) |
| 24–59 months | ||||
| Median 2012–2013 | 153 | 27 | 16 | ref |
| 2015 | 213 | 27 | 13 | 22 (P=.19) |
| 2016 | 440 | 68 | 15 | 5 (P=.52) |
DISCUSSION
We observed declines in acute all-cause diarrhea and rotavirus-related healthcare visits in Zimbabwe during the two years following national rotavirus vaccine introduction in May 2014. Active, prospective surveillance found the percentage of rotavirus-positive specimens among children <60 months of age was reduced by more than one-third in the two years following rotavirus vaccine introduction. These declines were particularly sharp in children 0–11 months and 12–23 months of age who were age-eligible for vaccination. These results are similar to those reported by other countries, including recent data from other African countries [8–13].
Our findings were consistent across data sources of different acuity levels and geographic scope in Zimbabwe. Prospective, active surveillance from three sentinel hospitals, a retrospective log book review of hospitalizations from the three sentinel hospitals, and national-level, diarrhea-related outpatient visits all showed annual peaks of diarrhea-related healthcare visits in May and June before vaccine introduction. Following rotavirus vaccine introduction, there were substantially lower levels of rotavirus EIA positivity and diarrhea-related healthcare visits. We also observed a shift in the peak season from the winter months of May and June to September and October. This shift in rotavirus peak season following vaccine introduction has been observed in some other countries including the US and Mexico, and may be due to a decrease in the number of unvaccinated, susceptible infants, resulting in reduced intensity of transmission of rotavirus infection among susceptible children [14, 15].
This evaluation has several limitations. First, active surveillance enrollment varied over the surveillance period, with much higher numbers of children enrolled after rotavirus vaccine introduction when surveillance had been enhanced at the sentinel facilities. Despite increased enrollment, declines in the percentage of rotavirus-positive visits were observed among all age groups during the post-introduction period. Additionally, when comparing the number of children enrolled in active surveillance with the number of children recorded in hospital registers during 2013–2016, the annual proportion of children enrolled in active surveillance ranged from 19% to 42%, with the highest proportions enrolled in 2015 and 2016 after enhanced surveillance was established. As stated in the methods section, it was anticipated that surveillance staff would not be able to enroll all eligible children given the high volume of patients seen at the sentinel hospitals, which is why the hospital register review was conducted. Despite not capturing all eligible children, the trends in diarrhea visits among children enrolled in active surveillance were very similar to those seen in both the passively reported national outpatient diarrhea data and the hospital register data. Thus, the changes in diarrhea and rotavirus disease seen among the children enrolled in active surveillance are likely similar to what is occurring on a national level. Second, visit type (i.e., hospitalization or A&E visit) may have been misclassified for children enrolled in active surveillance at HCH as some children admitted to the oral rehydration area may have been classified as receiving inpatient treatment even though they were discharged home. Conversely, hospitalizations may also have been misclassified as A&E visits, especially if children were transferred to the inpatient ward following a stay in the oral rehydration area. Nevertheless, we are reassured by the overall declines in rotavirus EIA positivity observed for both hospitalizations and A&E visits. Finally, we did not evaluate concurrent interventions to improve water, sanitation, and hygiene practices and their potential impact on diarrhea-related healthcare visits. However, no new national-level interventions were implemented during the post-introduction period. According to the National Action Committee on Water, Sanitation and Hygiene (WASH), there has been little change in WASH during this period [16, 17]. Given that substantial declines in diarrhea-related hospitalizations occurred specifically during the winter season when rotavirus predominates and since rotavirus infection occurs even with improvements in water, sanitation, and hygiene practices, it is likely that vaccine introduction is primarily responsible for the declines observed in this evaluation.
Initial reductions in diarrhea illness among children <60 months of age in Zimbabwe following rotavirus vaccine introduction are promising both in greater Harare and nationally. Declines were especially pronounced in children 0–11 months of age, and this swift impact on morbidity is likely connected to rapid uptake and high coverage of the target population with rotavirus vaccine Given that to date, there are only two full years of post-introduction data, rotavirus diarrhea surveillance should continue to confirm the positive impact of the rotavirus vaccination program. These early results are encouraging and provide evidence to support continued rotavirus vaccination and surveillance to inform immunization program planning in Zimbabwe. Additionally, these findings highlight the importance and value of establishing active disease surveillance programs for new vaccine introduction impact evaluations.
Supplementary Material
Supplemental Digital Content 1. Figure of monthly acute diarrhea hospitalizations among children <60 months of age, by year and age group — hospital register data from 3 sentinel surveillance hospitals, Zimbabwe, 1 January 2013—31 December 2016
Supplemental Digital Content 2. Figure of acute diarrhea outpatient visits among children <60 months of age as reported to the Ministry of Health and Child Care — 61 consistently reporting districts/organizational units in Zimbabwe, 1 January 2012—31 December 2016
Supplemental Digital Content 3. Figure if annual acute diarrhea A) hospitalizations and B) accident & emergency (A&E) visits among children <60 months of age enrolled in active surveillance, by rotavirus test result and proportion rotavirus test positive — Chitungwiza and Harare Central Hospitals, Zimbabwe, 1 January 2012—31 December 2016
Acknowledgments
Financial support: This work was supported by the United States Agency for International Development and the World Health Organization.
This work was support by the United States Agency for International Development (financial support for the impact evaluation and enhancement of rotavirus surveillance) and by the World Health Organization (financial support for routine rotavirus surveillance). We would like to thank Jane Murenga, Collin Madzima, Memory Mucheka, Trymore Mashamba, Audrey G. Shava, Rodrick Marozva, and Cashington Siameja for their assistance with hospital surveillance activities and hospital register reviews; Dr. Pasipanodya Nziramasanga, Head of the National Virology Laboratory, for providing vehicle access for transportation of samples from sites and other rotavirus surveillance-related activities and for signing off on all completed rotavirus tests; Paradzai Chibukira for timely transportation of samples, case investigation and outcome forms, and delivery of supplies to sites; the Secretary for Health and Child Care Brigadier General Gwinji for granting access to data; Collene Chigodo and Priscilla Chirisa from the MOHCC for their support and assistance; Tendai Mandebvu from the WHO Country Office for his program assistance; and Christine Jonesteller from CDC for her administrative support.
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
Conflicts of interest: The authors indicate that they do not have a commercial or other association that might pose a conflict of interest.
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official positions of the Ministry of Health and Child Care of Zimbabwe, the US Centers for Disease Control and Prevention, and the World Health Organization.
References
- 1.UNICEF. One is too many: Ending child deaths from pneumonia and diarrhoea. 2016 [Google Scholar]
- 2.World Health Organization. Rotavirus Vaccines WHO Position Paper. Weekly Epidemiological Record. 2013;88(5):49–64. [Google Scholar]
- 3.World Health Organization. Rotavirus vaccines: an update. Weekly Epidemiological Record. 2009;84(51):533–40. [Google Scholar]
- 4.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance N Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi: 10.1093/cid/civ1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukaratirwa A, Berejena C, Nziramasanga P, et al. Epidemiologic and genotypic characteristics of rotavirus strains detected in children less than 5 years of age with gastroenteritis treated at 3 pediatric hospitals in Zimbabwe during 2008–2011. Pediatr Infect Dis J. 2014;33(Suppl 1):S45–8. doi: 10.1097/INF.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health and Child Care of Zimbabwe. Health Management Information System database. Unpublished data. [Google Scholar]
- 7.World Health Organization. Generic protocols for (i) hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children: field test version. 2002 [Google Scholar]
- 8.Bar-Zeev N, Jere KC, Bennett A, et al. Population Impact and Effectiveness of Monovalent Rotavirus Vaccination in Urban Malawian Children 3 Years After Vaccine Introduction: Ecological and Case-Control Analyses. Clin Infect Dis. 2016;62(Suppl 2):S213–9. doi: 10.1093/cid/civ1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groome MJ, Zell ER, Solomon F, et al. Temporal Association of Rotavirus Vaccine Introduction and Reduction in All-Cause Childhood Diarrheal Hospitalizations in South Africa. Clin Infect Dis. 2016;62(Suppl 2):S188–95. doi: 10.1093/cid/civ1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mpabalwani EM, Simwaka CJ, Mwenda JM, et al. Impact of Rotavirus Vaccination on Diarrheal Hospitalizations in Children Aged <5 Years in Lusaka, Zambia. Clin Infect Dis. 2016;62(Suppl 2):S183–7. doi: 10.1093/cid/civ1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abeid KA, Jani B, Cortese MM, et al. Monovalent Rotavirus Vaccine Effectiveness and Impact on Rotavirus Hospitalizations in Zanzibar, Tanzania: Data From the First 3 Years After Introduction. J Infect Dis. 2016 doi: 10.1093/infdis/jiw524. [DOI] [PubMed] [Google Scholar]
- 12.Safadi MA, Berezin EN, Munford V, et al. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in Sao Paulo, Brazil. Pediatr Infect Dis J. 2010;29(11):1019–22. doi: 10.1097/INF.0b013e3181e7886a. [DOI] [PubMed] [Google Scholar]
- 13.Bayard V, DeAntonio R, Contreras R, et al. Impact of rotavirus vaccination on childhood gastroenteritis-related mortality and hospital discharges in Panama. Int J Infect Dis. 2012;16(2):e94–8. doi: 10.1016/j.ijid.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Aliabadi N, Tate JE, Haynes AK, Parashar UD, Centers for Disease C, Prevention Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64(13):337–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Esparza-Aguilar M, Gastanaduy PA, Sanchez-Uribe E, et al. Diarrhoea-related hospitalizations in children before and after implementation of monovalent rotavirus vaccination in Mexico. Bull World Health Organ. 2014;92(2):117–25. doi: 10.2471/BLT.13.125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimbabwe National Statistics Agency and ICF International. Zimbabwe Demographic and Health Survey 2015: Final Report. Rockville, Maryland, USA: Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International; 2016. [Google Scholar]
- 17.Manangazira P (co-author) personal communication. 2017 Feb 27; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figure of monthly acute diarrhea hospitalizations among children <60 months of age, by year and age group — hospital register data from 3 sentinel surveillance hospitals, Zimbabwe, 1 January 2013—31 December 2016
Supplemental Digital Content 2. Figure of acute diarrhea outpatient visits among children <60 months of age as reported to the Ministry of Health and Child Care — 61 consistently reporting districts/organizational units in Zimbabwe, 1 January 2012—31 December 2016
Supplemental Digital Content 3. Figure if annual acute diarrhea A) hospitalizations and B) accident & emergency (A&E) visits among children <60 months of age enrolled in active surveillance, by rotavirus test result and proportion rotavirus test positive — Chitungwiza and Harare Central Hospitals, Zimbabwe, 1 January 2012—31 December 2016


